Abstract
Blunt snout bream (Megalobrama amblycephala) were randomly assigned into three diets: normal-carbohydrate diet (NCD, 30% carbohydrate, w/w), high-carbohydrate diet (HCD, 43% carbohydrate), and HCB (HCD supplemented with 50 mg/kg berberine (BBR)). After 10 weeks’ feeding trial, the results showed that higher levels of plasma glucose, triglyceride, and total cholesterol were observed in HCD-fed fish than in NCD-fed fish, while HCB feeding significantly ameliorated this effect. Moreover, HCB feeding remarkably reversed HCD-induced hepatic glycogen and lipid contents. In insulin signaling, BBR inclusion restored HCD-induced suppression of insulin receptor substrate mRNA expression and elevation of forkhead transcription factor 1 mRNA expression. In glucose metabolism, upregulated glucose transporter 2 and glycogen synthase mRNA expressions in the HCD group were observed compared to the NCD group. However, BBR adding reduced the mRNA expressions of glycogen synthase, phosphoenolpyruvate carboxykinase, and glucose-6-phosphatase and increased the transcriptional levels of glucose transporter 2 and pyruvate kinase. In lipid metabolism, BBR supplementation could reverse downregulated hepatic carnitine palmitoyl transferase I mRNA expression and upregulated hepatic acetyl-CoA carboxylase and fatty acid synthetase mRNA expressions in the HCD group. Taken together, it demonstrates that BBR could improve glucose metabolism of this species via enhancing liver’s glycolysis and insulin signaling, while inhibiting liver’s glycogen synthesis and gluconeogenesis. It also indicates that BBR could reduce the metabolic burden of the liver by inhibiting fat synthesis and promoting lipid decomposition, and then enhance fat uptake in peripheral tissues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the increasing demand for aquatic feed, the booming aquaculture industry has been facing a big problem, how to replace expensive and limited animal-origin feed with cheap plant-origin feed. Carbohydrate, rich in sugar and starch, is an inexpensive and widely sourced energy substance. Adding carbohydrates appropriately in the diets can promote fish growth, save protein, reduce ammonia emissions, cut down lipid catabolism, provide metabolic intermediates, and etc. (Azaza et al. 2015; Li et al. 2015; Watanabe 2002). However, due to lower carbohydrate utilization rate of fish than mammal, long-term intake of a carbohydrate-rich diet would cause a glucose metabolic disorder to fish (Moon 2001; NRC 2011). Functional foods may have the potential to improve glycemic control (Bai et al. 2015). For example, adding specific nutrients exogenously, such as thiamine and specific amino acids, is a very simple and effective way to attenuate hyperglycemia (Andoh 2007; Hansen et al. 2015; Kamalam et al. 2017). Additionally, Chinese herbal medicine, as a natural medicine, has been showing the characteristics of mild hypoglycemic effect and low cost to solve this issue (El Kaissi and Sherbeeni 2011; Li et al. 2004).
Berberine (BBR) is a natural compound in many Chinese herbaceous plants such as Coptis chinensis and has been recognized as having various medical potentials (Tillhon et al. 2012). Traditionally, BBR is often used to treat gastrointestinal infections due to its antibacterial and anti-inflammatory properties (Chow et al. 2016; Neag et al. 2018; Tillhon et al. 2012). Nowadays, BBR is attracting much attention because of its outstanding regulation of glucose and lipid metabolism (Ilyas et al. 2020; Zhang et al. 2008), and has also been confirmed in clinical trials (Ni 1988; Wei et al. 2004; Xie et al. 2005). Not only that, BBR also shows a good potential of regulating glucolipid metabolism in fish. For example, in Ctenopharyngodon idella, BBR-supplemented feed can significantly reduce the levels of glucose, total cholesterol (TC), and triglyceride (TG) in the blood, which might be through affecting the diversity and structure of intestinal microorganisms (Pan et al. 2019). And it was also reported that BBR could also reduce lipids by activating genes related to fatty acid oxidation and genes related to reducing fatty acid intake (Lu et al. 2016; Zhou et al. 2019). However, there are limited reports about the molecular mechanism of BBR regulating glycometabolism so far.
Blunt snout bream (Megalobrama amblycephala) is a herbivorous freshwater fish of key economic and cultural importance in China. Its herbivory and relative tolerance to carbohydrate also make it possible to add more starch to its feed for saving costs, although excessive carbohydrate still causes disorder of glucose metabolism. Furthermore, adding glucose-lowering substances into the feed to alleviate the metabolic problems caused by the high-carbohydrate feed is thought to be a direct and effective strategy for blunt snout bream. Previous studies in our lab proofed that adding metformin or resveratrol can effectively reduce the blood glucose by regulating the liver glucose metabolism; however, it still causes an increase in liver glycogen and hepatosomatic index (HSI), and may elevate the liver metabolic burden in the long term (Shi et al. 2018; Xu et al. 2017). Therefore, it is very necessary to find a new candidate to reduce blood glucose as well as liver glycogen for fish. BBR has been shown great properties to anti-diabetes in rodents. However, the regulation of BBR on glucose metabolism in fish has only been limited to the study of gut microbiome currently.
Thus, the purpose of this study is to explore hypoglycemic and lipid-lowering effects of dietary BBR supplemented in high-carbohydrate diet of blunt snout bream. And it may help to understand the underlying mechanism of glycolipid metabolism control by BBR and provide some new insights for improving the carbohydrate utilization of aquatic animals.
Materials and methods
Experimental diets
Feed formulation and proximate composition were presented in Table 1. BBR (≥98%) used in this study was purchased from a local company (Spring and Autumn Biotechnology Company, Nanjing, China). The addition amount of BBR in the experimental diet is 50 mg/kg referenced from a previous study (Lu et al. 2016). A total of three isonitrogenous and isolipidic diets were formulated, including normal-carbohydrate diet (NCD, 30% carbohydrate), high-carbohydrate diet (HCD, 43% carbohydrate), and high-carbohydrate diet supplemented with BBR (HCB, 43% carbohydrate + 50 mg/kg BBR). As for the ingredients, fish meal, soybean meal, rapeseed meal, and cottonseed meal served as protein sources. Corn starch was adopted as the main carbohydrate source. Fish oil and soybean oil were applied to meet the lipid demand of this species. The experimental diets were produced by a pellet extruder (MUZL 180, Jiangsu Muyang Group Co., Ltd., Yangzhou, China), dried in a ventilation room for 24 h, and then stored in plastic bags at −20 °C until use. Proximate composition of each diet was analyzed following the protocol, which consists of moisture, crude lipid, ash, crude protein, crude fiber, nitrogen-free extract, and energy (AOAC 1995).
Fish and the feeding trial
Juvenile blunt snout bream were purchased from the Fish Hatchery of Yangzhou (Jiangsu, China). Before feeding experiment, fish were fed with commercial aquafeed (32% protein, 7% lipid, 33% nitrogen-free extract; Shuaifeng Aquafeed Co., Ltd., Nanjing, China) three times a day to a slight excess of satiety for a week. After acclimation, 288 fish (average initial weight of 20.36 ± 1.44 g) were randomly distributed into 24 floating net cages (1 × 1 × 1.5 m, L: W: H), and each cage houses 12 fish. Fish in each cage were randomly assigned to one of the three experimental diets (each treatment had eight replicates). Fish were hand-fed to an apparent visual satiety three times a day (7:00, 12:00, and 17:00), which lasted for 10 weeks. During feeding trial, the water quality was set up as follows: Temperature varied from 27 to 29 °C; dissolved oxygen was maintained above 5.0 mg/L; pH ranged from 7.3 to 7.6; and total ammonia nitrogen and nitrite were kept < 0.4 and 0.01 mg/L, respectively.
Sample collection
After the feeding trial, fish were starved for 24 h to empty the intestine, and then all fish were counted and weighed. Sampling was performed using 100 mg/L MS-222 (tricaine methanesulfonate; Sigma, USA) for anesthetization. Blood was rapidly drawn from the caudal vein into 1.5-mL heparinized tubes and centrifuged at 3000 × rpm for 10 min at 4 °C, and the supernatant plasma was collected and kept at −80 °C. Additionally, liver, muscle, and intraperitoneal fat tissue specimens were taken and immediately frozen in liquid nitrogen, and then kept at −80 °C.
Analytical procedures
Growth parameters
The growth parameters obtained in this study were calculated using the following formulas:
where W0 is the initial body weight, Wt is the final body weight, T is the feeding period in days, N0/E0 and Nt/Et are the initial and final nitrogen/energy contents in whole body, respectively, and Ndiet/Ediet are the nitrogen/energy contents in diets.
Proximate composition analysis
The methods of approximate compositions of diets and fish were as follows: The samples were dried at 105° until constant weight to measure its moisture; crude protein contents (nitrogen × 6.25) were assessed with a Kjeltec analyzer unit; crude lipid was determined by ether extraction using a Soxtec auto extraction unit; ash content was estimated through carbonizing and combusting in a muffle furnace at 550 °C for 2 h; gross energy was measured by a Bomb Calorimeter (PARR 1281; Parr Instrument Company); and crude fiber was determined by fritted glass crucible method using an automatic analyzer (ANKOM A2000i, USA).
Measurements of plasma metabolites and tissue glycogen and lipid contents
Plasma glucose levels were determined using the glucose oxidase method described by Asadi et al. (2009). Plasma insulin level was determined by radioimmunoassay kit and used guinea pig anti-insulin antibody (Beijing North Institute of Biotechnology Co., Ltd.). Plasma pyruvate levels were estimated according to the method of Nigam (1962). Levels of advanced glycation products (AGEs) in plasma were measured following the method of Monnier et al. (1986). Plasma triglyceride levels in lipoprotein fractions were evaluated using a colorimetric method (McNamara and Schaefer 1987). Plasma lactate levels were measured by injecting 10 μL of serum into a VITROSDT60II chemical analyzer (Kodak, NY, USA) using a DT pipette. Tissue glycogen levels were determined following the method of Pfleiderer and Bergmeyer (1974). According to the method of Floch (1957), the content of tissue lipid was extracted with a mixture of chloroform:methanol (2:1, V:V).
Oil red O staining
Liver samples (n = 3) were fixed in 40 g/kg paraformaldehyde solution for 24 h. The liver was then cut into 20-μm sections and neutral lipid staining with oil red O was performed (Jiang et al. 2018). Under the microscope, the lipid is red and the nucleus is light blue. The Image-Pro Plus 6.0 software was used to measure the red area representing lipid accumulation. Three areas in each sample were randomly selected to obtain the size and number of lipid droplets.
Quantitative RT-PCR
Total RNA was extracted from liver tissue with Trizol (Invitrogen, CA, USA) following the manufacturer’s instruction. The quantity and purity of the extracted RNA were determined by NanoDrop 2000 (Thermo Fisher Scientific, Wilmington, DE, USA), in terms of its absorbance at 260 nm and 280 nm. RNA integrity assessment was performed using denatured agarose gel with 1% formaldehyde. cDNA was prepared from 500 ng/μL RNA using a PrimeScript™ RT Master Mix Kit (Cat. No. RR036A, TaKaRa, Co. Ltd., Dalian, China). The resulting cDNA was diluted with DNase/RNase-free water and then worked as a template for quantitative PCR using a TB Green™ Premix Ex Taq™ Kit (Cat. No. RR820A, TaKaRa, Co. Ltd.) and a real-time PCR detection system (ABI7300; Applied Biosystems, Foster City, CA, USA). A 20-μL real-time PCR reaction volume was adopted, consisting of 10 μL of TB Green™ Premix Ex Taq™ II (Tli RNaseH Plus) (2×), 0.4 μL of PCR forward/reverse primer (10 μM) each, 0.4 μL of ROX reference dye II (50×), 2.0 μL of cDNA template, and 6.8 μL of DNase/RNase-free water. The PCR reaction conditions were as follows: 95 °C for 30 s, then followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s. Melting curves and electrophoresis were applied to judge the specificity of primers. The relative expression of the target gene is calculated by the 2−ΔΔCt method (Livak and Schmittgen, 2001). β-Action was used as an endogenous control for normalization of the gene expression levels of target genes. All primers are presented in Table 2.
Statistical analyses
Data in the present study were all analyzed by one-way ANOVA using the SPSS 25.0 statistical software package for Windows (SPSS, Michigan Avenue, Chicago, IL, USA). All data were firstly tested for the homogeneity of variances using the Levene’s test, and then analyzed by one-way ANOVA. Tukey’s honestly significant difference test was used to distinguish differences among treatments (P < 0.05). All data were expressed as mean ± S.E.M (mean standard error).
Results
Growth performance and feed utilization
To assess the effect of BBR on the growth performance of high-carbohydrate-fed fish, NCD, HCD, or HCB were fed to blunt snout bream for 10 weeks, and no mortality was observed during the trial. As was showed in Table 3, final weight, SGR, FI, FCR, PER, NRE, and HSI observed no significant difference among all groups, but the AFP value of fish-fed NCD was significantly lower than those of fish-fed HCD and HCB. HCB-fed fish had a higher ERE than that of NCD-fed fish. For the results of whole-body composition, there were no differences in moisture, ash, and crude protein among all groups, whereas HCB feeding resulted in significant increases in crude lipid and gross energy versus NCD feeding.
Plasma biochemistry parameters
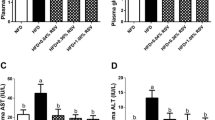
BBR addition significantly reversed the HCD-induced increases in plasma glucose, TG, and TC contents (Table 4). Higher AGE level was detected in HCD-fed fish than in NCD-fed fish, while BBR supplementation tended to reduce this effect. And dietary BBR inclusion was inclined to ameliorate the HCD-induced blood insulin impairment versus the HCD group. In addition, plasma pyruvate level of the HCB group was significantly lower than those of the other groups.
Tissue glycogen and lipid contents
HCD feeding resulted in significant higher hepatic glycogen and lipid contents than NCD feeding (Table 5), whereas HCB remarkably reversed this effect versus HCD (P < 0.05). The glycogen and lipid levels of intraperitoneal fat in NCD-feeding fish were significantly lower than those in HCD-feeding or HCB-feeding fish. However, there were no significant differences in muscular glycogen or lipid content among all groups.
Oil red O staining of the liver
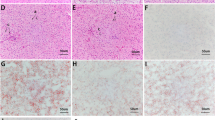
The accumulation of liver lipid was evaluated by oil red O staining (Fig. 1). According to the photomicrographs of the liver tissue, the lipid droplet clusters of fish-fed HCD were more than that of fish-fed NCD or HCB (Fig. 1d), whereas the addition of BBR showed an obvious lipid-reducing effect.
Hepatic lipid profiles of Megalobrama amblycephala fed different experimental diets. a Fish-fed NCD, b Fish-fed HCD. c Fish-fed HCB. d The lipid accumulation (red-stained area, oil red O staining). Red point lipid droplet, white point vacuoles, blue point nucleus. Photomicrographs (×400) and scale bar (20 μm). Each data represents the average of 3 replicates. The bars assigned with different lowercase letters are significantly different (P < 0.05)
Relative expression of genes related to insulin signaling
As can be seen from Fig. 2, to reveal the molecular mechanism associated with high-carbohydrate diet and BBR supplementation-induced insulin signaling, the relative gene expressions of insulin receptor substrate (irs), phosphatidylinositol 3-kinase (pi3k), protein kinase B (akt), and forkhead transcription factor 1 (foxo1) were detected in the liver of this species using RT-qPCR. BBR supplementation remarkably reversed the HCD-induced suppression of irs mRNA expression and the HCD-induced upregulation of foxo1 mRNA expression. The pi3k and akt mRNA expressions of HCB-fed fish were significantly higher than those of the other two groups.
Relative expressions of insulin signaling-related genes in the liver of blunt snout bream fed with different experimental diets. The mRNA levels of insulin receptor substrate (irs) (a), phosphatidylinositol 3-kinase (pi3k) (b), protein kinase B (akt) (c), and forkhead transcription factor 1 (foxo1) (d) were evaluated using real-time RT-PCR. Expression values were normalized using β-action as an endogenous control. Each data represents the average of 6-7 replicates. The bars assigned with different superscripts are significantly different (P < 0.05)
Relative expression of glucose metabolism-related genes
As was shown in Fig. 3, there were no differences of phosphoenolpyruvate carboxykinase (pepck), glucose-6-phosphatase (g6pase), and pyruvate kinase (pk) mRNA expressions between the NCD and HCD groups. The pepck and g6pase mRNA expressions in the liver of HCB-fed fish were significantly lower than those of NCD and HCD-fed fish. HCB feeding resulted in significant increases in glucose transporter 2 (glut2) and pk mRNA expressions versus NCD and HCD. HCB remarkably reduced HCD-induced glycogen synthase (gs) expression. There was no difference of hepatic fructose-1,6-biphosphatase (fbpase) mRNA expression among all the treatments.
Relative expressions of glucose metabolism-related genes in the liver of blunt snout bream fed with different experimental diets. The mRNA levels of phosphoenolpyruvate carboxykinase (pepck) (a), glucose-6-phosphatase (g6pase) (b), fructose-1,6-biphosphatase (fbpase) (c), glucose transporter 2 (glut2) (d), pyruvate kinase (pk) (e), and glycogen synthase (gs) (f) were evaluated using real-time RT-PCR. Expression values were normalized using β-action as an endogenous control. Each data represents the average of 6-7 replicates. The bars assigned with different superscripts are significantly different (P < 0.05)
Relative expression of lipid metabolism-related genes
In Fig. 4, HCD feeding resulted in significantly higher hepatic fatty acid synthetase (fas) and acetyl-CoA carboxylase (accα) expressions and a relatively lower hepatic carnitine palmitoyl transferase I (cpt I) expression than NCD feeding, while HCB remarkably ameliorated this effect versus HCD.
Relative expressions of lipid metabolism-related genes in the liver of blunt snout bream fed with different experimental diets. The mRNA levels of acetyl-CoA carboxylase α (accα) (a), carnitine palmitoyl transferase I (cpt I) (b), and fatty acid synthase (fas) (c) were evaluated using real-time RT-PCR. Expression values were normalized using β-action as an endogenous control. Each data represents the average of 6-7 replicates. The bars assigned with different superscripts are significantly different (P < 0.05)
Discussion
The previous studies demonstrated that fish exposed to a high-carbohydrate diet in a long term will suffer from the postprandial hyperglycemia, the metabolic disorder, and be subjected to the impaired health status eventually (Kamalam et al. 2017; Ren et al. 2015; Ren et al. 2013). Although some medicines, such as benfotiamine, metformin, and nicotinamide, can effectively lower blood glucose and improve the glucolipid metabolism, there still exist some side effects, say the increased liver glycogen or lipid content (Shi et al. 2020; Shi et al. 2018; Xu et al. 2017; Xu et al. 2018). As a plant-origin bioactive substance, BBR has been shown an ability to reduce glucose level with almost less side effects (Na et al. 2012). Therefore, this study aimed to explore the role and mechanism of BBR applied in high-carbohydrate diet-fed blunt snout bream, from the aspects of growth, body composition, plasma biochemical indicators, and glucolipid metabolism processes.
We first determined whether BBR supplementation imposes any effects on the growth performance of HCD-fed fish. We found that either dietary carbohydrate levels or the addition of BBR revealed no significant differences of SGR, FCR, and FI among all groups. The reason might be that blunt snout bream has better utilization of carbohydrates than most carnivorous and omnivorous species (Li et al. 2013; Ren et al. 2013; Shi et al. 2018), and in a certain range, BBR inclusion does not impair growth. It was also reported that the addition of BBR did not affect the body weight gain and fatness of Ctenopharyngodon idellus compared with the control group (Pan et al. 2019). In addition, HCD feeding significantly enhanced AFP level compared with NCD feeding, while BBR supplemented in HCD did not change this effect. According to previous research, high-carbohydrates diet could increase the synthesis of glycogen and fat (Li et al. 2019; Prisingkorn et al. 2017; Ren et al. 2011; Wang et al. 2016). And the abdomen is the main part of fat deposition, which is easy to cause the increase of AFP, and can lead to an increase in whole-body lipid. With the addition of BBR, the ERE, whole-body lipid, and gross energy in the HCB group increased significantly versus the NCD group. The reason might be related to BBR’s ability to inhibit gluconeogenesis pathway, which in turn improves energy retention efficiency (especially fat). The latest research demonstrated that BBR also could enrich the intestinal flora of fish and increase beneficial microorganisms, so that these effects might promote the absorption of nutrients and enhance the ERE in fish whole body (Pan et al. 2019).
For the evaluation of fish physiological health conditions, plasma indicators are considered reliable parameters (Habte Tsion et al. 2016). After 10 weeks’ feeding trial, HCD resulted in higher levels of plasma glucose, AGEs, TG, and TC contents compared with NCD (Table 4). According to previous research in fish, long-term excessive carbohydrate intake does cause a symptom of hyperglycemia (Hemre et al. 2002; Peres and Oliva Teles 1999; Wilson 1994), and increase lipogenesis (Zhou et al. 2017), which might lead to the enhanced levels of glucose, AGEs, TG, and TC in blood. Furthermore, in this study, BBR supplementation significantly reduced the levels of glucose, TG, and pyruvate in plasma versus HCD, and insulin secretion tended to be induced by BBR although no significant difference was observed. According to previous reports, these phenomenons might be explained by the following facts: (1) BBR can directly inhibit gluconeogenesis to lower blood glucose, and does not completely depend on the action of insulin (Xia et al. 2011); (2) BBR may reduce blood glucose TG by adjusting the intestinal microbial flora (Pan et al. 2019) or downregulating stearoyl-CoA desaturase in blunt snout bream (Zhou et al. 2019); (3) BBR may accelerate glycolysis and promote the use of pyruvate (Yin et al. 2008). Regarding the change of TC level, HCD tended to increase TC content compared to NCD, and BBR inclusion had a slight TC-lowering effect (Table 4). These phenomena might be explained that the additive amount of BBR in this study was not enough to lower plasma cholesterol.
In terms of the roles in reducing tissue glycogen and lipid content, BBR has been applied to treat fatty liver and obesity in humans (Lee et al. 2006). In accordance with our results, it was also confirmed that BBR could reduce the accumulation of lipid and glycogen in the liver caused by HCD feeding in blunt snout bream. Oil red O staining of liver tissue demonstrated that BBR addition can reduce lipid droplet in hepatocyte (Fig. 1). It is generally believed that fat is the main form of energy storage, and fish lipid is generally deposited in the peritoneum, mesentery, and muscle tissues besides the liver (Tessari et al. 2009). In this study, BBR promoted the transfer of liver lipid/glycogen to peripheral tissues storing as lipid (abdominal fat and muscle) and enhanced the ERE in fish whole body (Table 3), and alleviated liver injury caused by fatty liver. This effect may be related to the increase of BBR in plasma VLDL level, the promotion of lipoprotein secretion in the liver, and the increase of liver fat output (Lu et al. 2013; Richard et al. 2006; Zhou et al. 2019). Therefore, the accumulation of peripheric fat leads to an increase in lipid in the fish body (Table 3). Hepatic glucose production accounts for more than 90% of endogenous glucose production, and mostly comes from glycogenolysis and gluconeogenesis (Petersen et al. 2017). For liver glycogen, BBR may reduce its content by increasing the expression of PPAR-α and PPAR-δ proteins, and reduce the pathological process of liver to regulate the metabolism of glycolipids (Zhou et al. 2008). Of course, the decrease in glycogen content in the liver may be also related to the decreased expression of gs. Gs is one of the important regulating enzymes for glycogen synthesis, and the reduced expression of gs mRNA in the HCB group may also explain this phenomenon (Fig. 3). Surprisingly, the addition of BBR increased the glycogen content in intraperitoneal fat. This may be because BBR is mainly enriched in the liver, and then liver glycogen synthesis is inhibited, resulting in more glucose accumulation in intraperitoneal fat (Liu et al. 2010; Yan et al. 2015).
In the regulation of glycolipid metabolism, the insulin signaling IRS/ PI3K/AKT is a major signaling pathway (Kamalam et al. 2017; Nandipati et al. 2017). After insulin binds to a receptor in its target cell, a signal cascade is triggered. Activated insulin receptor promotes tyrosine phosphorylation of IRS, thereby activating PI3K and AKT and downstream glucose and lipid metabolism (Zhang et al. 2018). In this study, the high-carbohydrate diet downregulated irs expression significantly versus NCD (Fig. 2). Pi3k and akt expressions also decreased, although the difference was not significant. This phenomenon was similar with the results in the oral glucose test of blunt snout bream (Pan et al. 2017). Moreover, high level of carbohydrates can also reduce IRS expression by stimulating S6K1 expression, leading to the phosphorylation or degradation of IRS1, which limits the transmission of insulin signal from insulin receptor to PI3K, thus weakening the effect of insulin (Manning 2004). However, the addition of BBR could significantly increase the expression of irs, pi3k, and akt (Fig. 2). Liu et al. (2018) reported that BBR prevents LPS-induced TLR4/TNF-α activation and enhances IRS expression in the liver. Thereafter, IRS could activate and stimulate PI3K and AKT activity, and then further inhibits its downstream targets, such as foxo1. Furthermore, AKT could phosphorylate FOXO1 and inhibit its expression to inhibit gluconeogenesis (Datta et al. 1999; Kaestner et al. 2000; Kops and Burgering 1999), and promote glycolysis (Zhang et al. 2006). This is also one of the main ways for BBR to lower plasma glucose.
Regarding glycometabolism, the expressions of glut2 and gs in the HCD group were significantly upregulated compared with the NCD group, and the expressions of pepck, g6pase, and fbpase in the HCD group had no effect. This indicated that long-term high-carbohydrate intake promotes liver glycogen synthesis but has no significant effect on gluconeogenesis. Glucose in the blood moves into the liver mainly through glut2, and long-term HCD feeding upregulated glut2 to promote liver uptake of glucose, which enhanced glycogen synthesis by gs upregulation (Enes et al. 2009). Pepck, g6pase, and fbpase, the key genes for gluconeogenesis, were not significantly changed in the HCD group versus the NCD group. This phenomenon could be caused by insufficient insulin secretion (Table 4), which was also observed in rainbow trout (Panserat et al. 2000; Zhou et al. 2016). However, BBR adding could reduce the expressions of pepck, g6pase, and gs, and increase the transcriptional levels of glut2 and pk (Fig. 3). This indicated that BBR can inhibit liver glycogen synthesis and gluconeogenesis and promote glucose transport and glycolysis, which may also be the cause of lower plasma glucose and reduced liver glycogen contents observed in the HCB group (Tables 4 and 5). It was also supported by a previous study that upregulated hepatic AKT can result in the upregulation of liver Glut2, a downstream substrate of AKT, which in turn promotes glucose transport (Giorgino et al. 2000). With regard to the pk expression in the HCB group, BBR may enhance the expression of pk by demethylating PK promoter and increasing the level of acetylation of histones H3 and H4 of PK (Zhang et al. 2015). BBR may also reduce ATP levels by inhibiting mitochondrial function of hepatocytes or increase the expression of hnf-4α mRNA, thereby inhibiting the expressions of pepck and g6pase (Wang et al. 2008; Xia et al. 2011). The results show that BBR not only promotes the uptake and decomposition of liver glucose, but also inhibits the production of liver glucose.
Compared to mammals, fish are more susceptible to fatty liver because the liver is the main site of fat metabolism for fish (Hemre et al. 2002). In accordance with our results, liver cpt I mRNA expression was significantly downregulated in the HCD group, while accα and fas were opposite. And these changes would increase liver fat synthesis and reduce lipolysis, eventually leading to liver lipid accumulation (Shi et al. 2018; Xu et al. 2017; Xu et al. 2018). Cpt I is considered to be a key regulator of mitochondrial β-oxidation (Kerner and Hoppel 2000). The study of Lu et al. (2016) demonstrated BBR could upregulate the expression of cpt I by activating pparα, thereby regulating mitochondrial β-oxidation. Accα and fas are two key enzymes closely related to liver fatty acid synthesis (Qian et al. 2015). The plasma TG-lowering and liver lipid-declining effects of BBR might be partially modulated by downregulating gene expressions of fas and accα through activation of the AMPK signaling pathway (Cao et al. 2013). This can explain why the hepatic lipid content of the HCB group was decreased.
Conclusion
BBR inclusion in HCD could attenuate hyperglycemia of blunt snout bream, mainly through promoting liver’s glycolysis and insulin signaling, while inhibiting liver’s glycogen synthesis and gluconeogenesis. In addition, BBR could ameliorate the metabolic burden of the liver by inhibiting fat synthesis and promoting lipid decomposition, and then enhance fat uptake in peripheral tissues.
Data availability
The data sets supporting the results of this article are included within the article and its additional files,
References
Andoh T (2007) Amino acids are more important insulinotropins than glucose in a teleost fish, barfin flounder (Verasper moseri). Gen Comp Endocrinol 151:308–317
AOAC (1995) Official methods of analysis of official analytical chemists international, 16th edn. Association of Official Analytical Chemists, Arlington
Asadi F, Hallajian A, Asadian P, Shahriari A, Pourkabir M (2009) Serum lipid, free fatty acid, and proteins in juvenile sturgeons: Acipenser persicus and Acipenser stellatus. Comp Clin Pathol 18:287–289
Azaza MS, Khiari N, Dhraief MN, Aloui N, Kraϊem MM, Elfeki A (2015) Growth performance, oxidative stress indices and hepatic carbohydrate metabolic enzymes activities of juvenile Nile tilapia, O reochromis niloticus L., in response to dietary starch to protein ratios. Aquac Res 46:14–27
Bai SC, Katya K, Yun H (2015) 7 - Additives in aquafeed: an overview. In: Davis DA (ed) Feed and feeding practices in aquaculture. Woodhead Publishing, Oxford, pp 171–202. https://doi.org/10.1016/B978-0-08-100506-4.00007-6
Cao SJ et al (2013) Berberine metabolites exhibit triglyceride-lowering effects via activation of AMP-activated protein kinase in Hep G2 cells. J Ethnopharmacol 149:576–582. https://doi.org/10.1016/j.jep.2013.07.025
Chow YL, Sogame M, Sato F (2016) 13-Methylberberine, a berberine analogue with stronger anti-adipogenic effects on mouse 3T3-L1 cells. Sci Rep 6:38129. https://doi.org/10.1038/srep38129
Datta SR, Brunet A, Greenberg ME (1999) Cellular survival: a play in three Akts. Genes Dev 13:2905–2927
El Kaissi S, Sherbeeni S (2011) Pharmacological management of type 2 diabetes mellitus: an update. Curr Diabetes Rev 7:392–405
Enes P, Panserat S, Kaushik S, Teles O (2009) Nutritional regulation of hepatic glucose metabolism in fish. Fish Physiol Biochem 35:519–539
Floch J (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Giorgino F, De Robertis O, Laviola L, Montrone C, Perrini S, McCowen KC, Smith RJ (2000) The sentrin-conjugating enzyme mUbc9 interacts with GLUT4 and GLUT1 glucose transporters and regulates transporter levels in skeletal muscle cells. Proc Natl Acad Sci 97:1125–1130
Habte Tsion HM, Ren MC, Liu B, Ge XP, Xie J, Chen RL (2016) Threonine modulates immune response, antioxidant status and gene expressions of antioxidant enzymes and antioxidant-immune-cytokine-related signaling molecules in juvenile blunt snout bream (Megalobrama amblycephala). Fish Shellfish Immunol 51:189–199
Hansen AC, Waagbø R, Hemre GI (2015) New B vitamin recommendations in fish when fed plant-based diets. Aquac Nutr 21:507–527
Hemre GI, Mommsen TP, Krogdahl A (2002) Carbohydrates in fish nutrition: effects on growth, glucose metabolism and hepatic enzymes. Aquac Nutr 8:175–194. https://doi.org/10.1046/j.1365-2095.2002.00200.x
Ilyas Z et al (2020) The effect of Berberine on weight loss in order to prevent obesity: a systematic review. Biomed Pharmacother 127:110137. https://doi.org/10.1016/j.biopha.2020.110137
Jiang GZ, Zhou M, Zhang DD, Li XF, Liu WB (2018) The mechanism of action of a fat regulator: glycyrrhetinic acid (GA) stimulating fatty acid transmembrane and intracellular transport in blunt snout bream (Megalobrama amblycephala). Comp Biochem Physiol A Mol Integr Physiol 226:83–90. https://doi.org/10.1016/j.cbpa.2018.08.014
Kaestner KH, Knöchel W, Martínez DE (2000) Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev 14:142–146
Kamalam BS, Medale F, Panserat S (2017) Utilisation of dietary carbohydrates in farmed fishes: new insights on influencing factors, biological limitations and future strategies. Aquaculture 467:3–27. https://doi.org/10.1016/j.aquaculture.2016.02.007
Kerner J, Hoppel C (2000) Fatty acid import into mitochondria. Biochimica et Biophysica Acta (BBA) 1486:1–17. https://doi.org/10.1016/S1388-1981(00)00044-5
Kops GJ, Burgering BM (1999) Forkhead transcription factors: new insights into protein kinase B (c-akt) signaling. J Mol Med 77:656–665
Lee YS et al (2006) Berberine, a natural plant product, activates AMP-activated protein linase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes 55:2256–2264. https://doi.org/10.2337/db06-0006
Li WL, Zheng HC, Bukuru J, De Kimpe N (2004) Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J Ethnopharmacol 92:1–21
Li XF, Wang Y, Liu WB, Jiang GZ, Zhu J (2013) Effects of dietary carbohydrate/lipid ratios on growth performance, body composition and glucose metabolism of fingerling blunt snout bream Megalobrama amblycephala. Aquac Nutr 19:701–708
Li XY, Wang JT, Han T, Hu SX, Jiang YD (2015) Effects of dietary carbohydrate level on growth and body composition of juvenile giant croaker Nibea japonica. Aquac Res 46:2851–2858
Li SL, Sang CY, Wang A, Zhang JC, Chen NS (2019) Effects of dietary carbohydrate sources on growth performance, glycogen accumulation, insulin signaling pathway and hepatic glucose metabolism in largemouth bass, Micropterus salmoides. Aquaculture 513:734391. https://doi.org/10.1016/j.aquaculture.2019.734391
Liu YT, Hao HP, Xie HG, Lai L, Wang Q, Liu CX, Wang GJ (2010) Extensive intestinal first-pass elimination and predominant hepatic distribution of berberine explain its low plasma levels in rats. Drug Metab Dispos 38:1779–1784. https://doi.org/10.1124/dmd.110.033936
Liu D, Zhang Y, Liu Y, Hou L, Li S, Tian H, Zhao T (2018) Berberine modulates gut microbiota and reduces insulin resistance via the TLR4 signaling pathway. Exp Clin Endocrinol Diabetes 126:513–520
Lu KL, Xu WN, Li XF, Liu WB, Wang LN, Zhang CN (2013) Hepatic triacylglycerol secretion, lipid transport and tissue lipid uptake in blunt snout bream (Megalobrama amblycephala) fed high-fat diet. Aquaculture 408:160–168
Lu KL, Zhang DD, Wang LN, Xu WN, Liu WB (2016) Molecular characterization of carnitine palmitoyltransferase IA in Megalobrama amblycephala and effects on its expression of feeding status and dietary lipid and berberine. Comp Biochem Physiol B Biochem Mol Biol 191:20–25. https://doi.org/10.1016/j.cbpb.2015.08.010
Manning BD (2004) Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J Cell Biol 167:399–403
McNamara JR, Schaefer EJ (1987) Automated enzymatic standardized lipid analyses for plasma and lipoprotein fractions. Clin Chim Acta 166:1–8
Monnier VM, Vishwanath V, Frank KE, Elmets CA, Dauchot P, Kohn RR (1986) Relation between complications of type I diabetes mellitus and collagen-linked fluorescence. N Engl J Med 314:403–408
Moon TW (2001) Glucose intolerance in teleost fish: fact or fiction? Comp Biochem Physiol B: Biochem Mol Biol 129:243–249
Na Q, Zhao TY, He M, Tian C (2012) Effectiveness and safety of berberine in the treatment of type 2 diabetes: a systematic review. Chin J Evid Based Med 12:81–91
Nandipati KC, Subramanian S, Agrawal DK (2017) Protein kinases: mechanisms and downstream targets in inflammation-mediated obesity and insulin resistance. Mol Cell Biochem 426:27–45
Neag MA, Mocan A, Echeverría J, Pop RM, Bocsan CI, Crişan G, Buzoianu AD (2018) Berberine: botanical occurrence, traditional uses, extraction methods, and relevance in cardiovascular, metabolic, hepatic, and renal disorders. Front Pharmacol 9. https://doi.org/10.3389/fphar.2018.00557
Ni YX (1988) Therapeutic effect of berberine on 60 patients with type II diabetes mellitus and experimental research. Zhong xi yi jie he za zhi 8:711
Nigam VN (1962) An enzymatic method for the determination of pyruvate phosphoenolpyruvate, 2-and 3-phosphoglyceric acids. Can J Biochem Physiol 40:836–840
NRC N (2011) Nutrient requirements of fish and shrimp. The National Academies Press, Washington DC
Pan WJ et al (2017) Regulation mechanism of oxidative stress induced by high glucose through PI3K/Akt/Nrf2 pathway in juvenile blunt snout bream (Megalobrama amblycephala). Fish Shellfish Immunol 70:66–75. https://doi.org/10.1016/j.fsi.2017.09.005
Pan HJ, Li Z, Xie J, Liu D, Wang H, Yu D, Zhang Q, Hu Z, Shi C (2019) Berberine influences blood glucose via modulating the gut microbiome in grass carp. Front Microbiol 10. https://doi.org/10.3389/fmicb.2019.01066
Panserat S et al (2000) Hepatic glucokinase is induced by dietary carbohydrates in rainbow trout, gilthead seabream, and common carp. Am J Physiol Regul Integr Comp Physiol 278:R1164–R1170. https://doi.org/10.1152/ajpregu.2000.278.5.R1164
Peres H, Oliva Teles A (1999) Influence of temperature on protein utilization in juvenile European seabass (Dicentrarchus labrax). Aquaculture 170:337–348. https://doi.org/10.1016/S0044-8486(98)00422-0
Petersen MC, Vatner DF, Shulman GI (2017) Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol 13:572–587
Pfleiderer G, Bergmeyer H (1974) Glycogen: determination with amyloglucosidase. Methods Enzymat Analy 2:59–62
Prisingkorn W, Prathomya P, Jakovlic I, Liu H, Zhao YH, Wang WM (2017) Transcriptomics, metabolomics and histology indicate that high-carbohydrate diet negatively affects the liver health of blunt snout bream (Megalobrama amblycephala). BMC Genomics 18:856. https://doi.org/10.1186/s12864-017-4246-9
Qian Y, Li XF, Zhang DD, Cai DS, Tian HY, Liu WB (2015) Effects of dietary pantothenic acid on growth, intestinal function, anti-oxidative status and fatty acids synthesis of juvenile blunt snout bream Megalobrama amblycephala. PLoS One 10:e0119518. https://doi.org/10.1371/journal.pone.0119518
Ren MC, Ai QH, Mai KS, Ma HM, Wang XJ (2011) Effect of dietary carbohydrate level on growth performance, body composition, apparent digestibility coefficient and digestive enzyme activities of juvenile cobia, Rachycentron canadum L. Aquac Res 42:1467–1475. https://doi.org/10.1111/j.1365-2109.2010.02739.x
Ren MC et al (2013) Dietary arginine requirement of juvenile blunt snout bream, Megalobrama amblycephala. Aquaculture 414-415:229–234. https://doi.org/10.1016/j.aquaculture.2013.08.021
Ren MC et al (2015) Effects of dietary carbohydrate source on growth performance, diet digestibility and liver glucose enzyme activity in blunt snout bream, Megalobrama amblycephala. Aquaculture 438:75–81. https://doi.org/10.1016/j.aquaculture.2015.01.008
Richard N, Kaushik S, Larroquet L, Panserat S, Corraze G (2006) Replacing dietary fish oil by vegetable oils has little effect on lipogenesis, lipid transport and tissue lipid uptake in rainbow trout (Oncorhynchus mykiss). Brit J Nutr 96:299–309
Shi HJ et al (2018) Resveratrol improves the energy sensing and glycolipid metabolism of blunt snout bream Megalobrama amblycephala fed high-carbohydrate diets by activating the AMPK-SIRT1-PGC-1alpha network. Front Physiol 9:1258. https://doi.org/10.3389/fphys.2018.01258
Shi HJ, Li XF, Xu C, Zhang DD, Zhang L, Xia SL, Liu WB (2020) Nicotinamide improves the growth performance, intermediary metabolism and glucose homeostasis of blunt snout bream Megalobrama amblycephala fed high-carbohydrate diets. Aquac Nutr. https://doi.org/10.1111/anu.13088
Tessari P, Coracina A, Cosma A, Tiengo A (2009) Hepatic lipid metabolism and non-alcoholic fatty liver disease. Nutr Metab Cardiovas 19:291–302
Tillhon M, Guamán Ortiz LM, Lombardi P, Scovassi AI (2012) Berberine: new perspectives for old remedies. Biochem Pharmacol 84:1260–1267. https://doi.org/10.1016/j.bcp.2012.07.018
Wang ZQ et al (2008) Facilitating effects of berberine on rat pancreatic islets through modulating hepatic nuclear factor 4 alpha expression and glucokinase activity. World J Gastroenterol: WJG 14:6004
Wang JT et al (2016) Effects of different dietary carbohydrate levels on growth, feed utilization and body composition of juvenile grouper Epinephelus akaara. Aquaculture 459:143–147. https://doi.org/10.1016/j.aquaculture.2016.03.034
Watanabe T (2002) Strategies for further development of aquatic feeds. Fish Sci 68:242–252. https://doi.org/10.1046/j.1444-2906.2002.00418.x
Wei J, Wu JD, Jiag J (2004) Clinical study on improvement of type II diabetes mellitus complicated with fatty liver treated by berberine. Chin J Integ Tradition West Med Liver Dis 6:334–336
Wilson RP (1994) Utilization of dietary carbohydrate by fish. Aquaculture 124:67–80. https://doi.org/10.1016/0044-8486(94)90363-8
Xia XA et al (2011) Berberine improves glucose metabolism in diabetic rats by inhibition of hepatic gluconeogenesis. PLoS One 6:e16556–e16556. https://doi.org/10.1371/journal.pone.0016556
Xie P, Zhou H, Gao Y (2005) The clinical efficacy of berberine in treatment of type 2 diabetes mellitus. Chin J Clin Healthcare 8:402–403
Xu C, Liu WB, Dai YJ, Jiang GZ, Wang BK, Li XF (2017) Long-term administration of benfotiamine benefits the glucose homeostasis of juvenile blunt snout bream Megalobrama amblycephala fed a high-carbohydrate diet. Aquaculture 470:74–83. https://doi.org/10.1016/j.aquaculture.2016.12.025
Xu C, Liu WB, Zhang DD, Cao XF, Shi HJ, Li XF (2018) Interactions between dietary carbohydrate and metformin: implications on energy sensing, insulin signaling pathway, glycolipid metabolism and glucose tolerance in blunt snout bream Megalobrama amblycephala. Aquaculture 483:183–195. https://doi.org/10.1016/j.aquaculture.2017.10.022
Yan HM, Xia MF, Wang Y, Chang XX, Yao XZ, Rao SX, Zeng MS, Tu YF, Feng R, Jia WP, Liu J, Deng W, Jiang JD, Gao X (2015) Efficacy of berberine in patients with non-alcoholic fatty liver disease. PLoS One 10:e0134172. https://doi.org/10.1371/journal.pone.0134172
Yin J, Gao Z, Liu D, Liu Z, Ye J (2008) Berberine improves glucose metabolism through induction of glycolysis. Am J Phys Endocrinol Metab 294:E148–E156. https://doi.org/10.1152/ajpendo.00211.2007
Zhang WW et al (2006) FoxO1 regulates multiple metabolic pathways in the liver-effects on gluconeogenic, glycolytic, and lipogenic gene expression. J Biol Chem 281:10105–10117. https://doi.org/10.1074/jbc.M600272200
Zhang YF et al (2008) Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J Clin Endocrinol Metabol 93:2559–2565. https://doi.org/10.1210/jc.2007-2404
Zhang YH et al (2015) Berberine reverses abnormal expression of L-type pyruvate kinase by DNA demethylation and histone acetylation in the livers of the non-alcoholic fatty disease rat. Int J Clin Exp Med 8:7535–7543
Zhang YP et al (2018) Fucoxanthin ameliorates hyperglycemia, hyperlipidemia and insulin resistance in diabetic mice partially through IRS-1/PI3K/Akt and AMPK pathways. J Funct Foods 48:515–524. https://doi.org/10.1016/j.jff.2018.07.048
Zhou JY, Zhou SW, Zhang KB, Tang JL, Guang LX, Ying Y, Xu Y, Zhang L, Li DD (2008) Chronic effects of berberine on blood, liver glucolipid metabolism and liver PPARs expression in diabetic hyperlipidemic rats. Biol Pharm Bull 31:1169–1176
Zhou PP, Wang MQ, Xie FJ, Deng DF, Zhou QC (2016) Effects of dietary carbohydrate to lipid ratios on growth performance, digestive enzyme and hepatic carbohydrate metabolic enzyme activities of large yellow croaker (Larmichthys crocea). Aquaculture 452:45–51. https://doi.org/10.1016/j.aquaculture.2015.10.010
Zhou CP, Ge XP, Liu B, Xie J, Chen RL, Miao LH, Ren MC (2017) Comparative study on the effect of high dietary carbohydrate on the growth performance, body composition, serum physiological responses and hepatic antioxidant abilities in Wuchang bream (Megalobrama amblycephala) and black carp (Mylopharyngodon piceus Richardson, 1846). Aquac Res 48:1020–1030. https://doi.org/10.1111/are.12944
Zhou WH, Rahimnejad S, Lu KL, Wang LN, Liu WB (2019) Effects of berberine on growth, liver histology, and expression of lipid-related genes in blunt snout bream (Megalobrama amblycephala) fed high-fat diets. Fish Physiol Biochem 45:83–91
Code availability
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China (No. 31972801) and the earmarked fund for China Agriculture Research System (CARS-45-14).
Author information
Authors and Affiliations
Contributions
Chang He: writing—original draft, writing—reviewing and editing, software, data curation, investigation, and resources.
Xiaoyan Jia: software, conceptualization, and data curation.
Li Zhang: supervision and conceptualization.
Fan Gao: writing—reviewing and editing.
Weibo Jiang: investigation and resources.
Chuang Wen: investigation and resources.
Cheng Chi: software and methodology.
Xiangfei Li: conceptualization.
Guangzhen Jiang: conceptualization.
Haifeng Mi: feed formulation and consultation.
Wenbin Liu: funding acquisition and conceptualization.
Dingdong Zhang: funding acquisition, project administration, supervision, and writing—reviewing and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experimental procedures for animal care and handling in this study were approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University (Permit number: IACUC2020174).
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
He, C., Jia, X., Zhang, L. et al. Dietary berberine can ameliorate glucose metabolism disorder of Megalobrama amblycephala exposed to a high-carbohydrate diet. Fish Physiol Biochem 47, 499–513 (2021). https://doi.org/10.1007/s10695-021-00927-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-021-00927-8