Abstract
Clomazone is considered a potential contaminant of groundwater and is persistent in the environment. To verify the effects of clomazone in Cyprinus carpio and Rhamdia quelen, a method that combines biomarker responses into an index of “integrated biomarker response” (IBR) was used for observed biological alterations in these species. Thiobarbituric acid-reactive substances in liver of carp and silver catfish decreased at both concentrations tested. However, in muscle it increased in carp at 3 mg/L and silver catfish at 6 mg/L. Protein carbonyl increased in liver (3 and 6 mg/L) and muscle (6 mg/L) of carp. In carp, superoxide dismutase (SOD) increased at 3 mg/L and catalase at 6 mg/L. In silver catfish, SOD in liver decreased at 3 mg/L. Glutathione-S-transferase increased at 3 mg/L in muscle of carp. Nonprotein thiol levels decreased at both concentrations in liver of silver catfish and muscle of carp. In silver catfish, acetylcholinesterase (AChE) decreased in brain at 6 mg/L. Nevertheless, AChE in muscle of both species increased at 3 and 6 mg/L. IBR was standardized scores of biomarker responses and was visualized using star plots. The IBR values shown that in carp there was predominantly an induction of parameters, whereas in silver catfish there was inhibition of these responses. In this way, IBR may be a practical tool for the identification of biological alterations in fish exposed to pesticides. In the present study, IBR was efficient for comparisons of fish species using clomazone. This study may serve as a base for evaluation of other pesticides in the rice field, environment, or laboratory experiment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
In modern agriculture, a wide variety of pesticides are used to increase productivity. In 2013 in the south of Brazil, there were approximately one million hectares of irrigated rice fields. For control of pests during the cultivation period, several herbicides, insecticides, and fungicides are applied to these rice fields (IRGA 2013). The most used herbicide is clomazone, which is annually applied in >400,000 hectares of rice field. This intense use is due to fact that clomazone is efficient both before and after emergence alone or combined with other classes of pesticides (EMBRAPA 2011; Andres et al. 2013).
Clomazone belongs to the isoxazolidinone chemical group and acts as an inhibitor of carotenoid synthesis, has high solubility in water (1100 mg/L), is volatile (P v = 1.44 × 10−4 mm Hg), is resistant to hydrolysis under a wide range of pH values, and is weakly sorptive to soil (k D = 0.47–5.30) (Andres et al. 2013; Cao et al. 2013). The physicochemical characteristics indicate that clomazone is persistent in the environment with a half-life of 7.2 days in water and 4–14 weeks in soil. Clomazone presents properties that indicate potential for groundwater contamination and has been detected in monitoring studies of water sampled in the environment (Zanella et al. 2000, 2002; Sauco et al. 2010; Tomco et al. 2010). Clomazone accumulated in muscle of fish, such as Perca fluviatis and Cyprinus carpio, after the fish were fed with foods containing 13 pesticides including clomazone (Lazartigues et al. 2013). Clomazone has been the subject of laboratory studies in species such as Rhamdia quelen, C. carpio and Prochilodus lineatus showing alterations in oxidative and antioxidant status of tissues as well as metabolic and histological parameters in liver (Crestani et al. 2007; Menezes et al. 2011; Pereira et al. 2013).
In general, the herbicides induce oxidative stress in fish due to the formation of reactive oxygen species (ROS) and cause alterations in the antioxidant systems. These alterations are caused by an imbalance between ROS and the antioxidant activity or damage to biological systems (Üner et al. 2006; Glusczak et al. 2011). The oxidative damage is measured in the form of thiobarbituric acid-reactive substances (TBARS) and protein carbonyl in fish (Almroth et al. 2005; Parvez and Raisuddin 2006; Cattaneo et al. 2012). The antioxidant enzymes are important due to their detoxification capacity of toxic agents and their metabolites. The fish have an enzymatic antioxidant system composed of superoxide dismutase (SOD) and catalase (CAT, as well as nonenzymatic mainly represented by nonprotein thiols (NPSH) (Oropesa et al. 2009; Pereira et al. 2013). Another important enzyme is glutathione-S-transferase (GST), which catalyzes the conjugation of glutathione-reduced (GSH) with a variety of intracellular compounds that are less permeable to the membranes and easily eliminated by increasing their hydrophilicity (van der Oost et al. 2003; Modesto and Martinez 2010). Acetylcholinesterase (AChE) is used for monitoring carbamate and organophosphate effects in vertebrates including fish (Filho et al. 2004). Furthermore, other pesticide classes show alterations in AChE activity, for example, isoxalidinone clomazone (Miron et al. 2005, 2008; Cattaneo et al. 2012).
Although biomarkers can provide valuable information and can be used to measure a wide range of physiological responses to chemicals, their use is limited if they cannot be integrated into a general scheme that facilitates data analysis and interpretation. The biomarkers evaluated reflect a hierarchy of biological organization and were used to develop the index of “integrated biomarker response” (IBR) described by Beliaeff and Burgeot (2002) for the interpretation of biomarker responses. The IBR reflects the contamination levels measured at different concentrations regardless of the variability in the biomarker sets used for the index calculations (Serafim et al. 2012). Studies using an IBR for simplifying the interpretation of biomarker responses have not been verified previously in silver catfish and carp under laboratory conditions. In this context, the IBR constitutes a practical and robust tool to assess the susceptibility of clomazone. The carp (C. carpio) and silver catfish (R. quelen) were used in this study because they are extensively used for monitoring freshwater contamination. Both species are commercialized in southern Brazil due to the rapid growth of the carp and high quality of the fillet of silver catfish. The species are found in rivers near to rice fields or create in consortium rice-fish (Bongers et al. 1998; Mabuchi et al. 2006; Pretto et al. 2011).
Thus, this study aimed to analyze several biomarkers related to the parameters of oxidation and defense if C. carpio and R. quelen, and to use a method that combines these biomarkers, for compare of way integrated between the concentrations tested, biological alterations in this species.
Materials and Methods
Chemicals
Commercial formulation of the herbicide clomazone (2-(2-chlorophenyl)methyl-4,4-dimethyl-3-isoxazolidinone), which has a trade name in the Brazilian market—Gamit (FMC Corp., Philadelphia, Pennsylvania, USA)—was used in this experiment. Acetylthiocholine (ASCh), 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB), 1-chloro-2,4 dinhitrobenzene (CDNB), bovine serum albumin (BSA), Triton X-100, hydrogen peroxide (H2O2), malondialdehyde (MDA), 2-thiobarbituric acid (TBA), and sodium dodecyl sulfate (SDS) were obtained from Sigma (St. Louis, Missouri, USA).
Animals and Water Parameters
Silver catfish (R. quelen) and carp (C. carpio) were obtained (males and females) from the fish farm of the Federal University of Santa Maria n RS, Brazil. Silver catfish weighed, on average, 27.8 ± 1.0 g and measured 12.4 ± 1.0 cm in length. Carp weighed, on average, 18.5 ± 0.7 g and were 10.4 ± 1.0 cm in length. The fish were acclimated in boxes (250 L) for 10 days under laboratory conditions. They were kept in continuously aerated dechlorinated tap water in a static system and with a natural photoperiod (12 h of light to 12 h of dark). Water parameters were measured every day: Temperature was 24.0 ± 2.0 °C, pH was 7.0 ± 0.5, dissolved oxygen was 8.5 ± 0.4 mg/L, nonionized ammonia was 0.80 ± 0.02 µg/L, nitrite was 0.08 ± 0.01 mg/L, and alkalinity was 18.0 ± 2.0. During acclimation, the fish were fed once a day with commercial fish pellets (42 % crude protein; Supra, Brazil). Feces and pellet residues were removed by suction. Filter systems were used to maintain water quality. After the acclimation period, the fish were allocated to 45-L fiberglass boxes and divided into groups.
Experimental Design
The fish (R. quelen and C. carpio) were distributed into a control group (no clomazone) and two exposure groups (3 and 6 mg/L clomazone). Each experiment was performed using 8 fish/treatment in duplicate, and thus the final number was n = 16 fish per group. The time of exposure of 8 days was based on the half-life of clomazone in water, i.e., 7.2 days (Tomco et al. 2010). The concentrations of herbicide were chosen based on previous studies involving freshwater fish species and considering medium values for clomazone exposure at sublethal concentrations. Miron et al. (2005) exposed R. quelen to 5, 10, and 20 mg/L of clomazone for 96 h. Pereira et al. (2013) exposed P. lineatus to 1, 5, and 10 mg/L of clomazone for 96 h. Miron et al. (2008) exposed Leporinus obtusidens to 0.5 mg/L of clomazone for 8 days, and Cattaneo et al. (2012) exposed C. carpio to 0.5 mg/L of clomazone in a rice field.
To obtain the experimental concentrations (3 and 6 mg/L of clomazone), the herbicide was diluted in distilled water. The stock solution (800 mg/L of clomazone) was added with a micropipette at only the beginning of experiment at the boxes, and there was no herbicide replacement during the study. The concentration of the herbicide in the water of the boxes was monitored during the experiment (days 1 and 8) by high performance liquid chromatography/ultraviolet (HPLC/UV) using the method described by Zanella et al. (2002). After the period of exposure, fish were sampled, and organs (liver, brain, and muscle) were collected and frozen at −80 °C until analysis. All protocols used in this study were approved by the Committee on Ethics and Animal Welfare of the Federal University of Santa Maria, protocol number 42-2010.
Lipid Peroxidation Estimation Assay
Lipid peroxidation was estimated by a TBARS assay according to Buege and Aust (1978), performed by an MDA reaction with TBA, which was optically measured. Samples of liver (1:20) were homogenized in a Potter–Elvejhem glass/Teflon homogenizer with 20 mM of potassium phosphate buffer (pH 7.5), and muscle (1:8) was homogenized with 150 mM of NaCl after the samples were centrifuged at 10,000×g for 10 min at 4 °C. To homogenates of liver (250 µL) and muscle (400 µL) was added trichloroacetic acid 10 % (TCA) (250–100 µL, respectively) and 0.67 % of TBA (500 µL) to adjust to a final volume of 1.0 mL. The reaction mixture was placed in a microcentrifuge tube and incubated for 30 min at 95 °C. After cooling, it was centrifuged at 5000×g for 15 min, and after samples and standards curve of MDA (0.03 mM) (TBA) were read at 532 nm against the blank of the standard curve. TBARS levels were expressed as nmol MDA/mg protein.
Enzyme Assays
For SOD, CAT, and GST, the organs were homogenized in a Potter–Elvejhem glass/Teflon homogenizer with 20 mM of potassium phosphate buffer (pH 7.5) (1:20 dilution) and centrifuged at 10,000×g for 10 min at 4 °C. SOD activity was determined in liver and was based on inhibition of the radical superoxide reaction with adrenalin as described by Misra and Fridovich (1972). In this method, SOD present in the sample competes with the detection system for radical superoxide. A unit of SOD was defined as the amount of enzyme that inhibits by 50 % the speed of oxidation of adrenalin. The oxidation of adrenalin leads to the formation of the colored product, adrenochrome, which as detected by spectrophotometer. SOD activity is determined by measuring the speed of adrenochrome formation, as observed at 480 nm, in a reaction medium containing glycine–NaOH [50 mM (pH 10)] and adrenalin (1 mM). The activity was expressed in IU SOD/mg protein. CAT activity in liver was assayed by UV spectrophotometry (Nelson and Kiesow 1972). The assay mixture consisted of 2.0 mL of potassium phosphate buffer [50 mM (pH 7.0)], 0.05 mL of H2O2 (0.3 M), and 0.05 mL of homogenate. Change of H2O2 absorbance in 60 s was measured by spectrophotometry at 240 nm. CAT activity was calculated and expressed in µmol/min/mg protein. GST activity was measured in liver and muscle according to Habig et al. (1974) using 1-chloro-2,4-dinitrobenzene (CDNB) as substrate. The formation of DNPH was monitored by the increase in absorbance at 340 nm against blank. The molar extinction coefficient used for CDNB was 9.6 mM/cm. The activity was expressed as μmol GS-DNB/min/mg protein.
AChE activity was measured using the method described by Ellman et al. (1961). Brain and muscle (50 mg) were weighted and homogenized (1:10) in a Potter-Elvejhem glass/Teflon homogenizer with sodium phosphate buffer 50 mM (pH 7.2) and Triton X-100 1 %. The homogenate was then centrifuged for 10 min at 3000×g at 5 °C, and the supernatant was used as enzyme source. Aliquots of supernatant (50 µL of brain and 100 µL of muscle) were incubated at 30 °C for 2 min with a solution containing 0.1 M of sodium phosphate buffer (pH 7.5) and 1 mM of DTNB. After the incubation period, the reaction was initiated by the addition of ASCh (1 mM). The final volume was 2.0 mL. Absorbance was measured by spectrophotometry at 412 nm for 2 min. Enzyme activity was expressed as µmol ASCh hydrolyzed min/mg protein.
Nonprotein Thiols
NPSH levels were determined in liver and muscle by the method of Ellman (1959). The liver and muscle (150 mg) were homogenized (1:10) with 1.5 mL of Tris–HCl 50 mM (pH 7.5) followed by centrifugation at 3000×g for 10 min. An aliquot of supernatants (1.0 mL) was mixed (1:1) with 10 % trichloroacetic acid and then centrifuged. To determine NPSH of organs, an aliquot (400 mL) of supernatant was added in a phosphate buffer 0.5 mM (pH 6.8), 10 mM DTNB, and cysteine 0.5 mM. The color reaction was measured at 412 nm. NPSH levels were expressed as µmol NPSH/g of tissue.
Protein Carbonyl Assay
The liver and muscle samples were homogenized in 10 volumes (w/v) of 10 mM Tris–HCl buffer (pH 7.4) using a glass homogenizer. Protein carbonyl measurement was assayed by the method described by Yan et al. (1995). Soluble protein (1.0 mL) was reacted with 10 mM of DNPH in 2 N hydrochloric acid (HCl). After incubation at room temperature for 1 h in the dark, 0.5 mL of denaturing buffer (150 mM of sodium phosphate buffer (pH 6.8) containing SDS 3.0 %), 2.0 mL of heptane (99.5 %), and 2.0 mL of ethanol (99.8 %) were added sequentially, vortexed for 40 s, and centrifuged for 15 min. Then the protein isolated from the interface was washed twice by resuspension in ethanol and ethyl acetate (1:1), and suspended in 1.0 mL of denaturing buffer and the carbonyl content was measured spectrophotometrically at 370 nm. Assay was performed in duplicate, and two tubes blank incubated with 2 N HCl without DNPH were included for each sample. Total carbonylation was calculated using a molar extinction coefficient of 22.000 M/cm and expressed in nmol carbonyl/mg protein.
Protein Determination
The protein levels were estimated spectrophotometrically using the method described by Bradford (1976), and BSA was used as the standard.
IBR
The results obtained in this study were applied to the IBR index to characterize the effects of different concentrations of clomazone. The IBR was calculated based on the log-transformation to decrease variance and is represented as star plots (Beliaeff and Burgeot 2002; Sanchez et al. 2013). For the IBR calculation, the experimental data for each concentration was log-transformed (Yi), and the overall mean (μ) and SD (s) were further calculated. Then Yi values were standardized by the following formula—Z i = (Yi − μ)/s—and the difference between Z i and Z 0 (control) was calculated to determine the A values. The A values represent the result attributed for each biomarker calculated. The IBR was estimated for each concentration by the sum of the A values resulting in S values. Representative results are shown as star plot charts indicating the deviation of all biomarkers in relation to the control (0).
Statistical Analysis
The normal distribution and homogeneity of the data were confirmed by Kolmogorov–Smirnov and Bartlett’s tests, respectively. Statistical analyses were performed using analysis of variance and Tukey’s multiple comparisons test considering p ≤ 0.05 as statistically significant for all analyses. The data were compared with the control group and between the treatments of each species studied. The tests were performed in GraphPad 6.01 (Software, Califórnia, USA).
Results
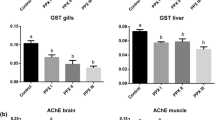
The clomazone concentrations in water were measured at the beginning and the end of the experimental period. The results showed a decrease of 48.5 % at 3 mg/L and 54.0 % at 6 mg/L in both experiments (Table 1). In carp and silver catfish, TBARS levels in liver decreased in both concentrations compared with the control group (Fig. 1a). In carp, TBARS levels in muscle increased at 3 mg/L and in silver catfish increased in 6 mg/L compared with the control group (Fig. 1b).
In carp, SOD activity in liver increased at 3 mg/L, whereas in silver catfish SOD activity decreased at 3 mg/L compared with the control group. In addition, CAT activity in liver of carp increased at 6 mg/L in relation to the control group, whereas silver catfish did not show significant results. In both species, GST activity in liver did not show significant differences. In carp, GST activity in muscle increased at 3 mg/L compared with the control group, whereas in silver catfish GST did not show significant results (Table 2).
In carp, NPSH levels in liver did not show significant results; however, in muscle, NPSH decreased in both concentrations compared with the control group. In silver catfish, NPSH levels in liver decreased at 3 and 6 mg/L compared with the control, whereas in muscle NPSH did not show any significant results (Table 2).
In carp, AChE activity in brain did not show significant results, but in muscle AChE increased in both concentrations compared with the control group. In silver catfish, AChE activity in brain decreased at 6 mg/L and in muscle increased in both concentrations compared with the control group (Table 2).
In carp, protein carbonyl in liver increased in both concentrations, whereas that in muscle increased only at 6 mg/L compared with the control group. In silver catfish, protein carbonyl did not show significant differences in any organ (Fig. 1c, d).
Values of the IBR for carp at 3 and 6 mg/L were 15.85 and 17.74, respectively. The parameters that most influenced this result at 3 mg/L were protein carbonyl, SOD, and GST of liver, AChE in brain, and GST and NPSH in muscle (Fig. 2a). The parameters that most contributed to this result at 6 mg/L were TBARS, CAT, GST, and NPSH of liver and protein carbonyl and AChE in muscle (Fig. 2b).
Values of IBR for silver catfish at 3 and 6 mg/L were 14.06 and 16.93, respectively. The parameters that most contributed to this result at 3 mg/L were TBARS, protein carbonyl, SOD, GST, and NPSH of liver and AChE of muscle (Fig. 3a). In contrast, at 6 mg/L the parameters that most influenced this result were TBARS, protein carbonyl, and NPSH of liver and muscle and AChE of brain and muscle (Fig. 3b).
Discussion
The reduction of clomazone for both experimental units ranged from 48.5 to 54.0 % in both experiments. The reduction observed could be attributed to the physicochemical characteristics of this herbicide as volatilization or by possible variation of pH (Cao et al. 2013). The results observed in the present study are in agreement with previous results obtained by our research group. Investigators have explained that the biotransformation of clomazone by fish metabolism probably results in damage to organs as evidenced by Moraes et al. (2009), Menezes et al. (2011), and Cattaneo et al. (2012). The clomazone reduction observed in water samples during the experimental period could be indicative of accumulation in fish tissues. Complementary studies are needed to better explain this hypothesis.
The results obtained in the present study show, in general, that these fish species show different results according to the organ analyzed. The TBARS evaluation could be used as a biomarker to identify damage in lipids for some tissues. Lipid peroxidation is a common response observed after herbicide exposure. However, for both species, TBARS levels in liver showed a decrease, thus indicating that lipid peroxidation did not occur in this organ probably due to the action of antioxidant enzymes. In liver of carp, the enzymes that possibly protected was SOD at 3 mg/L and CAT at 6 mg/L. In silver catfish, SOD at 3 mg/L may have contributed. These enzymes neutralize radicals, such as H2O2, superoxide anion (O−), and hydroxyl radicals (OH), which, due to reactivity, cause damage to lipids, protein, carbohydrates, and nucleic acids (Modesto and Martinez 2010). Therefore, damage to lipids was probably prevented by the action (increase or decrease of activity) of these enzymes in liver of these fish species.
TBARS levels in muscle increased in carp at 3 mg/L and in silver catfish at 6 mg/L. These results show that lipid peroxidation occurred in muscle due to clomazone exposure in both fish species studied. This result was previously reported by Cattaneo et al. (2012) when carp in rice fields exposed to clomazone (0.5 mg/L) for 7 days showed muscle lipid peroxidation and by Murussi et al. (2014) when silver catfish were exposed to penoxsulam (23 µg/L). The oxidative cell injury probably occurs due to the increase of free radicals mediated by the herbicide poisoning.
The enzymes SOD and CAT have a function in the conversion of ROS to harmless metabolites. It is important to consider that the antioxidant response related with these enzymes are capable of induction or inhibition under conditions of oxidative damage. The response observed for antioxidant enzymes in this study is intimately related to the protection shown by the liver against lipid peroxidation mediated by free radicals. The results of SOD were distinct according to the species analyzed. SOD in silver catfish showed a decrease at the low concentration, whereas SOD in carp increased in this same concentration. The SOD increase observed in carp may be justified by the process of adaptation in an attempt to detoxify the organism from oxygen free radicals and to prevent damage to macromolecules such as lipids. CAT activity is responsible for the detoxification of H2O2 into water as a way of cellular defense against oxidative stress (van der Oost et al. 2003; Mela et al. 2013). The observed absence of response for CAT activity and the SOD inhibition in liver of silver catfish could be explained by the increase in free radical formation due to clomazone exposure.
Increased GST activity in muscle of carp (3 mg/L) probably helped fish to prevent oxidative damage represented by the absence of protein carbonyl formation in this tissue. However, the increase in GST activity was not sufficient to neutralize the increase of TBARS levels in the muscle of carp in 3 mg/L clomazone. GST is especially important to the detoxification process after pesticide exposure. In contrast, GSH is considered the most abundant NPSH with important roles in antioxidant defense and signal transduction. In our study, NPSH was decreased in several organs of both fish species. These results could represent a loss of nonenzyme antioxidants (NPSH), thus decreasing the antioxidant capacity of fish exposed to clomazone. Another explanation is that GSH acts as a cofactor for GST or GPx enzymes, and in the context of the present study, could be an important NPSH contributor facilitating the removal of reactive molecules from the cells. Nevertheless, in silver catfish at 6 mg/L, other pathways—such as glutathione reductase (GR) and/or glutathione peroxidase—may play an important role in herbicide toxicity, but they were not measured in this study. This hypothesis was formed because SOD, CAT, GST, and NPSH did not show results that justify protection observed to liver for this concentration.
According to Almeida et al. (2005), AChE is related to tissue enervation, and in the present study, the brain and muscle activity of this enzyme was evaluated. The brain showed decreased activity only in silver catfish (6 mg/L). The inhibition of AChE activity in brain may result in hyperactivity and tremors and may lead the fish to consume greater quantities of energy due to the accumulation of acetylcholine in the synaptic cleft (Salbego et al. 2010). The induction of AChE in muscle was observed at the two concentrations of clomazone in both fish species analyzed. The activation of AChE activity in muscle after exposure to clomazone is not a common response. Moraes et al. (2007) showed this same effect when exposing L. obtusidens to clomazone (0.5 mg/L) and metsulfuron-methyl (0.002 mg/L). The activation of AChE activity in muscle may be due to the possible accumulation of acetylcholine in the brain, which leads to overstimulation of muscle fibers.
Measurements of protein damage could be made by protein carbonyl, thus quantifying the protein oxidation mediated by free radicals. Protein carbonyl increased in liver and muscle of carp. Parvez and Raisuddin (2005) affirmed that the increase in protein oxidation may lead to the loss of sulfhydryl groups due to the modification of amino acids, thus causing the formation of carbonyl and other oxidized moieties. In this way, the defenses (SOD and CAT) were efficient against lipid peroxidation in liver of carp, but they were not effective in controlling the increase in protein carbonyl in this organ. Carp seem to be more affected by protein oxidation, resulting in the disruption of the protein metabolism and changes in the enzyme activity, whereas changes in protein carbonyl did not occur in silver catfish.
The IBR was useful to differentiate, in a single value, the effects caused by clomazone exposure at each concentration in the fish species studied. In general, values >0 are considered induction and values <0 are considered inhibition for each score parameter. In carp, the response of induction was predominant. In contrast, in silver catfish, the values were <0 indicating inhibition. These results are in agreement with the responses observed for parameters analyzed in fish species.
Other studies with different species, including mussels (Damiens et al. 2007; Raftopoulou and Dimitriadis 2010) and fish (C. carpio; Kim et al. 2010), that used this index emphasized the potential of the IBR; however, some details must be carefully considered. Serafim et al. (2012) related that the IBR is a “dynamic” index and therefore s not able to give fixed values for a determined population or species when exposed to a determined contaminant; one must consider variation inherent in the species as well as other factors (temperature, pH, and nutritional conditions.) Another point, made by Raftopoulou and Dimitriadis (2010), is that the IBR may be used as a simple qualitative method but not for the quantitative evaluation of the degree of stress.
Considering the biomarkers studied as being relevant for each concentration and species, changes in more parameters were common at 6 mg/L. These biomarkers selected with the IBR might serve as a set of evaluating in studies of laboratory or in environmental monitoring. According to Beliaeff and Burgeot (2002), the IBR may be associated with a star or radar chart that shows the specific response at each concentration analyzed.
Conclusion
Clomazone herbicide caused oxidative damage in both fish species studied and in different organs in each species. The responses observed were different according to the fish species and clomazone concentration tested. The IBR value confirms this theory, showing that induction is prevalent in carp, whereas inhibition is more evident in silver catfish. In the present study, the IBR index showed that 6 mg/L of clomazone in both fish species may be causing more stress effects compared with 3 mg/L. This result indicates that fish exposed to high concentrations depend on defense mechanisms to eliminate the herbicide toxicity. In the laboratory, the use of the IBR was valid by the capacity of punctuate the biomarkers more relevant. To select a more complete set of biomarkers to apply in biomonitoring studies, more studies are necessary that involve other organs and that use the IBR as a tool for evaluation under different environmental conditions.
References
Almeida LC, Aguiar LH, Moraes G (2005) Methyl parathion effect in Matrinxã Brycon cephalus muscle and brain acetylcholinesterase activity. Ciência Rural 35:1412–1416
Almroth BC, Sturve J, Berglund A, Forlin L (2005) Oxidative damage in eelpout (Zoarces viviparus) measured as protein carbonyls and TBARS, as biomarkers. Aquat Toxicol 73:171–180
Andres A, Concenço G, Theisen G, Vidotto F, Ferrero A (2013) Selectivity and weed control efficacy of pre and post-emergence applications of clomazone in Southern Brazil. Crop Prot 53:103–108
Beliaeff B, Burgeot T (2002) Integrated biomarker response: a useful tool for ecological risk assessment. Environ Toxicol Chem 21:1316–1322
Bongers ABJ, Sukkel M, Gort G, Komen J, Richter JJ (1998) Development and use of genetically uniforms strain of common carp in experimental animal research. Lab Anim 32:349–363
Bradford MMA (1976) A rapid and sensitive method for the quantification of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem 72:248–254
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–309
Cao J, Diao X, Hu J (2013) Hydrolysis and photolysisc of herbicide clomazone in aqueous solutions and natural water under abiotic conditions. J Integr Agric 12:2074–2082
Cattaneo R, Moraes BS, Loro VL, Pretto A, Menezes C, Sartori GMS et al (2012) Tissue biochemical alterations of Cyprinus carpio exposed to commercial herbicide containing clomazone under rice-field conditions. Arch Environ Contam Toxicol 62:97–106
Crestani M, Menezes C, Glusczak L, Miron DS, Spanevello R, Silveira A et al (2007) Effect of clomazone herbicide on biochemical and histological aspects of silver catfish (Rhamdia quelen) and recovery pattern. Chemosphere 67:2305–2311
Damiens G, Gnassia-Barelli M, Loques F, Romeo M, Salbert V (2007) Integrated biomarker response index as a useful tool for environmental assessment evaluated using transplanted mussels. Chemosphere 66:574–583
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Ellman GL, Courtney KD, Andres V Jr (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
EMBRAPA. 2011. Empresa Brasileira de Pesquisa Agropecuária. Comunicado técnico 167: Dissipação do herbicida clomazone na cultura de arroz irrigado em Rio Brilhante, MS. http://www.infoteapproximatelycnptia.embrapa.br/bitstream/doc/901103/1/COT1672011.pdf. Accessed 21 Oct 2013
Filho MVS, Oliveira MM, Salles JB, Bastos VLFC, Cassano VPF, Bastos JC (2004) Methyl-paraoxon comparative inhibition kinetics from brain of neotropical fishes. Toxicol Lett 153:247–254
Glusczak L, Loro VL, Pretto A, Moraes BS, Raabe A, Duarte MF et al (2011) Acute exposure to glyphosate herbicide affects oxidative parameters in piava (Leporinus obtusidens). Arch Environ Contam Toxicol 61:624–630
Habig WH, Pabst MJ, Jacoby WB (1974) Glutathione S-transferase, the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Instituto Rio Grandense do Arroz (2013) Irga divulga estimativa da safra 2013/2014. http://www.irga.rs.gov.br/conteudo/4253/irga-divulga-estimativa-da-safra-2013/2014/termosbusca=produ%C3%A7%C3%A3o. Accessed 21 Oct 2013
Kim WK, Lee SK, Jung J (2010) Integrated assessment of biomarker responses in common carp (Cyprinus carpio) exposed to perfluorinated organic compounds. J Hazard Mater 180:395–400
Lazartigues A, Thomas M, Banas D, Brun-Bellet J, Cren-Olivé C, Feidt C (2013) Accumulation and half-lives of 13 pesticides in muscle tissue of freshwater fishes through food exposure. Chemosphere 91:530–535
Mabuchi K, Miya M, Senou H, Suzuki T, Nishida M (2006) Complete mitochondrial DNA sequence of the Lake Biwa wild strain of common carp (Cyprinus carpio L.): further evidence for an ancient origin. Aquaculture 257:68–77
Mela M, Guiloski IC, Doria HB, Randi MAF, Ribeiro MAFO, Pereira L et al (2013) Effects of the herbicide atrazine in neotropical catfish (Rhamdia quelen). Ecotoxicol Environ Saf 93:13–21
Menezes CC, Loro VL, Fonseca MB, Cattaneo R, Pretto A, Miron DS et al (2011) Oxidative parameters of Rhamdia quelen in response to commercial herbicide containing clomazone and recovery pattern. Pestic Biochem Physiol 100:145–150
Miron DS, Crestani M, Schetinger MR, Morsch VM, Baldisserotto B, Tierno MA et al (2005) Effects of the herbicide clomazone, quinclorac and metsulfuron methyl on acetylcholinesterase activity in the silver catfish (Rhamdia quelen) (Heptapteridae). Ecotoxicol Environ Saf 61:398–403
Miron DS, Pretto A, Crestani M, Glusczak L, Schetinger MR, Loro VL et al (2008) Biochemical effects of clomazone herbicide on piava (Leporinus obtusidens). Chemosphere 74:1–5
Misra HP, Fridovich I (1972) The role of superoxide anion in the auto-oxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Modesto KA, Martinez CBR (2010) Effects of Roundup Transorb on fish: hematology, antioxidant defenses and acetylcholinesterase activity. Chemosphere 81:781–787
Moraes BS, Loro VL, Glusczak L, Pretto A, Menezes C, Marchezan E et al (2007) Effects of four rice herbicides on some metabolic and toxicology parameters of teleost fish (Leporinus obtusidens). Chemosphere 68:1597–1601
Moraes BS, Loro VL, Pretto A, Fonseca MB, Menezes C, Marchesan E et al (2009) Toxicological and metabolic parameters of the teleost fish (Leporinus obtusidens) in response to commercial herbicides containing clomazone and propanil. Pestic Biochem Physiol 95:57–62
Murussi CR, Thorstenberg ML, Leitemperger J, Costa M, Clasen B, Santi A et al (2014) Toxic effects of penoxsulam herbicide in two fish species reared in southern Brazil. Bull Environ Contam Toxicol 92:81–84
Nelson DP, Kiesow LA (1972) Enthalpy of decomposition of hydrogen peroxide by catalase at 25 °C (with molar extinction coefficients of H2O2 solution in the UV). Anal Biochem 49:474–478
Oropesa AL, García-Cambero JP, Soler F (2009) Glutathione and malondialdehyde levels in common carp after exposure to simazine. Environ Toxicol Pharmacol 27:30–38
Parvez S, Raisuddin S (2005) Protein carbonyl: novel biomarkers of exposure to oxidative stress-inducing pesticides in freshwater fish Channa punctate (Bloch). Environ Toxicol Pharmacol 20:112–117
Parvez S, Raisuddin S (2006) Effects of paraquat on the freshwater fish Channa punctata (Bloch): nonenzymatic antioxidants as biomarkers of exposure. Arch Environ Contam Toxicol 50:392–397
Pereira L, Fernandes MN, Martinez CBR (2013) Hematological and biochemical alterations in the fish Prochilodus lineatus caused by the herbicide clomazone. Environ Toxicol Pharmacol 36:1–8
Pretto A, Loro VL, Menezes CC, Moraes BS, Reimche GB, Zanella R et al (2011) Commercial formulation containing quinclorac and metsulfuron-methyl herbicides inhibit acetylcholinesterase and induce biochemical alterations in tissues of Leporinus obtusidens. Ecotoxicol Environ Saf 74:336–341
Raftopoulou EK, Dimitriadis VK (2010) Assessment of the health status of mussels Mytilus galloprovincialis along Thermaikos Gulf (Northern Greece): an integrative biomarker approach using ecosystem health indices. Ecotoxicol Environ Saf 73:1580–1587
Salbego J, Pretto A, Gioda CR, Menezes CC, Lazzari R, Neto JR et al (2010) Herbicide formulation with glyphosate affects growth acetylcholinesterase activity, and metabolic and hematological parameters in piava (Leporinus obtusidens). Arch Environ Contam Toxicol 58:740–745
Sanchez W, Burgeot T, Porcher JM (2013) A novel “Integrated biomarker response” calculation based on reference deviation concept. Environ Sci Pollut Res 20:2721–2725
Sauco S, Eguren G, Heinzen H, Defeo O (2010) Effects of herbicides and freshwater discharge on water chemistry, toxicity and benthos in a Uruguayan sandy beach. Mar Environ Res 70:300–307
Serafim A, Company R, Lopes B, Fonseca VF, França S, Vasconcelos RP et al (2012) Application of an integrated biomarker response index (IBR) to assess temporal variation of environmental quality in two Portuguese aquatic systems. Ecol Indic 19:215–225
Tomco PL, Holstege DM, Zou W, Tjeederma RS (2010) Microbial degradation of clomazone under simulated California rice field conditions. J Agric Food Chem 58:3674–3680
Üner N, Oruç EO, Sevgiler Y, Sahin N, Durmaz H, Usta D (2006) Effects of diazinon on acetylcholinesterase activity and lipid peroxidation in the brain of Oreochromis niloticus. Environ Toxicol Pharmacol 21:241–245
van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149
Yan LJ, Traber MG, Packer L (1995) Spectrophotometric method for determination of carbonyls in oxidatively modified apolipoprotein B of human low-density lipoproteins. Anal Biochem 228:349–351
Zanella R, Primel EG, Gonçalves GG, Martins AF (2000) Development and validation of a high-chromatographic method for the determination of clomazone residues in surface water. J Chromatogr A 904:257–262
Zanella R, Primel EG, Machado SLO, Gonçalves FF, Marchezan E (2002) Monitoring of the herbicide clomazone in environmental water samples by solid-phase extraction and high-performance liquid chromatography with ultraviolet detection. Chromatographia 55:573–577
Acknowledgments
We thank the Federal University of Santa Maria for the support and facilities and the financial support and fellowships from the Brazilian agency Coordination for the Improvement of Higher Education Personnel.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murussi, C.R., Costa, M., Menezes, C. et al. Integrated Assessment of Biomarker Response in Carp (Cyprinus carpio) and Silver Catfish (Rhamdia quelen) Exposed to Clomazone. Arch Environ Contam Toxicol 68, 646–654 (2015). https://doi.org/10.1007/s00244-015-0145-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-015-0145-8