Abstract
Purpose
Nonalcoholic fatty liver disease (NAFLD) is currently the leading cause of chronic liver disease in developing countries. The pathogenesis is complex, and there is currently no effective treatment. Betaine is an essential intermediate in choline catabolism and an important component of the methionine cycle. Betaine deficiency is associated with NAFLD severity, and its mechanism needs to be further elaborated.
Methods
In this study, an NAFLD mouse model was established by feeding ApoE−/− mice a high-fat diet. The effects of betaine on NAFLD were investigated, including its mechanism.
Results
In this study, after treatment with betaine, blood lipid levels and liver damage were significantly decreased in the NAFLD mouse model. The fat infiltration of the liver tissues of high-fat diet (HFD)-fed mice after betaine administration was significantly improved. Betaine treatment significantly upregulated AMP-activated protein kinase (AMPK), fibroblast growth factor 10 (FGF10), and adipose triglyceride lipase (ATGL) protein levels both in vivo and in vitro and suppressed lipid metabolism-related genes. Furthermore, the overexpression of FGF10 increased the protein level of AMPK and decreased lipid accumulation in HepG2 cells.
Conclusion
Taken together, the data strongly suggest that betaine significantly prevents high-fat diet-induced NAFLD through the FGF10/AMPK signaling pathway in ApoE−/− mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common liver disease in developing countries. It also affects more than one-third of adults in Western countries [1]. NAFLD includes simple steatosis and insulin resistance, with or without fibrosis and cirrhosis. The initial characteristic of NAFLD is the excessive accumulation of triglycerides that sensitizes the liver to subsequent events leading to hepatic steatosis [2]. Currently, there are still no specific drugs for the treatment of NAFLD.
Betaine is abundant in many common foods, such as sugar beet, shellfish, spinach, and wheat [3]. Additionally, as an amino acid (trimethyl-glycine), betaine is a neutral zwitterionic compound found in most organisms [4]. Betaine is an essential intermediate in choline catabolism and an important component of the methionine cycle. It is derived from catalyzed choline, which is localized in the inner matrix of mitochondria. As an osmotic agent, it regulates the cell volume and maintains cell membrane integrity under hyperosmotic pressure [5]. As a methyl donor, betaine participates in the methionine-homocysteine cycle, which affects DNA and RNA methylation [6]. Indeed, betaine provides a methyl group to form creatine, which has a beneficial effect on improving cardiac function [7]. Several studies have revealed that betaine deficiency increases the severity of NAFLD [8, 9]. Moreover, one study reported that betaine significantly benefits the treatment of NAFLD in adults [10]. Betaine can reduce high-fat diet-induced hepatic lipid accumulation by enhancing hepatic lipid export and fatty acid oxidation in rats [11]. Nevertheless, it remains unknown whether betaine may alleviate NAFLD in ApoE−/− mice fed a high-fat diet.
Fibroblast growth factor 10 (FGF10) is one of the 22 members of the FGF family and is essential for the development of multiple organs. As a crucial paracrine signal, FGF10 plays important roles in epithelial-mesenchymal transition, the repair of tissue injury, and embryonic stem cell differentiation [12]. A previous study suggested that FGF10 is a therapeutic target for the treatment of obesity and metabolic diseases [15].
AMP-activated protein kinase (AMPK) increased fatty acid oxidation and the metabolic rate and inhibited hepatic lipid synthesis [16]. Woods et al. [17] demonstrated that activation of AMPK decreases hepatic triglyceride accumulation to ameliorate NAFLD. Rena et al. [18] reported that AMPK is indirectly activated by many compounds. Chau et al. [19] demonstrated that LKB1 is one of the phosphorylation targets of FGFR1, which activates the expression of AMPK by phosphorylating LKB1. Another study reported that the phosphorylation of AMPK was inhibited after FGFR2 inhibition in NCI-H716 cells [20]. Interestingly, FGF10 binds to FGFR1/2 to initiate signaling events that mediate biological functions in target cells [13, 14]. Fischer et al. [15] reported that the FGF10-FGFR2-mediated autocrine signaling mechanism induces white fat browning and increases the lipid metabolic rate. Therefore, it can be speculated that FGF10 ameliorates NAFLD by activating AMPK.
In this study, NAFLD models were established in vivo and in vitro and confirmed that betaine treatment prevents NAFLD by inhibiting lipogenesis and improving fatty acid oxidation. Then, we constructed an NAFLD model of FGF10 overexpression in HepG2 cells and found that FGF10 overexpression could reduce lipid accumulation and increase the expression of AMPK. Our data identify the effects of betaine on NAFLD and show that betaine controls lipid metabolism through the FGF10/AMPK signaling pathway.
Methods
Animal experimental design and treatments
The present study utilized a total of 27 male ApoE –/– mice were purchased from Beijing Biocytogen. The use of the mice was compliance with the guidelines established by the Animal Care Committee of Gannan Medical University. The experimental design to three groups, NFD (control) group mice fed a normal chow diet, which contained 4% fat, 18% protein. HFD (NAFLD) group mice were fed a high-fat diet, which contained 15% fat, 18% protein, 1.25% cholesterol and 0.5% cholate. HFD + B (NAFLD + Betaine) group mice were fed a high-fat diet with 2% (wt/v) betaine in drinking water. Food intake were alike in three groups (Table 1). The animals fasted for 12 h, and individual body weight were measured and following anesthesia with 3% pentobarbital and sacrifice via cervical dislocation. Blood samples were rapidly obtained by retro-orbital bleeding. Liver weight were measured immediately and samples were fixed with 4% formaldehyde at room temperature for 24 h.
Biochemical analyses
Alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglyceride (TG), cholesterol (TC), free fat acid (FFA), hydroxyproline (HYP), malondialdehyde (MDA), glutathione peroxidase ( GSH-Px), high density lipoprotein cholesterol (HDL-c), low density lipoprotein cholesterol (LDL-c), and superoxide dismutase (SOD) levels were measured using an enzyme-linked immunosorbent assay kit following the manufacturer’s instructions.
Histological analysis
The liver specimens were stained with Oil red O for histopathological examination. Liver specimens were in O.C.T. Compound (Tissue-TeK®, 4583), and 12 μm sections were stained with Oil red O. using an automated Leica TI-S microscope to observe, and quantified using Image J.
The cells were fixed with 4% formaldehyde for 10 min, soaked with 60% isopropanol, stained with Oil Red O for 10 min, and differentiated with 60% isopropanol. After washing with distilled water, using an automated Leica TI-S microscope to observe, and quantified using ImageJ.
The liver specimens were stained with H&E for histopathological examination. Formalin-fixed liver tissues were dehydrated in alcohol and embedded in paraffin wax sectioned were sliced at 5 μm, and were stained with H&E and observed using an automated Leica TI-S microscope. A single pathologist reviewed the liver histology according to the percentage of hepatocytes with fatty droplets by Ishak et al. Scoring of liver histology was according to the percent of hepatocytes containing fat droplets (0, < 1%; 1, 1–25%; 2, 26–50%; 3, 51–75%, and 4, 76–100%) [21].
HepG2 culture and experimental treatments
HepG2 cells were maintained in normal RPMI-1640 medium contained 10% fetal bovine serum (FBS), 1% penicillin streptomycin–neomycin antibiotics. The control group was maintained in normal RPMI-1640 medium. The oleic acid group (OA), oleic acid was added to the culture medium at the concentration of 0.2 mM to induced fatty liver cell models. Treatment group (OA + B), the betaine was added to the OA culture medium at the concentration of 20 mM, 40 mM, 80 mM and 160 mM, respectively. Treatment group (OA + FGF10), the FGF10 recombinant protein was added to the OA culture medium at the concentration of 25 ng/ml. Treatment group (OA + pFGF10), HepG2 cells were transfected using the Neon™ transfection system and 8 μg of a p-CMV3-FGF10-C-GFPSpark plasmid. Electroporation parameters: voltage (v) 1230, width (ms) 20, number 3, cell density (cells/ml) 5 × 106. Cells were seeded into a six well plates (2 × 105 cells/well) and grown to 70–80% confluence, incubated at 37 °C in a CO2 incubator for 48 h.
Quantitative real-time qPCR
Hepatic mRNA levels were analyzed by RT-qPCR using an CFX96 real-time PCR system (BIO-RAD, USA). Total RNA was isolated from liver tissues using QIAzol (QIAGEN cat # 79306) followed by DNAse treatment to remove genomic DNA. cDNA was synthesized using 40 ng of total RNA with SuperScript™ II Reverse Transcriptase (Invitrogen cat#18064014) at 37 ˚C for 30 min. Amplification reactions were performed using a TB Green® Premix Ex Taq™ (Takara cat#RR820A) according to the manufacturer’s protocol. Relative genes expression levels were analyzed using the 2−ΔΔCT method.
Western blotting
Approximately, 40 mg of the liver samples were homogenized in RIPA lysis buffer (Solarbio cat# R0010) containing protease inhibitors. Protein concentration were determined by the Bradford protein assay kit (TIANGEN PA115). Samples (40 μg) were separated by 10% sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF membranes. The membranes were then blocked with 5% non-fat dry milk in 0.05% Tween-20-PBS for 1.5 h at room temperature. Then, the membranes were incubated overnight at 4 ˚C with AMPKα (diluted 1:1000, proteintech cat# 66536-1-Ig), pAMPKα (diluted 1:1000, abcam ab23875), FASN (diluted 1:500, proteintech cat#10624-2-AP), NR1H3 (diluted 1:1000, proteintech cat# 14351-1-AP), SREBF1 (diluted 1:2000, proteintech cat#14088-1-AP), pSREBF1 (diluted 1:1000, abcam ab138663), GAPDH (diluted 1:3000, Proteintech cat#10494-1-AP), ACC (diluted 1:1000, abcam ab45174), pACC (diluted 1:5000, abcam ab68191), ATGL (diluted 1:5000, abcam ab109251), CPT1A (diluted 1:5000, abcam ab220789), PPARγ (diluted 1:1000, abcam ab178860), SIRT1 (diluted 1:1000, abcam ab32441), FGF10 (diluted 1:1000, Sino Biological 101637-T44) in 5% BSA (Sigm A4503). After washing with TBST, the membranes were incubated for 2 h at room temperature with secondary HRP conjugated antibody (diluted 1:5000 Proteintech cat#SA0001-A). Images were captured by ChemiDoc™ Imaging System (Bio-Rad, USA) and quantified with an Image-Lab software.
Statistical analysis
All data are expressed as mean ± standard error of mean. “n” denotes the sample size in each group. Between-group differences were assessed using one-way ANOVA with Bonferroni’s test. Statistical analyses were calculated using SPSS software (version 20.0). Differences were considered statistically significant at values of p < 0.05.
Results
Effects of betaine on lipid metabolism in NAFLD mice
Effects of betaine on lipid metabolism in vivo
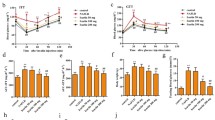
As shown in Fig. 1a–c, remarkably, NAFLD mice that were supplemented with betaine (HFD + B group) had lower weight gain and significantly lighter weight gain than NAFLD mice with vehicle (HFD + V group). Then, we tested the serum lipid levels (Table 2). When compared with those in the NFD group, the serum TC level in the HFD + V group was increased by 161%, serum TG by 57%, serum LDL-C by 46%, serum FFA by 90%, and serum HDL-C was decreased by 52%. When compared with that in the HFD + V group, the serum TC level in the HFD + B group was decreased by 9%, serum TG by 36%, serum FFA by − 20%, serum LDL-C by 28%, and serum HDL-C was increased by 91%. The data indicate that betaine can ameliorate dyslipidemia in NAFLD mice.
Effects of betaine on lipid metabolism in NAFLD mice. A schematic of the experiment to examine whether betaine could inhibit high-fat diet-induced NAFLD in ApoE–/– mice (a), NFD group mice were fed a normal chow diet, and HFD group mice were fed a high-fat diet with or without 2% (wt/v) betaine in drinking water for 8 weeks. Body weights of each group of mice every 2 weeks (b) and percent weight gain in each group of ApoE–/– mice at the end of the prevention study (c). Images of H&E (× 200) and oil red O (× 400)-stained livers from each group (d). Quantification of the oil red O staining area from each group of ApoE–/– mice (e). Data are expressed as the means ± SEM (n = 9). Model group: NAFLD nonalcoholic fatty liver disease, NFD group normal chow diet group, HFD + V group high-fat diet + vehicle group, HFD + B group high-fat diet + betaine group. SEM standard error of the mean, ANOVA analysis of variance
Effects of betaine on liver homogenate lipid levels
Then, we tested the lipid levels in the liver homogenate (Table 2). When compared with those in the NFD group, the liver homogenate TC level in the HFD group was increased by 350%, the liver homogenate TG by 160%, the liver homogenate LDL-C by 133%, and the liver homogenate FFA by 125%. There was no significant difference in the HDL-C between the groups of liver homogenate. The liver homogenate TC level in the HFD + B group was decreased by 22%, and the liver homogenate TG level was decreased by 38% as compared to those in the HFD group. There was no significant difference in the LDL-C, HDL-C, and FFA levels between the groups of liver homogenate. The data indicate that betaine can reduce TC and TG in the liver homogenate of NAFLD mice but does not affect LDL-C, HDL-C, and FFA levels.
Effects of betaine on hepatic steatosis
As shown in Fig. 1d and e, macroscopic samples showed visible yellow lipid plaque deposits on the surface of the liver in HFD + V mice. Yellow lipid plaque deposition can be diminished after treatment with betaine. Indeed, as compared to HFD + V mice, the HFD + B group mice had lower levels of hepatic lipid accumulation. When compared with those in the NFD group, H&E staining of liver tissue in the HFD + V group showed a disordered hepatic lobule structure, portal vein area—central vein dilatation, hepatic cell steatosis in hepatic lobules, mixed steatosis characterized by macrovascular steatosis, hepatocyte volume increase, and a hollow cytoplasm. However, as compared to that in HFD + V mice, in the HFD + B group, hepatic steatosis in the hepatic lobules was significantly reduced, and the visible cavities in the cytoplasm were also significantly reduced. The scoring of liver histology (Table 3) showed that the HFD + V mice had higher scores than the HFD + B mice. These results indicate that the histopathology of the liver tissues of HFD + V mice after betaine administration was significantly improved.
Effects of betaine on hepatic cell injury
As shown in Table 2, when compared with those in the NFD group, serum ALT, and AST levels were elevated by 108% and 51%, respectively. However, when compared with those in the HFD + V group, serum ALT was reduced by 44% and serum AST by 25% in the HFD + B group. Then, we checked other markers of hepatic cell injury, such as GSH-px, SOD, HYP, and MDA. When compared with those in the NFD group, the liver SOD level of mice was reduced by 79% in the HFD + V group, while the level was increased by 46% in the HFD + B group as compared to those in the HFD + V group. The liver MDA level of mice was increased by 57% in the HFD + V group and was reduced by 34% in the HFD + B group as compared to those in the HFD + V group. Liver GSH-px and HYP levels were not significantly different among the three groups. These findings suggest a hepatoprotective effect of betaine.
Effects of betaine on lipid metabolism in vitro
First, we need to determine a suitable concentration of betaine for the treatment of an oleic acid (OA + V group)-induced high-fat cell model. Cell lipid accumulation was increased significantly in the OA + V group (Fig. 2a, b). Interestingly, after treatment with betaine (OA + B group), as compared to that in the OA + V group, the cell lipid accumulation was decreased, especially in the OA + B (20 mM) group. The data indicate that betaine can reduce lipid accumulation in vitro at a concentration of 20 mM.
Effects of betaine on lipid metabolism in HepG2 cells. In the oleic acid group (OA), oleic acid was added to the culture medium at a concentration of 0.2 mM to induce fatty liver cell models. In the treatment group (OA + B), betaine was added to the OA culture medium at concentrations of 20 mM, 40 mM, 80 mM, and 160 mM. Representative images of oil red O-stained HepG2 cells from the control group and OA group treated with or without betaine for 48 h (a). Quantification of the oil red O staining area in cells from each group (b). Quantitative analysis of ALT and AST in the cell homogenates (c). Quantitative analysis of the total TC, TG, HDL-C, and LDL-C concentrations in the cell homogenate (d). The data are expressed as the means ± SEM (n = 9). Model group: TC cholesterol, TG triglyceride, LDL-C low-density lipoprotein cholesterol, HDL-C high-density lipoprotein cholesterol, ALT alanine transaminase, AST aspartate transaminase. Control group: OA + V group oleic acid + vehicle group, OA + B group oleic acid + betaine group SEM standard error of the mean, ANOVA analysis of variance
Then, we examined the effect of betaine on lipid metabolism in vitro. The aminotransferase level of the cell homogenate and ALT level were increased by 189% (Fig. 2c), and the AST level was increased by 52% (Fig. 2d). In the OA + V group, the cell homogenate TC level was increased by 32%, TG by 90%, HDL-C was decreased by 31%, and LDL-C was increased by 12% (Fig. 2e). After treatment with betaine, the ALT level was decreased by 13% (Fig. 2c) and the AST level was decreased by 16% (Fig. 2d) as compared to that in the OA + V group. The cell homogenate TC level in the OA + B group was decreased by 11% and TG by 20%, and HDL-C was increased by 14% (Fig. 2e). The data indicate that betaine can ameliorate dyslipidemia in vitro.
Effects of betaine on the mRNA expression of genes and proteins in vitro
We checked the mRNA expression levels of lipogenesis genes and the expression levels of regulatory factors for cholesterol synthesis. Many gene expression levels are too low to be detected. Only the expression levels of AMPK, LCAT, and Rxra could be detected and were significantly decreased in the OA + V group and increased after treatment with betaine (Fig. 3a).
Effects of betaine on the mRNA expression of genes and proteins in vivo. The mRNA expression of AMPK, LCAT, and Rxra (a) was tested. The protein expression levels of AMPK (d and g); the de novo lipogenesis-related proteins FASN (b and c), SREBF1 (d and f), NR1H3 (d and h), and ACC (d and e); and the β-oxidation-related proteins SIRT1, ATGL, CPT1A, and PPARγ (i and j) in the Con group and OA group of HepG2 cells treated with or without betaine for 48 h. Data are expressed as the means ± SEM (n = 9). Model group, Control group: OA + V group oleic acid + group, OA + B group oleic acid + betaine group. SEM standard error of the mean, ANOVA analysis of variance
We continued to check the AMPK protein level in vitro. As shown in Fig. 3d and g, the AMPK protein level of the liver in the OA + V group was decreased as compared to that in the control groups and can be increased after treatment with betaine. We then checked the AMPK target genes, including the FASN, SREBF1, NR1H3, ACC, SIRT1, ATGL, CPT1A, and PPARγ protein levels in vitro. As shown in Fig. 3d and f, the de novo lipogenesis-related proteins, including SREBF1, in the OA + V group were increased as compared to those in the control group and were decreased after treatment with betaine. The FASN and ACC (Fig. 3b, d) protein levels were not different between the control group and OA + V group; however, they were decreased after treatment with betaine. The levels of the fatty acid oxidation-related proteins SIRT1, CPT1A, and PPARγ (Fig. 3i, j) in the OA + V group were increased as compared to those in the control group and could be decreased after treatment with betaine. Interestingly, the ATGL (Fig. 3i, j) protein level was not different between the control group and OA group; however, it was increased after treatment with betaine. The NR1H3 (Fig. 3d, h) protein level was not different among the three groups. The data indicate that betaine can increase the protein expression levels of AMPK and ATGL and decrease SREBP1, FASN, ACC, SIRT1, and PPARγ expression levels in vitro.
Effects of betaine on the mRNA expression of genes and proteins in vivo
As shown in Fig. 4a, the mRNA expression levels of lipogenesis genes, including HMGCR, ACC2, and SREBP-1, were increased in the HFD group but inhibited after treatment with betaine. In addition, the expression levels of FASN, ACAT, CPT1A, SREBP-2, and HMGCS were significantly increased in the HFD + V mice (Fig. 4a, b). However, there were no significant differences in the HFD + B group. The mRNA expression of AMPK, which is another metabolism pathway-related enzyme for metabolic homeostasis (Fig. 4b), was significantly decreased in the HFD mice but increased after treatment with betaine. The expression of LCAT and SIRT3 was not significantly different.
Effects of betaine on the mRNA expression of genes and proteins in vivo. mRNA expression of the lipogenic enzymes FAS, ACC2, SREBP-1, and Nr1h3 (a) in the livers of ApoE–/– mice. mRNA expression of the cholesterol-related synthetic enzymes ACAT, HMGCR, HMGCS, and SREBP-2 (a and b) in the livers of ApoE–/– mice. mRNA expression of other metabolism pathway-related enzymes, including AMPK, CPT1A, SIRT3, and LCAT (b), in the livers of ApoE–/– mice. The protein expression of AMPK (c and f); the de novo lipogenesis-related proteins FASN (G and H), SREBF1 (c and e), NR1H3 (g and h), and ACC (c and d); and the lipid β-oxidation-related proteins SIRT1, ATGL, CPT1A, and PPARγ (i and j) levels in the livers of ApoE–/– mice. NFD group mice were fed a normal chow diet, and HFD group mice were fed a high-fat diet with or without 2% (wt/v) betaine in drinking water for 8 weeks. Data are expressed as the means ± SEM (n = 9). Model group: NAFLD nonalcoholic fatty liver disease, NFD group normal chow diet group, HFD + V group high-fat diet + vehicle group, HFD + B group high-fat diet + betaine group. SEM standard error of the mean, ANOVA analysis of variance
We then checked the AMPK protein level in vivo. As shown in Fig. 4c and f, the AMPK protein level of the liver in HFD mice was decreased as compared to that in NFD mice and can be increased after treatment with betaine. We then checked the AMPK target genes, including FASN, SREBF1, NR1H3, ACC, SIRT1, ATGL, CPT1A, and PPARγ protein levels, in vivo. As shown in Fig. 4c, e, g, and h, the de novo lipogenesis-related protein levels, including SREBF1 and NR1H3, in HFD mice were increased as compared to those in NFD mice and were decreased after treatment with betaine. The FASN and ACC (Fig. 4c, d, g, h) protein levels were not different between the NFD and HFD groups; however, they were decreased after treatment with betaine. The levels of the fatty acid oxidation-related proteins SIRT1 and PPARγ (Fig. 4i, j) in HFD mice were increased as compared to those in NFD mice and decreased after treatment with betaine. Interestingly, the ATGL (Fig. 4i, j) protein level of the liver in HFD mice was decreased as compared to that in NFD mice and was increased after treatment with betaine. However, the CPT1A (Fig. 4i, j) protein level was not different in each group. The data indicate that betaine can increase the protein expression levels of AMPK and ATGL and decrease SREBP1, FASN, NR1H3, ACC, SIRT1, and PPARγ expression levels in vivo.
Effects of betaine on lipid metabolism by increasing the expression of FGF10
Effects of betaine on FGF10 expression
We first collected the cell supernatants to detect FGF10 levels. When compared with that in the control group, the cell supernatant FGF10 level decreased in the OA + V group (Fig. 5a), and after treatment with betaine, the cell supernatant FGF10 level increased significantly. Moreover, to determine whether betaine increases the expression of FGF10, we then checked FGF10 protein levels in vivo and in vitro. As shown in Fig. 5b–d, the FGF10 protein level in HFD-fed mice was decreased as compared to that in control mice and was increased after treatment with betaine. This phenomenon is repeated in vitro. The data showed that betaine can increase the protein expression levels of FGF10.
Effects of betaine on lipid metabolism through the increase in expression of FGF10. The cell supernatant concentration (a) and protein expression (b and c) of FGF10 in the control group and OA group of HepG2 cells treated with or without betaine at a concentration of 20 mM for 48 h. The protein expression of FGF10 (b and d) in the livers of NFD and HFD ApoE–/– mice treated with or without 2% (wt/v) betaine in drinking water for 8 weeks. Representative images of oil red O staining (e), quantification of the oil red O-stained area (f), and AMPKα protein levels (g and h) in FGF10-overexpressing cells. The data are expressed as the means ± SEM (n = 9). Model group: NAFLD nonalcoholic fatty liver disease, NFD group normal chow diet, HFD + V high-fat diet + vehicle, HFD + B group high-fat diet + betaine group. Control group: OA + V group oleic acid + vehicle group, OA + B group oleic acid + betaine group, OA + pFGF10 group oleic acid + p-CMV3-FGF10-C-GFPSpark plasmid group, OA + FGF10 group oleic acid + FGF10 group. SEM standard error of the mean, ANOVA analysis of variance
The overexpression of FGF10 reduces lipid accumulation in HepG2 cells. To demonstrate whether FGF10 could reduce lipid accumulation, we then examined lipid accumulation using a fatty liver cell model. When compared with the OA + V group, the OA + FGF10-C-GFPSpark group and the OA + FGF10 group had lower levels of lipid accumulation, as evidenced by oil red O staining (Fig. 5e, f). These data showed that overexpression of FGF10 can reduce lipid accumulation in vitro.
Effect of FGF10 on AMPK expression. To further confirm that the effect of AMPK on NAFLD treatment is FGF10 dependent, we investigated the effect of FGF10 overexpression on AMPK. When compared with the control group, AMPK increased significantly in the FGF10 group (Fig. 5g, h). Moreover, when compared with the OA + V group, AMPK increased significantly in the OA + B, OA + FGF10-C-GFPSpark and OA + FGF10 groups. These results showed that AMPK protein levels increased with FGF10 overexpression. These results indicated that betaine could indirectly activate AMPK through FGF10. Overall, the effect of betaine on NAFLD treatment was mediated by the FGF10/AMPK pathway.
Discussion
The pathogenesis of NAFLD is complicated. Despite extensive research work, the molecular mechanisms involved in the pathogenesis of NAFLD are still only partially understood. Excessive lipid accumulation caused by disorders of lipid metabolism in hepatocytes plays an important role in the development of NAFLD. Liu et al. [22] reported that ApoE is involved in cholesterol transport and mediates high affinity binding of chylomicrons and vLDL particles to the LDL receptor, allowing for specific uptake of these particles by the liver. Interestingly, ApoE−/− mice were also used as a model of NAFLD because lipid deposition in the liver resulted in the onset of NAFLD after feeding a high-fat diet [23].
After 8 weeks of feeding a high-fat diet to ApoE−/− mice, we found that the levels of TC, TG, LDL-C and FFA in the serum of mice were increased significantly, and the level of HDL-C was significantly decreased, as was the case in liver homogenate. We checked the serum aminotransferase levels and some markers of hepatic cell injury and found that ALT and AST levels were significantly increased. The results showed that ApoE−/− mice developed NAFLD after feeding with a high-fat diet, and mouse pathological examination and hepatocyte injury marker examination supported this diagnosis. After administration of betaine in NAFLD mice, blood lipids and blood cholesterol esters were significantly reduced, liver function was also greatly restored, and pathological manifestations were also significantly improved, suggesting that betaine can significantly improve NAFLD. Moreover, we have also found that betaine can indeed reduce the incidence of atherosclerosis in mice and reduce serum homocysteine levels (data not shown), consistent with other experimental results [24].
Then, we wanted to determine whether betaine changes enzymes or signaling pathways involved in lipid metabolism or cholesterol metabolism. NAFLD is characterized by the excessive accumulation of liver triglycerides [25]. Fatty acids are derived from de novo lipogenesis by LXR, SREBP1, ACC, and FAS and then synthesized to triglycerides for storage [25, 26]. qPCR and western blot results indicate that some lipid metabolism- or cholesterol metabolism-related enzymes or signaling pathways are changed when ApoE−/− mice are fed a high-fat diet, which proves that these enzymes or signaling pathways are involved in the pathogenesis of NAFLD mice. Our data show that most of the genes have a corresponding trend with the improvement in symptoms, and a small number of genes have significant changes, including AMPK, SREBP-1, ACC2, LCAT, Rxra, and HMGCR. Furthermore, the protein expression of LXR, SREBF1, ACC, PPARγ, SIRT1, and FASN was increased in NAFLD mice. This suggests that a high-fat diet causes metabolic disorders and increases body weight by disturbing lipogenesis and lipolysis, which often leads to obesity-mediated NAFLD.
AMPK serves as a key energy sensor and ameliorates NAFLD by increasing fatty acid utilization and inhibiting hepatic lipid synthesis [16, 27]. Sterol regulatory element binding protein 1c (SREBP-1c) is a transcription factor that regulates the fatty production in rodent liver and plays a critical role in the fatty synthesis process [28]. Activation of AMPK reduces lipid accumulation by inhibiting SREBP-1c expression and its target genes, including acetyl-CoA carboxylase (ACC) and fatty acid synthase (FASN), in the liver [16]. Adipose triglyceride lipase (ATGL) catalyzes the lipolysis of triglycerides. Reduced expression of the ATGL gene in the liver aggravates insulin resistance and lipid accumulation in patients with NAFLD. Moreover, Wu et al. [29] reported that AMPK regulates lipid accumulation by increasing ATGL expression. In fact, AMPK activation protected against weight gain and obesity in high-fat diet-induced NAFLD mice [27]. This may be the reason we observed significant effects on reduced lipid content and lower body weight after betaine supplementation.
We then examined AMPK activation of betaine using NAFLD models. We observed that betaine treatment increased the expression of AMPK both in vivo and in vitro. Interestingly, AMPK has been demonstrated to be an upstream kinase of SREBP, ACC, FAS, HMGCR, HMGCS, LXR, and PPARγ [16, 26, 30,31,32,33]. Moreover, a previous study showed that AMPK significantly inhibits the activity of SIRT1 directly [34]. Indeed, our results showed that the protein expression of SREBF1, ACC, FASN, LXR (NR1H3), SIRT1 and PPARγ was inhibited and that ATGL was increased after betaine treatment. However, the protein expression of CPT1A decreased with betaine treatment in HepG2 cells. Therefore, betaine lowers body weight and reduces lipid accumulation through the regulation of AMPK and its target genes.
Interestingly, FGF10 plays an important role in liver development [35]. Moreover, one study reported that the cells secreting FGF10 can modulate whole-body metabolism and increase the whole-body metabolic rate [15]. Our results showed that the cell FGF10 concentration increased significantly after treatment with betaine. Moreover, we observed that overexpression of FGF10 decreased lipid accumulation in NAFLD model cells. Thus, we found that betaine treatment may induce the secretion of FGF10, which therapeutically improves NAFLD.
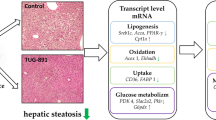
Then, we speculated that the potential therapeutic effect of betaine prevents NAFLD by activating the FGF10/AMPK signaling pathway. Our data showed that betaine activation of FGF10 and AMPK was sufficient to prevent high-fat diet-induced NAFLD. Moreover, FGF10/AMPK activation significantly reduced white adipose depots in response to NAFLD in mice. Indeed, FGF10 activation in betaine-treated NAFLD cells increased the expression of AMPK protein and reduced lipid accumulation. Thus, the FGF10/AMPK signaling pathway is a new target in the potential therapeutic treatment of NAFLD. Overall, our results demonstrated that betaine reduces lipid synthesis and improves fatty acid oxidation to prevent NAFLD by activating AMPK, which might provide an attractive therapeutic strategy for preventing NAFLD (Fig. 6).
Schematic representation of the signaling mechanisms that mediate betaine-induced activation of the FGF10/AMPK signaling pathway. Betaine treatment stimulates FGF10 secretion mainly from the liver. FGF10 binds the FGFR1/2 receptor to form the FGF10-FGFR1/2 complex, which subsequently activates AMPK through LKB1 activation. FASN, SREBF1, NR1H3, HMGCR, HMGCS, ACAT, LCAT, and ACC are essential downstream targets of FGF10-mediated AMPK activation, inhibiting de novo lipogenesis while activating the FGF10/AMPK signaling pathway. SIRT1, CPT1A, and PPARγ are essential downstream targets of FGF10-mediated AMPK activation, inhibiting fatty acid β-oxidation while activating the FGF10/AMPK signaling pathway. ATGL and Rxra are essential downstream targets of FGF10-mediated AMPK activation, increasing lipolysis while activating the FGF10/AMPK signaling pathway. ACC acetyl-CoA carboxylase, AMPK AMP-activated protein kinase, ATGL adipose triglyceride lipase, CPT1A carnitine palmitoyl transferase 1A, FASN fatty acid synthase, FFA free fatty acid, FGF10 fibroblast growth factor 10, FGFR1/2 fibroblast growth factor receptor 1/2, HMGCS 3-hydroxy-3-methylglutaryl-coenzyme A synthase, HMGCR 3-hydroxy-3-methylglutaryl-CoA reductase, LKB1 liver kinase B1, PPARγ peroxisome proliferator-activated receptor γ, SREBF1 sterol regulatory element binding factor 1, NR1H3 liver X receptor alpha gene, SIRT1 sirtuin 1
Abbreviations
- ACC:
-
Acetyl-CoA carboxylase
- AMPK:
-
AMP-activated protein kinase
- ATGL:
-
Adipose triglyceride lipase
- CPT1A:
-
Carnitine palmitoyl transferase 1A
- FASN:
-
Fatty acid synthase
- FFA:
-
Free fatty acid
- FGF10:
-
Fibroblast growth factor 10
- FGFR1/2:
-
Fibroblast growth factor receptor 1/2
- HCC:
-
Hepatocellular carcinoma
- HMGCS:
-
3-Hydroxy-3-methylglutaryl-Coenzyme A synthase
- HMGCR:
-
3-Hydroxy-3-methylglutaryl-CoA reductase
- LKB1:
-
Liver kinase B1
- PPARγ:
-
Peroxisome proliferator-activated receptor γ
- SREBP-1c:
-
Sterol regulatory element binding protein 1c
- SREBF1:
-
Sterol regulatory element binding factor 1
- SCD:
-
Stearoyl-CoA desaturase
- NR1H3:
-
Liver X receptors alpha gene
- SIRT1:
-
Sirtuin 1
- TC:
-
Cholesterol
- TG:
-
Triglyceride
- LDL-C:
-
Low-density lipoprotein cholesterol
- HDL-C:
-
High-density lipoprotein cholesterol
- FFA:
-
Free fatty acid
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate transaminase
- GHS-PX:
-
Homogenate glutathione peroxidase
- SOD:
-
Superoxide dismutase
- HYP:
-
Hydroxyproline
- MDA:
-
Malondialdehyde
- NAFLD:
-
Non-alcoholic fatty liver disease
References
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M (2016) Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64(1):73–84. https://doi.org/10.1002/hep.28431
Wan X, Xu C, Yu C, Li Y (2016) Role of NLRP3 Inflammasome in the progression of NAFLD to NASH. Can J Gastroenterol Hepatol 2016:7. https://doi.org/10.1155/2016/6489012
Filipcev B, Kojic J, Krulj J, Bodroza-Solarov M, Ilic N (2018) Betaine in cereal grains and grain-based products. Foods. https://doi.org/10.3390/foods7040049
Zhou Z, Garrow TA, Dong X, Luchini DN, Loor JJ (2017) Hepatic activity and transcription of betaine-homocysteine methyltransferase, methionine synthase, and cystathionine synthase in periparturient dairy cows are altered to different extents by supply of methionine and choline. J Nutr 147(1):11–19. https://doi.org/10.3945/jn.116.240234
Du J, Shen L, Tan Z, Zhang P, Zhao X, Xu Y, Gan M, Yang Q, Ma J, Jiang A, Tang G, Jiang Y, Jin L, Li M, Bai L, Li X, Wang J, Zhang S, Zhu L (2018) Betaine supplementation enhances lipid metabolism and improves insulin resistance in mice fed a high-fat diet. Nutrients. https://doi.org/10.3390/nu10020131
Musso M, Bocciardi R, Parodi S, Ravazzolo R, Ceccherini I (2006) Betaine, dimethyl sulfoxide, and 7-deaza-dGTP, a powerful mixture for amplification of GC-Rich DNA sequences. J Mol Diagn 8(5):544–550. https://doi.org/10.2353/jmoldx.2006.060058
Karunamuni G, Sheehan MM, Doughman YQ, Gu S, Sun J, Li Y, Strainic JP, Rollins AM, Jenkins MW, Watanabe M (2017) Supplementation with the methyl donor betaine prevents congenital defects induced by prenatal alcohol exposure. Alcoholism 41(11):1917–1927. https://doi.org/10.1111/acer.13495
Wang C-M, Yuan R-S, Zhuang W-Y, Sun J-H, Wu J-Y, Li H, Chen J-G (2016) Schisandra polysaccharide inhibits hepatic lipid accumulation by downregulating expression of SREBPs in NAFLD mice. Lipids Health Dis 15(1):195. https://doi.org/10.1186/s12944-016-0358-5
Hu Y, Sun Q, Liu J, Jia Y, Cai D, Idriss AA, Omer NA, Zhao R (2017) In ovo injection of betaine alleviates corticosterone-induced fatty liver in chickens through epigenetic modifications. Sci Rep 7(1):40251. https://doi.org/10.1038/srep40251
Chen Y-m, Liu Y, Zhou R-f, Chen X-l, Wang C, Tan X-y, Wang L-j, Zheng R-d, Zhang H-w, Ling W-h, Zhu H-l (2016) Associations of gut-flora-dependent metabolite trimethylamine-N-oxide, betaine and choline with non-alcoholic fatty liver disease in adults. Sci Rep 6:19076. https://doi.org/10.1038/srep19076
Xu L, Huang D, Hu Q, Wu J, Wang Y, Feng J (2015) Betaine alleviates hepatic lipid accumulation via enhancing hepatic lipid export and fatty acid oxidation in rats fed with a high-fat diet. Br J Nutr 113(12):1835–1843. https://doi.org/10.1017/S0007114515001130
Itoh N (2016) FGF10: A multifunctional mesenchymal–epithelial signaling growth factor in development, health, and disease. Cytokine Growth Factor Rev 28:63–69. https://doi.org/10.1016/j.cytogfr.2015.10.001
Flippot R, Kone M, Magné N, Vignot S (2015) FGF/FGFR signalling: implication in oncogenesis and perspectives. Bull Cancer 102(6):516–526. https://doi.org/10.1016/j.bulcan.2015.04.010
Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M, Ornitz DM (2006) Receptor specificity of the fibroblast growth factor family: the complete mammalian FGF family. J Biol Chem 281(23):15694–15700. https://doi.org/10.1074/jbc.M601252200
Fischer C, Seki T, Lim S, Nakamura M, Andersson P, Yang Y, Honek J, Wang Y, Gao Y, Chen F, Samani NJ, Zhang J, Miyake M, Oyadomari S, Yasue A, Li X, Zhang Y, Liu Y, Cao Y (2017) A miR-327–FGF10–FGFR2-mediated autocrine signaling mechanism controls white fat browning. Nat Commun 8(1):2079. https://doi.org/10.1038/s41467-017-02158-z
Chen Q, Liu M, Yu H, Li J, Wang S, Zhang Y, Qiu F, Wang T (2018) Scutellaria baicalensis regulates FFA metabolism to ameliorate NAFLD through the AMPK-mediated SREBP signaling pathway. J Nat Med 72(3):655–666. https://doi.org/10.1007/s11418-018-1199-5
Woods A, Williams JR, Muckett PJ, Mayer FV, Liljevald M, Bohlooly-Y M, Carling D (2017) Liver-specific activation of AMPK prevents steatosis on a high-fructose diet. Cell Rep 18(13):3043–3051. https://doi.org/10.1016/j.celrep.2017.03.011
Rena G, Hardie DG, Pearson ER (2017) The mechanisms of action of metformin. Diabetologia 60(9):1577–1585. https://doi.org/10.1007/s00125-017-4342-z
Chau MDL, Gao J, Yang Q, Wu Z, Gromada J (2010) Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK–SIRT1–PGC-1α pathway. Proc Natl Acad Sci 107(28):12553–12558. https://doi.org/10.1073/pnas.1006962107
Mathur A, Ware C, Davis L, Gazdar A, Pan B-S, Lutterbach B (2014) FGFR2 is amplified in the NCI-H716 colorectal cancer cell line and is required for growth and survival. PLoS ONE 9(6):e98515. https://doi.org/10.1371/journal.pone.0098515
Ishak K, Baptista A et al (1995) Histological grading and staging of chronic hepatitis. J Hepatol 22:696–699. https://doi.org/10.1016/0168-8278(95)80226-6
Liu C-C, Kanekiyo T, Xu H, Bu G (2013) Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol 9(2):106–118. https://doi.org/10.1038/nrneurol.2012.263
Zhang L, Yang M, Ren H, Hu H, Boden G, Li L, Yang G (2013) GLP-1 analogue prevents NAFLD in ApoE KO mice with diet and Acrp30 knockdown by inhibiting c-JNK. Liver Int 33(5):794–804. https://doi.org/10.1111/liv.12120
Lv S, Fan R, Du Y, Hou M, Tang Z, Ling W, Zhu H (2009) Betaine supplementation attenuates atherosclerotic lesion in apolipoprotein E-deficient mice. Eur J Nutr 48(4):205–212. https://doi.org/10.1007/s00394-009-0003-4
Postic C, Girard J (2008) Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Investig 118(3):829–838. https://doi.org/10.1172/JCI34275
Cai D, Yuan M, Liu H, Pan S, Ma W, Hong J, Zhao R (2016) Maternal betaine supplementation throughout gestation and lactation modifies hepatic cholesterol metabolic genes in weaning piglets via AMPK/LXR-mediated pathway and histone modification. Nutrients. https://doi.org/10.3390/nu8100646
Garcia D, Hellberg K, Chaix A, Wallace M, Herzig S, Badur MG, Lin T, Shokhirev MN, Pinto AFM, Ross DS, Saghatelian A, Panda S, Dow LE, Metallo CM, Shaw RJ (2019) Genetic liver-specific AMPK activation protects against diet-induced obesity and NAFLD. Cell Rep 26(1):192–208.e196. https://doi.org/10.1016/j.celrep.2018.12.036
Lee J, Hong S-W, Park SE, Rhee E-J, Park C-Y, Oh K-W, Park S-W, Lee W-Y (2015) AMP-activated protein kinase suppresses the expression of LXR/SREBP-1 signaling-induced ANGPTL8 in HepG2 cells. Mol Cell Endocrinol 414:148–155. https://doi.org/10.1016/j.mce.2015.07.031
Yang Y, Li W, Liu Y, Sun Y, Li Y, Yao Q, Li J, Zhang Q, Gao Y, Gao L, Zhao J (2014) Alpha-lipoic acid improves high-fat diet-induced hepatic steatosis by modulating the transcription factors SREBP-1, FoxO1 and Nrf2 via the SIRT1/LKB1/AMPK pathway. J Nutr Biochem 25(11):1207–1217. https://doi.org/10.1016/j.jnutbio.2014.06.001
Sun H, Zhu X, Lin W, Zhou Y, Cai W, Qiu L (2017) Interactions of TLR4 and PPARγ, dependent on AMPK signalling pathway contribute to anti-inflammatory effects of vaccariae hypaphorine in endothelial cells. Cell Physiol Biochem 42(3):1227–1239. https://doi.org/10.1159/000478920
Kim DH, Lee B, Kim MJ, Park MH, An HJ, Lee EK, Chung KW, Park JW, Yu BP, Choi JS, Chung HY (2016) Molecular mechanism of betaine on hepatic lipid metabolism: inhibition of forkhead Box O1 (FoxO1) binding to peroxisome proliferator-activated receptor gamma (PPARγ). J Agric Food Chem 64(36):6819–6825. https://doi.org/10.1021/acs.jafc.6b02644
Lee C-W, Wong LL-Y, Tse EY-T, Liu H-F, Leong VY-L, Lee JM-F, Hardie DG, Ng IO-L, Ching Y-P (2012) AMPK promotes p53 acetylation via phosphorylation and inactivation of SIRT1 in liver cancer cells. Can Res 72(17):4394–4404. https://doi.org/10.1158/0008-5472.can-12-0429
Wu W, Feng J, Jiang D, Zhou X, Jiang Q, Cai M, Wang X, Shan T, Wang Y (2017) AMPK regulates lipid accumulation in skeletal muscle cells through FTO-dependent demethylation of N6-methyladenosine. Sci Rep 7:41606. https://doi.org/10.1038/srep41606
Steinberg GR, Carling D (2019) AMP-activated protein kinase: the current landscape for drug development. Nat Rev Drug Discov 18(7):527–551. https://doi.org/10.1038/s41573-019-0019-2
Itoh N, Nakayama Y, Konishi M (2016) Roles of FGFs as paracrine or endocrine signals in liver development, health, and disease. Front Cell Dev Biol. https://doi.org/10.3389/fcell.2016.00030
Acknowledgements
The experimental work described was carried out by Weiqiang Chen and Xiaoli Zhang as part of his Marster project. The authors’ responsibilities were as follows-Yucai Wang, Qingyan Zou and Liefeng Wang conceived and designed the study and supervised; Weiqiang Chen, Xiaoli Zhang, Minwen Xu, Lixia Jiang, Min Zhou, Yi Zhang, Wenjun Liu, Zhijun Chen: conducted the research; Weiqiang Chen: wrote the first draft of the manuscript; liefeng wang: wrote the final draft of the manuscript; and all authors: read and approved the final manuscript.
Funding
This work was supported in part by the Science and Technology Project Foundation of Education Department of Jiangxi Provincial (GJJ150961) to MX, the Corporate Crossswise project funding of Jiangxi Xi Di biological science and Technology Co., Ltd (HX201801),The Open Project of Key Laboratory of Prevention and treatment of cardiovascular and cerebrovascular diseases, Ministry of Education (XN201803), the Natural Science Foundation of Jiangxi Province (20132BAB205032), and the National Natural Science Foundation of China (31660256 and 31860247) to LW. The Open Project of Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases, Ministry of Education (No. XN201803) to LW.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical statement
Our animal studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. You may have to state that specific national laws have been observed, too.
Rights and permissions
About this article
Cite this article
Chen, W., Zhang, X., Xu, M. et al. Betaine prevented high-fat diet-induced NAFLD by regulating the FGF10/AMPK signaling pathway in ApoE−/− mice. Eur J Nutr 60, 1655–1668 (2021). https://doi.org/10.1007/s00394-020-02362-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-020-02362-6