Abstract
Early mild stress (EMS) is like preparedness and might help fish deal with stress appropriately. This study investigated how EMS and photoperiod changes can impact growth, haematology, blood biochemistry, immunological response, antioxidant system, liver enzymes, and stress response of oscar (Astronotus ocellatus; 7.29 ± 0.96 g) before and after acute confinement stress (AC stress). Ten experimental treatments included five different photoperiods 8L16D (08:16 light to dark), 12L12D (12:12 light to dark), 16L8D (16:08 light to dark), 20L4D (20:04 light to dark), and 24L0D (24:00 light to dark), and these five photoperiod schedules were conducted in an EMS condition. After 9 weeks, no significant differences were found in growth parameters, survival rate, and body composition. At the end of the experiment and after AC stress, fish farmed in 24 light hours had the lowest haematocrit, white blood cells, total protein, blood performance, lysozyme, immunoglobulin M, complement C3, superoxide dismutase, and catalase. Fish that experienced EMS had significantly higher survival rates than those farmed in normal conditions (80.67% vs 61.33%). In conclusion, considering all measured parameters, 8-h light can be suggested as an optimum photoperiod for this fish species. Under 24L0D (no EMS) conditions, there were many negative effects apparent. In addition, a positive effect of EMS was evident in terms of survival after AC stress. AC stress decreased some health parameters under 24-h light treatment, while these results were not observed in EMS-exposed fish. Therefore, the EMS schedule can be a useful tool in preventing the negative effects of stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Photoperiod has been known since the past century as an external factor that affects the growth, reproduction, and health of fish (Harrington Jr 1956). Therefore, manipulation of photoperiod has been a common practice in modern farming systems, like indoor systems and aquariums. Aquatic animal species responded to a change of photoperiod differently. Photoperiod manipulation has been successfully applied to improve the growth of several finfish species. For example, red sea bream (Pagrus major) (Biswas et al. 2006a, b), rainbow trout (Oncorhynchus mykiss) (Valenzuela et al. 2006), tilapia (Oreochromis niloticus) (Biswas et al. 2004), gibel carp (Carassius auratus) (Wei et al. 2019), coho salmon (Oncorhynchus kisutch) (Fang et al. 2019), zebrafish (Danio rerio) (Abdollahpour et al. 2020), and beluga (Huso huso) (Bani et al. 2009) grew well under 24-h continuous light. However, other fish species experienced stress, aggressiveness, and reduced growth with manipulation of photoperiod, including but not limited to African catfish (Clarias gariepinus) (Almazán-Rueda et al. 2005) with increased light, blunt snout bream (Megalobrama amblycephala) (Tian et al. 2019), and golden mahseer (Tor putitora) (Akhtar et al. 2020) with 8L:16D. The effect of photoperiod in parameters such as growth (Tian et al. 2019; Abdollahpour et al. 2020; Akhtar et al. 2020), metabolic rate (Hvas 2022), haematological parameters, liver and antioxidant enzymes (Biswas et al. 2004; Valenzuela et al. 2006; Kitagawa et al. 2015; Malinovskyi et al. 2022), maturation (Hansen et al. 1992), and immune system (Ángeles Esteban et al. 2006) has been widely investigated.
The market size of ornamental fish species is trending upward, and this sector continues to play a central role in the aquaculture industry. This market is predicted to expand at a compound annual growth rate of 8.5% from 2022 to 2030 and to reach more than USD 11 billion by 2030 (Ghosi Mobaraki et al. 2020). However, the increasing production trend requires more stock from hatcheries and improvements to productivity. Oscars are popular ornamental species of freshwater aquariums known for their large size and sociable personalities. Their intensive production, which is common in ornamental aquaculture, can potentially result in stress (Martos-Sitcha et al. 2020).
Stress causes cellular instability and imbalance in homeostasis (Adam et al. 2011) and any factor (both internal and external) that disturbs homeostasis can be named stress. Photoperiod can affect the stress response of fish in different ways. Melatonin is responsible for night and day patterns, and as a result, changes in this hormone drive many physiological alterations and have key roles in the redistribution of energy to maximise fitness and survival (Walton et al. 2011). The molecular and biological functions and related pathways to melatonin metabolism were reviewed elsewhere (Reiter 2003). Melatonin has direct interactions with the immune and antioxidant systems, and therefore, photoperiods, which have a direct impact on melatonin, can directly affect the growth, immune, and antioxidant systems of fish.
Early mild stress can occur positively in early-life stages, which means that mild chronic stress in early life can potentially shape positive phenotypes later in life. EMS is defined as a situation whereby animals do not die or suffer significantly from chronic stress, and they have a short-term response. In aquatic species, studies related to EMS, which can be categorised as eustress, are limited to our previous work and other investigations that lower hypothalamus catecholaminergic; brainstem serotonergic responses to stress and cortisol responsiveness were reported (Auperin and Geslin 2008; Madaro et al. 2015; Pederzoli & Mola 2016; Vindas et al. 2016). Further, higher brain catecholaminergic signalling and neuronal activity were reported in fish (Vindas et al. 2018). In our works, the interaction of EMS with fish meal replacement (Zare et al. 2023a), temperature (Esmaeili et al. 2024), and different levels of lipid (Esmaeili et al. 2022) and protein (Zare et al. 2024) was tested. The relationship between the onset of EMS and final acute confinement stress (AC stress) was also investigated (Zare et al. 2023b). The highest survival rate after final acute stress in oscar (Astronotus ocellatus) exposed to 2 weeks of EMS out of 10 weeks of the trial was observed (Esmaeili et al. 2022; Zare et al. 2023a, b). These results indicated that 2 weeks of EMS had the highest survival rate in oscar regardless of lipid or protein contents in diets. To the best of our knowledge, no study has investigated the effect of photoperiod on the physiology of oscar species. Further, no research tested how EMS can interact with photoperiod in animals and affect survival rate after an acute stress event. Therefore, in follow-up research with the same experimental conditions, 2 out of 10 weeks of scheduled EMS stress and five photoperiods were considered to see their effects on growth performance, haematology, blood biochemistry, immune response, antioxidant activities, stress response, and liver enzymes of juvenile oscar. In addition, a final AC stress event was carried out for the assessment of fish survival rate and biomarkers.
Materials and methods
Animal ethics, fish, and experimental conditions
A National Animal Care and Committee approved all experimental protocols (281–1385) (Safavi et al. 2019; Tazikeh et al. 2020; Ahmadi-Noorbakhsh et al. 2021). For this experiment, 621 oscars (initial weight, 7.29 ± 0.96 g) were obtained from the Abzian Center (Mahallat, Markazi, Iran). The acclimatisation steps to the trial condition were for 2 weeks and fish was fed with a commercial diet (500 and 150 g/kg crude protein and lipid, respectively). Twenty-two fish were randomly distributed into 30 rectangular glass tanks (100 L), with three replicate aquaria per experimental treatment. The fish were provided with their respective diets three times per day (09:00 h, 14:00 h, and 19:00 h) for 10 weeks to apparent satiety. For the 8L16D group, we fed them twice per day (09:00 h, 14:00 h) to apparent satiety. The ten experimental treatments were 8L16D (08:16 light to dark), 12L12D (12:12 light to dark), 16L8D (16:08 light to dark), 20L4D (20:04 light to dark), and 24L0D (24:00 light to dark), and fish in these five photoperiods were farmed with or without an EMS schedule (Table 1). To remove faeces and debris, approximately 25% of the water in each tank was exchanged daily with dechlorinated water during the experiment. The water quality parameters (dissolved oxygen (6.8 ± 0.8 mg/L), temperature (24.7 ± 0.9 °C), ammonia (0.47 ± 0.1 mg/L), nitrite (0.027 ± 0.005 mg/L), nitrate (22.7 ± 2.0 mg/L), pH (7.3 ± 0.9)) were measured regularly and kept in standard levels. Water quality was maintained by aeration with compressed air and mechanical filtration, which was detailed in our earlier experiments from this project (Esmaeili et al. 2022; Zare et al. 2023a).
Diet formulation and experimental design
An optimal isonitrogenous (450 crude protein/kg feed and 180 g/kg fish meal) and isolipidic (180 g/kg lipid) (Zare et al. 2024) diet strategy was used for this experiment. The same diet preparation and formulation processes were previously reported (Hosseinpour Aghaei et al. 2018; Esmaeili et al. 2022). During the 9-week feeding experiment, two scheduled EMS stress events were conducted in weeks 2 and 8, on both Monday and Friday of that week (Table 1). This involved dragging an aquarium net around the tank for 5 min after a water exchange without actively chasing or removing any fish. This stress, for the first time, was applied in a previous study (Esmaeili et al. 2022) and then others (Zare et al. 2023a, 2023b).
Sample collection and growth performance
Prior to final measurements (end of week 9), the fish were fasted for 24 h and then anaesthetised using clove oil stock solution (50 ppm) before removal from tanks (Esmaeili et al. 2017). Standard methods and relationships were used to calculate weight gain, specific growth rate, feed conversion ratio (FCR), daily feed intake, hepatosomatic index (HSI), viscerosomatic index, and condition factor at the end of the experiment (Zaretabar et al. 2021). Further, four fish were chosen at random from each tank, and after collecting blood, their liver and viscera were sampled and weighed. The dissected fish were returned to a bag (minus the blood) and kept frozen until further analysis. The remaining fish were returned to their respective tanks for a further 1-week period of feeding with no EMS event prior to the application of AC stress below.
Chemical analysis of diets and fish body composition
The proximate composition of the diets and whole body samples was measured by AOAC methods (AOAC 2000). Briefly, nitrogen (nitrogen × 6.25) was determined using the Kjeldahl method and an automatic Kjeldahl system (Kjeltec Analyser unit 2300, Sweden). The Soxhlet extraction method was used to examine crude lipids (Soxtec 2050 FOSS Model, Switzerland). Moisture was determined gravimetrically by oven drying samples at 105 °C oven for 12 h. The ash content was determined using a Nabertherm muffle furnace (Model K, Germany) at 550 °C for 4 h.
Blood collection and sample preparation
The serum from four fish from each tank was tested for haematology, immune response, blood biochemistry, antioxidants, and serum enzymes at the end of the experiment (week 9 and week 10). The blood samples were quickly collected (within 2 min) via venipuncture of the caudal vein with an 18-G needle connected to a sterile 5-mL syringe. Aside from sampling blood with anticoagulant (EDTA) for haematology analysis, we sampled blood in tubes without anticoagulant for serum. Following that, blood was refrigerated for 2 h before serum was collected after centrifuging at 3000 g at 4 °C for 2 min (Esmaeili et al. 2017) and then stored at − 20 °C until further analyses.
Haematology profile
Red blood cells (RBCs) and white blood cells (WBCs) were counted in a Neubauer haemocytometer and the Neubauer chamber, respectively, as described earlier (Kenari et al. 2013; Esmaeili et al. 2022). Further, the haemoglobin (Hb) and haematocrit (Ht) were determined by cyanmethaemoglobin and the microhaematocrit method (Řehulka et al. 2004; Esmaeili et al. 2022). Mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC) (Wintrobe 1929), and blood performance (BP) (Esmaeili 2021) were calculated according to the below formulas:
Blood biochemistry, antioxidant enzyme activities, serum enzymes, and cortisol
Plasma biochemical parameters, glucose, total protein (TP), albumin, globulin, high-density lipoproteins (HDL), low-density lipoproteins (LDL), cholesterol, triglycerides, lactate, alkaline phosphatase (ALP), lactate dehydrogenase (LDH), aspartate transaminase (AST), and alanine aminotransferase (ALT), were analysed using commercial clinical investigation kits (Pars Azmoon Kit, Karaj, Iran). The antioxidant enzymes, including superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx), and malondialdehyde (MDA), were measured using ELISA kits, according to the kit protocol (ZellBio, GmbH, Germany) which were used in earlier studies (Esmaeili et al. 2022; Zare et al. 2023a, 2023b). Cortisol levels in serum were measured using fluorescence immunoassay (FIA) and a commercial kit (iCHROMA, South Korea) and the protocol provided with the kit. Linearity and parallelism were tested using serial dilutions of samples (0, 25, 50, and 75%). An R2 of more than 90% was considered to be the threshold to pass the limit for the linearity test. For the parallelism test, the coefficient of variation (% CV) between the slope of the standard curve and the samples was calculated (values of ≤ 20% passed the test). The recovery range of 80–120% CV was considered threshold. Intra‐assay and intra‐assay precision thresholds were 20% CV.
Nonspecific immune parameters
To determine serum lysozyme, gram-positive bacteria sensitive to the lysozyme enzyme method were used (Micrococcus lysodeikticus) (Clerton et al. 2001) as substrate. Alternative complement pathway hemolytic activity (ACH50) was determined by haemolysis of rabbit RBCs (RaABC) (Amar et al. 2000). Serum immunoglobulin M, complement C3 (C3), and complement C4 (C4) levels were measured by ELISA method using CUSABIO and MyBioSource kit companies (CUSABIO-CSB-E12045Fh and CUSABIO, CSB-E09727s) based on the protocol available in the kit package. The complete methods for measuring these parameters were described in our previous study (Hosseini et al. 2022). The qualitative and quantitative checks of data were done, as previously explained in the “Blood biochemistry, antioxidant enzyme activities, serum enzymes, and cortisol” section.
Acute confinement stress (AC stress)
After the 10 weeks of the photoperiod and EMS experiment, oscars were exposed to AC stress based on our previous studies to test fish’s ability to tackle stressful situations (Asgari et al. 2020; Esmaeili et al. 2022). After collecting samples at the end of week nine, ten fish per tank were biomass adjusted with three tanks per treatment. Then, the fish were fed as usual for 1 week to recover from stress, and we applied AC stress at the end of week 10. The acute stress was a succession of netting all of the fish in each tank followed by a 30-s air exposure before being transferred to a plastic mesh bucket at a density of 120 g/L in their original tank for 5 h. Aeration was maintained to prevent oxygen depletion and minimise any premature death as a result of the AC stress. Following the 5 h of confinement stress, blood sampling and serum extraction from fish that could survive were performed as previously described on three fish per tank (Esmaeili et al. 2022). The survival rate of fish after 48 h in various treatments is shown in Table 2. We needed to test the fish’s ability and our hypothesis to determine whether fish that experienced EMS could tolerate AC stress at the end of the experiment better or not. To do so, we had to design an AC stress severe enough to kill some fish.
Statistical analysis
This study used a completely randomised design with nine treatments and three replications. After testing the normality and homogeneity of data, two-way ANOVA was used to investigate the “photoperiod effect” and the “EMS effect”. When the P value of interaction was not significant, treatments were compared in different EMS groups and five photoperiod treatments in pooled data (Table 4). When the interaction was significant, the original data was unpacked, and treatments for each EMS group and photoperiod level were compared (figures). Further, data were compared before and after AC stress to see which parameter was changed with the AC stress in each treatment (Hosseini et al. 2022; Zare et al. 2024). In all analyses, a significant difference between treatments was defined as a difference of 5% or less. SPSS (version 21.0 for Windows) was used to analyse the data.
Results and discussion
It has been nearly half a century since the effect of photoperiod on fish growth, reproduction physiology, and health was investigated. As time passes, more fish species are farmed in controlled environmental conditions such as recirculation aquaculture systems and aquariums. It means that photoperiod manipulation to increase the growth of fish is possible and practical. Although many studies are available on different species, no studies have been conducted on the effect of photoperiod on oscar families. This study was a follow-up study from our EMS project. After four studies (Esmaeili et al. 2022; Zare et al. 2024, 2023a, 2023b), we understood that 2 out of 10-week stress events do not impair growth, stress response, and many blood parameters and even improved survival after AC stress. Further, the interaction of photoperiod and EMS and eventually their impacts on the final survival rate after acute AC stress was undiscovered. This study indicated that photoperiod does not affect fish growth, but consistent light schedule impaired fish health. This result shows that we can farm oscars even in the 8:16 (light to dark) regime without decreased growth. Unlike previously available studies (Esmaeili et al. 2022; Zare et al. 2023a, 2023b), EMS did not affect the growth and physiology of fish due to the probably stronger effect of photoperiod. As it is the first study on this topic, more studies are required to test the interaction of EMS and photoperiods on other species.
Growth performance and body composition
One of the most important consequences of photoperiod manipulation is its potential to affect the growth performance of fish. In the present research, the effect of photoperiod or EMS schedule on growth performance was not significant and showed that fish grew well on all five photoperiod schedules. Providing light for aquaculture systems can be costly, and even with 8 h of light, oscar can grow well. Similar to this output, other studies indicated fish growth was the same between 8:16 and 12:12 L:D, which has been considered the optimum for most fish species. While we fed fish twice per day in the 8:16 group, the growth was not affected, showing that fish were able to adjust their feeding with this photoperiod schedule. For example, in African catfish (Almazán‐Rueda et al. 2005) and channel catfish (Ictalurus punctatus) (Stickney and Andrews 1971), these results were observed. Photoperiod similarly did not affect the growth performance of flounder (Pleuronectes ferrugineus) (Purchase et al. 2000), rainbow trout (Reddy and Leatherland 2003), turbot (Psetta maxima) (Imsland et al. 1995), and sunshine bass (Morone chrysops × Morone saxatilis) (Davis and Mcentire 2006). Other studies reported the positive effect of continued or increased light on the growth of fish such as gilthead sea bream (Sparus aurata) (Ginés et al. 2004), which was due to increased food conversion efficiency and suppressed sexual maturation. Since photoperiod can affect the level of plasma growth hormone and eventual growth, it is reasonable to hypothesise that levels of hepatic insulin-like growth factor I mRNA are indirectly regulated by photoperiod (Vera Cruz and Brown 2009). While there are possible mechanisms to improve growth by photoperiod, oscar was not sensitive to light in the present study, similar to several other fish species noted above. In fish species like catfishes, longer periods of light can be stressful as more time is needed to show dominant behaviour or escape from dominant individuals by subordinate ones (Boeuf and Falcon 2001). Oscars may have less dominant-subordinate and social hierarchies, and because of that, continued light did not negatively affect fish growth. More behavioural studies may be warranted to better understand the relationship between social hierarchies and photoperiod.
In the present study, DFI, HSI, feed intake, and FCR were not affected by photoperiod (Table 2), which is unlike other studies that showed, for example, improved FCR with decreased and increased light period (Purchase et al. 2000; El-Sayed and Kawanna 2004). However, similar results were observed in tilapia (Wang et al. 2023), barramundi (Lates calcarifer) (Barlow et al. 1995), and yellowtail flounder (Purchase et al. 2000). Lack of any change in these parameters is in line with growth data indicating that oscar had no issue with different photoperiod schedules.
The survival rates in the current study were insignificant among treatments and greater than 90% at the end of the 9-week experiment, indicating that all fish were grown in a suitable environment. However, the effect of EMS on survival after final AC stress was significant, and those subjected to the EMS schedule had a higher survival (80.67%) than those grown in normal conditions without EMS (61.33%) (P < 0.05). The higher survival rate in EMS groups is consistent with our previous studies (Esmaeili et al. 2022; Zare et al. 2023a, 2023b) and is further evidence that the fish experienced adaptation and that allowed them to tolerate an acute stress condition better. This finding supports the notion that in a healthy population of high-performing fish, equal growth performance after an EMS or even AC stress does not necessarily translate to more robust fish. Nutritional status, cultural system, size of fish, and species are other variables that may invariably affect the results of growth performance. While both biotic (age, gender, and size) and abiotic factors (water quality, season, and geographical location) can affect the proximate body composition of aquatic species, the diet is most likely responsible for most of the changes (Shearer 1994). Unsurprisingly, as oscars in the present study were fed the same diets, there was no significant difference in protein, fat, ash, and moisture contents (Table 3). These results align with earlier studies investigating EMS (Esmaeili et al. 2022) and photoperiod (Biswas et al. 2006a, b; Biswas et al. 2008, 2016; Zolfaghari et al. 2011; Tian et al. 2019). However, other studies on marine species, such as gilthead sea bream, have reported changes to the proximate composition of fish in response to different photoperiod regimes (Ginés et al. 2004).
Haematology and blood biochemistry
Environmental factors such as photoperiod and stress drive alterations in haematology and biochemistry parameters, and these parameters can be indicators of the health status of fish. Our results indicated that some markers were affected by the photoperiod including haematocrit, WBC, and blood performance before and after AC stress (P < 0.05; Table 4). In detail, fish exposed to 24 h of light had lower haematocrit and WBC levels. It can be hypothesised that 24 h of light was a bit stressful for oscar, which eventually decreased their haematocrit and WBC. Decreasing these parameters with stress has been reported earlier (Gao et al. 2021; Shin et al. 2016). These results are consistent with our previous EMS works (Esmaeili et al. 2022). Other studies showed that photoperiod has significantly altered the haematology parameters. For example, in coho salmon (Fang et al. 2019) and piracanjubas (Brycon orbygnianus) (Machado et al. 2016), the same results were observed. In contrast, some other species, such as beluga (Bani et al. 2009), red sea bream (Biswas et al. 2006a, b; Biswas et al. 2006a, b; Biswas et al. 2010), and pacamã (Lophiosilurus alexandri) (Kitagawa et al. 2015), experienced no alterations in haematocrit under photoperiod treatments. The WBC is an important parameter showing the immune status of fish (Esmaeili 2021). A decrease in this parameter in the 24L0D group could be evidence of a suppressed immune system with continuous light. Photoperiods did not change the WBC in iridescent shark catfish (Pangasionodon hypophthalmus) (Windarti et al. 2021). However, this parameter was changed in other species such as pikeperch (Sander lucioperca) (Pourhosein Sarameh et al. 2013), Persian sturgeon (Acipenser persicus) (Falahatkar et al. 2012), rainbow trout (Valenzuela et al. 2022), tambaqui (Colossoma macropomum) (Pereira et al. 2021), and carp (Cyprinus carpio) (Ruchin 2006). The observed various outputs can highlight the fact that fish species respond differently to photoperiod regardless of trophic levels.
A blood performance (BP) marker has previously been used as an indicator of fish growth and health (Esmaeili 2021). In the current data, the photoperiod effect for BP before and after AC stress was significant, and accordingly, the 240LD group (14.22 and 13.88 for before and after AC stress, respectively) had a lower value than the others (Fig. 1). These results show that BP can be an indicator of immunity of fish as the immune parameters, WBC, and haematocrit were lower in continued light. To further validate, new investigations related to BP and photoperiod to compare with the current data are still required. Unlike the present data, in other studies from this project (Esmaeili et al. 2022; Zare et al. 2024, 2023a, 2023b) and also other studies (Esmaeili et al. 2017; Hosseini et al. 2021, 2022; Montazeri et al. 2021; Ravardshiri et al. 2021), the connection of growth and BP was observed. Earlier research found that a lower BP level can indicate a weaker fish in terms of its overall health status. Accordingly, when fish were fed too much soybean (Montazeri et al. 2021), total carbohydrate (Ravardshiri et al. 2021), and meat and bone meal (Esmaeili et al. 2017), the BP was lower when compared to the control group.
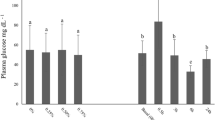
A blood biochemistry panel of markers is considered an indicator of fish metabolism as it is in other species and humans. Some blood biochemistry parameters like albumin and total protein are also markers of the immune system status. In the current study, total protein was the only parameter affected by photoperiod, with the 24L0D group having the lowest value before (5.02 g/dL) and after (4.75 g/dL) AC stress (Fig. 2). Proteins have key roles in almost all physiological metabolism, such as the transport of bilirubin, hormones, metals, vitamins, drugs, and lipid metabolism. Most importantly, almost all immune system components are protein-based, such as carrier proteins, enzymes, complements, and immunoglobulins. Some studies indicated a change in total protein with photoperiod. For example, in golden mahseer (Akhtar et al. 2020) and largemouth bass (Micropterus salmoides) (Malinovskyi et al. 2022), these results were observed. However, no change in this parameter was observed in Murray cod (Maccullochella peelii) (Di et al. 2023), Nile tilapia (Wang et al. 2023), and red sea bream (Biswas et al. 2006a, b).
Immune and stress response
The EMS can positively improve the immune and stress response and can act as a preparedness for the fish in terms of future stressful conditions. We observed the positive effect of EMS on the immune system and stress response of oscars in several prior studies (Esmaeili et al. 2022; Zare et al. 2023a, 2023b) but not in the present study where the effect of EMS was only significant for immunoglobulin M, being lower in the EMS group (Fig. 3). However, the effect of photoperiod before AC stress for lysozyme, immunoglobulin M, and complement C3 was significant. The oscar that was grown in the continued light and before AC stress had the lowest value of lysozyme (29.95 µ/mL), immunoglobulin M (15.25 mg/mL), and complement C3 (90.88 mg/dL). After the AC stress was applied, the fish followed the same trend, and the 24L0D group had the lowest values of immunoglobulin M (15.95 mg/mL), lysozyme (24.72 µ/mL) and ACH50 (159.15 µ/mL). The photoperiod and immune system are closely related to each other. It has been well-known that the immune system is affected by photoperiods and, in most cases, improved immunity in less light is observed. For example, increased spleen and thymus mass and numbers of lymphocytes and neutrophils are documented (Nelson et al. 1996). Melatonin influences the light–dark rhythm in most vertebrates, including fish, and eventually affects the humoral innate immune system (Ángeles Esteban et al. 2006). In the present study, the immune system was decreased in 24 h of light but with no change in stress response parameters like glucose and cortisol. This is potential evidence that it has not been due to suppression of immunity by the endocrine system. Chronic stress also prevents immune cells and signalling networks from communicating with each other properly (Bae and Shin 2019). It can be hypothesised that the relationship between immunity and photoperiod comes from melatonin. Unfortunately, we could not measure this parameter and more hormonal studies are required.
Immune response parameters of oscar to different photoperiods and early mild stress treatments during 10 weeks plus data related to after acute confinement stress (AC stress). Values were represented by means ± SDM of triplicate samples. Hashtag (#) indicates the significant difference in each treatment between farmed fish with and without early stress according to the independent sample T-test (P < 0.05) (for example, 24L0D compared to ES24L0D). X, Y, and Z indicated significant differences across different photoperiod groups after AC stress (the interaction effect was significant for this parameter)
Lysozyme plays a key role in the nonspecific immune response of fish and is one of the most commonly measured parameters in aquaculture. In other studies, 24 h of light during culture decreased lysozyme, erythrocytes, leucocytes, lymphocytes, monocytes, and polymorphonuclear in rainbow trout compared with the 12:12 light to dark cycle (Burgos et al. 2004; Valenzuela et al. 2022). Similar to our data, decreased lysozyme was observed in gilthead seabream and European sea bass (Dicentrarchus labrax) (Ceballos-Francisco et al. 2020) as well. However, other studies reported no change in lysozyme with photoperiod in other species including golden mahseer (Akhtar et al. 2020), Atlantic halibut (Hippoglossus hippoglossus) (Bowden et al. 2004), European sea bass (Ángeles Esteban et al. 2006), and Nile tilapia (Atwood et al. 2003). Immunoglobulin M was another parameter that was declined in the 24L0D group. Decreased immunoglobulin with increased light in gilthead seabream and European sea bass (Ceballos-Francisco et al. 2020) and also other animals (Guo et al. 2010; Park et al. 2015) was observed, which is in line with the current study.
The 24L0D groups had the lowest values of some haematological parameters and total proteins as well that can be connected to the immune system results. Although it was not significant, the 24L0D groups also had numerically lower growth (around 32 g compared to others, around 37 g), which can potentially be connected to these blood chemistry results. However, the 24L0D groups had the same survival rate compared to other pre-AC stresses, while the survival rate was significantly lower in the 24L0D groups after AC stress. It is possible that the stress events were not sufficient to affect the growth and survival rate significantly after EMS stress, but the immune parameters were changed. Our previous studies observed that decreased immunity was responsible for decreased growth and survival rate after AC stress (Esmaeili et al. 2022; Zare et al. 2023a, 2023b). To the best of our knowledge, no study has measured fish in a vast panel of immunity parameters under photoperiod treatments in oscar. Further research is needed to determine how different fish species’ immune systems react to EMS and photoperiod and how this eventually translates to growth performance and survival.
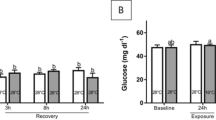
The present study indicated no significant differences in stress parameters’ levels such as glucose, lactate, and cortisol (Fig. 4). It should be noted that after AC stress, these parameters were higher in most treatments but not significant. The result of this study is unlike our previous studies that showed AC stress increased stress parameters (Esmaeili et al. 2022; Zare et al. 2023a, 2023b). However, as previously mentioned, the fish grown in the EMS schedule had a higher survival rate after AC stress. Aquaculture sustainability depends on growth performance and survival as key indicators, and reduced stress responsiveness can improve these parameters. The present study clearly demonstrates that glucose, lactate, and glucose cannot always be relied upon as the only indicators in fish studies to show stress responsiveness after acute stress. It has been reported that stress does not always cause an increase in cortisol, glucose, and lactate. The possible reasons can be impaired cortisol secretion by interrenal exhaustion and environmental factors or the HPI axis is no longer being stimulated, as the fish “acclimate” through gradual loss of awareness of the stress factor (Ellis et al. 2012).
Antioxidant enzyme activities
Antioxidant enzymes such as SOD, CAT, GPx, and MDA are also indicators of health status and are commonly employed in aquaculture studies. These enzymes protect cells from uncontrolled oxidative reactions that produce superoxide and H2O2 radicals (Hoseinifar et al. 2020). The current data indicated that before AC stress, SOD and catalase were affected by both EMS and photoperiod. SOD and catalase were higher and lower in the “without EMS” groups (Fig. 5). Like several other measured parameters explained in previous sections, 24L0D groups had the lowest values of SOD and catalase before (37.88 U/mL and 29.03 U/mL, respectively) and after AC stress (33.05 U/mL and 28.10 U/mL, respectively). After AC stress, GPx in the 24L0D and without EMS groups had the lowest level compared to other treatments. It can be seen that, generally, the 24L0D had a lower value of antioxidant system enzymes, which may indicate this group of fish was under oxidative stress. The link between growth and antioxidant activities was well-reviewed (Hoseinifar et al. 2020), and previously, we linked the lower survival rate to decreased cellular resistance to oxidative stress and impaired maintenance of the antioxidant-ROS balance (Esmaeili et al. 2022; Zare et al. 2023a, 2023b). In the present study, the photoperiod effect was more dominant and influential in changing antioxidant enzymes rather than EMS. Other studies similarly indicated that SOD, catalase, and GPx were changed with photoperiod in European sea bass (Li et al. 2021), blunt snout bream (Tian et al. 2019), golden hamster (Mukherjee and Haldar 2015), and gibel carp (Wei et al. 2019) where the lowest antioxidant status was observed in the groups with highest light exposure. However, no change was observed in black sea bass (Centropristis striata) (Ren et al. 2020). As we can see, most of the literature reported decreased antioxidant activities with higher or continued light. The possible reason for the lower antioxidant status in the 24L0D group of the present study can be related to a higher rate of metabolic oxygen consumption (Martínez-Álvarez et al. 2005). However, this group did not have a significantly lower growth or survival rate as a result of oxidative stress.
Antioxidant system parameters of oscar exposed to different photoperiods and early mild stress treatments during 10 weeks plus data related to after acute confinement stress (AC stress). Values were represented by means ± SDM of triplicate samples. Hashtag (#) indicates the significant difference in each treatment between farmed fish with and without early stress according to the independent sample T-test (P < 0.05) (for example, 24L0D compared to ES24L0D). x, y, and z and also, a, b, and c indicated significant differences across different photoperiod groups with and without early stress, respectively (the interaction effect was significant for this parameter)
Liver enzymes
Liver or serological enzymes (LDH, ALP, AST, and ALT) can show the health status of fish, especially the liver, which is the central metabolic organ. Consistent with other markers examined in this study, the AST in the 24L0D group before AC stress was higher than in the other groups (Fig. 6). Other parameters did not change with EMS or photoperiod and can be a positive sign that oscars were in a healthy/normal condition. In previous studies, higher liver enzymes in oscar were evidence of stress, leading to decreased growth (Esmaeili et al. 2022). The interaction effect for ALT before and after AC stress was significant, as shown in Fig. 6. In the without EMS group, before and after AC stress, and in EMS treatment before AC stress, the 24L0D group had the highest value among the others. Other studies indicated that liver enzymes were changed with photoperiod in common carp, largemouth bass (Malinovskyi et al. 2022), Malaysian red tilapia (Malambugi et al. 2020), and tiger puffer (Takifugu rubripes) (Ma et al. 2021) and no change in Murray cod (Di et al. 2023). These results are matched with other parameters, such as lower antioxidant enzymes and immune system parameters in the 24L0D group. It was earlier mentioned that a lower survival rate after AC stress and higher liver enzymes are further evidence that this treatment was not displaying normal physiological responses (Zare et al. 2023a). Aquaculture studies have reported elevated ALT and AST in response to different stresses in a wide range of fish species (Tejpal et al. 2009; Liu et al. 2016; Sun et al. 2019; Dawood et al. 2021; Hoseini et al. 2022). More research is necessary to demonstrate how EMS, photoperiod, and the liver’s physiological status react with each other.
Liver enzyme parameters of oscar to different photoperiods and early mild stress treatments during 10 weeks plus data related to after acute confinement stress (AC stress). Values were represented by means ± SDM of triplicate samples. Hashtag (#) indicates the significant difference in each treatment between farmed fish with and without early stress according to the independent sample T-test (P < 0.05) (for example, 20L4D compared to ES20L4D). Further, x, y, and z and also, a, b, and c indicated significant differences across different photoperiod groups with and without early stress, respectively (the interaction effect was significant for this parameter). In addition, X, Y, and Z indicated significant differences across different photoperiod groups after AC stress (patterned columns)
Conclusion
The result of the present research suggests that neither EMS nor photoperiod affected fish growth performance or survival rate after a 9-week experiment. Fish exposed to continued light (24L0D) showed negative responses to many markers examined, such as lower haematological parameters, immune system parameters, antioxidant enzyme activities, and higher liver enzymes. However, none of these alterations led to impaired fish growth performance. Survival rate was significantly changed after AC stress, with fish exposed to EMS regimes being more able to cope with stress. AC stress decreased some health parameters under 24-h light, while such results were not observed in the EMS-exposed fish. Therefore, the EMS schedule can be a useful tool in preventing the negative effects of acute stress. More programmed stresses and measurements of more parameters at the classical and molecular levels are needed, as well as a nutritional fortification, to illustrate the various mechanisms of EMS and its interaction with photoperiod in fish. EMS can directly influence the behaviour of fish; behavioural and learning mechanisms should be focused on in future studies.
Data Availability
Data available on request due to privacy/ethical restrictions (The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.)
References
Abdollahpour H, Falahatkar B, Lawrence C (2020) The effect of photoperiod on growth and spawning performance of zebrafish, Danio Rerio. Aquac Rep 17:100295. https://doi.org/10.1016/j.aqrep.2020.100295
Adam ML, Torres MFP, Franci AC, Sponchiado G, Torres RA, Correia MTDS (2011) On the stress by photoperiod, temperature and noise as possible causes of genomic damaging in an animal model. Stress Health 27:e152–e156. https://doi.org/10.1002/smi.1350
Ahmadi-Noorbakhsh S, Mirabzadeh Ardakani E, Sadighi J, Aldavood SJ, Farajli Abbasi M, Farzad-Mohajeri S, Ghasemi A, Sharif-Paghaleh E, Hatami Z, Nikravanfard N (2021) Guideline for the care and use of laboratory animals in Iran. Lab Anim 50:303–305. https://doi.org/10.1038/s41684-021-00871-3
Akhtar M, Rajesh M, Kamalam B, Ciji A (2020) Effect of photoperiod and temperature on indicators of immunity and wellbeing of endangered golden mahseer (Tor putitora) broodstock. J Therm Biol 93:102694. https://doi.org/10.1016/j.jtherbio.2020.102694
Almazán-Rueda P, Van Helmond AM, Verreth J, Schrama J (2005) Photoperiod affects growth, behaviour and stress variables in Clarias gariepinus. J Fish Biol 67:1029–1039. https://doi.org/10.1111/j.0022-1112.2005.00806.x
Amar EC, Kiron V, Satoh S, Okamoto N, Watanabe T (2000) Effects of dietary β-carotene on the immune response of rainbow trout Oncorhynchus mykiss. Fish Sci 66:1068–1075. https://doi.org/10.1046/j.1444-2906.2000.00170.x
Ángeles Esteban M, Cuesta A, Rodríguez A, Meseguer J (2006) Effect of photoperiod on the fish innate immune system: a link between fish pineal gland and the immune system. J Pineal Res 41:261–266. https://doi.org/10.1111/j.1600-079X.2006.00362.x
AOAC (2000) Official methods of analysis of the AOAC International Vol. 18. The Association
Asgari M, Abedian Kenari A, Esmaeili N, Rombenso A (2020) Effects of hydroalcoholic extract of honeybee pollen on growth performance, flesh quality, and immune and stress response response of rainbow trout (Oncorhynchus mykiss). Aquac Nutr 26:1505–1519. https://doi.org/10.1111/anu.13098
Atwood H, Tomasso J, Webb K, Gatlin Iii D (2003) Low-temperature tolerance of Nile tilapia, Oreochromis niloticus: effects of environmental and dietary factors. Aquac Res 34:241–251. https://doi.org/10.1046/j.1365-2109.2003.00811.x
Auperin B, Geslin M (2008) Plasma cortisol response to stress in juvenile rainbow trout is influenced by their life history during early development and by egg cortisol content. Gen Comp Endocrinol 158:234–239. https://doi.org/10.1016/j.ygcen.2008.07.002
Bae Y-S, Shin E-C, Bae Y-S, Van Eden W (2019) Frontiers Media SA 10:245. https://doi.org/10.3389/fimmu.2019.00245
Bani A, Tabarsa M, Falahatkar B, Banan A (2009) Effects of different photoperiods on growth, stress and haematological parameters in juvenile great sturgeon Huso huso. Aquac Res 40:1899–1907. https://doi.org/10.1111/j.1365-2109.2009.02321.x
Barlow C, Pearce M, Rodgers L, Clayton P (1995) Effects of photoperiod on growth, survival and feeding periodicity of larval and juvenile barramundi Lates calcarifer (Bloch). Aquac 138:159–168. https://doi.org/10.1016/0044-8486(95)01073-4
Biswas A, Maita M, Yoshizaki G, Takeuchi T (2004) Physiological responses in Nile tilapia exposed to different photoperiod regimes. J Fish Biol 65:811–821. https://doi.org/10.1111/j.0022-1112.2004.00487.x
Biswas AK, Seoka M, Takii K, Maita M, Kumai H (2006a) Stress response of red sea bream Pagrus major to acute handling and chronic photoperiod manipulation. Aquac 252:566–572. https://doi.org/10.1016/j.aquaculture.2005.06.043
Biswas AK, Seoka M, Tanaka Y, Takii K, Kumai H (2006b) Effect of photoperiod manipulation on the growth performance and stress response of juvenile red sea bream (Pagrus major). Aquac 258:350–356. https://doi.org/10.1016/j.aquaculture.2006.03.048
Biswas AK, Seoka M, Ueno K, Takii K, Kumai H (2008) Stimulation of growth performance without causing stress response in young red sea bream, Pagrus major (Temminck & Schlegel), by photoperiod manipulation. Aquac Res 39:457–463. https://doi.org/10.1111/j.1365-2109.2008.01897.x
Biswas A, Seoka M, Inagaki H, Takii K (2010) Reproduction, growth and stress response in adult red sea bream, Pagrus major (Temminck & Schlegel) exposed to different photoperiods at spawning season. Aquac Res 41:519–527. https://doi.org/10.1111/j.1365-2109.2009.02341.x
Biswas A, Takaoka O, Kumai H, Takii K (2016) Combined effect of photoperiod and self-feeder on the growth performance of striped knifejaw, Oplegnathus fasciatus. Aquac 452:183–187. https://doi.org/10.1016/j.aquaculture.2015.10.038
Boeuf G & Falcon J (2001) Photoperiod and growth in fish. Vie et Milieu/Life & Environ 247–266
Bowden T, Butler R, Bricknell I (2004) Seasonal variation of serum lysozyme levels in Atlantic halibut (Hippoglossus hippoglossus L.). Fish Shellfish Immunol 17:129–135. https://doi.org/10.1016/j.fsi.2003.12.001
Burgos A, Valenzuela A, Gonzalez M, Klempau A (2004) Nonspecific defence mechanisms of rainbow trout (Oncorhynchus mykiss) during artificial photoperiod. Bull Eur Assoc Fish Pathol 24:240–245
Ceballos-Francisco D, Cuesta A, Esteban MÁ (2020) Effect of light–dark cycle on skin mucosal immune activities of gilthead seabream (Sparus aurata) and European sea bass (Dicentrarchus labrax). Fishes 5:10. https://doi.org/10.3390/fishes5010010
Clerton P, Troutaud D, Verlhac V, Gabaudan J, Deschaux P (2001) Dietary vitamin E and rainbow trout (Oncorhynchus mykiss) phagocyte functions: effect on gut and on head kidney leucocytes. Fish Shellfish Immunol 11:1–13. https://doi.org/10.1006/fsim.2000.0287
Davis KB, Mcentire M (2006) Effect of photoperiod on feeding, intraperitoneal fat, and insulin-like growth factor-I in sunshine bass. J World Aquaculture Soc 37:431–436. https://doi.org/10.1111/j.1749-7345.2006.00056.x
Dawood MA, Gewaily MS, Monier MN, Younis EM, Van Doan H, Sewilam H (2021) The regulatory roles of yucca extract on the growth rate, hepato-renal function, histopathological alterations, and immune-related genes in common carp exposed with acute ammonia stress. Aquac 534:736287. https://doi.org/10.1016/j.aquaculture.2020.736287
Di Z, Li K, Li T, Yan L, Jiang H, Liu L (2023) Effects of light intensity and photoperiod on the growth performance of juvenile Murray cods (Maccullochella peelii) in recirculating aquaculture system (RAS). Aquac Fish 8:274–279. https://doi.org/10.1016/j.aaf.2021.12.009
Ellis T, Yildiz HY, López-Olmeda SMT, Tort L, Øverli Ø, Martins CIM (2012) Cortisol and finfish welfare. Fish Physiol Biochem 38:163–188. https://doi.org/10.1007/s10695-011-9568-y
El-Sayed A-FM, Kawanna M (2004) Effects of photoperiod on the performance of farmed Nile tilapia Oreochromis niloticus: I. Growth, feed sutilisation efficiency and survival of fry and fingerlings. Aquac 231:393–402. https://doi.org/10.1016/j.aquaculture.2003.11.012
Esmaeili N (2021) Blood performance: a new formula for fish growth and health. Biology 10:1236. https://doi.org/10.3390/biology10121236
Esmaeili N, Abedian Kenari A, Rombenso A (2017) Immunohematological status under acute ammonia stress of juvenile rainbow trout (Oncorhynchus mykiss Walbaum, 1792) fed garlic (Allium sativum) powder-supplemented meat and bone meal-based feeds. Comp Clin Pathol 26:853–866. https://doi.org/10.1007/s00580-017-2457-8
Esmaeili N, Hosseini H, Zare M, Akhavan SR, Rombenso A (2022) Early mild stress along with lipid improves the stress responsiveness of oscar (Astronotus ocellatus). Aquac Nutr 8991678 https://doi.org/10.1155/2022/8991678
Esmaeili N, Zare M, Choupani SMH, Kazempour M, Hosseini H, Akhavan S & Salini M (2024) Immunohaemtalogical changes of oscar (Astronotus ocellatus) in response to temperature and early mild stress. Fish Shellfish Immunol
Falahatkar B, Poursaeid S, Efatpanah I, Meknatkhah B, Biswas A (2012) Effect of photoperiod manipulation on growth performance, physiological and hematological indices in juvenile Persian sturgeon, Acipenser persicus. J World Aquac Soc 43:679–687. https://doi.org/10.1111/j.1749-7345.2012.00600.x
Fang Y, Chan VK, Hines CW, Stiller KT, Richards JG, Brauner CJ (2019) The effects of salinity and photoperiod on aerobic scope, hypoxia tolerance and swimming performance of coho salmon (Oncorhynchus kisutch) reared in recirculating aquaculture systems. Comp Biochem Physiol a: Mol Integr Physiol 231:82–90. https://doi.org/10.1016/j.cbpa.2019.01.026
Gao XQ, Fei F, Huang B, Meng XS, Zhang T, Zhao K, Chen H, Xing R, Liu B (2021) Alterations in hematological and biochemical parameters, oxidative stress, and immune response in Takifugu rubripes under acute ammonia exposure. Comp Biochem Physiol c: Toxicol Pharmacol 243:108978. https://doi.org/10.1016/j.cbpc.2021.108978
Ghosi Mobaraki MR, Abedian Kenari A, Bahrami Gorji S, Esmaeili N (2020) Effect of dietary fish and vegetable oil on the growth performance, body composition, fatty acids profile, reproductive performance and larval resistance in pearl gourami (Trichogaster leeri). Aquac Nutr 26:894–907. https://doi.org/10.1111/anu.13048
Ginés R, Afonso JM, Argüello A, Zamorano MJ, López JL (2004) The effects of long-day photoperiod on growth, body composition and skin colour in immature gilthead sea bream (Sparus aurata L.). Aquac Res 35:1207–1212. https://doi.org/10.1111/j.1365-2109.2004.01126.x
Guo BY, Li W, Chen J (2010) Influence of nutrient density and lighting regime in broiler chickens: effect on antioxidant status and immune function. Br Poult Sci 51:222–228. https://doi.org/10.1080/00071661003746503
Hansen T, Stefansson S, Taranger G (1992) Growth and sexual maturation in Atlantic salmon, Salmon salar L., reared in sea cages at two different light regimes. Aquac Res 23:275–280. https://doi.org/10.1111/j.1365-2109.1992.tb00770.x
Harrington RW Jr (1956) An experiment on the effects of contrasting daily photoperiods on gametogenesis and reproduction in the centrarchid fish, Enneacanthus obesus (Girard). J Exp Zool 131:203–223. https://doi.org/10.1002/jez.1401310302
Hoseini SM, Gupta SK, Yousefi M, Kulikov EV, Drukovsky SG, Petrov AK, Mirghaed AT, Hoseinifar SH, Van Doan H (2022) Mitigation of transportation stress in common carp, Cyprinus carpio, by dietary administration of turmeric. Aquaculture 564:737380. https://doi.org/10.1016/j.aquaculture.2021.737380
Hoseinifar SH, Yousefi S, Van Doan H, Ashouri G, Gioacchini G, Maradonna F, Carnevali O (2020) Oxidative stress and antioxidant defense in fish: the implications of probiotic, prebiotic, and synbiotics. Rev Fish Sci Aquac 29:198–217. https://doi.org/10.1080/23308249.2020.1795616
Hosseini H, Esmaeili N, Zare M, Rombenso A (2021) Egg enrichment with n-3 fatty acids in farmed hens in sub-optimum temperature: a cold-temperament additive mix alleviates adverse effects of stress on performance and health. J Anim Physiol Anim Nutr. https://doi.org/10.1111/jpn.13659
Hosseini H, Pooyanmehr M, Foroughi A, Esmaeili N, Ghiasi F, Lorestany R (2022) Remarkable positive effects of figwort (Scrophularia striata) on improving growth performance, and immunohematological parameters of fish. Fish Shellfish Immunol 120:111–121. https://doi.org/10.1016/j.fsi.2021.11.020
Hosseinpour Aghaei R, Abedian Kenari A, Yazdani Sadati MA, Esmaeili N (2018) The effect of time-dependent protein restriction on growth factors, nonspecific immunity, body composition, fatty acids and amino acids in the Siberian sturgeon (Acipenser baerii). Aquac Res 49:3033–3044. https://doi.org/10.1111/are.13764
Hvas M (2022) Influence of photoperiod and protocol length on metabolic rate traits in ballan wrasse Labrus bergylta. J Fish Biol 100:687–696. https://doi.org/10.1111/jfb.14981
Imsland AK, Folkvord A, Stefansson S (1995) Growth, oxygen consumption and activity of juvenile turbot (Scophthalmus maximus L.) reared under different temperatures and photoperiods. Neth J Sea Res 34:149–159. https://doi.org/10.1016/0077-7579(95)90023-3
Kenari AA, Mahmoudi N, Soltani M, Abediankenari S (2013) Dietary nucleotide supplements influence the growth, haemato-immunological parameters and stress responses in endangered Caspian brown trout (Salmo trutta caspius Kessler, 1877). Aquac Nutr 19:54–63. https://doi.org/10.1111/j.1365-2095.2012.00938.x
Kitagawa AT, Costa LS, Paulino RR, Luz RK, Rosa PV, Guerra-Santos B, Fortes-Silva R (2015) Feeding behavior and the effect of photoperiod on the performance and hematological parameters of the pacamã catfish (Lophiosilurus alexandri). Appl Anim Behav Sci 171:211–218. https://doi.org/10.1016/j.applanim.2015.08.025
Li X, Wei P, Liu S, Tian Y, Ma H, Liu Y (2021) Photoperiods affect growth, food intake and physiological metabolism of juvenile European sea bass (Dicentrachus labrax L.). Aquac Rep 20:100656 https://doi.org/10.1016/j.aqrep.2021.100656
Liu F, Shi H-Z, Guo Q-S, Yu Y-B, Wang A-M, Lv F, Shen W-B (2016) Effects of astaxanthin and emodin on the growth, stress resistance and disease resistance of yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol 51:125–135. https://doi.org/10.1016/j.fsi.2016.02.020
Ma H, Wei P, Li X, Liu S, Tian Y, Zhang Q, Liu Y (2021) Effects of photoperiod on growth, digestive, metabolic and non-special immunity enzymes of Takifugu rubripes larvae. Aquaculture 542:736840. https://doi.org/10.1016/j.aquaculture.2021.736840
Machado MRF, De Andrade EA, De Souza Andrade E, De Jesus Paula DA, Oliveira JA, Costa AC, Peconick AP & Murgas LDS (2016) Influence of photoperiod over morphometric and hematological parameters of juvenile piracanjubas (Brycon orbygnianus). J Agric Sci Technol B 6:350–359 https://doi.org/10.17265/2161-6264/2016.05.009
Madaro A, Olsen RE, Kristiansen TS, Ebbesson LO, Nilsen TO, Flik G, Gorissen M (2015) Stress in Atlantic salmon: response to unpredictable chronic stress. J Exp Biol 218:2538–2550. https://doi.org/10.1242/jeb.120535
Malambugi A, Yu Z, Zhu W, Wang L, Song F, Limbu SM, Dong Z (2020) Effects of photoperiod on growth performance and melanogenesis pathway for skin pigmentation of Malaysian red tilapia. Aquac Res 51:1824–1833. https://doi.org/10.1111/are.14531
Malinovskyi O, Rahimnejad S, Stejskal V, Boňko D, Stará A, Velíšek J, Policar T (2022) Effects of different photoperiods on growth performance and health status of largemouth bass (Micropterus salmoides) juveniles. Aquaculture 548:737631. https://doi.org/10.1016/j.aquaculture.2021.737631
Martínez-Álvarez RM, Morales AE, Sanz A (2005) Antioxidant defenses in fish: biotic and abiotic factors. Rev Fish Biol Fisheries 15:75–88. https://doi.org/10.1007/s11160-005-7846-4
Martos-Sitcha JA, Mancera JM, Prunet P & Magnoni LJ (2020) Frontiers Media SA 11:162 https://doi.org/10.3389/fphys.2020.00162
Montazeri H, Abedian Kenari A, Esmaeili N (2021) Soybean-based diets plus probiotics improve the profile of fatty acids, digestibility, intestinal microflora, growth performance, and the innate immunity of beluga (Huso huso). Aquac Res 52:152–166. https://doi.org/10.1111/are.14877
Mukherjee A, Haldar C (2015) Effect of 2-deoxy-d-glucose induced metabolic stress on testicular steroidogenesis and antioxidant status in golden hamster, Mesocricetus auratus: role of photoperiod. J Photochem Photobiol, B 153:40–50. https://doi.org/10.1016/j.jphotobiol.2015.09.004
Nelson RJ, Demas GE (1996) Seasonal changes in immune function. Q Rev Biol 71:511–548. https://doi.org/10.1086/419555
Park SH, Kim IG, Kim HC, Gang MJ, Son SE, Lee HJ (2015) Influence of various photoperiods on stress hormone production, immune function, and hematological parameters in ICR mice. Korean J Vet Res 55:111–116 https://doi.org/10.14405/kjvr.2015.55.2.111
Pederzoli A, Mola L (2016) The early stress responses in fish larvae. Acta Histochem 118:443–449. https://doi.org/10.1016/j.acthis.2016.03.001
Pereira LAL, Amanajás RD, De Oliveira AM, Da Silva MDNP, Val AL (2021) Health of the Amazonian fish tambaqui (Colossoma macropomum): effects of prolonged photoperiod and high temperature. Aquac 541:736836. https://doi.org/10.1016/j.aquaculture.2021.736836
Pourhosein Sarameh S, Falahatkar B, Azari Takami G, Efatpanah I (2013) Physiological changes in male and female pikeperch Sander lucioperca (Linnaeus, 1758) subjected to different photoperiods and handling stress during the reproductive season. Fish Physiol Biochem 39:1253–1266. https://doi.org/10.1007/s10695-013-9780-z
Purchase C, Boyce D, Brown J (2000) Growth and survival of juvenile yellowtail flounder Pleuronectes ferrugineus (Storer) under different photoperiods. Aquac Res 31:547–552. https://doi.org/10.1046/j.1365-2109.2000.00480.x
Ravardshiri M, Bahram S, Javadian SR, Bahrekazemi M (2021) Cinnamon promotes growth performance, digestive enzyme, blood parameters, and antioxidant activity of rainbow trout (Oncorhynchus mykiss) in low-carbohydrate diets. Turk J Fish Aquat Sci 21:309–322. https://doi.org/10.4194/1303-2712-v21_7_01
Reddy P, Leatherland J (2003) Influences of photoperiod and alternate days of feeding on plasma growth hormone and thyroid hormone levels in juvenile rainbow trout. J Fish Biol 63:197–212. https://doi.org/10.1046/j.1095-8649.2003.00144.x
Řehulka J, Minařík B, Řehulková E (2004) Red blood cell indices of rainbow trout Oncorhynchus mykiss (Walbaum) in aquaculture. Aquac Res 35:529–546. https://doi.org/10.1111/j.1365-2109.2004.01035.x
Reiter RJ (2003) Melatonin: clinical relevance. Best Pract Res Clin Endocrinol Metab 17:273–285. https://doi.org/10.1016/S1521-690X(03)00016-2
Ren X, Zhang J, Wang L, Wang Z, Wang Y (2020) Diel variation in cortisol, glucose, lactic acid and antioxidant system of black sea bass Centropristis striata under natural photoperiod. Chronobiol Int 37:176–188. https://doi.org/10.1080/07420528.2019.1675684
Ruchin A (2006) Effect of light on white blood cell count in carp Cyprinus carpio L. Biology Bulletin 33:517–520. https://doi.org/10.1134/S1062359006050153
Safavi SV, Abedian Kenari A, Tabarsa M, Esmaeili N (2019) Effect of sulfated polysaccharides extracted from marine macroalgae (Ulva intestinalis and Gracilariopsis persica) on growth performance, fatty acid profile, and immune response of rainbow trout (Oncorhynchus mykiss). J Appl Phycol 31:4021–4035. https://doi.org/10.1007/s10811-019-01902-w
Shearer KD (1994) Factors affecting the proximate composition of cultured fishes with emphasis on salmonids. Aquac 119:63–88. https://doi.org/10.1016/0044-8486(94)90444-8
Shin KW, Kim SH, Kim JH, Hwang SD, Kan JC (2016) Toxic effects of ammonia exposure on growth performance, hematological parameters, and plasma components in rockfish, Sebastes schlegelii, during thermal stress. Fish Aquat Sci 19:44. https://doi.org/10.1186/s41240-016-0044-6
Stickney RR, Andrews JW (1971) The influence of photoperiod on growth and food conversion of channel catfish. The Progressive Fish-Culturist 33:204–205. https://doi.org/10.1577/1548-8640(1971)33[204:TIOPOG]2.0.CO;2
Sun Z, Tan X, Liu Q, Ye H, Zou C, Xu M, Zhang Y, Ye C (2019) Physiological, immune responses and liver lipid metabolism of orange-spotted grouper (Epinephelus coioides) under cold stress. Aquac 498:545–555. https://doi.org/10.1016/j.aquaculture.2018.08.051
Tazikeh T, Abedian Kenari A, Esmaeili N (2020) Effects of fish meal replacement by meat and bone meal supplemented with garlic (Allium sativum) powder on biological indices, feeding, muscle composition, fatty acid and amino acid profiles of whiteleg shrimp (Litopenaeus vannamei). Aquac Res 51:674–686. https://doi.org/10.1111/are.14416
Tejpal C, Pal A, Sahu N, Kumar JA, Muthappa N, Vidya S, Rajan M (2009) Dietary supplementation of L-tryptophan mitigates crowding stress and augments the growth in Cirrhinus mrigala fingerlings. Aquac 293:272–277. https://doi.org/10.1016/j.aquaculture.2008.09.014
Tian H, Zhang D, Li X, Jiang G, Liu W (2019) Photoperiod affects blunt snout bream (Megalobrama amblycephala) growth, diel rhythm of cortisol, activities of antioxidant enzymes and mRNA expression of GH/IGF-I. Comp Biochem Physiol b: Biochem Mol Biol 233:4–10. https://doi.org/10.1016/j.cbpb.2019.03.007
Valenzuela AE, Silva VM, Klempau AE (2006) Effects of constant light on haematological parameters of cultured rainbow trout (Oncorhynchus mykiss) in the Southern Hemisphere. Fish Physiol Biochem 32:113–120. https://doi.org/10.1007/s10695-006-9103-8
Valenzuela A, Rodríguez I, Schulz B, Cortés R, Acosta J, Campos V, Escobar-Aguirre S (2022) Effects of Continuous Light (LD24: 0) Modulate the expression of lysozyme, mucin and peripheral blood cells in rainbow trout. Fishes 7:28. https://doi.org/10.3390/fishes7010028
Vera Cruz E, Brown C (2009) Influence of the photoperiod on growth rate and insulin-like growth factor-I gene expression in Nile tilapia Oreochromis niloticus. J Fish Biol 75:130–141. https://doi.org/10.1111/j.1095-8649.2009.02271.x
Vindas MA, Madaro A, Fraser TW, Höglund E, Olsen RE, Øverli Ø, Kristiansen TS (2016) Coping with a changing environment: the effects of early life stress. R Soc Open Sci 3:160382. https://doi.org/10.1098/rsos.160382
Vindas MA, Fokos S, Pavlidis M, Höglund E, Dionysopoulou S, Ebbesson LOE, Papandroulakis N, Dermon CR (2018) Early life stress induces long-term changes in limbic areas of a teleost fish: the role of catecholamine systems in stress coping. Sci Rep 8:5638. https://doi.org/10.1038/s41598-018-23950-x
Walton JC, Weil ZM, Nelson RJ (2011) Influence of photoperiod on hormones, behavior, and immune function. Front Neuroendocrinol 32:303–319. https://doi.org/10.1016/j.yfrne.2010.12.003
Wang K, Li K, Liu L, Tanase C, Mols R, Van Der Meer M (2023) Effects of light intensity and photoperiod on the growth and stress response of juvenile Nile tilapia (Oreochromis niloticus) in a recirculating aquaculture system. Aquaculture and Fisheries 8:85–90. https://doi.org/10.1016/j.aaf.2020.03.001
Wei H, Cai W-J, Liu H-K, Han D, Zhu X-M, Yang Y-X, Jin J-Y, Xie S-Q (2019) Effects of photoperiod on growth, lipid metabolism and oxidative stress of juvenile gibel carp (Carassius auratus). J Photochem Photobiol, B 198:111552. https://doi.org/10.1016/j.jphotobiol.2019.111552
Windarti W, Amin B & Simarmata AH (2021) Growth and health status of Pangasionodon hypophthalmus reared under manipulated photoperiod conditions. F1000Res 10 https://doi.org/10.12688/f1000research.28259.1
Wintrobe M (1929) The volume and hemoglobin content of the red blood corpuscle: simple method of calculation, normal findings, and value of such calculations in the anemias. Am J Med Sci 177:513–522. https://doi.org/10.1097/00000441-192904000-00006
Zare M, Esmaeili N, Hosseini H, Choupani SMH, Akhavan S, Rombenso A (2023a) Fish meal replacement and early mild stress improve stress responsiveness of oscar (Astronotus ocellatus) in future stressful events. Animals 13:1314. https://doi.org/10.3390/ani13081314
Zare M, Heidari E, Choupani SMH, Akhavan S, Rombenso A, Esmaeili N (2023b) The recovery time between early mild stress and final acute stress affects survival rate, growth, immunity, health physiology, and stress response of oscar (Astronotus ocellatus). Animals 13:1606. https://doi.org/10.3390/ani13101606
Zare M, Esmaeili N, Hosseini H, Choupani SMH, Akhavan S, Salini M, Rombenso A & Stejskal V (2024) How do optimum dietary protein and early mild stress events prepare fish for a stressful future? Stress responsiveness of oscar (Astronotus ocellatus) Aquac Rep
Zaretabar A, Ouraji H, Kenari AA, Yeganeh S, Esmaeili N, Amirkolaee AK (2021) One step toward aquaculture sustainability of a carnivorous species: fish meal replacement with barley protein concentrate plus wheat gluten meal in Caspian brown trout (Salmo trutta caspius). Aquac Rep 20:100714. https://doi.org/10.1016/j.aqrep.2021.100714
Zolfaghari M, Imanpour MR, Najafi E (2011) Effect of photoperiod and feeding frequency on growth and feed sutilisation of fingerlings Persian sturgeon (Acipenser persicus). Aquac Res 42:1594–1599. https://doi.org/10.1111/j.1365-2109.2010.02749.x
Acknowledgements
The authors would like to thank Abzian for their financial support as well as for providing space and fish for this research. Thank you is also extended to everyone for their invaluable practical assistance.
Author information
Authors and Affiliations
Contributions
Mahyar Zare: statistical analysis, investigation, resources, research; Mohammad Kazempour: student, research, lab works, investigation; Seyedeh Mahsa Hosseini: student, research, lab works, investigation; Sobhan R. Akhavan: validation, revision; Michael Salini: validation, revision; Artur Rombenso: validation, revision; Noah Esmaeili: ideas, supervision, validation, writing.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zare, M., Kazempour, M., Hosseini Choupani, S.M. et al. The crosstalk between photoperiod and early mild stress on juvenile oscar (Astronotus ocellatus) after acute stress. Fish Physiol Biochem 50, 1025–1046 (2024). https://doi.org/10.1007/s10695-024-01316-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-024-01316-7