Abstract
A 12-week experiment was conducted to explore the effects of betaine and/or TMAO on growth, hepatic health, gut microbiota, and serum metabolites in Megalobrama amblycephala fed with high-carbohydrate diets. The diets were as follows: CD group (control diet, 28.5% carbohydrate), HCD group (high-carbohydrate diet, 38.2% carbohydrate), HBD group (betaine-added diet, 38.3% carbohydrate + 1.2% betaine), HTD group (TMAO-added diet, 38.2% carbohydrate + 0.2% TMAO), and HBT group (diet added with both betaine and TMAO, 38.2% carbohydrate + 1.2% betaine + 0.2% TMAO). The results showed that the hepatosomatic index (HSI); whole-body crude fat; hepatic lipid accumulation; messenger RNA expression levels of gk, fpbase, g6pase, ahas, and bcat; serum branched-chain amino acids (BCAAs); ratio of Firmicutes-to-Bacteroidetes; and abundance of the genus Aeromonas were all significantly increased, while the abundance levels of the genus Lactobacillus and phyla Tenericutes and Bacteroidetes were drastically decreased in the HCD group. Compared with the HCD group, the HSI; whole-body crude fat; hepatic lipid accumulation; expression levels of fbpase, g6pase, pepck, ahas, and bcat; circulating BCAA; ratio of Firmicutes-to-Bacteroidetes; and abundance levels of the genus Aeromonas and phyla Tenericutes and Bacteroidetes were significantly downregulated in the HBD, HTD, and HBT groups. Meanwhile, the expression levels of pk were drastically upregulated in the HBD, HTD, and HBT groups as well as the abundance of Lactobacillus in the HBT group. These results indicated that the supplementation of betaine and/or TMAO in high-carbohydrate diets could affect the hepatic lipid accumulation and glycometabolism of M. amblycephala by promoting glycolysis, inhibiting gluconeogenesis and biosynthesis of BCAA, and mitigating the negative alteration of gut microbiota. Among them, the combination of betaine and TMAO had the best effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dietary carbohydrates was directly relevant to the sustainability of aquaculture, and which was economically indispensable in farmed fish species with commercial feeds due to their low cost and high abundance, saving protein and reducing the catabolism of lipids (Kamzlam et al. 2017; Prisingkorn et al. 2017). However, fishes were generally considered to have poor ability of utilizing carbohydrate and are low glucose intolerant (Callet et al. 2020; Wang et al. 2021a). Excess high-carbohydrate diets could negatively impact fish health through metabolic disturbances and stress responses such as hyperglycemia (Kamzlam et al. 2017; Lin et al. 2018), lipid deposition (Boonanuntanasarn et al. 2018; Liu et al. 2018), and liver damage (Prathomya et al. 2017; Wang et al. 2019). It had been reported that supplementation of betaine in high-carbohydrate diets could reduce liver lipid accumulation in Megalobrama amblycephala (Wang et al. 2019). In addition, dietary berberine could ameliorate glucose metabolism disorder and attenuate hyperglycemia of M. amblycephala exposed to a high-carbohydrate diet (He et al. 2021). Some studies for Micropterus salmoides indicated that supplementation of bile acids in high-carbohydrate diet could improve growth performance and glucose and lipid metabolism and could alleviate metabolic syndromes (Guo et al. 2020; Yu et al. 2019). These reports suggested that the specific exogenous supplementation had the potential to alleviate negative impacts caused by high-carbohydrate diet. Therefore, it had attracted more and more attention to find a new candidate to improve carbohydrate use in fish.
Trimethylamine N-oxide (TMAO) was an osmolyte and frequently found in a variety of marine biota (Lidbury et al. 2014), in which TMAO was known to protect against the adverse effects of temperature, salinity, osmolarity, hydrostatic pressure, or urea (Velasquez et al. 2016; Seibel and Walsh 2002; Yancey 2005). It was mainly formed from the metabolites of choline, carnitine, and betaine in the diet, which was absorbed and transformed to trimethylamine (TMA) by the gut microbiota (Wang et al. 2019), then TMA is converted into TMAO in the liver by flavin-containing monooxygenase 1 (FMO1) and FMO3 (Coutinho-Wolino et al. 2021; Gatarek and Kaluzna-Czaplinska 2021; Subramaniam and Fletcher 2018). The levels of TMAO, as an early biomarker of adipose dysfunction and non-alcoholic fatty liver disease (NAFLD), were positively associated with the visceral adiposity index and the fatty liver index and NAFLD (Barrea et al. 2018, 2019). After TMAO is orally consumed, it exhibits a fast turnover in the circulation with the majority being eliminated by urine (Taesuwan et al. 2017). TMAO had a special flavor and could improve nerve excitability by stimulating the olfactory organs of aquatic animals as a new type of aquatic animal food attractant (Yi et al. 2021). TMAO had a strong feeding attraction effect on Oreochromis mossambicus, and the optimal TMAO level was 0.2% (Sun et al. 2005). Moreover, the food intake, growth performances, body composition, and digestive ability of Hucho taimen were all improved by the supplementation of 0.2% TMAO (Wang et al. 2011, 2012). However, to the best of our knowledge, there were few studies investigating the molecular mechanism of TMAO regulating glycolipid metabolism so far.
M. amblycephala was a herbivorous freshwater fish with fast growth rate and low farming cost and had been the sixth largest freshwater cultured fish in China (Wang et al. 2021b; He et al. 2021). It was possible to supplement more starch to its diets for saving costs because of its relative tolerance to carbohydrate. Therefore, it was necessary to look for glucose-lowering substances to alleviate the metabolic problems caused by the high-carbohydrate feed of M. amblycephala (He et al. 2021). Betaine was a natural compound derived from animals and plants and was widely used as a food attractant in aquaculture (Kasumyan and Døving 2003; Sun et al. 2018). It had stable properties, small dosage, and low price and could be produced on a large scale. Similar as TMAO, betaine also played a role in the osmoregulation. Meanwhile, betaine could protect animals from nutritional fatty liver and is a highly competitive liver protection substance (Ge et al. 2016; Chen et al. 2021). Furthermore, previous studies in our lab had suggested that betaine supplementation could decrease the disruption of liver function and overall fat deposition in a high-carbohydrate diet (Xu et al. 2018). At the same time, the optimum betaine supplementation was 1.2% (Wang et al. 2020), and it was consistent with the previous report (Adjoumani et al. 2017). However, there were few studies investigating the combined effects of betaine and TMAO on hepatic lipid accumulation and glucose metabolism, and the molecular mechanisms behind these alterations were not fully clarified. Thus, this study aimed to explore the long-term effects of betaine and/or TMAO supplementation on the growth performance, body composition, health of liver, gut microbe composition, and serum metabolite alteration in M. amblycephala fed with high-carbohydrate diets. It is helpful for understanding the underlying mechanism of glycolipid metabolism controlled by betaine and/or TMAO, finding effective approaches to improve its carbohydrate utilization, and promoting the development of low-protein and high-energy feed for fish.
Materials and methods
Fish and diets
Healthy 4-month-old M. amblycephala (n = 600; average weight = 5.00 ± 0.50 g) were obtained from the experimental base of the Huazhong Agricultural University, Wuhan, China; distributed into six plastic tanks (25.00 ± 1.00 °C; dissolved oxygen of > 5.0 mg/l was kept); and fed the control diet during the acclimation period (14 days). Then, a total of 450 healthy fish that could feed normal diets stably were selected and divided into five treatments. Each treatment contained three plastic tanks (stocking density = 30 fish per tank) with 300 L water capacity each tank, water temperature of 25 ± 1.00 °C, dissolved oxygen of 9 ± 1.00 mg/l, and nitrate of 0.01 ± 0.01 mg/l, and the photoperiod is natural. The study consisted of the following five diets: CD group (control diet, 28.5% carbohydrate), HCD group (high-carbohydrate diet, 38.2% carbohydrate), HBD group (betaine-added diet, 38.3% carbohydrate + 1.2% betaine), HTD group (TMAO-added diet, 38.2% carbohydrate + 0.2% TMAO), and HBT group (diet added with both betaine and TMAO, 38.2% carbohydrate + 1.2% betaine + 0.2% TMAO). The detailed description of the ingredients and the composition of the diets are shown in Table 1. The carbohydrate content of diets was determined by the 3,5-dinitrosalicylic acid method (Yin et al. 2007). The period of the rearing trial was 12 weeks, during which the fry was fed twice daily quantitatively (the weight of each feeding was 1–2% of body weight), and the water was replaced daily from 25 to 50% in each tank.
Serum, liver, and intestinal content sampling
At the end of the 12-week trial, fish from each tank were anesthetized with 150 mg/l tricaine methanesulfonate buffered with sodium bicarbonate (MS-222; Sigma, USA) (Collymore et al. 2013; Welker et al. 2007) and 15 specimens were sampled randomly. The body weight and body length of fish were measured. Blood samples were collected from the caudal vein and centrifuged at 3000 × g at 4 °C for 10 min to separate the serum, which then stored at − 80 °C for analysis. After dissection, the weights of the viscera and liver were measured. Livers were divided into 2 parts: one part of the liver was placed in 10% paraformaldehyde solution for subsequent histological and lipid analysis, and the other part was kept at − 80 °C for RNA extraction. The intestinal contents were collected into sterile tubes and frozen in liquid nitrogen, then stored at − 80 °C for DNA extraction and intestinal microbial composition analysis.
Analysis of growth performance
The growth performance parameters were calculated as follows:
Analysis of proximate composition in whole fish
Ten fish from each replicate were used for the proximate composition analysis of whole fish with the official method of AOAC: Official Methods of Analysis (1995) (Cunniff et al. 1995). Moisture, crude protein, crude fat, and crude ash of whole body and diets were measured as previously described (Zhou et al. 2015a).
Histopathological and oil red O staining analyses of liver
Paraffin sections of liver were stained with hematoxylin–eosin (HE) and oil red O by Wuhan Servicebio Technology Co., Ltd. (Wuhan, China). Briefly, hepatic tissues were immersed in 10% paraformaldehyde, paraffin embedded, sliced, and dehydrated. Sections were subjected to HE staining, then liver tissue morphology and unstained areas of fat vacuoles were observed under a light microscope with × 200 magnification (Olympus, Tokyo, Japan). Morphological and pathological analyses of HE staining were conducted. For oil red O staining, liver tissues were made into frozen sections, dried in the air, stained with oil red solution, and sealed with glycerin gelatin, and finally, the grease drops were observed under a microscope with × 200 magnification (Olympus, Tokyo, Japan). The Olympus Image-Pro Plus 6.0 software was used to conduct the quantitative analysis and calculate the oil red O–stained areas.
Analysis of gut microbiota
Total genomic DNA of gut microbiota was obtained using the hexadecyltrimethylammonium bromide extraction method. The quantity and quality of extracted DNA were measured using a NanoDrop 2000 spectrophotometer (Thermo Scientific, Delaware, USA) and agarose gel electrophoresis, respectively. The extracted DNA was subjected to PCR amplification of the bacterial 16S ribosomal DNA (rDNA) V3–V4 region using the forward primer 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and the reverse primer 806R (5′-GGACTACHVGGGTWTCTAAT-3′). Pyrosequencing of 16S rDNA was performed using the Illumina MiSeq platform with MiSeq Reagent Kit v3 at Shanghai Personal Biotechnology Co., Ltd. (Shanghai, China). The Quantitative Insights Into Microbial Ecology (QIIME) v1.8.0 pipeline was employed to process the sequencing data (QIIME allows the analysis of high-throughput community sequencing data) and operational taxonomic unit (OTU) analysis under a 97% similar level (Edgar 2010). Sequence data analyses were mainly performed using QIIME and R packages (v3.2.0).
Quantification of metabolites in serum
The contents of related metabolites in serum were measured under the high-performance liquid chromatography-tandem mass spectrometry (HPLC–MS/MS) on the Ultimate3000 (Dionex, Sunnyvale, USA)-API 3200 Q-TRAP (AB Sciex, Framingham, MA, USA) system. Chromatographic analyses were performed by the Beijing Mass Spectrometry Medical Research Co. Ltd. (Beijing, China). In general, 50 µl of protein precipitant (containing NVL) was added to 50 µl of serum, mixed thoroughly, and centrifuged. The supernatant (10 µl) was mixed with 50 µl of methanol borate buffer thoroughly (for amino acids, the above solution was mixed with 20 µl of diethyl ethoxymethylenemalonate). This solution (50 µl) was analyzed by HPLC–MS/MS. Chromatographic separations were performed on an MS Lab C18 column (150 × 4.6 mm, 5 µm). The mobile phase A was 0.1% formic acid in water (v/v), and the organic mobile phase B was 0.1% ammonium formate in acetonitrile (v/v) with pH 5.8. Solvent was delivered to the column at a flow rate of 1 ml/min. The gradient elution program was as follows: 0 min, 90% A and 10% B; 1.4 min, 90% A and 10% B; 1.5 min, 40% A and 60% B; 3.4 min, 40% A and 60% B; 3.5 min, 5% A and 95% B; 5.9 min, 5% A and 95% B; 6.0 min, 90% A and 10% B; and 10.0 min, 90% A and 10% B. The sample injection size was 5 µl, and the flow rate was 1 ml/min. The mass spectrometry analysis was performed on an API 4000 Q-TRAP (Biosystems, USA). In the multiple reaction monitoring mode, nitrogen gas was used as the collision gas. The conditions for mass spectrometry detection were as follows: mode, positive ion mode; curtain gas pressure, 20 psi; ion spray voltage, 4800 V; collision gas pressure, medium; nebulizer gas pressure, 55 psi; turbo gas temperature, 500 °C; collision cell exit potential, 2 V; and entrance potential, 10 V. Data were processed using Analyst software version 1.5.1 (Applied Biosystems).
Analysis of real-time PCR
Total RNA was extracted from liver tissues using TRIzol reagent (Takara, Japan), and the concentration, qualification, and quality were measured on the NanoDrop 2000 spectrophotometer (Thermo Scientific, Delaware, USA) and by agarose gel electrophoresis. Qualified RNA reversely transcribed into cDNA by using a synthesis kit (Takara, Japan). Quantitative reverse transcription PCR was performed using the ChamQ Universal SYBR qPCR Master Mix (Vazyme, China) to determine the messenger RNA (mRNA) expression levels. β-Actin (F:CGGACAGGTCATCACCATTG; R:CGCAAGACTCCATACCCAAGA) was used as the reference gene to analyze the mRNA, and the relative levels of gene expression were calculated by the 2−ΔΔCt method (Adjoumani et al. 2017). Three enzyme genes, namely pyruvate carboxylase (pc), acetohydroxy acid synthase (ahas), and branched-chain aminotransferase (bcat), were selected in the KEGG database for expression pattern analyses. Primers of these genes were designed by Primier 5 software (Primier Biosoft, USA). Primers for this experiment are shown in Table 2.
Statistical analysis
GraphPad Prism 8.0 was used for data analyses and figure editing. Data were expressed as mean ± standard deviation. Statistical significance of the data was analyzed using one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) multiple comparison test (differences were significant if P < 0.05 and were extremely significant if P < 0.01).
Results
Growth performance and whole-body composition
WGR, SGR, FCR, CF, and SR had no significant difference among all groups (P > 0.05), but the HSI of the HCD group was significantly higher than that of other experimental groups (P < 0.05, Table 3). The HBT group had significantly lower VSI than the HCD group (P < 0.05). For the results of whole-body composition, there were no differences in moisture, ash, and crude protein among all groups (P > 0.05), whereas the crude fat of the HCD group was significantly higher than that of other experimental groups (P < 0.05).
Histopathological and oil red O staining of liver
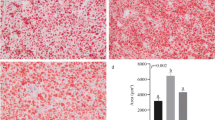
The hepatic hematoxylin–eosin-stained sections (Fig. 1A–E) illustrated the whole staining of the CD group was uniform with a regular and round nucleus, whereas the liver sections of the HCD group were severely damaged including swollen hepatocytes with fat vacuoles and liver cell nucleus becoming abnormal and/or absent. Some slightly damaged and disappearing nuclei could be observed in the hepatocytes of the HBD, HTD, and HBT groups, but these symptoms were not as severe as those in the HCD group. According to photomicrographs of oil red O staining in × 200 magnification (Fig. 1F–J), the accumulation of liver lipid for fish fed with HCD diet was significantly higher (P < 0.05) than that of all the other groups (Fig. 1K). Comparing with that in the HCD group, the accumulation of lipid droplets was significantly reduced in the HBD, HTD, and HBT groups (P < 0.05). Simultaneously, the accumulation of lipid droplets of the HBT group was significantly lower than that of the HBD and HTD groups (P < 0.05). These results were consistent with the above results of growth performance and whole-body composition, suggesting that the addition of betaine and/or TMAO alleviated hepatic steatosis and liver fat accumulation caused by high-carbohydrate diets.
Hematoxylin–eosin (A–E) and oil red O (F–J) staining of hepatic tissues of Megalobrama amblycephala fed with different experimental diets. A, F Fish fed CD (28.5% carbohydrate). B, G Fish fed HCD (38.2% carbohydrate). C, H Fish fed HBD (38.3% carbohydrate + 1.2% betaine). D, I Fish fed HTD (38.2% carbohydrate + 0.2% TMAO). E, J Fish fed HBT (38.2% carbohydrate + 1.2% betaine diet + 0.2% TMAO). K The quantitative results of the oil red O staining, presented as the means ± SD for each group, where different lowercase letters above the bars indicate a significant difference (P < 0.05). Photomicrographs in × 200 magnification. Arrows represent the examples of (a) a normal liver cell with a regular, round nucleus; (b) swollen hepatocytes with fat vacuoles; (c) an abnormal liver cell nucleus; and (d) an absent liver cell nucleus
Gut microbial community composition
The alpha diversity indices, including Good’s coverage index (P = 0.23), Shannon-Weiner index (P = 0.17), Simpson index (P = 0.19), and Pielou´s evenness index (P = 0.21), were not different among all these groups (Fig. 2E). Fusobacteria was an absolutely dominant phylum in all groups, and the top five phyla were Fusobacteria, Tenericutes, Bacteroidetes, Proteobacteria, and Firmicutes (Fig. 2A). Compared with those in the CD group, Tenericutes and Bacteroidetes were sharply decreased in the HCD groups (P < 0.05) and their abundance levels were recovered again for the supplementation of betaine and/or TMAO in the HBD, HTD, and HBT groups (P < 0.05) (Fig. 2B). In the HCD group, there was a significant increase in the Firmicutes-to-Bacteroidetes ratio compared to that in the CD, HBD, HTD, and HBT groups (P < 0.05) (Fig. 2B). Based on the results of genera, Cetobacterium was the most abundant, and Flavobacterium and Aeromonas were the second and third, respectively (Fig. 2C). Compared to the CD, HBD, HTD, and HBT groups, the HCD group showed to have significantly greater abundance of the genus Aeromonas (P < 0.05) (Fig. 2D). Simultaneously, the genus Lactobacillus was significantly more abundant in the CD and HBT groups than in the HCD, HBD, and HTD groups (P < 0.05) (Fig. 2D).
The relative abundance and analysis of the top 10 phyla (A, B), relative abundance and analysis of the top 20 genera (C, D), and alpha diversity of the intestinal microbiota (E) in Megalobrama amblycephala. Data were presented as the means ± SD for each group, where different lowercase letters above the bars indicate a significant difference (P < 0.05)
Metabolites and related gene expression levels of glucose metabolism
Compared with the CD group, the mRNA expression of glucokinase (gk, Fig. 3A) in the HCD group was increased significantly (P < 0.05), and it was not significantly different among the HCD, HBD, HTD, and HBT groups (P > 0.05). There were no greater differences in the mRNA expression levels of 6-phosphofructokinase-1 (pfk1, Fig. 3B) and pyruvate carboxylase (pc, Fig. 3D) among all the treatments (P > 0.05). The pyruvate kinase (pk, Fig. 3C) mRNA expression of the HCD group was significantly lower than that of the HBD, HTD, and HBT groups (P < 0.05), whereas the expression of phosphoenolpyruvate carboxykinase (pepck, Fig. 3E) in the HCD group was significantly higher than that in the HBD, HTD, and HBT groups (P < 0.05). The expression levels of fructose 1,6-biphosphatase (fbpase, Fig. 3F), glucose 6-phosphatase (g6pase, Fig. 3G), acetohydroxy acid synthase (ahas, Fig. 3H), and branched-chain aminotransferase (bcat, Fig. 3I) in the HCD group were significantly higher than those in the other groups (P < 0.05).
The relative expression levels of liver genes (A–I) and concentrations of serum metabolites (J–L) related to glycometabolism for Megalobrama amblycephala fed with different experimental diets. Expression values were evaluated by qPCR and normalized using β-actin as the reference gene. Values (means ± SE) embedded in the figure with different letters indicate a significant difference (P < 0.05). gk, glucokinase; pfk1, 6-phosphofructokinase-1; pk, pyruvate kinase; pc, pyruvate carboxylase; pepck, phosphoenolpyruvate carboxykinase; fpbase, fructose 1,6-biphosphatase; g6pase, glucose 6-phosphatase; ahas, acetohydroxy acid synthase; bcat, branched-chain aminotransferase
Furthermore, according to serum metabolites of M. amblycephala (Fig. 3J–L), high-carbohydrate diets in the HCD group resulted in significantly higher concentrations of valine, isoleucine, and leucine than those in the CD group (P < 0.05), while their concentrations were remarkably reduced in the HBD, HTD, and HBT groups compared to that in the HCD group (P < 0.05).
Discussion
In the present study, the influence of dietary carbohydrate levels on WGR, SGR, FCR, and CF of fish exerted a little difference. On the one hand, this result might be due to the fact that M. amblycephala had better utilization of carbohydrates than most carnivorous and omnivorous species (Shi et al. 2018); on the other hand, the supplementation of betaine and/or TMAO might be another reason that it could alleviate negative impacts of liver health caused by high-carbohydrate diets and was ultimately beneficial to the growth of fish. Hence, as reported earlier (Shi et al. 2020, 2018; Prathomya et al. 2017), high dietary carbohydrate did not lead to negative effect on growth performance for M. amblycephala in this research. Furthermore, both HSI and VSI were also increased when dietary carbohydrate was increased, while the supplementation of betaine and/or TMAO alleviated them in the current study. Similarly, previous investigations had provided compelling evidence that high-carbohydrate diets often resulted in increasing lipid accumulation and indicative symptoms of liver damage in M. amblycephala, including negative liver health indices, reduction and disappearance of nuclei, symptoms of steatosis, and a high number of lipid droplets (Prisingkorn et al. 2017; Zhou et al. 2015b). But, the TMAO-supplemented diets significantly reduced fat belching and the number of fat droplets in rainbow trout (Rørvik et al. 2015). And, betaine supplementation also ameliorated hepatic injury by decreased liver histopathological alterations in acute and chronic animal models of hepatic injury (Heidari et al. 2018). This was also supported by the fact that fish fed with betaine could reduce the accumulation of fat in the body and liver and notably improved liver health parameters (Xu et al. 2018). Therefore, the reduction of HSI and VSI was reasonable in M. amblycephala when the high-carbohydrate diets were supplemented with betaine and/or TMAO.
In accordance with the abovementioned reports (Prisingkorn et al. 2017; Zhou et al. 2015b; Heidari et al. 2018; Xu et al. 2018), according to HE and oil red O stains of hepatic tissues, there were large lipid droplets, cellular swelling, partial necrosis, and severe steatosis in M. amblycephala fed with high-carbohydrate diets, while the hepatic cells showed less degeneration and necrosis, and the degree of hepatocyte hypertrophy and intracellular lipid drop accumulation were reduced when high-carbohydrate diets were supplemented with betaine and/or TMAO. Furthermore, oil red O staining of the hepatic tissue revealed a much higher number of red-stained hepatocytes and higher oil red O–stained areas in the HCD group than in the other groups. These results had been confirmed in previous studies that betaine inhibited hepatic fat accumulation (Adjoumani et al. 2017; Xu et al. 2018; Zhang et al. 2019). Therefore, we concluded that these hepatocyte symptoms were significantly reduced and even completely abolished for M. amblycephala in betaine and/or TMAO intervention circumstances.
Moreover, the whole-body crude lipid was changed significantly in the present study: high dietary carbohydrate of the HCD group resulted in the increase of crude lipid compared to the CD group, but this phenomenon was recovered by the supplementation of betaine and/or TMAO. According to previous research, high-carbohydrate diets could increase the synthesis of glycogen and fat in fish (Li et al. 2019; Prisingkorn et al. 2017), thus enhancing lipid deposition in tissue (Enes et al. 2008; Shi et al. 2020). In addition, the diets with optimal TMAO reduced backfat thickness and tended to increase lean percentage of pork (Overland et al. 1999). Dietary betaine supplementation significantly inhibited the white fat production and reduced intramyocellular lipid accumulation in a high-fat diet (HFD)-induced obese mice (Du et al. 2018). Thereby, the result of whole-body crude lipid was consistent with the previous reports (Du et al. 2018; Overland et al. 1999; Prisingkorn et al. 2017) and our foregoing results that both hepatic lipid accumulation and liver health parameters were notably improved for M. amblycephala when high-carbohydrate diets were supplemented with betaine and/or TMAO.
A tremendous amount of gut microbiota inhabited the intestines of animals, which was associated with various physiological and metabolic diseases, such as obesity, diabetes, and nonalcoholic fatty liver (Guo et al. 2021; Uittenbogaart et al. 2019). However, the gut microbiota was susceptible to changes by many influencing factors, including environmental factors and dietary nutrients, and the latter was one of the most important factors (Tomasello et al. 2014; Maslowski and Mackay 2011). Although the top five phyla of the current study were all the core gut microbiota of fish as previously reported (Bereded et al. 2022; Fogarty et al. 2019), Tenericutes and Bacteroidetes were drastically decreased in the HCD group and recovered again in the HBD, HTD, and HBT groups compared to the CD group. The result confirmed the abovementioned view that dietary nutrients could affect the intestinal microbial composition (Tomasello et al. 2014; Maslowski and Mackay 2011) and indicated the supplementation of betaine and/or TMAO could recover the changes caused by high-carbohydrate diets.
In this study, compared to other groups, there was a greater increase in the Firmicutes-to-Bacteroidetes ratio and a significant decrease of Bacteroidetes for juvenile M. amblycephala in the HCD group. But, they were recovered to the levels of control group when betaine and/or TMAO were supplemented to high-carbohydrate diets. Since Bacteroidetes was related to many metabolic activities involving the fermentation of carbohydrates (Zhang et al. 2016) and the higher Firmicutes-to-Bacteroidetes ratio in the gut microbiota was associated with the efficient absorption of food energy (Murphy et al. 2010), it was deduced that the influence of high-carbohydrate diets on M. amblycephala might be conducted by remolding its gut microbiota to regulate carbohydrate metabolism and energy absorption. Simultaneously, the restoration of Bacteroidetes and Firmicutes-to-Bacteroidetes ratio in the HBD, HTD, and HBT groups indicated the supplementation of betaine and/or TMAO could alleviate or remove the alteration of gut microbiota caused by high carbohydrate.
A high-starch or high-carbohydrate diets caused disturbances in the intestinal microbiota and increased the abundance of conditioned bacterial pathogens (e.g., Aeromonas sp.) of Oncorhynchus mykiss (Geurden et al. 2014) and Trachinotus ovatus (Zhao et al. 2020). Except for the increased abundance of the genus Aeromonas, the abundance of beneficial bacteria (the genus Lactobacillus) was also decreased for Micropterus salmoides fed with a high-starch diet, which impaired the intestinal function of fish (Huang et al. 2021; Zhang et al. 2020; Zhou et al. 2020). Similarly, in the current study, the abundance levels of the genera Aeromonas and Lactobacillus in the HCD group were significantly increased and decreased respectively compared with those in the CD group, whereas Lactobacillus abundance was recovered in the HBT group as well as Aeromonas abundance in the HBD, HTD, and HBT groups. These results suggested that the high-carbohydrate diets caused the increase of harmful bacteria and the decrease of beneficial bacteria in fish intestine, and indicated that the supplementation of dietary betaine and/or TMAO could mitigate the gut negative change caused by high-carbohydrate diets in M. amblycephala.
The key enzymes are glucokinase (gk), phosphofructokinase-1 (pfk1), and pyruvate kinase (pk) in the glycolytic pathway as well as pyruvate carboxylase (pc), phosphoenolpyruvate carboxykinase (pepck), fructose 1,6-bisphosphatase (fpbase), and glucose 6-phosphatase (g6pase) in the gluconeogenesis pathway (Hamanaka and Chandel 2012; Litwack 2018; Matschinsky 1996). Additionally, acetohydroxy acid synthase (ahas) is the key enzyme and branched-chain aminotransferase (bcat) is the common enzyme and catalyzes the final step in the branched-chain amino acid (BCAA) biosynthetic pathway (Do et al. 2018; Liang et al. 2021). In the current study, the mRNA expression levels of gk, fbpase, g6pase, ahas, and bcat in the HCD group were significantly higher than those of the CD group, but the expression levels of pfk1, pk, pc, and pepck were not greatly changed (Fig. 4). Simultaneously, the serum concentrations of valine, isoleucine, and leucine were drastically increased in the HCD group (Fig. 4). These results indicated that long-term high-carbohydrate intake resulted in the two kinds of alterations: one was that it promoted liver gluconeogenesis synthesis, which was also observed in Siberian sturgeon (Liang et al. 2017), and the other was that fish tried their best to maintain the homeostasis of glucose, which led to promoting the glycolysis and increasing the plasma BCAA levels. Similarly, high dietary carbohydrates could significantly increase glucokinase activity in liver for European sea bass and gilthead sea bream (Liang et al. 2017), which was consistent with our results.
Overview of glycometabolism pathway in Megalobrama amblycephala fed with different experimental diets. Up arrow indicates a significant increase, down arrow indicates a significant decrease, and horizontal arrow indicates a nonsignificant change or unchanged concentration. Red arrows (HCD compared to CD), blue arrows (HTD compared to HCD), orange arrows (HBD compared to HCD), and purple arrows (HBT compared to HCD) indicate the direction of the change in the concentration of a metabolite. Up arrow indicates a significant increase, down arrow indicates a significant decrease, and horizontal arrow indicates a nonsignificant change or unchanged concentration. The region selected in the blue box represents that the reaction occurs through the corresponding enzyme ahas or bcat. Glu, glucose; G-6-P, glucose 6-phosphate; F-6-P, fructose 6-phosphate; F-1,6-P, fructose 1,6-bisphosphate; PEP, phosphoenolpyruvate; PA, pyruvic acid; OAA, oxaloacetic acid; ALT, 2-acetolactate; AHB, 2-aceto-2-hydroxybutanoate; MOPA, 3-methyl-2-oxopentanoic acid; MOBA, 3-methyl-2-oxobutanoic acid; MOPT, 4-methyl-2-oxopentanoate; Val, valine; Ile, isoleucine; Leu, leucine; gk, glucokinase; pfk1, 6-phosphofructokinase-1; pk, pyruvate kinase; pc, pyruvate carboxylase; pepck, phosphoenolpyruvate carboxykinase; fpbase, fructose 1,6-biphosphatase; g6pase, glucose 6-phosphatase; ahas, acetohydroxy acid synthase; bcat, branched-chain aminotransferase
Furthermore, compared with the HCD group, the expression levels of fbpase, g6pase, pepck, ahas, and bcat were significantly downregulated and the expression of pk was drastically upregulated in the HBD, HTD, and HBT groups, but gk, pfk1, and pc were not greatly changed (Fig. 4). Simultaneously, the serum concentrations of valine, isoleucine, and leucine were significantly decreased (Fig. 4). These results indicated that the supplementation of betaine and/or TMAO in high-carbohydrate diets could not only inhibit liver gluconeogenesis and biosynthesis of BCAA but also promote glycolysis by the regulation of abovementioned metabolites and enzyme gene expression levels, which finally made most intermediate metabolites forming oxaloacetic acid (OAA) that can enter the mitochondrial tricarboxylic acid (TCA) cycle directly. Thus, the dietary carbohydrates were converted into energy and protected fish liver from the high glycogen under the actions of betaine and/or TMAO.
Plasma BCAA concentrations were positively correlated with insulin resistance and severity of NAFLD (Gaggini et al. 2018; Grzych et al. 2020; Muhammed et al. 2019). The high-fructose diets could activate hepatic carbohydrate-responsive element-binding proteins (Kim et al. 2016), which upregulated the branched-chain keto acid dehydrogenase kinase (BDK) and downregulated the protein phosphatase m1K (PPM1K). And, the increased BDK-to-PPM1K ratio contributed to the obesity-linked rise in circulating BCAA (White et al. 2018). In the present study, the serum BCAA concentrations were increased and the symptoms of hepatocyte steatosis and lipid accumulation were found in the HCD group, while the BCAAs were recovered and the hepatic symptoms were alleviated or eliminated in the HBD, HTD, and HBT groups. The results were in line with the foregoing reports and indicated high carbohydrates could increase the circulating BCAAs that were positively correlated with hepatic lipid accumulation. Meanwhile, the dietary supplementation of betaine and/or TMAO could restore the BCAA concentrations and hepatic symptoms of lipid accumulations caused by high carbohydrates in M. amblycephala. In addition, compared with the HBD and HTD groups, the HSI and VSI and the accumulation of lipid droplets were all lowest in the HBT group, and the latter was significantly lower than that of the HBD and HTD groups (Fig. 1K), whereas the genus Lactobacillus was significantly more abundant in the HBT group than in the HBD and HTD groups (Fig. 2D). Therefore, the HBT group had the best effect.
Conclusion
Overall, these results of the current study demonstrated that the supplementation of betaine and/or TMAO in high-carbohydrate diets could affect the hepatic lipid accumulation and glycometabolism of M. amblycephala by promoting hepatic glycolysis, inhibiting gluconeogenesis and biosynthesis of circulating BCAA, and mitigating the negative alteration of gut microbiota caused by high-carbohydrate diets. Among them, the combination of betaine and TMAO had the best effect.
Data availability
The data used to support the study’s conclusions are contained in the publication; further details can be requested from the first author.
Code availability
Not applicable.
References
Adjoumani JY, Wang K, Zhou M, Liu W, Zhang D (2017) Effect of dietary betaine on growth performance, antioxidant capacity and lipid metabolism in blunt snout bream fed a high-fat diet. Fish Physiol Biochem 43:1733–1745. https://doi.org/10.1007/s10695-017-0405-9
Barrea L, Annunziata G, Muscogiuri G, Di Somma C, Laudisio D, Maisto M, Alteriis GD, Tenore GC, Colao A, Savastano S (2018) Trimethylamine-N-oxide (TMAO) as novel potential biomarker of early predictors of metabolic syndrome. Nutrients 10:1971–1989. https://doi.org/10.3390/nu10121971
Barrea L, Muscogiuri G, Annunziata G, Laudisio D, de Alteriis G, Tenore GC, Colao A, Savastano S (2019) A new light on vitamin D in obesity: a novel association with trimethylamine-N-oxide (TMAO). Nutrients 11:1310–1324. https://doi.org/10.3390/nu11061310
Bereded NK, Abebe GB, Fanta SW, Curto M, Waidbacher H, Meimberg H, Domig KJ (2022) The gut bacterial microbiome of Nile tilapia (Oreochromis niloticus) from lakes across an altitudinal gradient. BMC Microbiol 22:87–100. https://doi.org/10.1186/s12866-022-02496-z
Boonanuntanasarn S, Kumkhong S, Yoohat K, Plagnes-Juan E, Burel C, Marandel L, Panserat S (2018) Molecular responses of Nile tilapia (Oreochromis niloticus) to different levels of dietary carbohydrates. Aquaculture 482:117–123. https://doi.org/10.1016/j.aquaculture.2017.09.032
Callet T, Hu H, Larroquet L, Surget A, Liu J, Plagnes-Juan E, Maunas P, Turonnet N, Mennigen JA, Bobe J, Burel C, Corraze G, Panserat S, Marandel L (2020) Exploring the impact of a low-protein high-carbohydrate diet in mature broodstock of a glucose-intolerant teleost, the rainbow trout. Front Physiol 11:303–323. https://doi.org/10.3389/fphys.2020.00303
Chen W, Zhang X, Xu M, Jiang L, Zhou M, Liu W, Chen Z, Wang Y, Zou Q, Wang L (2021) Betaine prevented high-fat diet-induced NAFLD by regulating the FGF10/AMPK signaling pathway in ApoE-/- mice. Eur J Nutr 60:1655–1668. https://doi.org/10.1007/s00394-020-02362-6
Collymore C, Rasmussen S, Tolwani RJ (2013) Gavaging Adult Zebrafish J vis Exp 78:50691–50695. https://doi.org/10.3791/50691
Coutinho-Wolino KS, de Cardozo FLFM, de Oliveira Leal V, Mafra D, Stockler-Pinto MB (2021) Can diet modulate trimethylamine N-oxide (TMAO) production? What do we know so far? Eur J Nutr 60:3567–3584. https://doi.org/10.1007/s00394-021-02491-6
Cunniff P, Hirwitz W, Latimer G (1995) Official methods of analysis of AOAC International (16th edn). Trends Food Sci Technol 6(11):382–382. https://doi.org/10.1016/S0924-2244(00)89213-4
Do MH, Lee E, Oh MJ, Kim Y, Park HY (2018) High-glucose or -fructose diet cause changes of the gut microbiota and metabolic disorders in mice without body weight change. Nutrients 10:761–774. https://doi.org/10.3390/nu10060761
Du J, Shen L, Tan Z, Zhang P, Zhao X, Xu Y, Gan M, Yang Q, Ma J, Jiang A, Tang G, Jiang Y, Jin L, Li M, Bai L, Li X, Wang J, Zhang S, Zhu L (2018) Betaine supplementation enhances lipid metabolism and improves insulin resistance in mice fed a high-fat diet. Nutrients 10:131–147. https://doi.org/10.3390/nu10020131
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461. https://doi.org/10.1093/bioinformatics/btq461
Enes P, Panserat S, Kaushik S, Oliva-Teles A (2008) Nutritional regulation of hepatic glucose metabolism in fish. Fish Physiol Biochem 35(3):519–539. https://doi.org/10.1007/s10695-008-9259-5
Fogarty C, Burgess CM, Cotter PD, Cabrera-Rubio R, Whyte P, Smyth C, Bolton DJ (2019) Diversity and composition of the gut microbiota of Atlantic salmon (Salmo salar) farmed in Irish waters. J Appl Microbiol 127:648–657. https://doi.org/10.1111/jam.14291
Gaggini M, Carli F, Rosso C, Buzzigoli E, Marietti M, Della Latta V, Ciociaro D, Abate ML, Gambino R, Cassader M, Bugianesi E, Gastaldelli A (2018) Altered amino acid concentrations in NAFLD: impact of obesity and insulin resistance. Hepatology 67:145–158. https://doi.org/10.1002/hep.29465
Gao Z, Luo W, Liu H, Zeng C, Liu X, Yi S, Wang W (2012) Transcriptome analysis and SSR/SNP markers information of the blunt snout bream (Megalobrama amblycephala). PLoS ONE 7:e42637–e42646. https://doi.org/10.1371/journal.pone.0042637
Gatarek P, Kaluzna-Czaplinska J (2021) Trimethylamine N-oxide (TMAO) in human health. Excli J 20:301–319. https://doi.org/10.17179/excli2020-3239
Ge CX, Yu R, Xu MX, Li PQ, Fan CY, Li JM, Kong LD (2016) Betaine prevented fructose-induced NAFLD by regulating LXRα/PPARα pathway and alleviating ER stress in rats. Eur J Pharmacol 770:154–164. https://doi.org/10.1016/j.ejphar.2015.11.043
Geurden I, Mennigen J, Plagnes-Juan E, Veron V, Cerezo T, Mazurais D, Zambonino-Infante J, Gatesoupe J, Skiba-Cassy S, Panserat S (2014) High or low dietary carbohydrate:protein ratios during first-feeding affect glucose metabolism and intestinal microbiota in juvenile rainbow trout. J Exp Biol 217:3396–3406. https://doi.org/10.1242/jeb.106062
Grzych G, Vonghia L, Bout MA, Weyler J, Verrijken A, Dirinck E, Chevalier Curt MJ, Van Gaal L, Paumelle R, Francque S, Tailleux A, Haas JT, Staels B (2020) Plasma BCAA changes in patients with NAFLD are sex dependent. J Clin Endocrinol Metab 105:2311–2321. https://doi.org/10.1210/clinem/dgaa175
Guo JL, Kuang WM, Zhong YF, Zhou YL, Chen YJ, Lin SM (2020) Effects of supplemental dietary bile acids on growth, liver function and immunity of juvenile largemouth bass (Micropterus salmoides) fed high-starch diet. Fish Shellfish Immunol 97:602–607. https://doi.org/10.1016/j.fsi.2019.12.087
Guo L, Yang K, Zhou P, Yong W (2021) Gut microbiota in obesity and nonalcoholic fatty liver disease. Surgery in Practice and Science 5:100030–100047. https://doi.org/10.1016/j.sipas.2021.100030
He C, Jia X, Zhang L, Gao F, Jiang W, Wen C, Chi C, Li X, Jiang G, Mi H, Liu W, Zhang D (2021) Dietary berberine can ameliorate glucose metabolism disorder of Megalobrama amblycephala exposed to a high-carbohydrate diet. Fish Physiol Biochem 47:499–513. https://doi.org/10.1007/s10695-021-00927-8
Heidari R, Niknahad H, Sadeghi A, Mohammadi H, Ghanbarinejad V, Ommati MM, Hosseini A, Azarpira N, Khodaei F, Farshad O, Rashidi E, Siavashpour A, Najibi A, Ahmadi A, Jamshidzadeh A (2018) Betaine treatment protects liver through regulating mitochondrial function and counteracting oxidative stress in acute and chronic animal models of hepatic injury. Biomed Pharmacother 103:75–86. https://doi.org/10.1016/j.biopha.2018.04.010
Huang X, Zhong L, Kang Q, Liu S, Feng Y, Geng Y, Chen D, Ou Y, Yang S, Yin L, Luo W (2021) A high starch diet alters the composition of the intestinal microbiota of largemouth bass Micropterus salmoides, which may be associated with the development of enteritis. Front Microbiol 12:696588–696898. https://doi.org/10.3389/fmicb.2021.696588
Kamzlam BS, Medale F, Panserat S (2017) Utilisation of dietary carbohydrates in farmed fishes: new insights on influencing factors, biological limitations and future strategies. Aquaculture 467:3–27. https://doi.org/10.1016/j.aquaculture.2016.02.007
Kasumyan AO, Døving KB (2003) Taste preferences in fish. Fish Fish 4:289–347. https://doi.org/10.1046/j.1467-2979.2003.00121.x
Kim MS, Krawczyk SA, Doridot L, Fowler AJ, Wang JX, Trauger SA, Noh HL, Kang HJ, Meissen JK, Blatnik M, Kim JK, Lai M, Herman MA (2016) ChREBP regulates fructose-induced glucose production independently of insulin signaling. J Clin Invest 126:4372–4386. https://doi.org/10.1172/JCI81993
Li SL, Sang CY, Wang A, Zhang JC, Chen NS (2019) Effects of dietary carbohydrate sources on growth performance, glycogen accumulation, insulin signaling pathway and hepatic glucose metabolism in largemouth bass, Micropterus salmoides. Aquaculture 513:734391–734397. https://doi.org/10.1016/j.aquaculture.2019.734391
Li S, Sang C, Wang A, Zhang C, Chen N (2019) Effects of dietary carbohydrate sources on growth performance, glycogen accumulation, insulin signaling pathway and hepatic glucose metabolism in largemouth bass, Micropterus salmoides. Aquaculture 513:734391–734391. https://doi.org/10.1016/j.aquaculture.2019.734391
Liang X, Wang J, Gong G, Xue M, Dong Y, Wu X, Wang X, Chen C, Liang X, Qin Y (2017) Gluconeogenesis during starvation and refeeding phase is affected by previous dietary carbohydrates levels and a glucose stimuli during early life in Siberian sturgeon (Acipenser baerii). Anim Nutr 3:284–294. https://doi.org/10.1016/j.aninu.2017.06.001
Lidbury I, Murrell JC, Chen Y (2014) Trimethylamine N-oxide metabolism by abundant marine heterotrophic bacteria. Proc Natl Acad Sci USA 111:2710–2715. https://doi.org/10.1073/pnas.1317834111
Lin SM, Shi CM, Mu MM, Chen YJ, Luo L (2018) Effect of high dietary starch levels on growth, hepatic glucose metabolism, oxidative status and immune response of juvenile largemouth bass, Micropterus salmoides. Fish Shellfish Immunol 78:121–126. https://doi.org/10.1016/j.fsi.2018.04.046
Liu CZ, He AY, Ning LJ, Luo Y, Li DL, Zhang ML, Chen LQ, Du ZY (2018) Leptin selectively regulates nutrients metabolism in Nile tilapia fed on high carbohydrate or high fat diet. Front Endocrinol (lausanne) 9:574–586. https://doi.org/10.3389/fendo.2018.00574
Maslowski KM, Mackay CR (2011) Diet, gut microbiota and immune responses. Nat Immunol 12:5–9. https://doi.org/10.1038/ni0111-5
Mora-Sánchez B, Balcázar JL, Pérez-Sánchez T (2020) Effect of a novel postbiotic containing lactic acid bacteria on the intestinal microbiota and disease resistance of rainbow trout (Oncorhynchus mykiss). Biotechnol Lett 42:1957–1962. https://doi.org/10.1007/s10529-020-02919-9
Muhammed M, Meghan M, Zhang C, Nathan K, Vaishna M, Chaitra S, Nishanth S (2019) Impact of branched chain amino acid supplementation on hepatic mitochondrial metabolism in mice with non-alcoholic fatty liver disease (P08–136–19). Current Developments in Nutrition, Supplement_1. https://doi.org/10.1093/cdn/nzz044.P08-136-19
Murphy EF, Cotter PD, Healy S, Marques TM, O’Sullivan O, Fouhy F, Clarke SF, O’Toole PW, Quigley EM, Stanton C, Ross PR, O’Doherty RM, Shanahan F (2010) Composition and energy harvesting capacity of the gut microbiota: relationship to diet, obesity and time in mouse models. Gut 59:1635–1642. https://doi.org/10.1136/gut.2010.215665
Overland M, Rørvik KA, Skrede A (1999) Effect of trimethylamine oxide and betaine in swine diets on growth performance, carcass characteristics, nutrient digestibility, and sensory quality of pork. J Anim Sci 77:2143–2153. https://doi.org/10.2527/1999.7782143x
Park JS, Seo JH, Youn HS (2013) Gut microbiota and clinical disease: obesity and nonalcoholic Fatty liver disease. Pediatr Gastroenterol Hepatol Nutr 16:22–27. https://doi.org/10.5223/pghn.2013.16.1.22
Prathomya P, Prisingkorn W, Jakovli I, Deng FY, Zhao YH, Wang WM (2017) 1H NMR-based metabolomics approach reveals metabolic alterations in response to dietary imbalances in Megalobrama amblycephala. Metabolomics 13:17–29. https://doi.org/10.1007/s11306-016-1158-7
Prisingkorn W, Prathomya P, Jakovlić I, Liu H, Zhao YH, Wang WM (2017) Transcriptomics, metabolomics and histology indicate that high-carbohydrate diet negatively affects the liver health of blunt snout bream (Megalobrama amblycephala). BMC Genomics 18:856–870. https://doi.org/10.1186/s12864-017-4246-9
Rørvik KA, Steien SH, Saltkjelsvik B, Thomassen MS (2015) Urea and trimethylamine oxide in diets for seawater farmed rainbow trout: effect on fat belching, skin vesicle, winter ulcer and quality grading. Aquaculture Nutri 6:247–254
Seibel BA, Walsh PJ (2002) Trimethylamine oxide accumulation in marine animals: relationship to acylglycerol storage. J Exp Biol 205:297–306. https://doi.org/10.1242/jeb.205.3.297
Sesay DF, Habte-Tsion HM, Zhou Q, Ren M, Xie J, Liu B, Chen R, Pan L (2017) The effect of dietary folic acid on biochemical parameters and gene expression of three heat shock proteins (HSPs) of blunt snout bream (Megalobrama amblycephala) fingerling under acute high temperature stress. Fish Physiol Biochem 43:923–940. https://doi.org/10.1007/s10695-016-0311-6
Shi HJ, Xu C, Liu MY, Wang BK, Liu WB, Chen DH, Zhang L, Xu CY, Li XF (2018) Resveratrol improves the energy sensing and glycolipid metabolism of blunt snout bream Megalobrama amblycephala fed high-carbohydrate diets by activating the AMPK-SIRT1-PGC-1α network. Front Physiol 9:1258–1274. https://doi.org/10.3389/fphys.2018.01258
Shi HJ, Li XF, Xu C, Zhang DD, Liu WB (2020) Nicotinamide improves the growth performance, intermediary metabolism and glucose homeostasis of blunt snout bream Megalobrama amblycephala fed high-carbohydrate diets. Aquac Nutr 26:1311–1328. https://doi.org/10.1111/anu.13088
Subramaniam S, Fletcher C (2018) Trimethylamine N-oxide: breathe new life. Br J Pharmacol 175:1344–1353. https://doi.org/10.1111/bph.13959
Sun HX, Xia MS, Hu CH (2005) Effect of trimethylamine oxide on growth and nutrient composition of tilapia. Freshwater Fisheries 3:17–19. (Published in Chinese)
Sun CX, Xu WN, Zhang DD, Li XF, Li PF, Jiang GZ, Liu WB (2018) Different preference is modulated by the feeding stimulant supplementation in different Chinese soft-shelled turtle (Pelodiscus sinensis) basic diets. Aquacult Nutr 24:195–203. https://doi.org/10.1111/anu.12547
Taesuwan S, Cho CE, Malysheva OV, Bender E, King JH, Yan J, Thalacker-Mercer AE, Caudill MA (2017) The metabolic fate of isotopically labeled trimethylamine-N-oxide (TMAO) in humans. J Nutr Biochem 45:77–82. https://doi.org/10.1016/j.jnutbio.2017.02.010
Tomasello G, Tralongo P, Damiani P, Sinagra E, Di Trapani B, Zeenny MN, Hussein IH, Jurjus A, Leone A (2014) Dismicrobism in inflammatory bowel disease and colorectal cancer: changes in response of colocytes. World J Gastroenterol 20:18121–18130. https://doi.org/10.3748/wjg.v20.i48.18121
Uittenbogaart M, Leclercq WK, Bonouvrie D, Romeijn MM, Luijten AA, Olde Damink SW, van Dielen FM, Rensen SS (2019) Diet-induced alteration of microbiota and development of obesity, nonalcoholic fatty liver disease, and diabetes: study protocol of a prospective study. JMIR Res Protoc 8:e11553–e11564. https://doi.org/10.2196/11553
Velasquez MT, Ramezani A, Manal A, Raj DS (2016) Trimethylamine N-oxide: the good, the bad and the unknown. Toxins (basel) 8(11):326–336. https://doi.org/10.3390/toxins8110326
Wang F (2020) Pathways of betaine supplement in high-carbohydrate diets increasing plasma taurine in Megalobrama amblycephala. (Master’s thesis, Huazhong Agricultural University) DOI:https://doi.org/10.27158/d.cnki.ghznu.2020.000286. (Published in Chinese)
Wang CA, Xu QY, Chang YP, Xu HP, Yin JS (2011) Effects of feeding attractants on growth and body composition, enzyme and serum indices of Hucho taimen. J Anhui Agri Univ 38:65-71. https://doi.org/10.13610/j.cnki.1672-352x.2011.01.030. (Published in Chinese)
Wang CA, Xu QY, Xu H, Yin JS, Wang Y (2012) Trimethylamine oxide in diets for taimen (Hucho taimen): effects on growth performance, muscle composition, gastrointestinal lipase activity and serum biochemical indices. Journal of Animal Nutrition 24:2279–2286. (Published in Chinese)
Wang F, Xu J, Jakovlić I, Wang WM, Zhao YH (2019) Dietary betaine reduces liver lipid accumulation via improvement of bile acid and trimethylamine-N-oxide metabolism in blunt-snout bream. Food Funct 10:6675–6689. https://doi.org/10.1039/c9fo01853k
Wang G, Sun Q, Wang H, Liu H (2021a) Identification and characterization of circRNAs in the liver of blunt snout bream (Megalobrama amblycephala) infected with Aeromonas hydrophila. Developmental & Comparative Immunology 124:104185. https://doi.org/10.1016/j.dci.2021.104185
Wang T, Zhang N, Yu X, Qiao F, Chen L, Du Z, Zhang M (2021b) Inulin alleviates adverse metabolic syndrome and regulates intestinal microbiota composition in Nile tilapia (Oreochromis niloticus) fed with high-carbohydrate diet. British Journal of Nutrition 126(2):161–171. https://doi.org/10.1017/S000711452000402X
Welker TL, Lim C, Yildirim-Aksoy M, Klesius PH (2007) Effect of buffered and unbuffered tricaine methanesulfonate (MS-222) at different concentrations on the stress responses of channel catfish, Ictalurus punctatus Rafinesque. J Appl Aquac 19:1–18. https://doi.org/10.1300/J028v19n03_01
White PJ, McGarrah RW, Grimsrud PA, Tso SC, Yang WH, Haldeman JM, Grenier-Larouche T, An J, Lapworth AL, Astapova I, Hannou SA, George T, Arlotto M, Olson LB, Lai M, Zhang GF, Ilkayeva O, Herman MA, Wynn RM, Chuang DT, Newgard CB (2018) The BCKDH kinase and phosphatase integrate BCAA and lipid metabolism via regulation of ATP-citrate lyase. Cell Metab 27:1281–1293. https://doi.org/10.1016/j.cmet.2018.04.015
Xu J, Wang F, Jakovlić I, Prisingkorn W, Li JT, Wang WM, Zhao YH (2018) Metabolite and gene expression profiles suggest a putative mechanism through which high dietary carbohydrates reduce the content of hepatic betaine in Megalobrama amblycephala. Metabolomics 14:1–13. https://doi.org/10.1007/s11306-018-1389-x
Yancey PH (2005) Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol 208:2819–2830. https://doi.org/10.1242/jeb.01730
Yi HM, Ye YT, Lv H, Lv B, Shi Y, Sun F, Yu N, Xiao PZ (2021) Effects of three feeding agents on growth and physiology of channel catfish (Letalurus Punetaus). Feed Industry 42(18):50–56. https://doi.org/10.13302/j.cnki.fi.2021.18.008. (Published in Chinese)
Yin JX, Hong LU, Xie Q, Ding JL, Ni-Hang LI (2007) A study on rapid colorimetric determination of water soluble total sugar, reducing sugar and starch in tobacco with 3,5-dinitrosalicylic acid. J Yunnan Agri Univ 22:829–838. https://doi.org/10.16211/j.issn.1004-390x(n).2007.06.001
Yu H, Zhang L, Chen P, Liang X, Cao A, Han J, Wu X, Zheng Y, Qin Y, Xue M (2019) Dietary bile acids enhance growth, and alleviate hepatic fibrosis induced by a high starch diet via AKT/FOXO1 and cAMP/AMPK/SREBP1 pathway in Micropterus salmoides. Front Physiol 10:1430–1444. https://doi.org/10.3389/fphys.2019.01430
Zhang CJ, Yu M, Yang YX, Mu CL, Su Y, Zhu WY (2016) Effect of early antibiotic administration on cecal bacterial communities and their metabolic profiles in pigs fed diets with different protein levels. Anaerobe 42:188–196. https://doi.org/10.1016/j.anaerobe.2016.10.016
Zhang L, Qi Y, Aluo Z, Liu S, Zhang Z, Zhou L (2019) Betaine increases mitochondrial content and improves hepatic lipid metabolism. Food Funct 10:216–223. https://doi.org/10.1039/c8fo02004c
Zhang Y, Liang XF, He S, Chen X, Wang J, Li J, Zhu Q, Zhang Z, Li L, Alam MS (2020) Effects of High Carbohydrate Diet-Modulated Microbiota on Gut Health in Chinese Perch. Front Microbiol 11:575102–575118. https://doi.org/10.3389/fmicb.2020.575102
Zhao W, Xie JJ, Fang HH, Liu YJ, Niu J (2020) Effects of corn starch level on growth performance, antioxidant capacity, gut morphology and intestinal microflora of juvenile golden pompano, Trachinotus ovatus. Aquaculture 524:735197–735206. https://doi.org/10.1016/j.aquaculture.2020.735197
Zhou C, Ge X, Niu J, Lin H, Huang Z, Tan X (2015a) Effect of dietary carbohydrate levels on growth performance, body composition, intestinal and hepatic enzyme activities, and growth hormone gene expression of juvenile golden pompano, Trachinotus ovatus. Aquaculture 437:390–397. https://doi.org/10.1016/j.aquaculture.2014.12.016
Zhou C, Ge X, Liu B, Xie J, Chen R, Miao L, Ren M (2015b) Comparative study on the effect of high dietary carbohydrate on the growth performance, body composition, serum physiological responses and hepatic antioxidant abilities in Wuchang bream (Megalobrama amblycephala) and black carp (Mylopharyngodon piceus Richardson). Aquac Res 48:1020–1030. https://doi.org/10.1111/are.12944
Zhou CP, Ge XP, Liu B, Xie J, Chen RL, Miao LH, Ren MC (2017) Comparative study on the effect of high dietary carbohydrate on the growth performance, body composition, serum physiological responses and hepatic antioxidant abilities in Wuchang bream (Megalobrama amblycephala) and black carp (Mylopharyngodon piceus Richardson, 1846). Aquac Res 48:1401–1409. https://doi.org/10.1111/are.12944
Zhou YL, He GL, Jin T, Chen YJ, Lin SM (2020) High dietary starch impairs intestinal health and microbiota of largemouth bass, Micropterus salmoides. Aquaculture 534:736261–736268. https://doi.org/10.1016/j.aquaculture.2020.736261
Funding
This study was supported by the Fundamental Research Funds for the Central Universities (No. 2662021SCPY002) and Natural Science Foundation of China (No. 31401976).
Author information
Authors and Affiliations
Contributions
The authors’ responsibilities were as follows: Wangwang Huang: breeding experiments, formal analysis, data curation, and article writing; Yizhuo Hua: breeding experiments, formal analysis, and data curation; Fan Wang, Jia Xu, Lv Yuan, and Zhao Jing: formal analysis and data curation; Yuhua Zhao: conceptualization, funding acquisition, methodology, supervision, and writing and editing; Weimin Wang: conceptualization and methodology.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This research was approved by the Ethics Committee of the Institute of Laboratory Animal Centre, Huazhong Agriculture University (Ethical code: HZAUFI-2016–009). All animal-handling procedures and experiments were conducted under the Guidance of the Care and Use of Laboratory Animals in China (No. F20190101).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, W., Hua, Y., Wang, F. et al. Dietary betaine and/or TMAO affect hepatic lipid accumulation and glycometabolism of Megalobrama amblycephala exposed to a high-carbohydrate diet. Fish Physiol Biochem 50, 59–75 (2024). https://doi.org/10.1007/s10695-022-01160-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-022-01160-7