Abstract
Pikeperch broodstocks were exposed to different photoperiods: constant light (24L:0D), constant darkness (0L:24D), and 12 h light, 12 h darkness (12L:12D), for 40 days. Half of the broodstocks of each photoperiod were exposed to handling stress at a specific time of the day. Results showed that cortisol and lactate did not reveal any significant difference. However, glucose levels in females increased in the stress-free darkness period in comparison with stressful darkness photoperiods (0L:24D-s). Red blood cells in males and white blood cells in females showed a significant difference under different photoperiod regimes. Both sexes showed no significant difference in the differential count of leukocytes under different photoperiods and handling stress. Constant photoperiods and handling stress affected the hematological parameters, particularly, the number of lymphocytes and neutrophils in females. Our findings revealed that due to a long-term exposure to stressors, pikeperch brooders become adapted to stressful conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pikeperch, Sander lucioperca (Linnaeus, 1758), is a predatory fish in temperate waters of Eurasia (Rennert et al. 2004), and is a valuable species for aquaculture due to its rapid growth, flesh quality and high commercial value. According to FAO (2011), the amount of production exceeded 690 tons in 2010, but the total pikeperch production in aquaculture was 4 % of the caught amount in open waters (17,300 tons). As a new potential candidate for intensive aquaculture in Europe (Hilge and Steffens 1996), the interest to develop methods for commercial pikeperch culture has increased during the last decade (Kestemont and Melard 2000), and the aquaculturists have shown legitimate interest in intensive culture technologies for pikeperch. As basic preconditions for controlled cultivation, researchers succeeded in establishing protocols for artificial reproduction of pikeperch spawners (Zakes and Szczepkowski 2004; Ronyai 2007; Muller-Belecke and Zienert 2008; Hermelink et al. 2011).
The reproduction cycle of pikeperch depends on annual photoperiod and temperature (Wang et al. 2010). Relevant research has proven that photoperiod and daily variations of light intensity changes are involved in the environmental induction and quality of perch reproduction which include the timing and rate of spawning, fertilization and the mortality of broodstock (Fontaine et al. 2006; Migaud et al. 2006; Pourhosein Sarameh et al. 2012).
One of the main reasons for controlling the body clock is to change the reproduction cycle and allow rapid egg production along with larva outside the natural cycle (Bowden 2008). Migaud et al. (2006) reported that photoperiod conditions highly influence gametogenesis, female fecundity, spawning and fertilization rates, spawning time and breeder mortality in Eurasian perch broodstock. They showed that seasonal variations of photoperiod and daily variations of light intensity are important factors involved in the control and timing of gametogenesis and spawning, respectively. Photoperiod manipulation of broodstock is used to achieve year-round production of gametes in a number of teleost species that are commercially cultured, e.g., rainbow trout (Oncorhynchus mykiss), Eurasian perch (Perca fluviatilis), Atlantic cod (Gadus morhua) and sea bass (Dicentrarchus labrax) (Carrillo et al. 1989; Davies et al. 1999: Norberg et al. 2004; Migaud et al. 2004, 2006; Fontaine et al. 2006). Amano et al. (2001) examined the effects of photoperiod on pituitary levels of two types of gonadotropin (GTH), FSH and LH, in Masu salmon Oncorhynchus masou to find the mechanism of synthesis. Their results indicated that the synthesis of FSH and LH are differently regulated by photoperiod. Some studies have shown that constant photoperiods (24L:0D and 0L:24D) influenced percids gametogenesis and spawning time (Migaud et al. 2006; Pourhosein Sarameh et al. 2012).
Generally, fish are sensitive to acute and chronic environmental changes and react by stress responses (Pickering and Duston 1983; Wedemeyer et al. 1990; Barton and Iwama 1991; Iwama et al. 1995; Wendelaar Bonga 1997; Barton 1997, 2000). Although photoperiod changes do not regularly affect hormone levels and the diel activity, stress is not regarded as an evident outcome (Pickering and Pottinger 1983; Audet et al. 1986; Biswas et al. 2004). However, Leonardi and Klempau (2003) found that photoperiod manipulation induced a significant surge in stress responses of rainbow trout.
It has been found that photoperiod changes allow the animal to trace daily and seasonal rhythms and, accordingly, change its physiology in which the immune system is included as well (Bowden 2008). Similarly, hematological parameters such as the physiological condition indicators which help prevent and control stress-related pathogens are of great significance. These parameters are used as health and stress conditions in fish (Valenzuela et al. 2007).
Studies related to the effect of photoperiod on hematological parameters have shown different results, probably due to the differences of the illumination protocols utilized, species specificity, and the duration of light exposure (Melingen et al. 2002; Leonardi and Klempau 2003; Biswas et al. 2004). Typically, hematological parameters are recognized as secondary stress indicators when a growth in erythrocyte numbers, hematocrit, hemoglobin concentration, and leucopenia is observed (Wedemeyer et al. 1990; Wojtaszek et al. 2002; Pierson et al. 2004). Esteban et al. (2006) demonstrated that the humoral innate immune system has a circadian rhythm based on the light–dark cycle and this cycle might be affected by the pineal gland.
Wild fish caught for artificial reproduction purposes are frequently exposed to multiple stressors, but the influence of previous or ongoing stress on an animal’s subsequent response is poorly understood (McConnachie et al. 2012a, b). Fish exposed to stressful stimuli during transport, e.g., handling, netting, unloading and inadequate water exchange, usually suffer from a fight and flight stress response and it may cause adverse physiological reactions affecting the essential life functions (Erikson et al. 1997). Handling is a common practice for checking the breeders for ovulation, and we considered this practice as a main stressor during the reproductive season. This factor is unavoidably stressful cases in fish breeding and culture centers (Belanger et al. 2001) which can cause physiological and hematological changes (Acerete et al. 2004). Of course, the fact should be considered that maintaining the safety of brooders during vitellogenesis and oogenesis is of great significance in reducing mortality at larval and post-larval stages as well (Swain and Nayak 2009). The results of Valenzuela et al. (2012) implied that trout under an artificial photoperiod may be more susceptible to disease; this could be related to the immunosuppressive effect of cortisol on the defense system or to reproductive aspects. So, it seems necessary to study stress responses and hematological parameters of fish when subjected to typical handling stressor in aquaculture practices.
Considering those parameters and the studies which stated percids are specifically sensitive to lighting factors (Hokanson 1977; Migaud et al. 2002), it is necessary to investigate whether photoperiod manipulation causes significant stress and hematological changes in pikeperch before suggesting the use of photoperiods in different stages of the reproduction cycle.
Previous experiments which focused on fish hematological parameters to different photoperiods reported that those parameters changed under a constant photoperiod (24L:0D) (Valenzuela et al. 2006a; Bani et al. 2009; Falahatkar et al. 2012b). Also, Migaud et al. (2003) suggested Eurasian perch is especially sensitive to lighting conditions. We focused on evaluating whether pikeperch is especially sensitive to lighting or if it is more sensitive to constant photoperiod (darkness and light). So, constant light and darkness were established to investigate the effect of light severity on pikeperch, and we considered those photoperiods versus natural 12L:12D for comparison of some changes in the blood and immune system in pikeperch at the final stage of reproduction.
Practically, data about the “photoperiod * stress” interaction on pikeperch broodstocks in the final stage of reproduction, stress response and immune system are scarce. So, it is necessary to investigate whether photoperiod influences stress or maybe that photoperiod alters hematological parameters and immune system through stress. Therefore, the aim of this study was to examine the changes of hematological parameters and stress responses in broodstocks photo-manipulated during the final stage of pikeperch reproduction. Determining how pikeperch male and female respond to normal photoperiod (12L:12D), constant photoperiods (light/darkness), and interactions between photoperiod and stress will allow researchers/aquaculturists to understand the nature of the physiological changes in this species.
Materials and methods
Fish and rearing facilities
In autumn (December), brooders were captured by seine net from the lake behind the Aras dam located in northwestern Iran, between Iran and Nakhjavan Republic, which is the most important pikeperch habitat in the southwestern basin of the Caspian Sea. After 2 days, they were transferred by a truck equipped with an oxygen injector to the Dr. Yousefpour Fish Hatchery Center, located in Siahkal, Guilan province (north of Iran). For 3 months, they were kept in wintering 4 ha earthen ponds and were fed with baitfish (carp fries). Fish were held under natural photoperiod condition before starting the trial in March with an appropriate water temperature. The natural spawning season of pikeperch usually occurs in April/May (Teletchea et al. 2009), but naturally occurs during March/May in Iran when the temperature is 8.0–14 °C (Razavi et al. 1972). In March, 72 fish were harvested from the pond and sexually separated based on fish shape and genital papilla characteristics, and then they were transferred to 18 concrete tanks (4 fish per tank, 4–5 years old and 1:1 sex ratio) with 1,490 L in capacity, 50 cm depth and an average water flow of 20.2 ± 0.9 L min−1, in a non-recirculated system. Brooders had an average weight and length of 1,367.4 ± 55.3 g and 53.7 ± 0.6 cm (mean ± SE), respectively. The density of fish in each tank was 0.92 ± 0.05 kg m−3. They were adapted to the rearing condition for 10 days. Temperature and dissolved oxygen were controlled by a thermometer and oxymeter. During the experiment, temperature was kept at 13.1 ± 0.5 °C and dissolved oxygen at 9.7 ± 0.4 mg L−1. The age of the broodstocks was determined by the scales taken from the line of demarcation between the lateral line and dorsal fin. The scales were examined under microscope with magnification ×20 and the age was determined by counting the dark and light circles (Biswas 1993). Due to low temperature and prevention of eggs feeding by baitfish, broodstocks were not fed during the experimental period.
Photoperiod
To study the effect of light severity or the direction of the changes of photoperiod on stress indicators and hematological parameters, pikeperch broodstocks with the same natural photoperiod history were examined for 40 days with three different photoperiods: constant light (24L:0D), constant darkness (0L:24D), and half day of light followed by an abrupt change to half day of darkness (12L:12D) photoperiod that represents the normal photoperiod. The light required for 24L:0D and 12L:12D photoperiods was supplied by low-consumption fluorescent 100 W, 630 lux lamps (Pars Shahab, Rasht, Iran) installed 40 cm above the water surface for each tank. Black plastic was used for covering the tanks during the darkness periods for 0L:24D and 12L:12D photoperiods. The mean distance between each tank was 55.5 cm and there was no light interference between the tanks.
Handling stress
Each day at 9:15 AM, the water level in the tank of the fish exposed to handling stress was reduced to 10 cm from the bottom. Then, they were quickly captured by a scoop net and kept out of the water for 20 s (as simulation for checking the fish reproduction status), and later they were transferred back to the tank with water level increased back to the first level. These fish were called the fish of stressful photoperiods, and they were under the influence of handling stress for 40 days.
Experimental design
Each photoperiod consisted of three stressful and three stress-free replications. So, there were two groups of fish in each photoperiod. In one group, the fish were not exposed to any stress so they were called the fish of stress-free photoperiods. In the other group, they were exposed to handling stress and called the fish of stressful photoperiods.
Sampling and analysis
All parts of sampling and fish care during the experiments were conducted in accordance with the Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching (Granstrom 2003). Blood sampling was done after anaesthetizing brooders with a 300 mg L−1 of clove powder (Dadkhah, Rasht, Iran). All the procedure for blood sampling took less than 5 min for each fish. The blood sampling was taken immediately after spawning. In fish which did not spawn, the blood sampling was conducted at the end of the 40 days. Blood (2 mL) was taken from the caudal vein using 5 mL heparinized syringes and then divided into two aliquots. The first aliquot was used for hematological analysis, and the second one was centrifuged at 1,500×g for 10 min for separating the plasma, then it was kept in tubes at −25 °C until the time of analysis.
Hematological parameters were determined within 4 h after sampling. Red blood cell (RBC) and white blood cell (WBC) counts were calculated using a Neubauer hemocytometer. In order to count RBC, the blood was diluted 200 times using Natt and Herrick’s solution, and numbers were manually determined by Neubauer counting chamber. Also, the WBC counts were done in the same counting chamber of the RBC with the exception that WBC obtained from four squares of the large square were counted in replications that were averaged. Hematocrit was determined using microhematocrit technique (Brown 1980) immediately after sampling and placing the fresh blood in glass capillary tubes and centrifuging in a microhematocrit centrifuge for 10 min at 3,500×g. Hemoglobin concentration was determined by cyan-methemoglobin method (Van Kampen and Zijlstra 1961). Differential white blood cell counts were obtained from smears stained with May Grunwald-Giesma (Valenzuela et al. 2008). At least 200 WBCs were counted for differential WBC counts from two smears of each fish.
Plasma cortisol level was measured by radioimmunoassay (RIA) using a commercial antibody kit (cortisol-3-OCMO-antiserum, Immunotech, Prague, Czech Republic) described by Ruane et al. (2001). Plasma glucose level was measured using analytical kits (Greiner Diagnostic, Bahlingen, Germany) (Biswas et al. 2008). Plasma lactate was determined colorimetrically based on Barton et al. (2005).
Statistical analyses
All data collected at the location of the experiment along with those of the laboratory were recorded in Excel software and analyzed by SPSS software (Version 13, Chicago, IL, USA). The normality of the data was checked through Kolmogorov–Smirnov test. The two-way ANOVA test was used to study the effect of photoperiods (3 levels) and stress (2 levels) as independent variables on all biochemical and hematological parameters. Moreover, Tukey’s test was used for determining the significant difference between variance means. Comparison of the two stresses in each treatment was performed using the independent samples t test. The significance level in this study was P < 0.05. All data is presented as mean ± SE.
Results
Stress responses
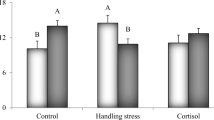
Average plasma cortisol and lactate levels showed no significant differences in male and female broodstocks under different photoperiods and handling stress (Fig. 1a, b). In females, no significant differences were observed between glucose levels in different photoperiods; however, glucose level in stressed fish under the 0L:24D-s photoperiod (the stressfully dark photoperiods) had a significant difference (P < 0.05) to that of the unstressed fish (0L:24D) (Fig. 1a). This means that we observed a significant difference in glucose levels between stressful and stress-free conditions in the 0L:24D photoperiod. Average glucose levels in male broodstocks showed no significant difference in the different photoperiods and handling stress (Fig. 1b). Overall, the interaction of light and stress in terms of stress responses was not observed in male and female broodstocks.
Mean values (± SE) of stress indices (cortisol, lactate and glucose) of female (a) and male (b) pikeperch (Sander lucioperca) broodstocks in different photoperiods: constant light (n = 12, 24L:0D), 12 h light–12 h darkness (n = 12, 12L:12D), constant darkness (n = 12, 0L:24D), constant light with stress (n = 12, 24L:0D-s), 12 h light–12 h darkness with stress (n = 12, 12L:12D-s), constant darkness with stress (n = 12, 0L:24D-s). *Significant difference between stressful and stress-free conditions in a photoperiod
Hematological parameters
In male and female brooders, hematocrit and hemoglobin showed no significant differences under different photoperiods and handling stress (Fig. 2a, b). Females did not display any significant differences in RBC under different photoperiods and handling stress (Fig. 2a). RBC of male brooders showed a significant difference under different photoperiods at which 12L:12D had a significant rise in the number of RBC and showed a significant difference in comparison with other photoperiods; no significant difference was observed between stressful and stress-free conditions under different photoperiods (Fig. 2b). WBC of females showed a significant difference under different photoperiods and handling stress so that 0L:24D had a significant rise compared to the 24L:0D and 0L:24D-s photoperiods (Fig. 2a). WBC of male brooders indicated no significant difference under different photoperiods and handling stress (Fig. 2b). In terms of stress condition, a significant difference was observed in the number of WBC between stressful and stress-free conditions in the 0L:24D photoperiod.
Mean values (± SE) of hematological parameters (hematocrit, hemoglobin, RBC and WBC) of female (a) and male (b) pikeperch (Sander lucioperca) broodstocks in different photoperiods: constant light (n = 12, 24L:0D), 12 h light–12 h darkness (n = 12, 12L:12D), constant darkness (n = 12, 0L:24D), constant light with stress (n = 12, 24L:0D-s), 12 h light–12 h darkness with stress (n = 12, 12L:12D-s), constant darkness with stress (n = 12, 0L:24D-s). *Significant difference between stressful and stress-free conditions in a photoperiod. Different letters (a, ab, b) denote significant difference (P < 0.05) among different photoperiods
Differential count of leukocytes
In males, the number of lymphocytes, neutrophils, eosinophils and monocytes showed no significant differences under different photoperiods and handling stress (Table 1). Females did not display any significant difference in the number of monocytes and eosinophils under different photoperiods and handling stress (Table 2). The number of lymphocytes in females showed no significant difference under different photoperiods and handling stress; however, lymphocytes showed an interaction between light and stress. Also an interaction between light and stress on the number of neutrophils was observed.
Discussion
Results showed that different photoperiods and handling stress in the final stage of reproduction do not lead to a significant difference in pikeperch male and female broodstocks concerning cortisol and lactate concentrations. In male and female broodstocks, maximum average level of cortisol was observed during the 24L:0D-s and 24L:0D photoperiod, respectively. The reason why cortisol levels did not change in the stressed fish could be explained by the fish becoming adapted to stressful conditions. Further, it might be possible that pikeperch has the ability to clear cortisol out of the system very quickly. Therefore, fish were not quite capable of responding to stress at the end of the experiment because according to other studies long periods of exposure to a stressor could lead to allostasis which is the body’s ability to return to its physiological levels observed before applying a stress (McEwen 1998; Schreck 2000; Fast et al. 2008). According the observation of Jentoft et al. (2005), the Eurasian perch stressed once had significantly higher cortisol levels than the repeatedly stressed fish after 24 h. Barton et al. (1987) showed habituated reductions in post-stress levels of plasma cortisol and glucose in juvenile rainbow trout stressed daily. Thus, the observed habituation in our study was apparently sufficient to explain absence of significant difference between the repeatedly handling stressed fish and the unstressed fish. Fast et al. (2008) showed that the continual application of an acute stressor over a 4-week period did not result in chronically elevated level of cortisol in Atlantic salmon (Salmo salar). Alternatively, the lack of increase in cortisol in stressed individuals may be due to a negative feedback of daily increase in cortisol on the hypothalamus–pituitary–interrenal axis (Pickering and Pottinger 1987).
The baseline level of cortisol in our fish was higher compared to the basal levels of other similar species. According to the observation by Falahatkar et al. (2012a) juvenile pikeperch showed high cortisol levels (261.7 ± 47.1 ng mL−1) at the resting time of the experiment. This high cortisol level indicates that pikeperch is highly sensitive. It seems that high basal cortisol level is species-specific and also related to the history background of fish.
In our experiment, all photoperiods indicated an increase in cortisol level; however, no significant difference was observed between different photoperiods, stressful, and stress-free conditions under different photoperiods. Biswas et al. (2008) found that different photoperiods do not cause a considerably significant stress response (glucose-cortisol) in striped knifejaw, Oplegnathus fasciatus. In their study, applying handling stress significantly increased plasma cortisol and glucose levels. However, these levels returned to those of the control group within 24 h. In other studies, manipulation of a photoperiod did not result in a considerably induced stress response in different fish species, either (Pickering and Pottinger 1983; Audet et al. 1986; Biswas et al. 2004, 2006a, b, 2008). Similar to our findings, the results obtained by Fast et al. (2008) on Atlantic salmon and those from Wang et al. (2004) on striped bass Morone saxatilis, brooders showed that continuous use of an acute handling stressor did not result in a long-term increase in glucose and cortisol levels.
In our study, a significant increase in glucose levels of female brooders of the 0L:24D photoperiod compared to the 0L:24D-s photoperiod is probably due to the fact that long exposure to darkness with no exhausting of fish under handling stress which showed reduction of glucose. It seems that female pikeperch brooders are sensitive to darkness and finally react to constant darkness by an increased glucose level as secondary stress response. Because stress responses might change in different photoperiods, light regimes may cause higher or lower sensitivity (Biswas et al. 2006a). In addition, the exposure time of the stressor can influence the magnitude of the physiological responses and the sensitivity of physiological response depends on stressor type (Acerete et al. 2004), which justifies the reason for a significant decrease of the glucose level in brooders during 0L:24D-s (stressful conditions) compared to that of the 0L:24D (stress-free conditions). Our results suggest that female pikeperch would be sensitive to continuous darkness (0L:24D) in the final stages of reproduction. Additionally, in this study, pikeperch in 24L:0D and 12L:12D photoperiods did not show significant stress responses, suggesting that the fish adapted to the stressor. But in females, continuous darkness caused secondary stress responses.
Spawning and fasting have effects on cortisolemia (Milla et al. 2009). It seems that long exposures to darkness during the 0L:24D photoperiod (with consideration of delayed spawning during 0L:24D photoperiod compared with other photoperiods) and lack of spawning in half of the brooders which were kept in 0L:24D-s photoperiods (Pourhosein Sarameh et al. 2012) caused an increase in the intensity of stress and consequently more intense stress responses. Photophase and illumination play a fundamental role in the daily behavior of fish, and many fish activity patterns vary with photophase and light intensity (Eriksson 1978; Levine et al. 1980). In fact, the intensity of the stressor caused secondary stress responses through increasing glucose levels of the stress-free dark photoperiod (0L:24D) and decreasing glucose levels of the stressful dark photoperiod (0L:24D-s).
Broodstocks in our study were not fed during the experimental period but the glucose levels increased in all photoperiods (with or without handling stress); however, no significant differences were observed between different photoperiods. Also, no changes of cortisol were found among the treatments. It seems that fasting pikeperch did not affect cortisol and glucose levels. Findings by Czesny et al. (2003) have shown that food deprivation did not affect the level of plasma cortisol in walleye (Stizostedion vitreum). Also, the glucose levels in food-deprived Arctic charr remained almost constant throughout the whole post-handling period (Jorgensen et al. 2002). As food deprivation increases glucose turnover in fish (Vijayan and Moon 1994), the lack of change in handling-induced plasma glucose concentration in the food-deprived fish may also be due to enhanced glucose production offsetting the increased clearance (Vijayan and Moon 1994).
In our study, the plasma lactate levels showed no significant differences in male and female broodstocks under different photoperiods and handling stress. This could be related to the low level of activity of fish observed during the experiment. It is perhaps due to lower muscle glycogen content which argue for a diminished capacity for anaerobic metabolism in pikeperch broodstocks. Some studies demonstrated that photoperiod manipulation caused no significant chronic and acute stress response in striped knifejaw and red sea bream (Biswas et al. 2006a, 2008). According to the observation of Bani et al. (2009), lactate levels of juvenile beluga sturgeon (Huso huso) were higher in the 0L:24D and 24L:0D photoperiods compared with 12L:12D and 18L:6D photoperiods. In contrast to the beluga sturgeon, Clarias gariepinus under 12L:12D and 18L:6D showed higher lactate levels compared to fish reared under 0L:24D and 6L:18D photoperiods (Almazan-Rueda et al. 2005).
The plasma lactate levels in Eurasian perch did not show significant changes after handling stress (Acerete et al. 2004). Recently, Falahatkar et al. (2012a), however, observed a significant decrease in lactate concentrations during the transportation of pikeperch juveniles. One possible explanation for decreases in lactate concentrations may be found in the age differences and duration of exposure to stressor. Also, environmental hypoxia would stimulate lactate appearance by activating anaerobic glycolysis in muscle and possibly liver, and that it would decrease lactate disposal by reducing the capacity of tissues to oxidize lactate (Omlin and Weber 2010). But in our study, no significant differences were observed that showed less sensitivity of the pikeperch brooders during the long exposure to stressors.
Results of the present study confirmed that different photoperiods and handling stress in the final stage of reproduction did not cause significant changes in hematocrit and hemoglobin levels in brooders. Adeyemo et al. (2009) did not find any significant difference in hematocrit and hemoglobin levels after applying handling stress in African catfish. When rainbow trout was held under the constant light regime (24L:0D) for 2 months, fish showed lower concentration in hemoglobin (Valenzuela et al. 2006a). In our findings in male brooders, RBC showed a significant difference during different photoperiods; males treated under 12L:12D had a significant increase of these cells relative to 24L:0D and 0L:24D photoperiods, but females did not display any significant difference in RBC under different photoperiods and handling stress. It is probable that sex-related characteristics are the reason for these differences because variation in erythrocyte number in terms of sex has already been documented (Lane 1979). Females and males of landlocked sockeye salmon showed different endocrine response to stress (Kubokawa et al. 2001). Moreover, the previous report indicated that the reproduction condition of fish causes changes in hematological parameters (Cech and Wohlschlag 1982). Therefore, differences in sexual maturity and reproduction performance account for the RBC count changes in males and females during different photoperiods. Fagundes and Urbinati (2008) showed that RBC counts of Pintado catfish Pseudoplatystoma corruscans were at the highest levels immediately after applying a light or dark photoperiod. In a study by Valenzuela et al. (2006b), constant light caused a decrease in immature erythrocyte numbers of rainbow trout, after an initial increase. Bani et al. (2009) found the effect of 12L:12D and 24L:0D photoperiods on juvenile beluga which had the highest and lowest erythrocyte counts, respectively, compared to other light regimes. Since a study has shown that Eurasian perch as a percid representative is sensitive to a 1-h photoperiod decrease (Fontaine et al. 2006), exposure to constant light (24L:0D) and darkness (0L:24D) photoperiods account for its significant decrease compared to the 12L:12D photoperiod used in this study. However, we found that RBC counts did not show any significant difference under various photoperiods with stressful condition. Our results suggest that during the breeding season, male pikeperch respond to photoperiod with changes of erythrocyte number, while females respond to photoperiod with changes of immunity parameters.
In the present study, results of WBC suggested that it may be possible that change in responses of fish under different photoperiod regimes to stressor might occur (Biswas et al. 2006a). The interaction between light and stress was only observed in the number of WBC in males and in the number of WBC and RBC in females. Some factors such as age, maturation, reproductive behavior and nutrition may influence the immunity parameters in brood fish (Swain and Nayak 2009). Seasonal changes such as photoperiod, adverse environmental conditions, and stress conditions like handling can also affect the immunity (Swain and Nayak 2009). Seasonal adaptation can be both initiated and/or terminated by changes in photoperiod (Bowden et al. 2007). Results of our study show that sensitivity of the immune system of pikeperch to darkness was evident as a significant increase in the number of WBC in female brooders. In this regard, researchers stated that seasonal changes in the environment induce variations in the immune capacity of poikilothermic vertebrates (Zapata et al. 1992; Yellon et al. 1999; Leu et al. 2001). Reversely, WBC of Jundia (Rhamdia quelen Quoy and Gaimard Pimelodidae) was not significantly affected by handling stress (Barcellos et al. 2004). Acerete et al. (2004) reported that the magnitude and duration of the physiological response in Eurasian perch are related to the stressor applied, and the dynamics of response depend on the type of stressor. In addition, Schreck et al. (2001) stated that the stress responses can be polymorphic with regard to species of fish, stage of maturity, type and severity of stressor. Consequently, differences in sexual maturity and the reproduction performance of fish explain WBC count changes in male and female brooders exposed to different photoperiods.
With regards to the effect of different photoperiods and handling stress on different leukocyte populations, Melingen et al. (2002) exposed Atlantic salmon to photoperiods and found that the fish had fewer lymphocytes (B-cells) and more neutrophils under continuous light conditions. Similarly, Valenzuela et al. (2008) observed fewer numbers of lymphocytes in rainbow trout under continuous light conditions. They stated that longer exposures to light (6:6–12:12–24:0) have a reverse relationship with the number of lymphocytes. In the present study, however, no significant difference was observed in leukocytes between different photoperiods and handling stress in females; the number of neutrophils and lymphocytes showed interaction between light and stress that could be due to stress induced by photoperiods or reproductive state that might be the probable cause of these changes. Pickering (1986) described lymphopenia in sexually mature fish toward spawning time. In the study of Biswas et al. (2004), tilapias Oreochromis niloticus exposed to a repeated photoperiod (6L:6D) had a larger number of lymphocytes compared to 12L:12D, but no change was observed in the number of neutrophils. Moreover, in our study, the number of neutrophils in male brooders did not show significant differences between different photoperiods and handling stress, which was probably due to functional activation of the hemopoietic tissues (Peters and Schwarzer 1985) that releases more monocytes and neutrophils to encounter an external stressor. Rowley et al. (1988) reported that the granulocytic function of neutrophils and monocytes is a very important non-specific defense in fish which serves as a first line of the cell-mediated defense in cases of encountering any external stressor. This means lymphopenia and neutrophilia, being the outcomes of exposing fish to stress, lead to either decreasing lymphocytes or increasing immunological cells in lymphoid tissues (Espelid et al. 1996). This result shows that the immune system of females is more sensitive to constant photoperiod, especially constant darkness. Since WBC and glucose levels of females during the 0L:24D exhibited a significant rise compared to the 0L:24D-s, also with consideration of the interaction between light and stress in the number of neutrophils and lymphocytes, these results have led to the view that female pikeperch are more sensitive to constant darkness. So darkness photoperiod changed the physiological and immune responses to handling stress. In addition, the sensitivity of the immune system of brooders to constant photoperiod suggests that photoperiods influence immunity via stress. The stress response can be polymorphic with regard to species of fish, stage of maturity, and type and severity of stressor (Schreck et al. 2001). Moreover, with consideration of the delayed or absence of spawning in half of the fish exposed to handling stress (see Pourhosein Sarameh et al. 2012), it is concluded that the handling process induces stress in pikeperch and leads to a poor reproduction performance. Maximum average level of cortisol in males was observed during the 24L:0D-s photoperiod, and in females it was seen in the 24L:0D. Also, the interaction between light and stress in the number of neutrophils and lymphocytes showed sensitivity of immune system encounter the stressors, such as photoperiod or handling stress.
In summary, handling stress in pikeperch may affect the immune responses to photoperiod. These results indicate the importance of the proper management of brooders to prevent the deleterious effects of handling stress in fish. So, the importance of preventing harmful effects of handling stress and identifying methods which can minimize the adverse effects of handling should be considered. This work demonstrates that pikeperch immune system and hematological parameters is affected by photoperiod and handling stress, but female broodstocks are far more sensitive to different photoperiods. The psychological aspects of stress appear to be important in terms of physiological responses to stressful situations and perhaps the ability of the fish to resist exhaustion or achieve compensation. Regarding S. lucioperca, it seems, from the present results, that pikeperch either needs a longer exposure to this type of stressor to break out cortisol response or more likely that ability of this fish to recover rapidly from handling stressor. Even though animals chronically stressed seem to compensate physiologically to the stressor, their ability to perform important functions at the whole organism level may be affected (Schreck 2000).
Conclusions
This study allowed distinguishing the effect of light and stress in terms of stress responses on the induction of the reproductive cycle, changes in the blood parameters and immune system in pikeperch at the final stage of reproduction. Constant light and darkness were identified as playing a major role and the main inductive cues in this species. The immune system of pikeperch females was further aggravated by the application of constant photoperiod. Also, with consideration to the sensitivity of the immune system (the interaction between light and stress in the number of neutrophils and lymphocytes), we can mention that photoperiods influence immunity via stress, and especially females are sensitive to darkness during their final stages of reproduction.
To conclude, the sensitivity of hematological parameters, especially the immune system of pikeperch brooders to constant photoperiods in this reproductive stage, is pointed out here and the effects of intensity, duration and time of applying stressors in different reproductive stages on physiological responses are taken into account. In addition, brooders becoming adapted to acute handling stress are found to be related to long-term exposure to stressor. To have a complete understanding of these effects and sensitivities more research is necessary. Furthermore, it is well known that stress also influences immunity, but because of scarcity of data it is unknown whether photoperiod influences stress or not. The results obtained here along with those of future studies might help develop a suitable photoperiod regime or change photoperiods in different stages of maturity until the spawning of pikeperch. Furthermore, conducting studies on the effects of photoperiod on altering the timing of maturation and various maturational processes and the effects of light intensity, duration, and different photoperiod applications during different reproductive stages of pikeperch on hematological parameters, immune system, stress response, and reproductive performance are necessary.
References
Acerete L, Balasch JC, Espinosa E, Josa A, Tort L (2004) Physiological responses in Eurasian Perch (Perca fluviatilis L) subjected to stress by transport and handling. Aquaculture 237:167–178
Adeyemo OK, Naigaga I, Alli RA (2009) Effect of handling and transportation on haematology of African catfish Clarias gariepinus. J Fish Sci 3:333–341
Almazan-Rueda P, Van Helmond ATM, Verreth JAJ, Schrama JW (2005) Photoperiod affects growth, behaviour and stress variables in Clarias gariepinus. J Fish Biol 67:1029–1039
Amano M, Ikuta K, Kitamura S, Aida K (2001) Effects of photoperiod on pituitary gonadotropin levels in masu salmon. J Exp Zool 289:449–455
Audet C, FitzGerald GJ, Guderley H (1986) Photoperiod effects on plasma cortisol levels in Gasterosteus aculeatus. Gen Comp Endocrinol 61:76–81
Bani A, Tabarsa M, Falahatkar B, Banan A (2009) Effects of different photoperiods on growth, stress and haematological parameters in juvenile great sturgeon Huso huso. Aquac Res 40:1899–1907
Barcellos LJG, Kreutz LC, de Souza C, Rodrigues LB, Fioreze I, Quevedo RM, Cericato L, Soso AB, Fagundes M, Conrad J, de Almeida Lacerda L, Terra S (2004) Hematological changes in jundia (Rhamdia quelen Quoy and Gaimard Pimelodidae) after acute and chronic stress caused by usual aquacultural management, with emphasis on immunosuppressive effects. Aquaculture 237:229–236
Barton BA (1997) Stress in finfish: past, present and future a historical perspective. In: Iwama GK, Pickering AD, Sumpter JP, Schreck CB (eds) Fish stress and health in aquaculture, Seminar Series Society for Experimental Biology. Cambridge University Press, Cambridge, 62:1–33
Barton BA (2000) Salmonid fishes differ in their cortisol and glucose responses to handling and transport stress. N Am J Aquac 62:12–18
Barton BA, Iwama GK (1991) Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu Rev Fish Dis 1:3–26
Barton BA, Schreck CB, Barton LD (1987) Effects of chronic cortisol administration and daily acute stress on growth, physiological conditions, and stress responses in juvenile rainbow trout. Dis Aquat Org 2:173–185
Barton BA, Ribas L, Acerete L, Tort L (2005) Effects of chronic confinement on physiological responses of juvenile gilthead sea bream, (Sparus aurata L) to acute handling. Aquac Res 36:172–179
Belanger JM, Son JH, Laugero KD, Moberg GP, Doroshov SI, Lankford SE, Cech JJ Jr (2001) Effects of short-term management stress and ACTH injections on plasma cortisol levels in cultured white sturgeon, Acipenser transmontanus. Aquaculture 203:165–176
Biswas SP (1993) Manual of methods in fish biology. South Asian Publishers, PVT Ltd, New Delhi, p 157
Biswas AK, Maita M, Yoshizaki G, Takeuchi T (2004) Physiological responses in Nile tilapia exposed to different photoperiod regimes. J Fish Biol 65:811–821
Biswas AK, Seoka M, Takii K, Maita M, Kumai H (2006a) Stress response of red sea bream Pagrus major to acute handling and chronic photoperiod manipulation. Aquaculture 252:566–572
Biswas AK, Seoka M, Tanaka Y, Takii K, Kumai H (2006b) Effect of photoperiod manipulation on the growth performance and stress response of juvenile red sea bream Pagrus major. Aquaculture 258:350–356
Biswas AK, Seoka M, Ueno K, Yong ASK, Biswas BK, Kim YS, Takii K, Kumai H (2008) Growth performance and physiological responses in striped knifejaw Oplegnathus fasciatus held under different photoperiods. Aquaculture 279:42–46
Bowden TJ (2008) Modulation of the immune system of fish by their environment. Fish Shellfish Immunol 25:373–383
Bowden TJ, Thompson KD, Morgan AL, Gratacap RML, Nikoskelainen S (2007) Seasonal variation and the immune response: a fish perspective. Fish Shellfish Immunol 22:695–706
Brown BA (1980) Routine hematology procedures. In: Brown BA (ed) Hematology, principles and procedures. Lea and Fiebiger, Philadelphia, pp 71–112
Carrillo M, Bromage N, Zanuy S, Serrano R, Prat F (1989) The effect of modifications in photoperiod on spawning time, ovarian development and egg quality in the sea bass (Dicentrarchus labrax L.). Aquaculture 81:351–365
Cech JJ, Wohlschlag DE (1982) Seasonal patterns of respiration, gill ventilation, and hematological characteristics in the striped mullet, (Mugil cephalus L). Bull Mar Sci 32:130–138
Czesny S, Rinchard J, Abiado MA, Dabrowski K (2003) The effect of fasting, prolonged swimming, and predator presence on energy utilization and stress in juvenile walleye (Stizostedion vitreum). Physiol Behav 79:597–603
Davies B, Bromage N, Swanson P (1999) The brain-pituitary-gonadal axis of female rainbow trout Oncorhynchus mykiss: effects of photoperiod manipulation1. Gen Comp Endocrinol 115:155–166
Erikson U, Sigholt T, Seland A (1997) Handling stress and water quality during live transportation and slaughter of Atlantic salmon (Salmo salar). Aquaculture 149:243–252
Eriksson LO (1978) Nocturnalism versus diurnalism, dualism within fish individual: rhythmic activity of fishes. Bull Am Mus Nat Hist 117:399–481
Espelid S, Lokken GB, Steiro K, Bogwald J (1996) Effects of cortisol and stress on the immune system in Atlantic salmon (Salmo salar L). Fish Shellfish Immunol 6:95–110
Esteban MA, Cuesta A, Rodríguez A, Meseguer J (2006) Effect of photoperiod on the fish innate immune system: a link between fish pineal gland and the immune system. J Pineal Res 41:261–266
Fagundes M, Urbinati EC (2008) Stress in pintado Pseudoplatystoma corruscans during farming procedures. Aquaculture 276:112–119
Falahatkar B, Akhavan S, Efatpanah I, Meknatkhah B (2012a) Primary and secondary responses of juveniles of a teleostean, pikeperch Sander lucioperca, and a chondrostean, Persian sturgeon Acipenser persicus, to handling during transport. N Am J Aquac 74:241–250
Falahatkar B, Poursaeid S, Efatpanah I, Meknatkhah B, Biswas A (2012b) Effect of photoperiod manipulation on growth performance, physiological and hematological indices in juvenile Persian sturgeon, Acipenser persicus. J World Aquac Soc 43:692–700
FAO (2011) Culture aquatic species information programme Sander lucioperca (Linnaeus, 1758). http://www.fao.org/fishery/species/3098/en. Accessed on 24 June 2012
Fast MD, Hosoya S, Johnson SC, Afonso LOB (2008) Cortisol response and immune-related effects of Atlantic salmon Salmo salar Linnaeus subjected to short- and long-term stress. Fish Shellfish Immunol 24:194–204
Fontaine P, Pereira C, Wang N, Marie M (2006) Influence of pre-inductive photoperiod variations on Eurasian perch Perca fluviatilis broodstock response to an inductive photothermal program. Aquaculture 255:410–416
Granstrom DE (2003) Agricultural (non biomedical) animal research outside the laboratory: a review of guidelines for institutional animal care and use committees. ILAR 44:206–210
Hermelink B, Wuertz S, Trubiroha A, Rennert B, Kloas W, Schulz C (2011) Influence of temperature on puberty and maturation of pikeperch, Sander lucioperca. Gen Comp Endocrinol 172:282–292
Hilge V, Steffens W (1996) Aquaculture of fry and fingerling of pike-perch (Stizostedion lucioperca L) a short review. J Appl Ichthyol 12:167–170
Hokanson KEF (1977) Temperature requirements of some percids and adaptations to the seasonal temperature cycle. J Fish Res Board Can 34:1524–1550
Iwama GK, Morgan JD, Barton BA (1995) Simple field methods for monitoring stress and general condition of fish. Aquac Res 26:273–282
Jentoft S, Aastveit AH, Torjesen PA, Andersen O (2005) Effects of stress on growth, cortisol and glucose levels in non-domesticated Eurasian perch (Perca fluviatilis) and domesticated rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol 141:353–358
Jorgensen EH, Vijayan MM, Aluru N, Maule AG (2002) Fasting modifies Aroclor 1254 impact on plasma cortisol, glucose and lactate responses to a handling disturbance in Arctic charr. Comp Biochem Physiol C 132:235–245
Kestemont P, Melard C (2000) Aquaculture. In: Craig JF (ed) Percid fishes: systematics, ecology and exploitation. Blackwell Science, Oxford, pp 191–224
Kubokawa K, Yoshioka M, Iwata M (2001) Sex-specific cortisol and sex steroids responses in stressed Sockeye salmon during spawning period. Zool Sci 18:947–954
Lane HC (1979) Progressive changes in the haematology and tissue water of sexually mature trout, Salmo gairdneri Richardson during the autumn and winter. J Fish Biol 15:425–436
Leonardi MO, Klempau AE (2003) Artificial photoperiod influence on the immune system of juvenile rainbow trout Oncorhynchus mykiss in the southern hemisphere. Aquaculture 221:581–591
Leu SL, Shiah IS, Yatham LN, Cheu YM, Lam RW (2001) Immune-inflammatory markers in patients with seasonal affective disorder: effects of light therapy. J Affect Disord 63:27–34
Levine JS, Lobel PS, ManNichols EF (1980) Visual communication in fishes. In: Ali MA (ed) Environmental physiology of fishes. Plenum, New York, pp 447–475
McConnachie SH, O’Connor CM, Gilmour KM, Iwama GK, Cooke SJ (2012a) Supraphysiological cortisol elevation alters the response of wild bluegill sunfish to subsequent stressors. J Exp Zool 317:321–332
McConnachie SH, Cook KV, Patterson DA, Gilmour KM, Hinch SG, Farrell AP, Cooke SJ (2012b) Consequences of acute stress and cortisol manipulation on the physiology, behavior, and reproductive outcome of female Pacific salmon on spawning grounds. Horm Behav 62:67–76
McEwen BS (1998) Protective and damaging effects of stress mediators. N Engl J Med 338:171–179
Melingen GO, Pettersen EF, Wergeland HI (2002) Leucocyte populations and response to immunization and photoperiod manipulation in Atlantic salmon (Salmo salar L) 0 + smolt. Aquaculture 214:381–396
Migaud H, Fontaine P, Sulistyo I, Kestemont P, Gardeur JN (2002) Induction of out-of-season spawning in Eurasian perch Perca fluviatilis: effects of cooling and chilling periods on female gametogenesis and spawning. Aquaculture 205:253–267
Migaud H, Mandiki R, Gardeur JN, Kestemont P, Bromage NR, Fontaine P (2003) Influence of photoperiod regimes on the Eurasian perch gonadogenesis and spawning. Fish Physiol Biochem 28:395–397
Migaud H, Wang N, Gardeur JN, Fontaine P (2006) Influence of photoperiod on reproductive performances in Eurasian perch Perca fluviatilis. Aquaculture 252:385–393
Miguad H, Fontaine P, Kestemont P, Wang N, Brun-Bellut J (2004) Influence of photoperiod on the onset of gonadogenesis in Eurasian perch Perca fluviatilis. Aquaculture 241:561–574
Milla S, Wang N, Mandiki SNM, Kestemont PK (2009) Corticosteroids: friends or foes of teleost fish reproduction? Comp Biochem Physiol 153:242–251
Muller-Belecke A, Zienert S (2008) Out-of-season spawning of pikeperch (Sander lucioperca L) without the need for hormonal treatments. Aquac Res 39:1279–1285
Norberg B, Brown CL, Halldorsson O, Stensland K, Bjornsson BT (2004) Photoperiod regulates the timing of sexual maturation, spawning, sex steroid and thyroid hormone profiles in the Atlantic cod Gadus morhua. Aquaculture 229:451–467
Omlin T, Weber JM (2010) Hypoxia stimulates lactate disposal in rainbow trout. J Exp Biol 213:3802–3809
Peters G, Schwarzer R (1985) Changes in hemopoietic tissue of rainbow trout under influence of stress. Dis Aquat Org 1:1–10
Pickering AD (1986) Changes in blood cells composition of the brown trout (Salmo trutta L) during spawning season. J Fish Biol 29:335–347
Pickering AD, Duston J (1983) Administration of cortisol to brown trout (S. trutta) and its effects on the susceptibility to Saprolegnia infection and furunculosis. J Fish Biol 23:163–175
Pickering AD, Pottinger TG (1983) Seasonal and diel changes in plasma cortisol levels of brown trout, (Salmo trutta L). Gen Comp Endocrinol 49:232–239
Pickering AD, Pottinger TG (1987) Crowding causes prolonged leucopenia in salmonid fish, despite interrenal acclimation. J Fish Biol 30:701–712
Pierson PM, Lamers A, Flik G, Mayer-Gostan N (2004) The stress axis, stanniocalcin and ion balance in rainbow trout. Gen Comp Endocrinol 137:263–271
Pourhosein Sarameh S, Falahatkar B, Azari Takami G, Efatpanah I (2012) Effects of different photoperiods and handling stress on spawning and reproductive performance of pikeperch Sander lucioperca. Anim Reprod Sci 132:213–222
Razavi B, RaLonde R, Walczak P (1972) Report on stock assessment and composition of the commercial bony fishes of the southern Caspian Sea. Report of the Fisheries Research Institute, Bandar Anzali, p 32
Rennert B, Wirth M, Gunther S, Schulz C (2004) Influence of nutritional status of zander fingerlings (Sander lucioperca) on survival during wintering. Fisch Teichwirt 55:688–690
Ronyai A (2007) Induced out-of-season and seasonal tank spawning and stripping of pikeperch (Sander lucioperca L). Aquac Res 38:1144–1151
Rowley AF, Hunt TC, Page M, Mainwaning G (1988) Fish blood cells. In: Rowley AF, Ratcliff NA (eds) Vertebrate blood cells. Cambridge University Press, Cambridge, pp 19–127
Ruane NM, Huisman EA, Komen J (2001) Plasma cortisol and metabolite profiles in two isogenic strains of common carp during confinement. J Fish Biol 59:1–12
Schreck CB (2000) Accumulation and long-term effects of stress in fish. In: Moberg GP, Mench JA (eds) The biology of animal stress: basic principles and implications for animal welfare. CAB International, Wallingford, pp 147–158
Schreck CB, Contreras-Sanchez W, Fitzpatrick MS (2001) Effects of stress on fish reproduction, gamete quality, and progeny. Aquaculture 197:3–24
Swain P, Nayak SK (2009) Role of maternally derived immunity in fish. Fish Shellfish Immunol 27:89–99
Teletchea F, Gardeur JN, Psenicka M, Kaspar V, Dore YL, Linhart O, Fontaine P (2009) Effects of four factors on the quality of Male reproductive cycle in pikeperch (Sander lucioperca). Aquaculture 291:217–223
Valenzuela AE, Silva VM, Klempau AE (2006a) Effects of constant light on haematological parameters of cultured rainbow trout Oncorhynchus mykiss in the southern hemisphere. Fish Physiol Biochem 32:113–120
Valenzuela AE, Silva VM, Klempau AE (2006b) Qualitative and quantitative effects of constant light photoperiod on rainbow trout Oncorhynchus mykiss peripheral blood erythrocytes. Aquaculture 251:596–602
Valenzuela AE, Silva VM, Klempau AE (2007) Some changes in the haematological parameters of rainbow trout Oncorhynchus mykiss exposed to three artificial photoperiod regimes. Fish Physiol Biochem 33:35–48
Valenzuela AE, Silva VM, Klempau AE (2008) Effects of different artificial photoperiods and temperatures on haematological parameters of rainbow trout Oncorhynchus mykiss. Fish Physiol Biochem 34:159–167
Valenzuela AE, Campos V, Yanẽz F, Alveal K, Gutiérrez P, Rivas M, Contreras N, Klempau A, Fernandez I, Oyarzun C (2012) Application of artificial photoperiod in fish: a factor that increases susceptibility to infectious diseases? Fish Physiol Biochem 38:943–950
Van Kampen EJ, Zijlstra WG (1961) Standardization of hemoglobinometry, II. The hemiglobincyanide method. Clin Chim Acta 6:538–544
Vijayan MM, Moon TW (1994) The stress response and the plasma disappearance of corticosteroid and glucose in a marine teleost the sea raven. Can J Zool 72:379–386
Wang C, King VW, Woods LC III (2004) Physiological indicators of divergent stress responsiveness in male striped bass broodstock. Aquaculture 232:665–678
Wang N, Teletchea F, Kestemont P, Milla S, Fontaine P (2010) Photothermal control of the reproductive cycle in temperate fishes. Rev Aquacult 2:209–222
Wedemeyer GA, Barton BA, McLeay DJ (1990) Stress and acclimation. In: Schreck CB, Moyle PB (eds) Methods in fish biology. American Fisheries Society, Bethesda, pp 451–489
Wendelaar Bonga SE (1997) The stress response in fish. Physiol Rev 77:591–625
Wojtaszek J, Dziewulska-Szwajkowska D, Lozinska-Gabska M, Adamowicz A, Dzugaj A (2002) Hematological effects of high dose of cortisol on the carp (Cyprinus carpio L): cortisol effect on the carp blood. Gen Comp Endocrinol 125:176–183
Yellon SM, Fagoaga OR, Nehlsen-Cannarella SL (1999) Influence of photoperiod on immune cell functions in the male Siberian hamster. Am J Physiol 276:97–102
Zakes Z, Szczepkowski M (2004) Induction of out-of-season spawning of pikeperch, (Sander lucioperca L). Aquac Int 12:11–18
Zapata G, Varas A, Torroba M (1992) Seasonal variations in the immune system of lower vertebrates. Immunol Today 13:142–147
Acknowledgments
Thanks are extended to the staff at the Dr. Yousefpour Fish Hatchery Center for their help during the experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pourhosein Sarameh, S., Falahatkar, B., Azari Takami, G. et al. Physiological changes in male and female pikeperch Sander lucioperca (Linnaeus, 1758) subjected to different photoperiods and handling stress during the reproductive season. Fish Physiol Biochem 39, 1253–1266 (2013). https://doi.org/10.1007/s10695-013-9780-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-013-9780-z