Abstract
Ni and Cd are widely used together in the manufacture of cells and batteries. The incorrect disposal of these products can result in environmental contamination, posing risks to the organisms exposed to these contaminants. However, the effects of the mixture of Ni and Cd in freshwater fishes are still unclear in the current literature, especially in relation to biomarkers of oxidative stress. Thus, the objective of the current work was to compare the sublethal effects caused by the mixture of cadmium (Cd) and nickel (Ni) with the effects of these metals individually, in the fish Astyanax altiparanae. The animals were exposed for 72 h to 20 μg L-1 of Cd, 1.5 mg L-1 of Ni, or a mixture of these two metals at the concentrations mentioned. After exposure, tissue samples were collected to evaluate hematological and plasma parameters, biomarkers of oxidative stress in the gills, and acetylcholinesterase (AChE) activity in brain and muscle. Exposure to the mixture caused alterations that were not observed in the groups exposed to the metals individually, such as increased activity of catalase and glutathione-S-transferase, and a reduction in AChE activity in the brain. Thus, we concluded that exposure to the mixture of Cd and Ni is more harmful to A. altiparanae than exposure to these metals separately.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Several human activities, such as industrial and agricultural production, urbanization, and tourism, contribute to environmental contamination (Amiard-Triquet et al., 2015). Cadmium (Cd) and nickel (Ni) are among the main contaminants of aquatic ecosystems and are already known to have harmful effects in aquatic organisms (Palermo et al., 2015; Pereira et al., 2016). Although Ni is an essential metal for plants and terrestrial animals, studies with fish have not yet proven its essentiality (Wood, 2012). Exposure to this metal can cause several alterations in fish, such as oxidative stress, genotoxicity (Palermo et al., 2015), morphological damage to the gills, and neurotoxicity (Topal et al., 2015, 2017). Cadmium is a non-essential metal and can cause toxicity in concentrations as low as 1 μg L-1 (Pereira et al., 2016; Silva & Martinez, 2014). The main effects of this metal in fish include oxidative stress, genotoxicity (Pereira et al., 2016), osmoregulatory disorders (Silva & Martinez, 2014), and neurotoxicity (Naïja et al., 2017).
However, contaminants in the natural environment are not limited to one type in isolation, but instead, a combination of contaminants that can result in different consequences for the health of the exposed organisms (Nikinmaa, 2014; Niyogi et al., 2015). A metal mixture can cause different effects from those observed after exposure to the metals separately, causing greater toxicity (Abdalla et al., 2019; Oliveira et al., 2018). In addition, the sum of the individual effects is often not enough to infer the effect of the mixtures, and exposure tests are required to evaluate possible interactions between contaminants (Pillet et al., 2019).
Ni and Cd are widely used together in the manufacture of cells and batteries, and the incorrect disposal of these products can result in simultaneous contamination of the environment by these two metals (Carolin et al., 2017; Conte, 2016). Previous works have already demonstrated that Cd and Ni can be found together in aquatic ecosystems across the globe (Dourado et al., 2016; Rahman et al., 2022; Tegu et al., 2023; Wang et al., 2023). In Brazilian surface waters, cadmium can be found in concentrations ranging from 6 to 46.8 μg L-1 (Dourado et al., 2016; Viana et al., 2018). For nickel, the values range from 77 to 870 μg L-1 (Dourado et al., 2016; Melo et al., 2017; Viana et al., 2018).
According to the available literature, the effects of the mixture of Ni and Cd are still unclear. In zebrafish (Danio rerio), Ni reduced Cd uptake in the gills, but increased Cd uptake in the whole body (Komjarova & Blust, 2009). After a 3-h exposure, no interaction was observed between Cd and Ni at the binding sites in the gills of Oncorhynchus mykiss (Niyogi et al., 2015). In another study, Ni attenuated olfactory dysfunctions caused by Cd in O. mykiss after 96 h of exposure (Dew et al., 2016). Bezerra et al. (2022) evaluated the effects of a Ni and Cd mixture on plasma, histological, and biochemical biomarkers in the fish Prochilodus lineatus. In this species, all effects caused by exposure to the metal mixture were observed in the exposures to single metals, with the exception of hyperglycemia. However, it should be noted that none of these works evaluated biomarkers of oxidative stress, which are normally very sensitive to exposure to Cd and Ni, as already mentioned.
Oxidative stress occurs when there is an imbalance between the production and elimination of reactive oxygen species (ROS). Aerobic organisms have several defense mechanisms to avoid oxidative stress, since it can cause damage to macromolecules such as lipids, proteins, and DNA. Among these mechanisms we can mention enzymatic antioxidants, such as catalase (CAT), superoxide dismutase (SOD), and glutathione-S-transferase (GST), in addition to non-enzymatic antioxidants, such as glutathione (GSH) (Lushchak, 2016; Nikinmaa, 2014).
In addition to oxidative stress biomarkers, hematological parameters are important tools to assess the general health status of fish. These parameters may indicate physiological alterations resulting from exposure to various contaminants, such as metals (Shahjahan et al., 2022). Previous studies have shown that exposure to metals can cause reductions in hematocrit and hemoglobin concentration (Kim et al., 2019; Lee et al., 2022).
Acetylcholinesterase (AChE) activity is a neurotoxicity biomarker commonly used in ecotoxicology. Decreased activity of this enzyme is usually associated with exposure to pesticides (Araújo et al., 2018). However, many studies have shown that exposure to metals can also interfere with AChE activity. Different fish species exposed to Cu (Simonato et al., 2016), Cd (Naik et al., 2020), Ni (Topal et al., 2015), and Pb (Vicari et al., 2018) presented changes in AChE activity, which demonstrates the relevance of this biomarker for the evaluation of neurotoxic effects after exposure to metals.
Changes in biomarkers of oxidative damage, hematological parameters, and neurotoxicity biomarkers are warning signs for future effects at higher levels of biological organization (Rimoldi, 2021). Oxidative damage, for example, can cause loss of cell function and impair the health status of animals in the long term (Monserrat, 2021). The analysis of these parameters may be useful for future updates on the maximum limits for metals in aquatic ecosystems, since the current regulations were established mainly based on studies with exposure to single metals (Brasil, 2005).
The fish species chosen as the experimental model for this work is Astyanax altiparanae. This species is native to the Neotropical region, belonging to the order Characiformes, and is found in South America (Lima et al., 2003). The genus Astyanax is the most diverse of the Characidae family in the Neotropical region, with a hundred species abundantly distributed in Brazilian hydrographic basins (Lima et al., 2003; Ornelas-García et al., 2008; Prado et al., 2011). In addition, this genus has been shown to be very sensitive to various metals, such as aluminum, manganese, copper, and zinc (Abdalla et al., 2019; Santos et al., 2012; Tincani et al., 2019), and can be easily maintained under laboratory conditions, due to its small size. Therefore, the objective of the current work is to compare the hematological, plasma, and biochemical effects caused by cadmium and nickel, individually and in a mixture, in the freshwater fish A. altiparanae.
2 Material and Methods
Juveniles of A. altiparanae (n=56, 4.95 ± 0.42 g, 7.16 ± 0.25 cm, mean ± SD) were collected from the Fish Farming Station of the State University of Londrina (Londrina, Paraná, Brazil). All procedures involving these animals were approved by the Ethics Committee on Animal Experimentation of the State University of Londrina (protocol number: 13642.2019.46). The fish were acclimated in 300 L tanks containing dechlorinated water under constant aeration, and fed with commercial feed (Guabi®, Brazil) every 48 h. After acclimation, a 72-h exposure test was performed. Four 100 L glass aquariums were used, with dechlorinated water under constant aeration. Each aquarium contained 14 fish: one aquarium represented the control group (CTR), with only dechlorinated water; one contained 20 μg L-1 of cadmium (Cd group); one 1.5 mg L-1 of nickel (Ni group); and one aquarium contained a mixture of these two metals in the aforementioned concentrations (Mix group). The concentrations of Ni and Cd chosen are close to concentrations that caused sublethal effects in previous studies with a Neotropical fish (Palermo et al., 2015; Silva & Martinez, 2014). To reach the nominal concentrations of Ni and Cd in the exposure media, solutions with nickel chloride (NiCl2.6H2O) or cadmium chloride (CdCl2.H2O) dissolved in ultrapure water were used. During the exposure test, two water samples were collected from each aquarium every 24 h. One sample was only acidified (1% HNO3), to determine the total metal concentration, and the second sample was filtered (0.45 μm) and acidified, to evaluate the dissolved metal concentration. During acclimation and the exposure test, the water parameters remained adequate (temperature: 23.1 ± 0.3°C, pH: 8.6 ± 0.2, dissolved oxygen: 7.7 ± 1.3 mg L-1, mean ± SD).
After the exposure, the animals were removed from the aquariums and immediately anesthetized with benzocaine (0.1 g L-1), for blood collection via the caudal vein. The fish were then euthanized by medullar sectioning for the collection of gills, brain, and muscle samples, which were stored at -70°C until biochemical analysis. For all the tissues collected, samples were pooled (two animals per pool) for the evaluation of the biomarkers.
Blood samples were used to evaluate the number of erythrocytes (RBC), the mean corpuscular volume (MCV), the hematocrit (Hct), and the concentration of hemoglobin (Hb). The remainder of the blood was centrifuged to obtain the plasma, which was stored at -20°C. To assess the RBC, 5 μL of blood were diluted in 1 mL of formaldehyde citrate buffer (130 mM sodium citrate in 0.4% formaldehyde). This mixture was placed in a Neubauer chamber for cell counting under an optical microscope. Hct was determined according to the microhematocrit technique by centrifugation (1200 g, 7 min), for cell and plasma separation, followed by analysis on a standardized card. Hb concentration was determined in a spectrophotometer at 540 nm, using a commercial kit (Labtest Diagnóstica, Brazil). The MCV was calculated using the following formula:
Plasma glucose concentration was determined using a commercial kit (Doles, Brazil) in a spectrophotometer at 505 nm. Part of the gill samples were diluted (1:5, m/v), homogenized in potassium phosphate buffer (0.1 M, pH 7.0), and centrifuged (20 min, 16000 g, 4°C). The supernatant was used to evaluate the concentration of GSH, lipid peroxidation (LPO), and the activity of CAT and GST. GSH concentration was determined by the quantification of thiolate, in a spectrophotometer at 412 nm, as described by Beutler et al. (1963). LPO was evaluated through the TBARS assay in a fluorimeter (ex/em: 535/590 nm), using a malondialdehyde standard curve (Camejo et al., 1998). CAT activity was determined by the decomposition rate of H2O2 in a spectrophotometer at 240 nm (Beutler, 1975). The GST activity was evaluated in a spectrophotometer at 340 nm, by the complexation reaction of 1-chloro-2,4-dinitrobenzene (CDNB) with reduced glutathione, according to the method described by Gagné (2014). The remaining gill samples were homogenized (1:2, m/v) in buffer (0.5 M sucrose, 26 mM TRIS, 0.5 mM phenylmethylsulfonyl fluoride, 1.3 mM β-mercaptoethanol) and centrifuged (45 min, 18000 g, 4 °C). The supernatant was resuspended in ethanol/chloroform and the sulfhydryl groups (–SH) were quantified in a spectrophotometer at 412 nm (Viarengo et al., 1997).
Brain and muscle samples were diluted (1:10, m/v), homogenized in potassium phosphate buffer (0.1 M, pH 7.5), and centrifuged (20 min, 16000 g, 4°C). The supernatant was used to determine AChE activity according to Ellman et al. (1961), with adaptations by Costa et al. (2007). The concentration of total proteins was evaluated in gills, brain, and muscle samples by the method of Bradford (1976), for standardization of the expression of the results.
For the statistical analysis, the results of each parameter were evaluated for normality (Shapiro-Wilk test) and homogeneity of variance (Levene test). When the data showed normal distribution and homogeneity of variance, parametric tests were used (one-way ANOVA and Student-Newman-Keuls). Otherwise, the data were analyzed using non-parametric tests (Kruskal-Wallis and Dunn). In all tests, a significance level of 5% was adopted.
3 Results and discussion
The objective of this work was to compare the effects of Ni and Cd, alone and in a mixture, in A. altiparanae fish. When in a mixture, the interaction between two compounds that exert an adverse effect can be described as additive, antagonistic, or synergistic. An additive interaction refers to the sum of the effects of the two compounds. In an antagonistic (or less-than-additive) interaction, the presence of one compound reduces the toxicity of the other compound. We can say that there is synergism when the effect caused by the mixture is greater than the sum of the effects of the isolated compounds (also called more-than-additive). In addition, we can also mention the concept of potentiation. If a substance causes toxic effects when isolated and the other one does not induce toxicity alone, but increases the toxicity of the first substance in a mixture, we can say that there is a potentiation interaction (Carriquiriborde, 2021; Martin et al., 2021).
In the present work, the effective Cd concentration was very close to the nominal value in the Cd and Mix groups (Table 1). However, Ni concentration presented a variation of approximately -30% in the Ni group, and -20% in the Mix group. Similar variations have already been observed in other studies with Ni exposure (Bezerra et al., 2022) and may be related to the accumulation of the metal in fishes or to the formation of complexes between the metal and the organic matter present in the exposure medium, which would result in Ni deposition at the bottom of aquariums (Bezerra et al., 2022; Couture & Pyle, 2012).
Regarding hematological and plasma biomarkers, the RBC decreased (F = 4.44; p = 0.014) in the Cd and Mix groups (Table 2). In addition, MCV increased (H = 10.47; p = 0.015) in the Cd group. The concentration of Hb (F = 1.60; p = 0.217), Hct (F = 0.57; p = 0.643), and plasma glucose (F = 0.07; p = 0.974) did not differ between groups. The change in hematological parameters in the Mix group is probably more related to Cd, as the animals exposed to this single metal also showed a reduction in RBC and MCV. In another work, the fish Rhamdia quelen showed a reduction in Htco and Hb concentration after a 15-day exposure to 100 μg L-1 of Cd (Pereira et al., 2016). The same effect was observed in the fish Paralichthys olivaceus after 5 and 10 days of exposure to the same Cd concentration (100 μg L-1) (Lee et al., 2022). The reduction in RBC after exposure to a contaminant indicates a state of anemia, and is usually related to a reduction in the lifespan of the erythrocytes and a consequent increase in the rate of hemolysis, due to the interaction of the xenobiotic with the circulating erythrocytes (Witeska, 2015). The contaminant could also compromise the erythropoiesis processes, however, the increase in the rate of hemolysis is a more likely hypothesis for the present study, as the exposure period was only 72 h. The increase in MCV, which may be accompanied by an increase in intracellular pH, is a compensatory response commonly observed in fish during anemia, as it causes improvements in the oxygen-carrying capacity of the erythrocyte (Witeska, 2015). Furthermore, the increase in MCV explains why the reduction in RBC did not cause a reduction in hematocrit in the present study.
The presence of Ni did not interfere in the RBC reduction response caused by Cd, since this effect was observed in both the Cd and Mix groups, with no statistical difference between them. However, we observed an antagonistic/less-than-additive interaction regarding MCV, as the increase in this parameter was observed only in the Cd group.
In the gills, the activity of GST (F = 5.95; p = 0.005) and CAT (F = 4.75; p = 0.013) increased in the animals exposed to the metal mixture (Table 3). There were no differences between the groups regarding LPO (F = 1.71; p = 0.199) and the concentrations of GSH (F = 1.27; p = 0.309) and MT (F = 1.39; p = 0.275). Metals, such as Ni and Cd, can induce oxidative stress in fish through exacerbated production of ROS and changes in the antioxidant defense mechanisms (Santos Carvalho et al., 2015). In the animals exposed to single metals, the absence of changes may indicate that the basal activity of antioxidants was sufficient to prevent oxidative stress, since there was also no increase in LPO. The fish Galaxias maculatus also did not show oxidative damage or changes in the antioxidant defenses evaluated in the gills, after 96 h of exposure to 2 and 9 μg L-1 of Cd (McRae et al., 2019).
The increase in CAT and GST activity in the animals exposed to the metal mixture may indicate a response to increased ROS production caused by the metal exposure. CAT is responsible for converting hydrogen peroxide into water and oxygen, while GST can act as an antioxidant by conjugating GSH with electrophilic compounds (Lushchak, 2016; Nikinmaa, 2014). This increase in the activity of antioxidant enzymes observed in the gills in A. altiparanae was effective in preventing oxidative stress, as there was no increase in LPO in the Mix group.
Other studies with Ni exposure have already reported similar results regarding oxidative stress parameters. In the Neotropical fish P. lineatus, exposure to 2.5 mg L-1 of Ni for 96 h did not cause an increase in LPO in the gills, probably due to the increase in GSH concentration and greater activity of GST and SOD, as discussed by the authors (Palermo et al., 2015). Oncorhynchus mykiss exposed for 21 days to 1 mg L-1 of Ni showed increased GSH concentration, increased CAT activity, and no changes in LPO (Topal et al., 2017). Since the gills are in direct contact with the water and with the contaminants present there, this tissue may present faster initial activation of antioxidant defenses, and consequent greater efficiency in preventing oxidative stress (Palermo et al., 2015).
The increase in CAT and GST activity in the Mix group indicates a synergistic/more-than-additive effect on the oxidative stress parameters evaluated in the gills, since this response was not observed in the exposure to the single metals. In another work, P. lineatus exposed to a mixture of iron, zinc, and manganese showed an increase in the concentration of GSH in the liver, however this response was not sufficient to prevent an LPO increase in the tissue, an effect that was not observed in the exposure to the single metals (Oliveira et al., 2018). This study corroborates the results of the present work, indicating that exposure to a metal mixture can be more harmful than exposure to single metals, regarding oxidative stress parameters.
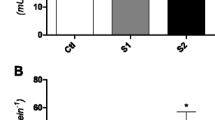
In relation to the neurotoxicity biomarker evaluated in A. altiparanae, AChE activity decreased (H = 18.66; p < 0.001) in the muscle of the Cd and Mix groups. In the brain, the activity of this enzyme was reduced (H = 13.46; p = 0.004) only in the Mix group (Fig. 1). AChE is an enzyme present in the synaptic cleft and neuromuscular junctions, being responsible for the hydrolysis of acetylcholine and interruption of nerve impulse transmission (Pereira et al., 2019). Inhibition or stimulation of this enzyme can promote physiological and behavioral changes that can interfere with swimming performance and reproduction, for example (Pretto et al., 2010).
The reduction in AChE activity in the muscle of the animals exposed only to Cd and to the metal mixture indicates a relation between the presence of Cd and the alteration in AChE activity. Several authors have already reported reductions in AChE activity in fishes after Cd exposure (Naïja et al., 2017; Naik et al., 2020; Pan et al., 2017). This effect may be caused by the binding of Cd to the active site of the enzyme, making it more difficult to break down acetylcholine (Pretto et al., 2010).
The reduction in AChE activity in the muscle also indicated that Ni and Cd presented an interaction similar to the one observed in the reduction in RBC, in which the presence of Ni did not interfere with the effect of Cd. However, in the brain we observed a synergistic effect of the mixture of Ni and Cd, since the reduction in AChE activity was not observed in this tissue after exposure to the single metals. In the work by Bezerra et al. (2022), P. lineatus exposed for 96 h to a mixture of Ni and Cd, at the same concentrations used in the present work, did not show a reduction in AChE activity in the brain. This indicates that in different species of fish the same tissue may present greater or lesser sensitivity after exposure to a metal mixture.
4 Conclusion
Together, the results of this work demonstrate that the mixture of Ni and Cd can exhibit different types of interaction depending on the biomarker evaluated, however, most of the interactions were additive or synergistic. In general, exposure to the mixture of Ni and Cd was more harmful to A. altiparanae, as it caused imbalances in antioxidant activity and reduced AChE activity in the brain, effects not observed after exposure to the single metals. In addition, we showed that acute exposure to this metal mixture can cause alterations that are harmful for this fish species, requiring further studies to assess whether these effects are intensified or modified in chronic exposures.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Abdalla, R. P., Kida, B. M. S., Pinheiro, J. P. S., Oliveira, L. F., Martinez, C. B. F., & Moreira, R. G. (2019). Exposure to aluminum, aluminum+manganese and acid pH triggers different antioxidant responses in gills and liver of Astyanax altiparanae (Teleostei: Characiformes: Characidae) males. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 215, 33–40. https://doi.org/10.1016/j.cbpc.2018.09.004
Amiard-Triquet, C., Amiard, J., & Mouneyrac, C. (2015). Aquatic ecotoxicology: advancing tools for dealing with emerging risks (p. 503). Academic Press.
Araújo, M. C., Assis, C. R. D., Silva, K. C. C., Souza, K. S., Azevedo, R. S., Alves, M. H. M. E., Silva, L. C., Silva, V. L., Adam, M. L., Carvalho Junior, L. B., Bezerra, R. S., & Oliveira, M. B. M. (2018). Characterization of brain acetylcholinesterase of bentonic fish Hoplosternum littorale: Perspectives of application in pesticides and metal ions biomonitoring. Aquatic Toxicology, 205, 213–226. https://doi.org/10.1016/j.aquatox.2018.10.017
Beutler, E. (1975). Red Cell Metabolism: A manual of biochemical methods (2nd ed.). Grune & Straton.
Beutler, E., Durom, O., & Kelly, B. M. (1963). Improved method for the determination of blood glutathione. Journal of Laboratory and Clinical Medicine, 61, 882–890.
Bezerra, V., Risso, W. E., Martinez, C. B. R., & Simonato, J. D. (2022). Can Lemna minor mitigate the effects of cadmium and nickel exposure in a Neotropical fish? Environmental Toxicology and Pharmacology, 92, 103862. https://doi.org/10.1016/j.etap.2022.103862
Bradford, M. M. (1976). A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry, 72(1–2), 248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Brasil. (2005). Ministério do Meio Ambiente, Conselho Nacional do Meio Ambiente. Resolução CONAMA n° 357, de 17 de março de 2005. Diário Oficial da União, Brasília, (053), 58–63.
Camejo, G., Wallin, B., & Enojärvi, M. (1998). Analysis of oxidation and antioxidants using microtiter plates. In D. Armstrong (Ed.), Free radical and antioxidant protocols (Vol. 108, pp. 377–387). Humana Press.
Carolin, C. F., Kumar, P. S., Saravanan, A., Joshiba, G. J., & Naushad, M. (2017). Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. Journal of Environmental Chemical Engineering, 5(3), 2782–2799. https://doi.org/10.1016/j.jece.2017.05.029
Carriquiriborde, P. (2021). Bases sobre los efectos tóxicos inducidos por los contaminantes. In P. Carriquiriborde (Ed.), Principios de Ecotoxicología (pp. 94–115). Libros de Cátedra, Editorial de la Universidad Nacional de La Plata (EDULP) http://sedici.unlp.edu.ar/handle/10915/118183
Conte, A. A. (2016). Reverse logistic, recycling and eco-efficiency of the batteries: review. Brazilian Journal of Environmental Sciences (Online), 39, 124–139. https://doi.org/10.5327/Z2176-947820167114
Costa, J. R. M. A., Mela, M., de Assis, H. C. D. S., Pelletier, É., Randi, M. A. F., & de Oliveira Ribeiro, C. A. (2007). Enzymatic inhibition and morphological changes in Hoplias malabaricus from dietary exposure to lead (II) or methylmercury. Ecotoxicology and Environmental Safety, 67(1), 82–88. https://doi.org/10.1016/j.ecoenv.2006.03.013
Couture, P., & Pyle, G. (2012). Field studies on metal accumulation and effects in fish. In C. M. Wood, A. P. Farrell, & C. J. Brauner (Eds.), Fish Physiology, v 31 A, Homeostasis and Toxicology of Essential Metals (pp. 417–473). Academic Press. https://doi.org/10.1016/S1546-5098(11)31009-6
Dew, W. A., Veldhoen, N., Carew, A. C., Helbing, C. C., & Pyle, G. G. (2016). Cadmium-induced olfactory dysfunction in rainbow trout: Effects of binary and quaternary metal mixtures. Aquatic Toxicology, 172, 86–94. https://doi.org/10.1016/j.aquatox.2015.12.018
Dourado, P. L. R., Rocha, M. P. D., Roveda, L. M., Raposo, J. L., Cândido, L. S., Cardoso, C. A. L., Morales, M. A. M., Oliveira, K. M. P., & Grisolia, A. B. (2016). Genotoxic and mutagenic effects of polluted surface water in the midwestern region of Brazil using animal and plant bioassays. Genetics and Molecular Biology, 40, 123–133. https://doi.org/10.1590/1678-4685-GMB-2015-0223
Ellman, G. L., Coutney, K. O., Andres, V., & Featherstone, R. M. (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemistry Pharmacology, 7, 88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Gagné, F. (2014). Xenobiotic Biotransformation. In F. Gagné (Ed.), Biochemical Ecotoxicology - Principles and Methods (pp. 117–130). Academic Press.
Kim, J. H., Choi, H., Sung, G., Seo, S. A., Kim, K. I., Kang, Y. J., & Kang, J. C. (2019). Toxic effects on hematological parameters and oxidative stress in juvenile olive flounder, Paralichthys olivaceus exposed to waterborne zinc. Aquaculture Reports, 15, 100225. https://doi.org/10.1016/j.aqrep.2019.100225
Komjarova, I., & Blust, R. (2009). Multimetal interactions between Cd, Cu, Ni, Pb, and Zn uptake from water in the zebrafish Danio rerio. Environmental Science & Technology, 43(19), 7225–7229. https://doi.org/10.1021/es900587r
Lee, D. C., Choi, Y. J., & Kim, J. H. (2022). Toxic effects of waterborne cadmium exposure on hematological parameters, oxidative stress, neurotoxicity, and heat shock protein 70 in juvenile olive flounder, Paralichthys olivaceus. Fish & Shellfish Immunology, 122, 476–483. https://doi.org/10.1016/j.fsi.2022.02.022
Lima, F. C. T., Malabarba, L. R., Buckup, P. A., da Silva, J. F. P., Vari, R. P., Harold, A., Benine, R., Oyakawa, O. T., Pavanelli, C. S., Menezes, N. A., Lucena, C. A. S., Malabarba, M. C. S. L., Lucena, Z. M. S., Reis, R. E., Langeani, F., Cassati, L., Bertaco, V. A., Moreira, C., & Lucinda, P. H. F. (2003). Genera Incertae Sedis in Characidae. In R. E. Reis, S. O. Kullander, & C. J. Ferraris Júnior (Eds.), Checklist of the Freshwater Fishes of South and Central America (pp. 106–168). EDIPUCRS.
Lushchak, V. I. (2016). Contaminant-induced oxidative stress in fish: a mechanistic approach. Fish Physiology and Biochemistry, 42(2), 711–747. https://doi.org/10.1007/s10695-015-0171-5
Martin, O., Scholze, M., Ermler, S., McPhie, J., Bopp, S. K., Kienzler, A., Parissisb, N., & Kortenkamp, A. (2021). Ten years of research on synergisms and antagonisms in chemical mixtures: A systematic review and quantitative reappraisal of mixture studies. Environment International, 146, 106206. https://doi.org/10.1016/j.envint.2020.106206
McRae, N. K., Gaw, S., Brooks, B. W., & Glover, C. N. (2019). Oxidative stress in the galaxiid fish, Galaxias maculatus, exposed to binary waterborne mixtures of the pro-oxidant cadmium and the anti-oxidant diclofenac. Environmental Pollution, 247, 638–646. https://doi.org/10.1016/j.envpol.2019.01.073
Melo, W. A., Braga, C. A. D. S. B., & Carneiro, L. C. (2017). Occurrence of heavy metals and contaminants on the surface of adjacent rivers. Journal of Water and Health, 15(1), 50–57. https://doi.org/10.2166/wh.2016.135
Monserrat, J. M. (2021). Estrés Oxidativo. In P. Carriquiriborde (Ed.), Principios de Ecotoxicología (pp. 116–125). Libros de Cátedra, Editorial de la Universidad Nacional de La Plata (EDULP) http://sedici.unlp.edu.ar/handle/10915/118183
Naïja, A., Kestemont, P., Chénais, B., Haouas, Z., Blust, R., Helal, A. N., & Marchand, J. (2017). Cadmium exposure exerts neurotoxic effects in peacock blennies Salaria pavo. Ecotoxicology and Environmental Safety, 143, 217–227. https://doi.org/10.1016/j.ecoenv.2017.05.041
Naik, A. P., Shyama, S. K., & D'Costa, A. H. (2020). Evaluation of genotoxicity, enzymatic alterations and cadmium accumulation in Mozambique tilapia Oreochromis mossambicus exposed to sub lethal concentrations of cadmium chloride. Environmental Chemistry and Ecotoxicology, 2, 126–131. https://doi.org/10.1016/j.enceco.2020.07.006
Nikinmaa, M. (2014). An introduction to aquatic toxicology (p. 240). Academic Press.
Niyogi, S., Nadella, S. R., & Wood, C. M. (2015). Interactive effects of waterborne metals in binary mixtures on short-term gill–metal binding and ion uptake in rainbow trout (Oncorhynchus mykiss). Aquatic Toxicology, 165, 109–119. https://doi.org/10.1016/j.aquatox.2015.05.016
Oliveira, L. F., Santos, C., Risso, W. E., & Martinez, C. B. R. (2018). Triple-mixture of Zn, Mn, and Fe increases bioaccumulation and causes oxidative stress in freshwater Neotropical fish. Environmental Toxicology and Chemistry, 37(6), 1749–1756. https://doi.org/10.1002/etc.4133
Ornelas-García, C. P., Domínguez-Domínguez, O., & Doadrio, I. (2008). Evolutionary history of the fish genus Astyanax Baird & Girard (1854) (Actinopterygii, Characidae) in Mesoamerica reveals multiple morphological homoplasies. BMC Evolutionary Biology, 8(1), 1–17. https://doi.org/10.1186/1471-2148-8-340
Palermo, F. F., Risso, W. E., Simonato, J. D., & Martinez, C. B. R. (2015). Bioaccumulation of nickel and its biochemical and genotoxic effects on juveniles of the neotropical fish Prochilodus lineatus. Ecotoxicology and Environmental Safety, 116, 19–28. https://doi.org/10.1016/j.ecoenv.2015.02.032
Pan, H., Zhang, X., Ren, B., Yang, H., Ren, Z., & Wang, W. (2017). Toxic assessment of cadmium based on online swimming behavior and the continuous AChE activity in the gill of zebrafish (Danio rerio). Water, Air, & Soil Pollution, 228(9), 1–9. https://doi.org/10.1007/s11270-017-3540-0
Pereira, B. V., Silva-Zacarin, E. C., Costa, M. J., Dos Santos, A. C. A., do Carmo, J. B., & Nunes, B. (2019). Cholinesterases characterization of three tropical fish species, and their sensitivity towards specific contaminants. Ecotoxicology and Environmental Safety, 173, 482–493. https://doi.org/10.1016/j.ecoenv.2019.01.105
Pereira, L. S., Ribas, J. L. C., Vicari, T., Silva, S. B., Stival, J., Baldan, A. P., Valdez Domingos, F. X., Grassi, M. T., Cestari, M. M., & Silva de Assis, H. C. (2016). Effects of ecologically relevant concentrations of cadmium in a freshwater fish. Ecotoxicology and Environmental Safety, 130, 29–36. https://doi.org/10.1016/j.ecoenv.2016.03.046
Pillet, M., Castaldo, G., De Weggheleire, S., Bervoets, L., Blust, R., & De Boeck, G. (2019). Limited oxidative stress in common carp (Cyprinus carpio, L., 1758) exposed to a sublethal tertiary (Cu, Cd and Zn) metal mixture. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 218, 70–80. https://doi.org/10.1016/j.cbpc.2019.01.003
Prado, P. S., Souza, C. C., Bazzoli, N., & Rizzo, E. (2011). Reproductive disruption in lambari Astyanax fasciatus from a Southeastern Brazilian reservoir. Ecotoxicology and Environmental Safety, 74(7), 1879–1887. https://doi.org/10.1016/j.ecoenv.2011.07.017
Pretto, A., Loro, V. L., Morsch, V. M., Moraes, B. S., Menezes, C., Clasen, B., Hoehne, L., & Dressler, V. (2010). Acetylcholinesterase activity, lipid peroxidation, and bioaccumulation in silver catfish (Rhamdia quelen) exposed to cadmium. Archives of Environmental Contamination and Toxicology, 58(4), 1008–1014. https://doi.org/10.1007/s00244-009-9419-3
Rahman, M., Rima, S. A., Saha, S. K., Saima, J., Hossain, M. S., Tanni, T. N., Bakar, M. A., & Siddique, M. A. M. (2022). Pollution evaluation and health risk assessment of heavy metals in the surface water of a remote island Nijhum Dweep, northern Bay of Bengal. Environmental Nanotechnology, Monitoring & Management, 18, 100706. https://doi.org/10.1016/j.enmm.2022.100706
Rimoldi, F. (2021). Efectos de los contaminantes sobre poblaciones. In P. Carriquiriborde (Ed.), Principios de Ecotoxicología (pp. 209–231). Libros de Cátedra, Editorial de la Universidad Nacional de La Plata (EDULP) http://sedici.unlp.edu.ar/handle/10915/118183
Santos Carvalho, C., Bernusso, V. A., & Fernandes, M. N. (2015). Copper levels and changes in pH induce oxidative stress in the tissue of curimbata (Prochilodus lineatus). Aquatic Toxicology, 167, 220–227. https://doi.org/10.1016/j.aquatox.2015.08.003
Santos, D. C. M., da Matta, S. L. P., de Oliveira, J. A., & dos Santos, J. A. D. (2012). Histological alterations in gills of Astyanax aff. bimaculatus caused by acute exposition to zinc. Experimental and Toxicologic Pathology, 64(7–8), 861–866. https://doi.org/10.1016/j.etp.2011.03.007
Shahjahan, M., Islam, M. J., Hossain, M. T., Mishu, M. A., Hasan, J., & Brown, C. (2022). Blood biomarkers as diagnostic tools: An overview of climate-driven stress responses in fish. Science of the Total Environment, 843, 156910. https://doi.org/10.1016/j.scitotenv.2022.156910
Silva, A. O., & Martinez, C. B. R. (2014). Acute effects of cadmium on osmoregulation of the freshwater teleost Prochilodus lineatus: Enzymes activity and plasma ions. Aquatic Toxicology, 156, 161–168. https://doi.org/10.1016/j.aquatox.2014.08.009
Simonato, J. D., Mela, M., Doria, H. B., Guiloski, I. C., Randi, M. A., Carvalho, P. S. M., Meletti, P. C., Assis, H. C. S., Bianchini, A., & Martinez, C. B. R. (2016). Biomarkers of waterborne copper exposure in the Neotropical fish Prochilodus lineatus. Aquatic Toxicology, 170, 31–41. https://doi.org/10.1016/j.aquatox.2015.11.012
Tegu, T. B., Ekemube, R. A., Ebenezer, S. O., & Atta, A. T. (2023). Monitoring the Variability of the Pollutants Level in Urban Water Front during Dry and Wet Seasons. European Journal of Applied Sciences, 11(1). https://doi.org/10.14738/aivp.111.13747
Tincani, F. H., Santos, G. S., & Azevedo, A. C. B. (2019). Climbing the taxonomic ladder: Could a genus be used as bioindicator? The ecotoxicological relationship between biomarkers of Astyanax altiparanae, Astyanax bifasciatus and Astyanax ribeirae. Ecological Indicators, 106, 105474. https://doi.org/10.1016/j.ecolind.2019.105474
Topal, A., Atamanalp, M., Oruç, E., & Erol, H. S. (2017). Physiological and biochemical effects of nickel on rainbow trout (Oncorhynchus mykiss) tissues: assessment of nuclear factor kappa B activation, oxidative stress and histopathological changes. Chemosphere, 166, 445–452. https://doi.org/10.1016/j.chemosphere.2016.09.106
Topal, A., Atamanalp, M., Oruç, E., Halıcı, M. B., Şişecioğlu, M., Erol, H. S., Gergit, A., & Yılmaz, B. (2015). Neurotoxic effects of nickel chloride in the rainbow trout brain: assessment of c-Fos activity, antioxidant responses, acetylcholinesterase activity, and histopathological changes. Fish Physiology and Biochemistry, 41(3), 625–634. https://doi.org/10.1007/s10695-015-0033-1
Viana, L. F., Súarez, Y. R., Cardoso, C. A. L., Crispim, B. D. A., Cavalcante, D. N. D. C., Grisolia, A. B., & Lima-Junior, S. E. (2018). The response of neotropical fish species (Brazil) on the water pollution: metal bioaccumulation and genotoxicity. Archives of Environmental Contamination and Toxicology, 75, 476–485. https://doi.org/10.1007/s00244-018-0551-9
Viarengo, A., Ponzano, E., Dondero, F., & Fabbri, R. (1997). A simple spectrophotometric method for metallothionein evaluation in marine organisms: an application to Meditarranean and Antartic mollusks. Marine Environmental Research, 44, 69–84. https://doi.org/10.1016/S0141-1136(96)00103-1
Vicari, T., Dagostim, A. C., Klingelfus, T., Galvan, G. L., Monteiro, P. S., da Silva Pereira, L., Assis, H. C. S., & Cestari, M. M. (2018). Co-exposure to titanium dioxide nanoparticles (NpTiO2) and lead at environmentally relevant concentrations in the Neotropical fish species Hoplias intermedius. Toxicology Reports, 5, 1032–1043. https://doi.org/10.1016/j.toxrep.2018.09.001
Wang, Z., Hua, P., Zhang, J., & Krebs, P. (2023). Bayesian-Based Approaches to Exploring the Long-Term Alteration in Trace Metals of Surface Water and Its Driving Forces. Environmental Science & Technology, 57, 1658–1669. https://doi.org/10.1021/acs.est.2c07210
Witeska, M. (2015). Anemia in teleost fishes. Bulletin of the European Association of Fish Pathologists, 35(4), 148–160.
Wood, C. M. (2012). An introduction to metals in fish physiology and toxicology: basic principles. In C. M. Wood, A. P. Farrell, & C. J. Brauner (Eds.), Fish Physiology, v 31 A, Homeostasis and Toxicology of Essential Metals (pp. 1–51). Academic Press. https://doi.org/10.1016/S1546-5098(11)31001-1
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
D G.M.C.: Project administration, investigation, formal analysis, writing - original draft, and writing - review & editing. B V.: Investigation, formal analysis, writing - review & editing, and visualization. R W.E.: Investigation and writing - review & editing. M C.B.R.: Resources and writing - review & editing. S J.D.: Supervision, conceptualization, project administration, resources, and writing - review & editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethics Approval
All procedures performed were approved by the Ethics Committee on Animal Experimentation at the State University of Londrina, Brazil (protocol number: 13642.2019.46).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dias, G.M.C., Bezerra, V., Risso, W.E. et al. Hematological and Biochemical Changes in the Neotropical Fish Astyanax altiparanae after Acute Exposure to a Cadmium and Nickel Mixture. Water Air Soil Pollut 234, 307 (2023). https://doi.org/10.1007/s11270-023-06325-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06325-5