Abstract
The ability of diphenyl diselenide [(PhSe)2] to attenuate oxidative damage was evaluated in the liver, gills, brain, and muscle of carp (Cyprinus carpio) and silver catfish (Rhamdia quelen) experimentally exposed to fipronil (FPN). Initially, the fish were fed a diet without (PhSe)2 or a diet containing 3.0 mg/kg of (PhSe)2 for 60 days. After the 60-day period, the fish were exposed to 0.65 µg/L of FPN for 192 h. The results showed that carp exposed to FPN and not fed with (PhSe)2 exhibited acetylcholinesterase (AChE) inhibition in brain and muscle, and increased thiobarbituric acid-reactive substance (TBARS) in liver, gills, and brain. Furthermore, FPN decreased nonprotein thiols (NPSH) and δ-aminolevulinate dehydratase (δ-ALA-D) in carp liver and gills, and increased plasma glucose and protein levels. In silver catfish, FPN inhibited AChE and increased TBARS levels in muscle. In addition, glutathione S-transferase (GST) decreased in liver and muscle, and plasma glucose was increased. (PhSe)2 reversed some of these effects. It prevented the increase in TBARS levels in liver, gills, and brain in carp and in silver catfish muscle, and reversed the increase in plasma glucose levels in both species. Additionally, (PhSe)2 increased the NPSH levels in carp and silver catfish that had decreased in response to FPN exposure. However, (PhSe)2 was not effective in reversing the AChE inhibition in brain and muscle or the δ-ALA-D decrease in carp liver. Thus, (PhSe)2 protects tissues of both species of fish, mainly by preventing or counteracting the effects of FPN, on TBARS levels, antioxidants, and present anti-hyperglycemic property.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Elemental minerals are important nutrients because they are involved in many biological processes as cofactors or parts of numerous enzymes. Selenium (Se) is a constituent of various selenoproteins, which are important components of antioxidant systems that actively protect from damage caused by free radicals and reactive oxygen species (ROS) (Hamilton 2004). Interest in the chemistry and biochemistry of organoselenium compounds has increased in the last three decades due to the antioxidant activity exhibited by these compounds (Ribeiro et al. 2013). Diphenyl diselenide [(PhSe)2], the simplest of the diaryl diselenides, exhibits glutathione peroxidase-like activity and antioxidant properties in different experimental models (Nogueira and Rocha 2010). This compound has already demonstrated antioxidant properties in tissues of fish exposed to herbicides, such as quinclorac and clomazone (Menezes et al. 2012, 2013, 2014a).
Thus, it is interesting to determine whether this Se compound has effects on the toxicity caused by other environmental pollutants in aquatic organisms. The growing use of chemicals in agriculture, including fertilizers and pesticides, has contributed to environmental pollution. Fipronil (FPN) is a broadspectrum phenylpyrazole insecticide, widely used to control pests in urban areas and in agriculture (Beggel et al. 2012). This insecticide interferes with the insect central nervous system by selectively binding to gamma-aminobutyric acid (GABA)-gated chloride channels and antagonizing the action of GABA. GABA receptor inhibition disrupts the chloride ion control of neuronal signaling, causing central nervous system toxicity (Stehr et al. 2006). FPN has been detected in surface water at concentrations up to 12.6 μg/L in residential areas (Gan et al. 2012) and greater than 9 μg/L in treated fields (Schlenk et al. 2001). Clasen et al. (2012) observed that this insecticide caused altered production of antioxidant enzymes such as superoxide dismutase (SOD) and catalase (CAT), and increased lipid peroxidation and protein carbonylation in carp exposed to 90 days of rice field conditions.
In aquatic toxicology studies, fish are important indicators for the impact of toxic substances (Glusczak et al. 2011; Stara et al. 2013). The carp, Cyprinus carpio, and silver catfish, Rhamdia quelen, were the fish species chosen for this study because they are extensively used for monitoring freshwater contamination in southern Brazil (Mabuchi et al. 2006; Murussi et al. 2015). The carp is the longest cultured and is of significant economic importance in several countries; furthermore, it is an abundant species that is easy to capture (Vandeputte 2003). The silver catfish is a species that adapts well to different environments and artificial diets, is easy to handle, and has good commercial acceptance (Baldisserotto 2009).
Previous studies have shown that Se-containing compounds may protect against the toxic effects caused by pesticides in fish (Monteiro et al. 2009; Menezes et al. 2012, 2013). Hence, the present work was designed to evaluate whether (PhSe)2 supplementation could prevent or reduce biochemical alterations caused by exposure to the insecticide FPN, in two different species of fish.
Materials and methods
Chemicals
The FPN insecticide (CAS 120068-37-3) used was an available commercial formulation (Standak®-BASF), containing 25 % fipronil [(7)-5-amino-1-(2,6-dichloro-α-α-α-trifluoro-p-tolyl)-4-trifluoromethylsulfinylpyrazole-3-carbonitrile]. Malondialdehyde (MDA), 2-thiobarbituric acid (TBA), sodium dodecyl sulfate (SDS), and 2,4-dinitrophenylhydrazine (DNPH) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). All other chemicals were of analytical reagent grade and purchased from Merck (Rio de Janeiro, Brazil). Diphenyl diselenide [(PhSe)2] was synthesized according to the literature methods (Paulmier 1986).
Fish
Carp (weight, 18.28 ± 1.65 g; length, 10.20 ± 1.0 cm) and silver catfish (weight, 17.90 ± 1.44 g; length, 12.30 ± 0.31 cm) were obtained from a fish farm (RS, Brazil). Fish were acclimated to laboratory conditions for 15 days, in 250-L boxes of fiberglass prior to experiments. They were kept in continuously aerated tap water with a static system and with a natural photoperiod (12-h light/12-h dark). Water conditions were as follows: temperature 23.0 ± 1.0 °C, pH 7.3 ± 0.1 units, dissolved oxygen 7.1 ± 1.0 mg/L, non-ionized ammonia 0.3 ± 0.01 μg/L, and nitrite 0.05 ± 0.01 mg/L.
Diet preparation and experimental design
The experiment was performed first with the carp and after with silver catfish. Initially, the fish were divided into two groups: (1) control group (fish fed with a diet without (PhSe)2, n = 24) and (2) Se group (fish fed with a diet supplemented with 3.0 mg/Kg (PhSe)2, n = 24) and were fed with these diets for 60 days. The diet composition used in the experiment was based on other studies of the group, and the concentration of (PhSe)2 used was 3 mg/kg (Menezes et al. 2012). Briefly, to obtain the diets containing (PhSe)2, this compound was added to control diet, and all the ingredients were completely mixed before further homogenization. Control and (PhSe)2 diet pellets were stored at 4 °C until they were used. In this experimental protocol, the fish were fed 3 % biomass per day. The daily ration was divided into two equal meals fed at 09:00 and 16:00 hours. The concentration of (PhSe)2 chosen was based on previous studies, which demonstrated that 3.0 mg/Kg (PhSe)2 did not cause overt signals of toxicity in carp and silver catfish (Menezes et al. 2013, 2014b, 2016). Water quality parameters were monitored daily and were maintained equal to those registered during the acclimation period of both species.
After feeding period of 60 days, groups were subdivided and allocated in 45-L boxes of fiberglass into four experimental groups of five or seven fish each group: (1) control group [fish fed with a diet without (PhSe)2], (2) (PhSe)2 group [fish fed with a diet supplemented with 3.0 mg/kg of (PhSe)2], (3) FPN group [fish fed with a diet without (PhSe)2 and exposed to 0.65 µg/L fipronil for 192 h], (4) (PhSe)2 + FPN group [fish fed with a diet supplemented with 3.0 mg/kg of (PhSe)2 and exposed to 0.65 µg/L fipronil for 192 h]. The concentration of the insecticide, used in this study, was selected from other experiments performed by our group using this chemical compound (Clasen et al. 2012). The concentration of insecticide in water was measured in the beginning, middle, and final of experimental period by gas chromatography with electron capture detection (GC-ECD) according to Marchesan et al. (2010). Water analysis, at the end of experimental period, in the experiment of carp showed reduction in approximately 20 % in the concentration of FPN (0.52 µg/L) in relation to the concentration initial (0.65 µg/L). In the experiment with silver catfish reduced about of 30 % the concentration of FPN (0.45 µg/L) in relation to the concentration initial (0.64 µg/L).
Sample preparation
At the end of the exposure period, the fish were anesthetized with 50 mg/L clove oil for 3 min. After blood collection from caudal vein using syringe with heparin as anticoagulant, the plasma was obtained by centrifugation to analysis of metabolic parameters (glucose and protein). Fish were euthanized by spinal section, and liver, gills, brain, and muscle were removed. The tissues were washed with 150 mM saline solution, placed in Teflon tubes and kept at −80 °C for until analysis. The investigation was authorized by the Board on Experimentation on Animals of the Federal University of Santa Maria, Reference Number: 84/2009.
Chemical analysis
Acetylcholinesterase (AChE) activity
Tissue samples (brain and muscle) were weighed and homogenized with 150 mM NaCl (1:10 dilution). Homogenate were centrifuged for 15 min at 3000×g at 5 °C, and the supernatant was used for the determination of the enzyme. AChE activity was measured as described by Ellman et al. (1961). Aliquots of supernatant (50–100 µL for brain and muscle, respectively) were pre-incubated at 30 °C for 2 min with 0.1 M phosphate buffer, pH 7.5, 1 mM DTNB as chromogen. After 2 min, the reaction was initiated by the addition of acetylthiocholine (1 mM) as substrate for the reaction mixture. The final volume was 2.0 mL. Absorbances were determined at 412 nm during 2 min. Enzyme activity was expressed as µmol of acetylthiocholine (ASCh) hydrolyzed/mg protein/min.
Lipid peroxidation
Lipid peroxidation was estimated by thiobarbituric acid-reactive species (TBARS) production, performed by malondialdehyde reaction with 2-thiobarbituric acid, which was optically measured according to Buege and Aust (1978). In homogenates of the liver, gills, brain, and muscle (100–400 µL) were added TCA 10 and 0.67 % thiobarbituric acid, totalizing a volume of 1.0 mL. The reaction mixture was incubated for 30 min at 95 °C, and optical density was measured in a spectrophotometer at 532 nm. TBARS levels were expressed as nmol MDA/mg protein.
Glutathione S-transferase (GST) activity
Liver, gills, and muscle were homogenized with 20 mM potassium phosphate buffer, pH 7.5 (1:20 dilution), and centrifuged at 10,000×g for 10 min at 4 °C. GST activity was measured according to Habig et al. (1974) using 1-chloro-2,4-dinitrobenzene (CDNB) as a substrate. The formation of S-2, 4-dinitrophenyl glutathione was monitored by the increase in absorbance at 340 nm against blank. The molar extinction coefficient used for CDNB was 9.6 mM/cm. The activity was expressed as μmol GS-DNB/min/mg protein.
Nonprotein thiols (NPSH) and ascorbic acid (AA) levels
NPSH and AA contents were determined in liver, gills, and muscle by the method of Ellman (1959) and Roe (1954), respectively. The preparation of homogenate for the two determinations was the same. The tissues were homogenized with 1.5 mL Tris HCl 50 mM (pH 7.5) followed by centrifugation at 3000×g for 10 min. An aliquot of supernatants (1.0 mL) was mixed (1:1) with 10 % trichloroacetic acid and then centrifuged. To determine NPSH levels of tissues, an aliquot (400 µL) of supernatant was added in a phosphate buffer 0.5 mM (pH 6.8), 10 mM 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB), cysteine 0.5 mM. The color reaction was measured at 412 nm. NPSH levels were expressed as µmol NPSH/g tissue. For determination of AA, an aliquot of the supernatants (300 µL) was mixed with 2,4-dinitrophenylhydrazine (4.5 mg/mL), 0.6 mg/mL thiourea, CuSO4 (0.075 mg/mL), and trichloroacetic acid 13.3 % and incubated for 3 h at 37 °C. After, H2SO4 65 % (v/v) was added to the medium. AA levels were expressed as µg ascorbic acid/g tissue.
δ-Aminolevulinate dehydratase (δ-ALA-D) activity
δ-ALA-D activity was analyzed in liver and gills according to the method of Sassa (1982) by measuring the rate of product porphobilinogen (PBG) formation, except that 100 mM phosphate buffer (pH 6.8) and 2.5 mM delta aminolevulinic acid (ALA) were used. The tissues were homogenized in 150 mM NaCl at the dilution of 1:10 (liver) and 1:5 (gills). The homogenate was centrifuged at 3000 g for 10 min at 4 °C, and supernatant was used for enzyme assay. Incubations were carried out for 1 h at 37 °C. The reaction product was determined using modified Ehrlich’s reagent at 555 nm, with a molar absorption coefficient of 6.1 × 104 M/cm for Ehrlich-porphobilinogen salt. The reaction was stopped by adding 10 % of trichloroacetic acid (with 10 mM HgCl2). Enzyme activity was expressed as nmol of porphobilinogen (PBG)/h/mg protein.
Protein determination
Protein was determined by the Coomassie blue method using bovine serum albumin as standard. Absorbance of samples was measured at 595 nm (Bradford 1976).
Glucose and protein
Plasma glucose was measured by the glucose oxidase method with a Bioclin test kit, and total protein levels were measured according to Bradford (1976) using bovine serum albumin as standard.
Statistical analysis
The values in all determinations are presented as mean ± standard error of mean (SEM). Normality and homogeneity of variances in the samples were tested with the Kolmogorov–Smirnov and Levene tests, respectively. Statistical analysis was performed by two-way analysis of variance (ANOVA) followed by Newman–Keuls post hoc comparison. Analysis was performed using Graphpad Prism (version 6.0), and the minimum significance level was set at p < 0.05.
Results
AChE activity
A two-way ANOVA of AChE activity in brain and muscle of carp revealed a significant effect of FPN exposure (F 1,16 = 24.42; p = 0.0001 and F 1,16 = 43.94; p < 0.0001, respectively). Post hoc comparisons showed that (PhSe)2 supplementation per se did not change AChE activity in carp brain and muscle. The fish fed a diet without (PhSe)2 and exposed to FPN presented decreased AChE activity in brain and muscle as compared with the control group. Supplementation with (PhSe)2 was not able to reverse the inhibition caused by FPN in brain and muscle of carp (Fig. 1a).
A two-way ANOVA of AChE activity in brain of silver catfish demonstrated a significant effect of (PhSe)2 (F 1,20 = 43.13; p < 0.0001), and a significant effect of (PhSe)2 and FPN exposures was observed in muscle (F 1,20 = 16.90; p < 0.001 and F 1,20 = 21.28; p < 0.001, respectively). Post hoc comparisons showed that (PhSe)2 per se increased AChE activity in brain but did not change AChE activity in muscle in silver catfish. On the other hand, silver catfish exposed to FPN presented a decrease in AChE activity only in muscle when compared with the control group, but this decrease was not present in the (PhSe)2 + FPN group (Fig. 1b).
TBARS levels
A two-way ANOVA of TBARS levels in carp liver revealed a significant effect of both (PhSe)2 and FPN exposures (F 1,16 = 18.27; p < 0.001 and F 1,16 = 52.16; p < 0.0001, respectively). In gills, a significant effect of (PhSe)2 and FPN exposures was also demonstrated (F 1,16 = 34.41; p < 0.0001 and F 1,16 = 8.52; p = 0.01, respectively). Furthermore, a significant effect of (PhSe)2 and FPN exposures was revealed in brain (F 1,16 = 27.33; p < 0.0001 and F 1,16 = 19.55; p < 0.001, respectively). In muscle of carp, a two-way ANOVA demonstrated a significant (PhSe)2 × FPN exposure interaction (F 1,16 = 6.90; p < 0.05). Post hoc comparisons showed that carp fed a diet containing (PhSe)2 per se presented decreased TBARS levels in liver, gills, brain, and muscle. In carp exposed to FPN insecticide and fed a diet without (PhSe)2, the TBARS levels increased in liver, gills, and brain and no change in muscle when compared to control group. In addition, supplementation with (PhSe)2 was able to reverse the increase in TBARS levels caused by FPN in liver, gills, and brain of carp (Fig. 2a).
A two-way ANOVA of TBARS levels in liver of silver catfish demonstrated a significant effect of (PhSe)2 (F 1,20 = 10.35; p < 0.05). In the gills, a two-way ANOVA revealed a significant effect of (PhSe)2 and FPN exposures on TBARS levels (F 1,20 = 5.29; p < 0.05 and F 1,20 = 4.47; p < 0.05, respectively). In addition, the TBARS levels in brain yielded the significant effect of (PhSe)2 (F 1,16 = 6.19; p < 0.05) and in muscle of silver catfish, a two-way ANOVA demonstrated a significant effect of (PhSe)2 and FPN exposures (F 1,20 = 9.05; p < 0.05 and F 1,20 = 9.46; p < 0.01). Post hoc comparisons showed that supplementation with (PhSe)2 per se did not change TBARS levels in any tissue analyzed in comparison with the control group (Fig. 2b). Furthermore, exposure to FPN increased the TBARS levels in this species in muscle but did not change TBARS levels in liver, gills, or brain in comparison with the control group (Fig. 2b). In the silver catfish, reduced TBARS levels also were observed in gills and muscle in the (PhSe)2 + FPN group, and no change was observed in liver and brain when compared with the FPN group (Fig. 2b).
GST activity
A two-way ANOVA of GST activity in liver of carp yielded a significant effect of (PhSe)2 × FPN exposure interaction and FPN exposure (F 1,16 = 32.27; p < 0.0001 and F 1,16 = 8.32; p < 0.05, respectively). In gills, two-way ANOVA demonstrated in GST activity a significant effect of (PhSe)2 × FPN exposure interaction, (PhSe)2 and FPN exposures (F 1,16 = 27.29; p < 0.0001, F 1,16 = 15.12; p < 0.05 and F 1,16 = 43.13; p < 0.0001, respectively). In muscle of carp, a two-way ANOVA demonstrated a significant effect of FPN exposure (F 1,16 = 5.20; p < 0.05). Post hoc comparisons showed that a diet with (PhSe)2 per se increased GST activity in liver and gills of carp. FPN exposure increased GST activity in carp liver, whereas it did not modify this parameter in gills and muscle of this species. The GST activity was decreased in liver of the carp in the (PhSe)2 + FPN group when compared with the FPN group, returning values near to control (Fig. 3a).
A two-way ANOVA of GST activity in liver of silver catfish revealed a significant effect of (PhSe)2 and FPN exposures (F 1,20 = 19.86; p < 0.001 and F 1,20 = 9.07; p < 0.05, respectively). In gills, a two-way ANOVA demonstrated a significant effect of FPN exposure (F 1,20 = 5.52; p < 0.05), and in muscle, there was a significant (PhSe)2 × FPN exposure interaction (F 1,20 = 13.40; p < 0.05). Post hoc comparisons demonstrated that (PhSe)2 per se increased GST activity in liver of silver catfish and did not change GST activity in gills and muscle. Exposure to FPN insecticide decreased GST activity in liver and muscle and no change in gills in comparison with the control group. (PhSe)2 treatment restored GST activity that was diminished by FPN exposure in liver and muscle of silver catfish (Fig. 3b).
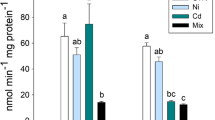
NPSH levels
A two-way ANOVA of NPSH levels in liver of carp revealed a significant effect of (PhSe)2 × FPN exposure interaction, (PhSe)2 and FPN exposure (F 1,16 = 11.20; p < 0.05, F 1,16 = 70.76; p < 0.0001 and F 1,16 = 40.66; p < 0.0001, respectively). In gills, a two-way ANOVA demonstrated a significant effect of (PhSe)2 and FPN exposures (F 1,16 = 24.34; p = 0.0001, F 1,16 = 75.86; p < 0.0001, respectively), and in carp muscle, a two-way ANOVA demonstrated a significant effect of (PhSe)2 (F 1,16 = 7.98; p < 0.05). Post hoc comparisons showed that supplementation with (PhSe)2 per se caused a significant increase in NPSH levels in liver and gills of carp, and no change was observed in muscle when compared with the control group. On the other hand, carp exposed to FPN insecticide and fed a diet without (PhSe)2 showed reduced NPSH levels in liver and gills and no change in muscle compared with the control group. In carp liver and gills, an increase in NPSH levels was observed in the (PhSe)2 + FPN group compared with the FPN group (Fig. 4a).
A two-way ANOVA of NPSH levels in the liver of silver catfish revealed a significant effect of (PhSe)2 × FPN exposure interaction, (PhSe)2 and FPN exposures (F 1,20 = 5.65; p < 0.05, F 1,20 = 9.08; p < 0.01 and F 1,20 = 33.36; p < 0.0001, respectively). In gills, two-way ANOVA demonstrated a significant effect of (PhSe)2 × FPN exposure interaction and (PhSe)2 (F 1,20 = 11.62; p < 0.05, F 1,20 = 43.98; p < 0.0001, respectively). In muscle of silver catfish, a two-way ANOVA of NPSH levels did not demonstrate a significant effect of (PhSe)2 × FPN exposure interaction, (PhSe)2 or FPN exposure. Post hoc comparisons showed (PhSe)2 per se increased the NPSH levels in liver and gills and did not change NPSH in muscle of silver catfish when compared with the control group. FPN exposure did not change NPSH levels in any of the analyzed tissues when compared with the control group in this species of fish. Furthermore, the (PhSe)2 + FPN group in silver catfish caused an increase in NPSH levels in gills in comparison with the control and FPN groups (Fig. 4b).
AA levels
A two-way ANOVA of AA levels in liver of carp showed a significant effect of (PhSe)2 and FPN exposures (F 1,16 = 28.00; p < 0.0001, F 1,16 = 8.12; p < 0.05, respectively). In gills, a two-way ANOVA revealed a significant effect of (PhSe)2 × FPN exposure interaction, (PhSe)2 and FPN exposures (F 1,16 = 22.25; p < 0.001, F 1,16 = 94.60; p < 0.0001, F 1,16 = 49.94; p < 0.0001, respectively). In muscle of carp, a two-way ANOVA demonstrated a significant effect of (PhSe)2 (F 1,16 = 52.49; p < 0.0001). Post hoc comparisons showed that (PhSe)2 per se in carp increased AA levels in liver, gills, and muscle. In addition, carp exposed to FPN and fed a diet without (PhSe)2 had no change in AA levels in the three tissues analyzed. In carp liver, gills, and muscle, an increase in AA levels was observed for the (PhSe)2 + FPN group when compared with the control and FPN groups (Table 1).
A two-way ANOVA of AA levels in liver of silver catfish revealed a significant effect of (PhSe)2 × FPN exposure interaction, (PhSe)2 and FPN exposures (F 1,16 = 66.93; p < 0.0001, F 1,16 = 40.33; p < 0.0001, F 1,16 = 55.66; p < 0.0001, respectively). In gills, a two-way ANOVA demonstrated a significant effect of FPN exposure (F 1,16 = 5.13; p < 0.05). In muscle of silver catfish, a two-way ANOVA of AA levels did not demonstrate a significant effect of (PhSe)2 × FPN exposure interaction, (PhSe)2 or FPN exposure. Post hoc comparisons showed that (PhSe)2 per se and FPN exposures did not change AA levels in liver, gills, and muscle of silver catfish when compared with the control group. In the liver of silver catfish, an increase in AA levels was observed in the (PhSe)2 + FPN group when compared with the control, (PhSe)2 and FPN groups (Table 1).
δ-ALA-D activity
A two-way ANOVA of δ-ALA-D activity in liver of carp yielded a significant effect of FPN exposure (F 1,16 = 25.50; p = 0.0001). In gills revealed a significant effect of (PhSe)2 × FPN exposure interaction, (PhSe)2 and FPN exposures (F 1,16 = 8.01; p < 0.05, F 1,16 = 112.90; p < 0.0001, F 1,16 = 7.65; p < 0.05, respectively). Post hoc comparisons showed that carp fed a diet containing (PhSe)2 per se presented increased δ-ALA-D activity in liver and gills in comparison with the control group. On the other hand, carp exposed to FPN and fed a diet without (PhSe)2 showed a decrease in δ-ALA-D activity in liver and gills. In liver of the carp, the diet containing (PhSe)2 was not effective in reversing the inhibition caused by FPN, whereas in gills an increase in the δ-ALA-D activity was observed in the (PhSe)2 + FPN group in comparison with the control and FPN groups (Table 2).
In liver of silver catfish, a two-way ANOVA of δ-ALA-D activity did not demonstrate a significant effect of (PhSe)2 × FPN exposure interaction, (PhSe)2 or FPN exposure. In gills revealed a significant effect of FPN exposure (F 1,20 = 35.39; p < 0.0001). Post hoc comparisons showed that the diet containing (PhSe)2 per se did not change the activity of δ-ALA-D in liver or gills of silver catfish. However, FPN exposure caused an increase in δ-ALA-D activity in gills, and no change was observed in liver when compared with the control group. In addition, an increase in the δ-ALA-D activity in the (PhSe)2 + FPN group was observed in gills of the silver catfish when compared with the control group (Table 2).
Glucose and protein
A two-way ANOVA of glucose levels in plasma of carp showed significant effects of (PhSe)2 and FPN exposures (F 1,16 = 30.33; p < 0.0001, F 1,16 = 82.79; p < 0.0001). Similarly, a two-way ANOVA of silver catfish plasma glucose levels demonstrated significant effects of (PhSe)2 and FPN exposures (F 1,16 = 42.74; p < 0.0001, F 1,16 = 380.20; p < 0.0001). Post hoc comparisons showed that carp and silver catfish fed a diet containing (PhSe)2 per se showed a decrease in plasma glucose levels. Both species of fish fed a diet without (PhSe)2 and exposed to FPN presented an increase in glucose levels. However, (PhSe)2 ameliorated the increased plasma glucose level induced by FPN in carp and silver catfish, but the glucose levels were still higher than the control values for both species (Table 3).
A two-way ANOVA of plasma protein levels in carp yielded a significant effect of FPN exposure (F 1,16 = 18.29; p < 0.001). Similarly, a two-way ANOVA of plasma protein levels in silver catfish demonstrated a significant effect of FPN exposure (F 1,16 = 4.80; p < 0.05). Post hoc comparisons demonstrated that the two species of fish fed a diet containing (PhSe)2 per se did not show a change in plasma protein levels when compared with the control group. FPN exposure increased the plasma protein levels in carp and did not changed these levels in silver catfish. Supplementation with (PhSe)2 in carp was not effective in returning the values of plasma protein to control values (Table 3).
Discussion
FPN is an insecticide commonly used in rice fields in southern Brazil. This insecticide was introduced to the consumer market in 1996 and was shown to be less toxic than the standard insecticides used in rice cultivation. Clasen et al. (2012) evaluated the effects of FPN in rice field conditions on carp and observed that this insecticide caused alterations in biochemical parameters in different tissues of this species. Thus, in the present study, we assessed the effects of (PhSe)2 in carp and silver catfish to determine whether this selenium-containing compound is able to reverse changes caused by FPN. Studies have shown that (PhSe)2 can interact in the body of fish to reduce the toxicity of pesticides (Menezes et al. 2012, 2013, 2014a). In this study, (PhSe)2 was effective in protecting against some damage caused by FPN in tissues of carp and silver catfish, mainly by acting on lipid peroxidation and antioxidant defenses. It was unable to reverse the effects of FPN on AChE activity in brain and muscle and δ-ALA-D activity in liver of carp. Furthermore, (PhSe)2 was able to reduce plasma glucose levels.
Alterations of AChE enzyme activity are used widely as a rapid method for the detection of pesticide toxicity. AChE catalyzes the hydrolysis of acetylcholine into choline and acetate in the synaptic cleft (Roex et al. 2003; Moraes et al. 2009). In this study, FPN caused inhibition of AChE activity in brain and muscle of carp and in muscle of silver catfish. Inhibition of AChE can influence cholinergic neurotransmission and promote undesirable effects due to an increased amount of acetylcholine in the synaptic cleft, which may lead to overactivation of tissue. (PhSe)2 per se only changed AChE activity in brain of silver catfish demonstrating that this selenium-containing compound shows few direct effects on this parameter. In addition, (PhSe)2 was not effective against FPN-induced AChE inhibition in carp tissues of carp, whereas (PhSe)2 reverted the effect of FPN in muscle of silver catfish.
The typical damage caused by reactive species involves peroxidation of lipids. Consistent with this, our results showed increased TBARS levels in liver, gills, and brain of carp and in muscle of silver catfish exposed to FPN. The TBARS results indicate the lipid peroxidation generated by FPN exposure caused oxidative damage in both species. Our results are in agreement with those obtained by Clasen et al. (2012) where TBARS levels significantly increased in brain, liver, and muscle of carp after 7, 30, and 90 days of exposure to FPN under rice field conditions. In addition, (PhSe)2 per se decreased TBARS levels in carp liver, gills, brain, and muscle, whereas no significant change was observed in the TBARS in silver catfish. In agreement, Menezes et al. (2014b) observed that carp fed a diet containing 3.0 mg/kg of (PhSe)2 had decreased TBARS levels in liver, gills, brain, and muscle. Furthermore, (PhSe)2 reduced lipid peroxidation in liver, gills, and brain of carp and in muscle of silver catfish caused by FPN. This effect is probably due to the antioxidant potential showed by this organoselenium compound. In accordance with this result, studies have shown that fish fed with dietary Se supplementation had reduced TBARS levels in different tissues after exposure to other pesticides (Monteiro et al. 2009; Menezes et al. 2012, 2013, 2014a). This response demonstrates that (PhSe)2 was effective in ameliorating oxidative damage induced by FPN exposure.
GST is a detoxifying enzyme that catalyzes the conjugation of a variety of electrophilic substrates to the thiol group of GSH, thereby decreasing the toxicity of the substrate. In the current study, we found that carp exposed to FPN had an increased level of GST activity in liver, but no significant difference in gills and muscle in comparison with the control group. However, in silver catfish, inhibition of this enzyme was observed in liver and muscle, but no change was observed in gills. The enzyme activity can be activated or inhibited by pesticide exposure. The exact effect depends on different factors, such as intensity and duration of stress, as well as the susceptibility of exposed species. Thus, we can see that the two species of fish used in this study show different effects of FPN insecticide exposure on GST activity. In silver catfish, supplementation with (PhSe)2 reverted the inhibition of GST activity in liver and muscle caused by FPN. This result reinforces the hypothesis that the mechanism of the protective action of (PhSe)2 is derived from its antioxidant properties. Moreover, (PhSe)2 per se increased GST activity in liver and gills of carp and in liver of silver catfish, suggesting a likely mechanism by which (PhSe)2 may act as a protective agent.
Carp exposed to FPN showed a decrease in NPSH levels in liver and gills, whereas no change was observed in the silver catfish. The decrease in NPSH levels in carp after FPN exposure could be associated with the elevation of TBARS levels. A carp diet containing (PhSe)2 reversed the reduction in NPSH levels observed in liver and gills. In addition, (PhSe)2 per se increased NPSH levels in liver and gills of both species. The results concerning the effects of (PhSe)2 per se are in agreement with Menezes et al. (2013), where carp and silver catfish showed increased NPSH levels in liver and gills. Some protective effects were observed, which may relate to an interaction between organic selenium and sulfhydryl groups of thiols. This fact was important in protecting the fish from FPN toxicity. Another non-enzymatic antioxidant evaluated in the present study was AA. Carp and silver catfish exposed to FPN did not show any change in AA levels in liver, gills, and muscle, so a protective effect of (PhSe)2 could not be determined. Menezes et al. (2012) also observed no change in AA levels in carp fed with (PhSe)2 and exposed to quinclorac herbicide. On the other hand, we observed an increase in the AA levels in liver, gills, and muscle of carp fed (PhSe)2 per se and in the (PhSe)2 + FPN. The increase in AA levels could be interpreted as part of the antioxidant response as a consequence of pre-administration of (PhSe)2.
Pesticides exposure can also cause inhibition of sulfhydryl enzymes like δ-ALA-D (Menezes et al. 2014c). In this study, we observed inhibition of δ-ALA-D activity in liver and gills of carp, whereas FPN exposure increased the activity of this enzyme in gills of silver catfish. Inhibition of δ-ALA-D activity can produce an increase in its substrate (ALA), which is accepted as a pro-oxidant compound (Barbosa et al. 1998). Menezes et al. (2014c) observed that exposure to the herbicide clomazone caused a significant reduction in δ-ALA-D activity in gills of carp exposed to concentrations of 5 and 10 mg/L. Oxidant agents may interact with the thiol group of this enzyme in particular and thereby impair its activity. Additionally, activation of δ-ALA-D in liver and gills was observed in carp fed with a (PhSe)2-supplemented diet per se. The increase in the activity of this enzyme may be associated with the increase in NPSH levels in these tissues. On the other hand, the diet containing (PhSe)2 failed to reverse the inhibition of δ-ALA-D activity caused by FPN in liver of carp. The combination of (PhSe)2 and FPN increased the δ-ALA-D activity, and this increase could offer an additional protection against FPN toxicity. Thus, (PhSe)2 may not be efficient in protecting against δ-ALA-D inhibition, but may on its own cause an increase in activity. Studies have previously observed that although (PhSe)2 protects against pro-oxidant effects caused by xenobiotics, it did not reverse the δ-ALA-D inhibition (Barbosa et al. 2008; Fiuza et al. 2015).
FPN exposure induced hyperglycemia in carp and silver catfish, which was demonstrated by increased glucose levels in plasma. Plasma glucose is used as a sensitive indicator of stress in fish, and an increase in this parameter may result from an increase in gluconeogenesis to provide energy for the increased metabolic demands imposed by FPN. On the other hand, (PhSe)2 per se caused a decrease in plasma glucose levels in carp and silver catfish. (PhSe)2 attenuated the increase caused by FPN in plasma glucose in carp and silver catfish, indicating its anti-hyperglycemic property. The protein levels in plasma were increased only in carp after exposure to FPN, and this increase persisted in the (PhSe)2 + FPN group. This result could be attributed to an increase in protein synthesis to suit the high energy demand caused by FPN exposure.
The present study demonstrated that treatment with (PhSe)2 restored oxidative damage (evidenced by TBARS levels) and change in defenses antioxidants (NPSH levels and GST activity) caused by FPN exposure in tissues of carp and silver catfish. The exact mechanism of the antioxidant action of (PhSe)2 is not clear at the moment, particularly with respect to the anti-hyperglycemic activity. Furthermore, (PhSe)2 caused an increase per se in NPSH levels and GST activity in liver and gills of both fish species. These results reinforce the involvement of the glutathione system in the antioxidant effect of (PhSe)2. Thus, the protective influence of (PhSe)2 was partly associated with its free radical scavenging and antioxidant actions. In addition to this benefit, the lack of adverse effect in carp and silver catfish fed with (PhSe)2 diet alone suggests its safety which is crucial if (PhSe)2 is considered for validation as a promising agent to minimize the toxicity of pesticides like FPN.
References
Baldisserotto B (2009) Piscicultura continental no Rio Grande do Sul: situação atual, problemas e perspectivas para o futuro. Ciênc Rural 39:291–299
Barbosa NBV, Rocha JBT, Zeni G, Emanuelli T, Beque MC, Braga AL (1998) Effect of organic forms of selenium on δ-aminolevulinate dehydratase from liver, kidney, and brain of adult rats. Toxicol Appl Pharmacol 149:243–253
Barbosa NBV, Rocha JBT, Soares JCM, Wondracek DC, Goncalves JF, Schetinger MRC, Nogueira CW (2008) Dietary diphenyl diselenide reduces the STZ induced toxicity. Food Chem Toxicol 46:186–194
Beggel S, Werner I, Connon RE, Geist JP (2012) Impacts of the phenylpyrazole insecticide fipronil on larval fish: time-series gene transcription responses in fathead minnow (Pimephales promelas) following short-term exposure. Sci Total Environ 426(1):160–165
Bradford MMA (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–309
Clasen B, Loro VL, Cattaneo R, Moraes B, Lópes T, Avila LA, Zanella R, Reimche GB, Baldisserotto B (2012) Effects of the commercial formulation containing fipronil on the non-target organism Cyprinus carpio: implications for rice-fish cultivation. Ecotoxicol Environ Saf 77:45–51
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem 82:70–77
Ellman GL, Courtney KD, Andres V Jr (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Fiuza TL, Oliveira CS, Costa M, Oliveira VA, Zeni G, Pereira ME (2015) Effectiveness of (PhSe)2 in protect against the HgCl2 toxicity. J Trace Elem Med Biol 29:255–262
Gan J, Bondarenko S, Oki L, Haver D, Li JX (2012) Occurrence of fipronil and its biologically active derivatives in urban residential runoff. Environ Sci Technol 46(3):1489–1495
Glusczak L, Loro VL, Pretto A, Moraes BS, Raabe A, Duarte MF, Fonseca MB, Menezes CC, Valladão DMS (2011) Acute exposure to glyphosate herbicide affects oxidative parameters in piava (Leporinus obtusidens). Arch Environ Contam Toxicol 61:624–630
Habig WH, Pabst MJ, Jacoby WB (1974) Glutathione S-transferase, the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hamilton SJ (2004) Review of selenium toxicity in the aquatic food chain. Sci Total Environ 326:1–21
Mabuchi K, Miya M, Senou H, Suzuki T, Nishida M (2006) Complete mitochondrial DNA sequence of the Lake Biwa wild strain of common carp (Cyprinus carpio L.): further evidence for an ancient origin. Aquaculture 257:68–77
Marchesan E, Sartori GMS, Avila LA, Machado SLO, Zanella R, Primel EG, Macedo VRM, Marchezan MG (2010) Resíduos de agrotóxicos na água de rios da Depressão Central do Estado do Rio Grande do Sul, Brasil. Ciênc Rural 40:1053–1059
Menezes CC, Leitemperger J, Santi A, Lópes T, Veiverberg CA, Peixoto S, Adaime MB, Zanella R, Barbosa NBV, Loro VL (2012) The effects of diphenyl diselenide on oxidative stress biomarkers in Cyprinus carpio exposed to herbicide quinclorac (Facet). Ecotoxicol Environ Saf 81:91–97
Menezes C, Leitemperger J, Toni C, Santi A, Lópes T, Barbosa NBV, Neto JR, Loro VL (2013) Comparative study on effects of dietary with diphenyl diselenide on oxidative stress in carp (Cyprinus carpio) and silver catfish (Rhamdia sp.) exposed to herbicide clomazone. Environ Toxicol Pharmacol 36:706–714
Menezes C, Ruiz-Jarabo I, Martos-Sitcha JA, Leitemperger J, Baldisserotto B, Mancera JM, Rosemberg DB, Loro VL (2014a) Diet with diphenyl diselenide mitigates quinclorac toxicity in silver catfish (Rhamdia quelen). PLoS ONE 9(12):e114233
Menezes C, Leitemperger J, Santi A, Dias G, Pedron FA, Neto JR, Salman SM, Barbosa NBV, Loro VL (2014b) Evaluation of the effects induced by dietary diphenyl diselenide on common carp Cyprinus capio. Fish Physiol Biochem 40:141–149
Menezes C, Leitemperger J, Murussi C, Toni C, Araújo MCS, Farias IL, Perazzo GX, Barbosa NV, Loro VL (2014c) Herbicide clomazone effects on δ-aminolevulinic acid activity and metabolic parameters in Cyprinus carpio. Bull Environ Contam Toxicol 92:393–398
Menezes C, Marins A, Murussi C, Pretto A, Leitemperger J, Loro VL (2016) Effects of diphenyl diselenide on growth, oxidative damage, and antioxidant response in silver catfish. Sci Total Environ 542:231–237
Monteiro DA, Rantin FT, Kalinin AL (2009) The effects of selenium on oxidative stress biomarkers in the freshwater characid fish matrinxã, Brycon cephalus (Gunther, 1869) exposed to organophosphate insecticide Folisuper 600 BR® (methyl parathion). Comp Biochem Physiol C 149:40–49
Moraes BS, Loro VL, Pretto A, Fonseca MB, Menezes C, Marchezan E, Reimche GB, Avila LA (2009) Toxicological and metabolic parameters of the teleost fish (Leporinus obtusidens) in response to commercial herbicides containing clomazone and propanil. Pestic Biochem Physiol 95:57–62
Murussi CR, Costa M, Menezes C, Leitemperger J, Guerra L, Lópes T, Severo E, Zanella R, Loro VL (2015) Integrated assessment of biomarker response in carp (Cyprinus carpio) and silver catfish (Rhamdia quelen) exposed to clomazone. Arch Environ Contam Toxicol 68:646–654
Nogueira CW, Rocha JBT (2010) Diphenyl diselenide a Janus-Faced molecule. J Braz Chem Soc 21:2055–2071
Paulmier C (1986) Selenium reagents and intermediates in organic synthesis. Pergamon Books, Oxford, pp 25–51
Ribeiro MCP, Ávila DS, Schiar VPP, dos Santos DB, Meinerz DF, Duarte MMF, Monteiro R, Puntel R, de Bem AF, Hassan W, Barbosa NBV, Rocha JBT (2013) Diphenyl diselenide supplementation reduces biochemical alterations associated with oxidative stress in rats fed with fructose and hydrochlorothiazide. Chem Biol Interact 204:191–199
Roe JH (1954) Chemical determination of ascorbic, dehydroascorbic and diketogulonic acids. In: Glick D (ed) Methods of biochemical analysis. Interscience Publishers, New York, pp 115–139
Roex EWM, Keijzers R, van Gestel CAM (2003) Acetylcholinesterase inhibition and increased food consumption rate in the zebrafish, Danio rerio, after chronic exposure to parathion. Aquat Toxicol 64:451–460
Sassa S (1982) δ-Aminolevulic acid dehydratase assay. Enzyme 28:133–145
Schlenk D, Huggett DB, Allgood J, Bennett E, Rimoldi J, Beeler AB, Block D, Holder AW, Hovinga R, Bedient P (2001) Toxicity of fipronil and its degradation products to Procambarus sp.: field and laboratory studies. Arch Environ Contam Toxicol 41(3):325–332
Stara A, Kristan J, Zuskova E, Velisek J (2013) Effect of chronic exposure to prometryne on oxidative stress and antioxidant response in common carp (Cyprinus carpio L.). Pestic Biochem Physiol 105:18–23
Stehr CM, Linbo TL, Incardona JP, Scholz NL (2006) The developmental neurotoxicity of fipronil: notochord degeneration and locomotor defects in zebrafish embryos and larvae. Toxicol Sci 92(1):270–278
Vandeputte M (2003) Selective breeding of quantitative traits in the common carp (Cyprinus carpio): a review. Aquat Living Resour 16:399–407
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Menezes, C., Leitemperger, J., Murussi, C. et al. Effect of diphenyl diselenide diet supplementation on oxidative stress biomarkers in two species of freshwater fish exposed to the insecticide fipronil. Fish Physiol Biochem 42, 1357–1368 (2016). https://doi.org/10.1007/s10695-016-0223-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-016-0223-5