Abstract
The sustainability of aquaculture is subject to environmental changes, such as changes in temperature, which affect the physiological homeostasis of fish. Thermal shock (natural or anthropogenic) is a stress condition that results in a sequence of physiological responses and even mortality. Considering the impact of sudden reductions in temperature on the biological systems and that this occurrence is common in regions where pacu (Piaractus mesopotamicus) is farmed, we evaluated the stress, immune, and antioxidant responses of the species exposed to temperature drop. Two groups of fish were exposed to 28 °C or 16 °C for 24 h, and then fish were kept at 28 °C to recover and be sampled: before the temperature drop (baseline); after 24 h of cold exposure; and 3, 8, and 24 h after the return to 28 °C (recovery). The exposure of pacu to 16 °C did not alter the stress biomarkers after 24 h, but it activated the innate immune system (leukocyte respiratory activity, hemolytic activity of the complement system, and serum lysozyme concentration). It also promoted oxidative stress without altering most of the antioxidant system indicators, except for an initial reduction of glutathione peroxidase (GPx) activity after exposure to 16 °C and a late activation, besides the late activation of glutathione transferase (GST), probably as a compensatory process to reduce tissue damage. Pacu was sensitive to temperature changes, but it was able to activate immune and compensatory responses to mitigate oxidative stress. The results will help producers in the management of pacu during periods of thermal changes, such as in autumn and winter.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The growing demand for protein sources for human consumption has driven the growth of aquaculture. It is estimated that the total production of fish in the world will increase to 204 million tons in 2030 (FAO 2020), and Brazil is following this trend, with production reaching 551.9 thousand tons in 2020 (IBGE 2020). However, the success of this activity depends on environmental conditions, such as temperature, which can impact the regulation of events such as the neuroendocrine, oxidative, metabolic, osmotic, molecular, and immunological responses of fish (Islam et al. 2021). The dependence of aquaculture production on the environment suggests its vulnerability to climate change (Mugwany et al. 2022).
Within a limited range, fish can adjust to the gradual temperature changes common in natural systems. However, heat shock (natural or anthropogenic) is a common stress condition that occurs when fish acclimatized to a certain range of water temperature are exposed to a sudden decrease in temperature, resulting in a sequence of behavioral responses, sublethal physiological conditions, and even mortality (Donaldson et al. 2018). Temperature is an important stress factor during fish farming (Islam et al. 2021), and changes can impair biochemical reactions (Wen et al. 2017); physiological functions, including the immune system, which is essential for protection against pathogens (Ahmad et al. 2011; Abram et al. 2017; Abram et al. 2019; Zhou et al. 2019); and fish growth (Milstein et al. 2000; Mello et al. 2022). Elevated cortisol levels have been found in fish acutely exposed to low temperatures (Chen et al. 2002; Liu et al. 2011; Arslan et al. 2015; Alzaid et al. 2015). Stress is an animal’s response to any extrinsic or intrinsic stimulus that alters its body homeostasis (Wendelaar Bonga 1997). In acute conditions, the mobilization of biological systems involved in the adjustment response, adaptation, and resistance to altered conditions occurs, while biological exhaustion and a loss of adaptive capacity result from the chronicity of the adjustment process (Wendelaar Bonga 1997; Mommsen et al. 1999; Barton et al. 2002), causing losses in the growth, reproduction, and immune functions of fish (Wendelaar Bonga 1997; Mommsen et al. 1999). Biochemical changes in blood parameters can occur in response to changes in metabolic demand, which can vary with temperature (Ficke et al. 2007; Xie et al. 2017) and can be considered a physiological stress response.
Among other functions, temperature can determine the speed of chemical reactions and/or cause changes in the conformation of the three-dimensional structure of enzymes and thus affect the catalytic capacity of reactions (Miyazaki et al. 2000), thereby interfering with biological processes, altering the respiratory rate, and causing physiological disturbances (Pörtner et al. 2002; Vinagre et al. 2012). Temperature can modulate oxygen consumption with a consequent increase in the production of reactive oxygen species (Lushchak and Bagnyukova 2006; Lushchak 2011; Welker et al. 2013), which results in oxidative stress.
The target fish of our study is the pacu (Piaractus mesopotamicus) due to its economic value in fish farming in South America (Valladão et al. 2018). In regions where the average water temperature is below 22 °C, the rearing of pacu is unfeasible, demonstrating the difficulty in producing this species at low temperatures (Ferrari and Bernardino 1986). According to Garcia et al. (2008), pacu is not able to tolerate temperatures lower than 16 °C, and temperatures between 20 and 32 °C were used in studies using pacu as the target fish (Borghetti and Canzi 1993; Assis et al. 2004; Paula et al. 2013). Previous studies with pacu have addressed the interaction of acute (Bacchetta et al. 2020; Ale et al. 2021) or chronic (Pinto et al. 2019; Nitz et al. 2020) changes in temperature with metabolism and oxidative stress, but they did not focus on stress and immune system responses. Considering the impact of sudden temperature reduction on fish biological systems and that this occurrence is common in regions where pacu is raised (Sant’Anna Neto et al. 2015), this study evaluated stress responses and the immune and antioxidant systems of species acutely exposed to temperature reduction.
Material and methods
Experimental fish and sample collection
Fifty pacu juveniles (159.83 ± 26.08 g, mean ± SD), supplied by Aquaculture Center of UNESP-CAUNESP, were checked regarding their health condition following guidelines from the Conselho Nacional de Controle de Experimentação Animal. They were randomly distributed into 10 indoor plastic tanks (100 L, rectangular box, 41 × 56 × 78 cm) (5 fish per tank), acclimated to the laboratory conditions for 7 days in a running flow water system at 28 °C (light regime 12L:12D; open flow from a well water, water flow 50 L/h; supplemental aeration), and sorted into two treatments: control temperature/28 °C, using thermostat heater, (28.1 °C ± 0.28; dissolved oxygen 6.3 ± 0.34 ml L−1; total ammonia 0.49 ± 0.017 mg L−1) and low temperature/16 °C (16.13 °C ± 0.11, dissolved oxygen 7.8 ± 0.1 ml L−1; total ammonia 0.46 ± 0.02 mg L−1). For the temperature exposure, the running water flow was closed in both treatments, keeping the water recirculating separately in both treatments compounded by five tanks each. A cooling equipment (Chiller, Gelaqua® Split) connected to the system was used to gradually reduce (1 °C/h) the temperature until reaching 16 °C. Following this, the fish were maintained for 24 h at this condition. After this period, running water flow was reopened, and it took 2 h to return from 16 to 28 °C (beginning of the recovery period). Fish were sampled before the temperature decreases (baseline sampling); 24 h after the 16 °C exposure; and 3, 8, and 24 h after the return of 28 °C (recovery period) (5 fish/treatment in each sampling). In each sampling, fish were captured and anesthetized with MS222 (50 mg L−1), and blood was collected by caudal vessels puncture and stored in microtubes containing or not containing anticoagulant. During the experiment, fish were not fed.
Stress biomarkers
Plasma was extracted from blood with fluoride EDTA (Glistab-Labtest) to quantify the concentration of glucose and cortisol with commercial kits (Labtest Ref.133 and DRG-Cortisol ELISA — enzyme-linked immunosorbent assay — 1887, respectively). The inter-assay coefficient of variation of the cortisol analysis was 5.4%, and intra-assay varied from 2.7 to 3.8%.
Innate immune biomarkers
An aliquot of whole blood with heparin was used to determine the respiratory activity of leukocytes (RAL) according to the protocol by Anderson and Siwicki (1995) and modified for pacu (Biller-Takahashi et al. 2013). The method is based on colorimetric determination of reactive oxygen species using nitroblue tetrazolium dye (NBT — Sigma Aldrich, N6876). The optical density of the solution was determined using a spectrophotometer — Thermo Scientific®, Genesys 10S UV–vis at 540 nm.
From blood stored without anticoagulant, serum was separated and used to determine the concentration of lysozyme and hemolytic activity of the complement system. Lysozyme concentration was determined according to the protocol of Demers and Bayne (1997) and modified for pacu (Zanuzzo et al. 2015) based on the lysis of the gram-positive bacterium Micrococcus lysodeikticus (Sigma-4698) at 450 nm. The complement system hemolytic activity (HAC50) was determined according to Zanuzzo et al. (2017) using a kinetic assay at 700 nm.
Antioxidant system and oxidative stress indicators
After blood collection, the fish were euthanized in a concentrated solution of MS222 for liver removal, which was immediately frozen in liquid nitrogen and kept at −80 °C.
Liver samples were homogenized (1:10 w/v) in a phosphate buffer solution 0.1 M, pH 7.0 (superoxide dismutase — SOD, catalase — CAT, reduced glutathione — GSH, glutathione transferase — GST, lipid peroxidation — LPO) and 7.6 (glutathione peroxidase — GSH-Px), centrifuged, and the supernatants were stored at −80 °C for further analysis. For the calculation of the enzyme activity, the protein content was determined according to Bradford (1976).
The activity of SOD was determined according to McCord and Fridovich (1969) based on the inhibition of the reduction rate of cytochrome c by the superoxide radical at 550 nm. SOD activity was expressed in units of mg protein−1, with one unit of SOD corresponding to the quantity of enzyme that promoted the inhibition of 50% of the reduction rate of cytochrome c.
The activity of CAT was determined according to Beutler (1975) based on the consumption of exogenous hydrogen peroxide (H2O2) by CAT, generating water and oxygen. The absorbance of the solution was read at 240 nm every 15 s for 5 min, and activity was expressed as nmol/mg protein/min.
The activity of GPx was measured according to Hopkins and Tudhope (1973) and estimated indirectly through the oxidation of NADPH in the presence of glutathione reductase and the substrate oxidized glutathione produced by the action of GPx at 340 nm.
The activity of GST activity was determined in a method based on the conjugation reaction of GSH with 1-chloro-2-4-dinitrobenzene substrate, which is broadly specific for the various isoforms of the enzyme with reading at 340 nm (Gagné 2014).
The concentration of GSH was determined according to Beutler et al. (1963). Glutathione reacts with 5,50-dithiobis-2-nitrobenzoic acid, forming thiolate, measurable at 412 nm against a GSH standard curve. Lipid peroxidation (LPO) was determined by thiobarbituric acid reactive substances (TBARS) assay according to Camejo et al. (1998). TBARS concentrations were expressed in nmol MDA mg protein−1.
Statistics
The experiment was set up in a completely randomized design in a factorial scheme 2 × 1, with 5 longitudinal factors (sampling times). The data were evaluated by normality tests (Cramer–von Mises), homoscedasticity (Brown–Forsythe), and two-way analysis of variance, and when the differences were significant (p < 0.05), a Tukey test was applied for comparison of the means using SAS software (version 9.3). N = 5. Values are means ± standard error.
Results
Stress biomarkers
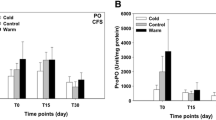
After 24 h of exposure, when the running water system was closed, the fish at both temperatures (28 °C and 16 °C) showed an increase in cortisol concentrations, and at 3 h into the recovery period, after the return to 28 °C, there was a reduction in cortisol in fish from both treatments (control, p = 0.0004; 16 °C, p = 0.0013) (Fig. 1A).
Cortisol (A) and glucose (B) concentration in the plasma of juvenile pacu (Piaractus mesopotamicus) exposed to 28 °C and 16 °C. Sampling times were 24 h of exposure and 3, 8, and 24 h of recovery. Lowercase letters indicate differences between the same treatment at different sampling times. The letters A and B indicate differences between treatments at the same sampling time (p < 0.05). The absence of symbols indicates the absence of statistical difference
Glucose concentrations did not differ, along the samplings, in control fish, while fish exposed to 16 °C showed the lowest values at 8 h of the recovery period (p = 0.1530), when the control group values were higher (p = 0.0233) (Fig. 1B).
Innate immune biomarkers
After 24 h with the water running system closed, RAL increased in fish exposed to both temperatures (28 °C and 16 °C), without differences between them, and reduced at 3 h of recovery in control fish and after 8 h in 16 °C fish (control, p < 0.0001; 16 °C, p < 0.0001) (Fig. 2A). At 3 h of recovery, the 16 °C fish had a higher RAL than the control fish (p = 0.0066) (Fig. 2A).
Respiratory activity of leukocytes (RAL) (A), hemolytic activity of the complement system in serum (HAC50) (B) serum concentrations of lysozyme (C) of juvenile pacu (Piaractus mesopotamicus) exposed to 28 °C and 16 °C. Sampling times were 24 h of exposure and 3, 8, and 24 h of recovery. Lowercase letters indicate differences between the same treatment at different sampling times. The letters A and B indicate differences between treatments at the same sampling time (p < 0.05)
Fish exposed to 16 °C showed higher HAC50 after the low temperature exposure, with a peak value at 8 h of recovery, while in the control group, there was a fluctuation of values without significant change (control, p = 0.1435; 16 °C, p = 0.1454) (Fig. 2B). At all sampling times, the 16 °C group had higher HAC50 than the control group, except in the baseline sampling (24 h exp., p = 0.0185; 3 h, p = 0.0012; 8 h, p = 0.0174) (Fig. 2B).
Regarding the serum lysozyme concentrations, they increased in the control fish at 8 h of recovery compared to the values observed at 24 h of exposure and at 3 h of recovery (p = 0.0414), while the 16 °C fish presented the highest value at 24 h of recovery (p = 0.0002) (Fig. 2C). Comparing the groups, fish exposed to 16 °C always showed higher lysozyme concentrations than the control fish, except in the baseline sampling and at 8 h (24 h exp., p = 0.0016; 3 h, p = 0.0410; 24 h, p = 0.0004) (Fig. 2C).
Antioxidant system and oxidative stress indicators
The activity of the superoxide dismutase (SOD) did not differ between treatments (28 °C, p = 0.9224; 16 °C, p = 0.3596) (Fig. 3A). Catalase activity (CAT) decreased in control fish after 24 h with the water running system closed, while the 16 °C fish showed a similar profile but without statistical significance (control, p = 0.002; 16 °C, p = 0.3863) (Fig. 3B).
Activity of superoxide dismutase (SOD) (A), catalase (CAT) (B), glutathione peroxidase (GPx) (C), glutathione transferase (GST) (D), concentration of reduced glutathione (GSH) (E), and lipoperoxidation (LPO) (F) in the liver of juvenile pacu (Piaractus mesopotamicus) exposed to 28 °C and 16 °C. Sampling times were 24 h of exposure and 3, 8, and 24 h of recovery. Lowercase letters indicate differences between the same treatment at different sampling times. Letters A and B indicate differences between treatments at the same sampling time (p < 0.05). The absence of symbols indicates the absence of statistical difference
Glutathione peroxidase (GPx) activity decreased in fish exposed to 16 °C for 24 h (p = 0.2086), when the activity was lower than that observed in the control fish (p = 0.0289). At 24 h of recovery, this pattern inverted and the 16 °C fish showed higher activity (p = 0.0095) (Fig. 3C).
Regarding glutathione transferase (GST) activity (Fig. 3D), the control fish showed small fluctuations, with an increase at 8 h and 24 h of recovery (p = 0.0666), while the 16 °C fish showed the highest activity at 8 h (p = 0.0031), when the activity was higher than in the control fish (p = 0.0215).
Reduced glutathione (GSH) concentrations did not differ between treatments (control, p = 0.5539; 16 °C, p = 0.5325) (Fig. 3E). There was an increase in LPO in fish exposed to 16 °C for 24 h, which remained until 8 h of recovery (p = 0.0010) (Fig. 3F). In the control fish, LPO increased only after 24 h of observation, when the running water system was closed (p = 0.0322). Compared to the control, the 16 °C fish always presented higher values, except at the baseline sampling and at 24 h of exposure (24 h exp., p = 0.0284; 3 h recovery, p = 0.0076; 8 h recovery, p = 0.0162).
Discussion
Physiological processes in fish are closely influenced by temperature fluctuations, as fish respond to suboptimal temperatures in an adaptive manner to maintain physiological homeostasis (Yang et al. 2018; Islam et al. 2021). The pacu exposure to 16 °C for 24 h did not modify the stress biomarkers, but it promoted activation of the innate immune system (leukocyte respiratory activity, HAC50, and lysozyme). In addition, it promoted oxidative stress without altering most indicators of the antioxidant system, except for an initial reduction in GPx activity, after exposure to 16 °C, followed by a late activation. GST was also activated late, probably as a compensatory process to reduce tissue damage.
The absence of changes in cortisol and glucose levels at the end of 24 h of exposure to 16 °C in relation to the control group does not rule out the occurrence of stress caused by cold, as reported in other studies. According to Chen et al. (2002), exposure of Oreochromis aureus to a temperature reduction, from 25 to 12 °C, increased cortisol concentrations 1 and 2 h after exposure. The same was observed in Larimichthys crocea, which were acutely exposed to a reduction in temperature, from 23 to 9 °C (1 °C every hour) and showed an increase in cortisol concentrations in the following 48 h (Liu et al. 2012). The increase in cortisol observed at 24 h after the interruption of water flow also occurred in the control group, which suggests that the increase in circulating hormone in that sampling was associated with the flow interruption. However, we must consider that the determination of biomarkers was not carried out at more acute times. Since the beginning of the water cooling, the fish were exposed to the changes, and cortisol levels could have increased before the samplings. The temperature reduction took 12 h, and fish were exposed to 16 °C for an additional 24-h period in which cortisol could have been cleared from the blood. Faced with a short-term stressor, fish have the natural ability to respond adaptively (Wendelaar Bonga 1997), which may explain the result observed in this study after 24 h of exposure to 16 °C. Regarding the increase of circulating cortisol in control fish, as well as in cold-exposed fish, as commented above, it can be associated with the running flow interruption, though the indicators of the water quality were within levels appropriate to the pacu (control group — 28.1 °C ± 0.28; dissolved oxygen 6.3 ± 0.34 ml L−1; total ammonia 0.49 ± 0.017 mg L−1; and 16 °C group — 16.13 °C ± 0.11, dissolved oxygen 7.8 ± 0.1 ml L−1; total ammonia 0.46 ± 0.02 mg L−1) (Urbinati and Takahashi 2021).
Variations in water temperature promote changes in body temperature and can affect key physiological processes for health and survival, such as the immune system of fish (Bowden 2008; Ahmad et al. 2011; Abram et al. 2017; Abram et al. 2019; Zhou et al. 2019). In the case of a short-term stressor, the response can have an activation phase that enhances innate responses (Tort 2011). This can explain what was observed in the present study. Fish exposed to 16 °C showed the activation of RAL at 3 h after the reestablishment of flow, activation of the complement system, and increase in serum lysozyme levels throughout the experiment from thermal exposure.
AHC50 reached peak values at 8 h of recovery, and lysozyme reached peak values later, at 24 h. The complement system is an indicator of the immune condition, and its activity, by the alternative pathway, is determined by the serum hemolytic activity when in contact with foreign erythrocytes (Yano 1992; Biller-Takahashi et al. 2012; Zanuzzo et al. 2017; Mello et al. 2019; Assis and Urbinati 2020; Faria et al. 2021; Lopes et al. 2022). Serum lysozyme concentrations are also used to express the immune status of fish (Zanuzzo et al. 2017; Mello et al. 2019; Assis and Urbinati 2020; Faria et al. 2021; Lopes et al. 2022).
In contrast with our findings, in Epinephelus coioides exposed to 19, 27, and 35 °C for 96 h, the respiratory burst decreased at 3, 48, and 96 h, after fish were transferred to 19 and 35 °C, while HAC50 and lysozyme activity decreased at 3–96 h after fish were transferred to 19 °C and 35 °C (Cheng et al. 2009). In Sparus aurata exposed to low temperatures for 2 weeks, both complement activity and lysozyme activity decreased after the reduction of temperature from 18 to 11 °C, being 1 °C per day (Tort et al. 2004).
Regarding the hepatic antioxidant system, our results suggest that the system was impaired by exposure to cold. The activities of SOD and CAT, which are important enzymes of oxidative metabolism, did not change. SOD, by reacting with the superoxide ion, forms hydrogen peroxide, which is decomposed into water and oxygen by CAT (Aebi 1984). Thus, the data suggest that this pathway was not activated, contributing to the installation of oxidative stress. In addition, low temperature negatively modulated GPx activity, which decreased after 24 h of exposure to 16 °C. However, in the last sampling, 24 h of recovery, GPx increased its activity and contributed to reducing the LPO. The enzyme glutathione peroxidase works by reducing different types of peroxides, including hydroperoxides, taking them to their corresponding alcohols, using GSH as a cofactor and generating oxidized glutathione (GSSG) as a product (Halliwell and Gutteridge 2007). Regarding the activity of GST, it increased from 3 h after the return to the control temperature in fish exposed to cold, concomitant with a gradual increase in LPO observed in the same group of fish. The GST acts in the detoxification of the organism, in the elimination of xenobiotics and other metabolites derived from lipid peroxidation through conjugation with reduced GSH (Raza 2011; Croom 2012), and in the reduction of hydroperoxides (Regoli et al. 2011). Higher GST activity in the liver of cold-exposed fish may indicate a compensatory mechanism to reduce tissue damage. A consequence of the increase in GST may be the reduction in LPO at 24 h, when an increase in GPx activity was also observed. According to Depledge (1989), an initial disturbance caused by a toxic exposure generates responses to regulate the cellular and tissue homeostasis. GSH concentrations were also unchanged. Glutathione levels are regulated by several enzymes (Meister and Anderson 1983) but mainly depend on the balance between the rate of GSH synthesis (by glutamate:cysteine ligase), rate of conjugation (by GSTs), rate of oxidation (not enzymatically or by GPx), and reduction of GSSG to GSH (by glutathione reductase). In fish, GSH levels can increase through biosynthesis or by regeneration by glutathione reductase as a protective response (Peña-Llopis et al. 2003). The concentration of GSH present in cells is used as a non-enzymatic marker of oxidative stress (Halliwell and Gutteridge 2007; Grim et al. 2013).
LPO increased after exposure of pacu to 16 °C for 24 h and only decreased 24 h after the temperature returned to 28 °C, when GPx and GST were increased. The characterization of oxidative stress is evidenced by changes in the levels of oxidative damage markers that can be products of protein and lipid oxidation (Halliwell and Gutteridge 1999; Stadtman and Levine 2000; Hermes-Lima 2004; Lushchak 2011). Lipoperoxidation starts with the reaction of a free radical with an unsaturated fatty acid and the consequent formation of peroxyl radicals (Halliwell and Gutteridge 1999). In the present study, increases in LPO indicated the occurrence of oxidative stress in fish exposed to 16 °C. Using a different cold exposure protocol than the one used in our study, Pinto et al. (2019) exposed pacu to a gradual temperature variation for 5 days. The GST activity in liver was reduced at 18 °C compared to that observed at 27 °C, while TBARS increased as the temperature increased from 18 to 21 °C and 27 °C. In another study, Bacchetta et al. (2020) submitted pacu to a cold challenge by transferring fish from 24 to 14 °C, without gradual reduction, and observed an increase only in the hepatic activity of SOD and glutathione reductase, the latter of which is not determined in our study. Using a protocol similar to that of Bacchetta et al. (2020), Ale et al. (2021) exposed pacu to thermal shock (from 24 to 14 °C), a condition in which they were kept for 24 h. The SOD activity increased in the liver, but other enzyme activities increased in other tissues, while oxidative stress was observed in the liver and intestine of fish exposed to 14 °C. In the aforementioned studies, the experimental protocol differs from that used in our study, which makes it difficult to compare the results, but, as observed in the different studies, temperature fluctuations lead to metabolic changes and, consequently, to the production of reactive oxygen species (Pavlović et al. 2010).
Conclusions
The present study showed that the drastic reduction in the comfort temperature and the exposure to cold (16 °C) for 24 h induced several biological changes in pacu, which were, on the one hand, protective, such as activation of the innate immune system (RAL, HAC50, and lysozyme). On the other hand, they showed that the hepatic antioxidant system had difficulty in combating oxidative stress, with a limited response of its components, that is, the reduction of GPx activity soon after exposure to cold and a lack of response of SOD, CAT, and GSH, with the late activation of GPx and activation of GST soon after exposure, when oxidative stress had already occurred. The results showed that pacu was sensitive to temperature reduction, but it was able to activate immune responses and compensatory responses to mitigate oxidative stress. The results will help producers in the management of pacu during periods of thermal change, such as autumn and winter.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Abram Q, Dixon B, Katzenback B (2017) Impacts of low temperature on the teleost immune system. Biology 6(4):39. https://doi.org/10.3390/biology6040039
Abram QH, Rodriguez-Ramos T, Bols NC, Katzenback BA, Dixon B (2019) Effect of suboptimal temperature on the regulation of endogenous antigen presentation in a rainbow trout hypodermal fibroblast cell line. Dev Comp Immunol 100:103–423. https://doi.org/10.1016/j.dci.2019.103423
Aebi H (1984) Catalase in vitro. In: Colowick SP, Kaplan NO (eds) Methods enzymology, vol 105. Academic Press, pp 121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Ahmad SM, Shah FA, Bhat FA, Bhat JIA, Balkhi MH (2011) Thermal adaptability and disease association in common carp (Cyprinus carpio communis) acclimated to different (four) temperatures. J Therm Biol 36:492–497. https://doi.org/10.1016/j.jtherbio.2011.08.007
Ale A, Bacchetta C, Rossi AS, Scarabotti PA, Cazenave J (2021) Low temperature stress in a cultured fish (Piaractus mesopotamicus) fed with Pyropia columbina red seaweed-supplemented diet. Fish Physiol Biochem 47:829–839. https://doi.org/10.1007/s10695-021-00944-7
Alzaid A, Hori TS, Hall JR, Rise ML, Gamperl AK (2015) Low-induced changes in stress hormone and steroidogenic transcript levels in cunner (Tautogolabrus adspersus), a fish capable of metabolic depression. Gen Comp Endocrinol 224:126–135. https://doi.org/10.1016/j.ygcen.2015.07.007
Anderson DP, Siwicki AK (1995) Basic haematology and serology for fish health programs. In: Shariff M, Arthur JR, Subasinghe RP (eds) Diseases in Asian Aquaculture II. Fish Health Section, Asian Fisheries Society, Manila, pp 185–202
Arslan G, Sahin T, Hisar O, Hisar SA (2015) Effects of low temperature and starvation on plasma cortisol, triiodothyronine, thyroxine, thyroid-stimulating hormone and prolactin levels of juvenile common carp (Cyprinus carpio). Mar Sci Tech Bull 4:5–9
Assis RWS, Urbinati EC (2020) Physiological activity of Aloe vera in pacu (Piaractus mesopotamicus) inoculated with Aeromonas hydrophila. Fish Physiol Biochem 46:1421–1430. https://doi.org/10.1007/s10695-020-00800-0
Assis JMF, Carvalho RF, Barbosa L, Agostinho CA, Dal Pal-Silva M (2004) Effects of incubation temperature on muscle morphology and growth in the pacu (Piaractus mesopotamicus). Aquaculture 237:251–267. https://doi.org/10.1016/j.aquaculture.2004.04.022
Bacchetta C, Ale A, Rossi AS, Karakachoff M, Cazenave J (2020) Effects of cold stress on juvenile Piaractus mesopotamicus and the mitigation by β-carotene. J Therm Biol 88:102497. https://doi.org/10.1016/j.jtherbio.2019.102497
Barton BA, Morgan JD, Vijayan MM (2002) Physiological and condition-related indicators of environmental stress in fish. American Fisheries Society, Bethesda, pp 111–148
Beutler E (1975) Red cell metabolism: a manual of biochemical methods. Grune & Stron, New York
Beutler E, Durom O, Kelly BM (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61:882–890
Biller-Takahashi JD, Takahashi LS, Marzocchi-Machado CM, Zanuzzo FS, Urbinati EC (2012) Hemolytic activity of alternative complement system as an indicator of innate immunity in pacu (Piaractus mesopotamicus). Rev Bras Zoot 41:237–241. https://doi.org/10.1590/S1516-35982012000200001
Biller-Takahashi JD, Takahashi LS, Saita MV, Gimbo RY, Urbinati EC (2013) Leucocytes respiratory burst activity as indicator of innate immunity of pacu Piaractus mesopotamicus. Braz J Biol 73:425–429. https://doi.org/10.1590/S1519-69842013000200026
Borghetti JR, Canzi C (1993) The effect of water temperature and feeding rate on the growth rate of pacu (Piaractus mesopotamicus) raised in cages. Aquaculture 114:93–101. https://doi.org/10.1016/0044-8486(93)90253-U
Bowden TJ (2008) Modulation of the immune system of fish by their environment. Fish Shellfish Immunol 25:373–383. https://doi.org/10.1016/j.fsi.2008.03.017
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Camejo G, Wallin B, Enojärvi M (1998) Analysis of oxidation and antioxidants using microtiter plates. In: Armstrong D (ed) Methods in molecular biology, Free Radical and Antioxidant Protocols, vol 108. Humana Press, Totowa, NJ, pp 377–387
Chen WH, Sun LT, Tsai CL, Song YL, Chang CF (2002) Cold-stress induced the modulation of catecholamines, cortisol, immunoglobulin M, and leukocyte phagocytosis in tilapia. Gen Comp Endocrinol 126:90–100. https://doi.org/10.1006/gcen.2001.7772
Cheng A, Cheng S-A, Chen Y-Y, Chen J-C (2009) Effects of temperature change on the innate cellular and humoral immune responses of orange-spotted grouper Epinephelus coioides and its susceptibility to Vibrio alginolyticus. Fish Shellfish Immunol 26:768–772. https://doi.org/10.1016/j.fsi.2009.03.011
Croom E (2012) Metabolism of xenobiotics of human environments. Prog Mol Biol Transl Sci 112:31–88. https://doi.org/10.1016/B978-0-12-415813-9.00003-9
Demers NE, Bayne CJ (1997) The immediate effects of stress on hormones and plasma lysozyme in rainbow trout. Dev Comp Immunol 21:363–373. https://doi.org/10.1016/s0145-305x(97)00009-8
Depledge MH (1989) The rational basis for detection of the early indicators of marine pollutants using physiological indicators. Ambio 18:301–302
Donaldson MR, Cooke SJ, Patterson DA, MacDonald JS (2018) Cold shock and fish. J Fish Biol 73:1491–1530. https://doi.org/10.1111/j.1095-8649.2008.02061.x
FAO Food and Agriculture Organization of the United Nations (2020) Global Aquaculture Production Statistics database update to 2020. Fisheries and Aquaculture Department
Faria FP, Martinez CBR, Takahashi LS, Mello MMM, Martins TP, Urbinati EC (2021) Modulation of the innate immune and antioxidant responses by cortisol in pacu (Piaractus mesopotamicus). Fish Physiol Biochem 47:895–905. https://doi.org/10.1007/s10695-021-00940-x
Ferrari VA, Bernardino G (1986) Efeitos da temperatura e densidade de estocagem na segunda alevinagem de pacu Colossoma mitrei. Simpósio Brasileiro de Aqüicultura, IV, ANAIS, Cuiabá, MT, p 87
Ficke AD, Myrick CA, Hansen LJ (2007) Potential impacts of global climate change on freshwater fisheries. Rev Fish Biol Fish 17:581–613. https://doi.org/10.1007/s11160-007-9059-5
Gagné F (2014) Xenobiotic biotransformation. In: Gagné F (ed) Biochemical ecotoxicology—principles and methods, vol Chap 7, 1st edn. Academic Press Elsevier
Garcia LDO, Copatti CE, Wachholz F, Pereira Filho W, Baldisserotto B (2008) Freshwater temperature in the state of Rio Grande do Sul, Southern Brazil, and its implication for fish culture. Neotrop Ichthyol 6:275–281. https://doi.org/10.1590/S1679-62252008000200016
Grim JM, Simonik EA, Semones MC, Kuhn DE, Crockett EL (2013) The glutathione-dependent system of antioxidant defense is not modulated by temperature acclimation in muscle tissues from striped bass, Morone saxatilis. Comp Biochem Physiol A 164:383–390. https://doi.org/10.1016/j.cbpa.2012.11.018
Halliwell B, Gutteridge JCM (1999) Free radicals in biology and medicine, 3rd edn. Oxford University Press
Halliwell B, Gutteridge JMC (2007) Free radicals in biology and medicine, 4th edn. Oxford University Press, Oxford
Hermes-Lima M (2004) Oxygen in biology and biochemistry: role of free radicals. In: Storey KB (ed) Functional metabolism: regulation and adaptation. John Wiley & Sons, New Jersey, pp 319–368
Hopkins J, Tudhope GR (1973) Glutathione peroxidase in human red cells in health and disease. J Haematol 25:563–575
IBGE - Instituto Brasileiro de Geografia e Estatística (2020) Censo agropecuário 2020. Disponível em: https://biblioteca.ibge.gov.br/visualizacao/periodicos/pdf
Islam MJ, Kunzmann A, Slater MJ (2021) Responses of aquaculture fish to climate change-induced extreme temperatures: a review. J World Aquac Soc 53(2):314–366. https://doi.org/10.1111/jwas.12853
Liu B, Wang MY, Xie J, Xu P, Ge XP, He YJ, Miu LH, Pan LS (2011) Effects of acute low stress on serum biochemical and immune parameters and liver Hsp70 gene expression in gift strain of Nile tilapia (Oreochromis niloticus). Acta Ecologica Sinica 31:4866–4873
Liu C, Ding J, Gao X, Du C, Hou C, Wu X, Shen W, Zhu J (2012) Effects of acute low temperature stress on the hormones and gene expression of glucocorticoid receptor of large yellow croaker Larimichthys crocea. J Therm Biol 99:103018. https://doi.org/10.1016/j.jtherbio.2021.103018
Lopes LMF, Mello MMM, Urbinati EC (2022) Dietary yeast β-glucan reduces cortisol plasma levels, enhances respiratory burst of leukocytes and serum lysozyme levels after Aeromonas hydrophila inoculation, and has lipolytic effects on the pacu fish (Piaractus mesopotamicus). Aquaculture 546(8):737411. https://doi.org/10.1016/j.aquaculture.2021.737411
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquatic Toxicol 101:13–30. https://doi.org/10.1016/j.aquatox.2010.10.006
Lushchak VI, Bagnyukova TV (2006) Temperature increase results in oxidative stress in goldfish tissues. 2. Antioxidant and associated enzymes. Comp Biochem Physiol C 143:36–41. https://doi.org/10.1016/j.cbpc.2005.11.018
McCord JE, Fridovich I (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). Journal of Biological Chemistry 244:6049–6055
Meister A, Anderson ME (1983) Glutathione. Ann Rev. Biochem 52:711–760. https://doi.org/10.1146/annurev.bi.52.070183.003431
Mello MMM, Faria CFP, Zanuzzo FS, Urbinati EC (2019) β-glucan modulates cortisol levels in stressed pacu (Piaractus mesopotamicus) inoculated with heat-killed Aeromonas hydrophila. Fish Shellfish Immunol 93:1076–1083. https://doi.org/10.1016/j.fsi.2019.07.068
Mello PH, Divanach P, Papadakis IE (2022) Temperature influences growth, digestive system ontogeny and lipids deposition in the liver in gilthead seabream (Sparus aurata) larvae and juveniles. Aquac Res 53(9):3416–3429. https://doi.org/10.1111/are.15849
Milstein A, Zoran M, Peretz Y, Joseph D (2000) Low temperature tolerance of pacu, Piaractus mesopotamicus. Environ Biol Fishes 58(4):455–460. https://doi.org/10.1023/A:1007672401544
Miyazaki K, Wintrode PL, Grayling RA, Rubingh DN, Arnold FH (2000) Directed evolution study of temperature adaptation in a psychrophilic enzyme. J Mol Biol 297(4):1015–1026. https://doi.org/10.1006/jmbi.2000.3612
Mommsen TP, Vijayan MM, Moon TW (1999) Cortisol in teleosts: dynamics, mechanisms of action, and metabolic regulation. Rev Fish Biol Fish 9:211–268. https://doi.org/10.1023/A:1008924418720
Mugwany M, Dawood MAO, Kimera F, Sewilam H (2022) Anthropogenic temperature fluctuations and their effect on aquaculture: a comprehensive review. Aquac Fish 7:223–243. https://doi.org/10.1016/j.aaf.2021.12.005
Nitz LF, Pellegrin L, Maltez LC, Pinto D, Sampaio LA, Monserrat JM, Garcia L (2020) Temperature and hypoxia on oxidative stress responses in pacu Piaractus mesopotamicus. J Therm Biol 92:102682. https://doi.org/10.1016/j.jtherbio.2020.102682
Paula TG, Almeida FLA, Carani FR, Vechetti-Júnior I, Padovani CR, Salomão RAS, Mareco EA, Santos VB, Dal-Pai-Silva M (2013) Rearing temperature induces changes in muscle growth and gene expression in juvenile pacu (Piaractus mesopotamicus). Comp Biochem Physiol B 169:31–37. https://doi.org/10.1016/j.cbpb.2013.12.004
Pavlović S, Borković S, Radovanović TB, Perendija BR, Despotovic S, Gavric J, Saičić ZS (2010) Seasonal variations of the activity of antioxidant defense enzymes in the red mullet (Mullus barbatus l.) from the Adriatic Sea. Marine Drugs 8(3):413–428. https://doi.org/10.3390/md8030413
Peña-Llopis S, Ferrando MD, Peña JB (2003) Fish tolerance to organophosphate-induced oxidative stress is dependent on the glutathione metabolism and enhanced by N-acetylcysteine. Aquatic Toxicol 65:337–360. https://doi.org/10.1016/S0166-445X(03)00148-6
Pinto D, Pellegrin L, Nitz LF, Costa ST, Monserrat JM, Garcia L (2019) Haematological and oxidative stress responses in Piaractus mesopotamicus under temperature variations in water. Aquac Res 50:3017–3027. https://doi.org/10.1111/are.14260
Pörtner HO (2002) Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp Biochem Physiol A 132:739–761. https://doi.org/10.1016/S1095-6433(02)00045-4
Raza H (2011) Dual localization of glutathione S-transferase in the cytosol and mitochondria: implications in oxidative stress, toxicity and disease. FEBS J 278:4243–4251. https://doi.org/10.1111/j.1742-4658.2011.08358.x
Regoli F, Giuliani ME, Benedetti M, Arukwe A (2011) Molecular and biochemical biomarkers in environmental monitoring: a comparison of biotransformation and antioxidant defense systems in multiple tissues. Aquatic Toxicol 105S:56–66. https://doi.org/10.1016/j.aquatox.2011.06.014
Santa’Anna Neto JL, Galvani E, Vieira BC (2015) Climates of Brazil: past and present. In Vieira BC, Salgado AAR, Santos LJC (ed), Landscapes and landforms of Brazil Dordrecht, The Netherlands: Springer, pp. 33–41
Stadtman ER, Levine RL (2000) Protein oxidation. Ann N Y Acad Sci 899:191–208. https://doi.org/10.1111/j.1749-6632.2000.tb06187.x
Tort L (2011) Stress and immune modulation in fish. Dev Comp Immunol 35:1366–1375. https://doi.org/10.1016/j.dci.2011.07.002
Tort L, Rotllant J, Liarte C, Acerete L, Hernández A, Ceulemans S (2004) Effects of temperature decrease on feeding rates, immune indicators and histopathological changes of gilthead sea bream Sparus aurata fed with an experimental diet. Aquaculture 229:55–65. https://doi.org/10.1016/S0044-8486(03)00403-4
Valladão GMR, Gallani SU, Pilarski F (2018) South American fish for continental aquaculture. Rev Aquac 10:351–369. https://doi.org/10.1111/raq.12164
Vinagre C, Madeira D, Narciso L, Cabral HN, Diniz M (2012) Effect of temperature on oxidative stress in fish: lipid peroxidation and catalase activity in the muscle of juvenile seabass, Dicentrarchus labrax. Ecol Indic 23:274–279. https://doi.org/10.1016/j.ecolind.2012.04.009
Welker AF, Moreira DC, Campos EG, Hermes-Lima M (2013) Role of redox metabolism for adaptation of aquatic animals to drastic changes in oxygen availability. Comp Biochem Physiol A 165:384–404. https://doi.org/10.1016/j.cbpa.2013.04.003
Wen B, Jin S-R, Chen Z-Z, Gao J-Z, Wang L, Liu Y, Liu H-P (2017) Plasticity of energy reserves and metabolic performance of discus fish (Symphysodon aequifasciatus) exposed to low-temperature stress. Aquaculture 481:169–176. https://doi.org/10.1016/j.aquaculture.2017.09.002
Wendelaar Bonga SE (1997) The stress response in fish. Physiol Rev 77:591–625. https://doi.org/10.1152/physrev.1997.77.3.591
Xie H, Lü X, Zhou J, Shi C, Li Y, Duan T, Li G, Luo Y (2017) Effects of acute temperature change and temperature acclimation on the respiratory metabolism of the snakehead. TrJFAS 17:535–542. https://doi.org/10.4194/1303-2712-v17_3_10
Yang S, Yan TL, Zhao L, Wu H, Du Z, Yan T, Xiao Q (2018) Effects of temperature on activities of antioxidant enzymes and Na+/K+-ATPase, and hormone levels in Schizothorax prenanti. J Therm Biol 72:155–160. https://doi.org/10.1016/j.jtherbio.2018.02.005
Yano T (1992) Assays of hemolytic complement activity. In: Stolen JS, Fletcher TC, Anderson DP, Roberson BS, Van Muiswinkel WB (eds) Techniques in fish immunology, Fair Haven, New Jersey: SOS, vol 2, pp 131–141
Zanuzzo FS, Urbinati EC, Rise ML, Hall JR, Nash GW, Gamperl AK (2015) Aeromonas salmonicida induced immune gene expression in aloe vera fed steelhead trout, Oncorhynchus mykiss (Walbaum). Aquaculture 435:1–9. https://doi.org/10.1016/j.aquaculture.2014.09.010
Zanuzzo FS, Sabioni RE, Montoya LN, Favero G, Urbinati EC (2017) Aloe vera enhances the innate immune response of pacu (Piaractus mesopotamicus) after transport stress and combined heat killed Aeromonas hydrophila infection. Fish Shellfish Immunol 65:198–205. https://doi.org/10.1016/j.fsi.2017.04.013
Zhou T, Gui L, Liu M, Li W, Hu P, Duarte DFC, Niu H, Chen L (2019) Transcriptomic responses to low temperature stress in the Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol 84:1145–1156. https://doi.org/10.1016/j.fsi.2018.10.023
Acknowledgements
The authors thank the Aquaculture Center of UNESP for providing the fish.
Funding
This project was financed by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) [Grants Code 001].
Author information
Authors and Affiliations
Contributions
CDFPdF: conceptualization, investigation, methodology, validation, formal analysis, software, data curation, writing — original draft. AEP: methodology, investigation. ECU: supervision, conceptualization, resources, data curation, writing — review and editing, visualization.
Corresponding author
Ethics declarations
Ethical approval
The experimental fish, rearing procedures, and sampling protocols were carried out following ethical guidelines from the Conselho Nacional de Controle de Experimentação Animal and approved by the Comitê de Ética no Uso de Animais of the Faculdade de Ciências Agrárias and Veterinárias, Jaboticabal campus (Protocol 016291/19).
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Brian Austin
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pereira de Faria, C.d., Piedade, A.E. & Urbinati, E.C. Effect of low water temperature on the stress, innate immune, and antioxidant responses of pacu, Piaractus mesopotamicus, a sub-tropical fish. Aquacult Int 31, 3297–3310 (2023). https://doi.org/10.1007/s10499-023-01125-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-023-01125-4