Abstract
Wetlands provide numerous ecological services and are key habitats for aquatic flora and fauna. In the Beledanga wetland, the current study was conducted for 3 years, from July 2019 to June 2021, to evaluate the seasonal influence of physicochemical parameters on phytoplankton diversity and abundance patterns. Overall 48 genera of phytoplankton were observed. Bacillariophyceae (27%) contributed the maximum to the total phytoplankton density. The total abundance of phytoplankton was found utmost during monsoon (4.081 × 103 unit l−1) and least during post-monsoon (3.316 × 103 unit l−1). One-way analysis of variance indicated significant seasonal differences (p < 0.05) for some genera. The study gave the idea about the most influencing physic-chemical parameters (dissolved oxygen, turbidity, total hardness, Ca2+, and total nitrogen) on the growth of phytoplankton with the help of different multivariate and univariate analysis (canonical correspondence analysis and Karl Pearson’s correlation). The study again highlighted that climate parameters (temperature and rainfall) had some effect on the phytoplanktonic groups. Our study conceded that N:P in the studied wetland was less than the Redfield ratio (16:1) in all three seasons, while the Si:P ratio was noticed in the high range (15:1) during pre-monsoon. The value of the Shannon diversity index and Margalef’s species richness index were noticed to be > 3, which signified quite rich in phytoplankton diversity. But the value of Algal Pollution Index, which describes the ecological pollution level based on the present algal genera was observed high throughout all seasons, indicating organic load. So in future the studied wetland may get adversely affected with influence of anthropogenic activities. Therefore, for sustainable biodiversity of the waterbody, the anthropogenic activities (retting and intensification of agricultural farming) and macrophytes need to be controlled and regulated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
India is fortunate to have access to abundant resources of 0.55 million ha of floodplains spread throughout the Ganga and Brahmaputra river basins, which support about 85 fish species and have an estimated annual production potential of around 1000 kg/ha. These resources provide security for food and livelihood (Karnatak et al., 2022; Sarkar et al., 2020). These wetlands provide favourable habitats for various aquatic organisms like fishes, plankton, benthos, macrophytes, etc. (Karnatak et al., 2022) besides several other ecological services and work as a carbon sink (Nag et al., 2023). Wetlands are very diverse and productive waterbodies, providing many fishermen with numerous goods, ecological services, and livelihood security (MEA 2005). The water body gives several important commercial fish a safe feeding, breeding, and spawning ground (Jhingran & Pullin, 1985). Because of this, wetlands are among the best and most productive fisheries resources among all the other types of water bodies, with an annual output potential of 1500–2000 kg/ha (Sarkar et al., 2016). In recent years, the productivity and diversity richness of the wetlands have rapidly reduced due to amplified anthropogenic activities caused by industries, hydroelectric power plants, sewage systems, and other environmental challenges (Gogoi et al., 2019; Sarkar & Borah, 2018). This era’s industrialization, modernization, urbanization, and economic growth brought several stresses and degradation to the environment, mainly in the aquatic environment (Ukaogo et al., 2020). Due to this stressful condition, the physicochemical parameters of the water body have changed. This altered physicochemical profile of water has impacted various biotic organisms like plankton, benthos, and fishes. Therefore, this can serve as standard knowledge for biologists and ecologists to comprehend the current ecological link between biotic components like plankton with the surrounding environment in wetlands.
Plankton is known as an accurate biotic indicator of any aquatic body (Mohanty et al., 2022). Phytoplankton are the primary producer of the food web of waterbody; thus, they play a remarkable component in any water body (Haque et al., 2021). The density and diversity of phytoplankton in freshwater are influenced by several water variables of the aquatic ecosystem (Ahmed & Wanganeo, 2015). Thus, any changes in physicochemical properties, especially in water body nutrients, can modify phytoplankton’s diversity and density (Atique & An, 2020; Haque, 2006; Haque et al., 2021). Therefore, monitoring the existence and nonexistence of phytoplankton in an aquatic ecosystem must give essential information and common connections within a water body (Thakur et al., 2013). Besides water parameters, there are other several climatic conditions like temperature fluctuation, rainfall, light accessibility, and consumption (Cloern, 1996; Sommer, 1989). Changing the climatic factor can alter the environmental factor of the water body and can modify the phytoplankton structure in terms of diversity and density (Winder & Sommer, 2012).
Beledanga wetland is an oxbow lake in the lower Gangetic basin of North 24 Parganas district in West Bengal, with a total area of 113.67 acres (46.00 ha) (Karnatak et al., 2022). The studied wetland’s area gradually decreased by 47.73 acres (19.15 ha) in 40 years due to several reasons like macrophyte plague, water stress, and sedimentation aggravated by climatic change. The wetland provides livelihood security to fishermen (nearly 176 families) (Karnatak et al., 2022). The reduction of area causes alteration in the habitat of many biotic components.
There are several kinds of literature available on the dynamics of phytoplankton diversity and density of lower Gangetic basin wetlands. However, no such reports are available on the present community-managed semi-impacted wetlands. Therefore, this study attempts to document the seasonal variation of phytoplankton richness and diversity along the influential climatic and physicochemical parameters and identify some pollution-indicating species for sustainable management of small-scale wetland fisheries.
Materials and methods
Study area
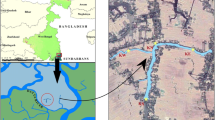
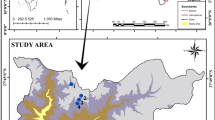
The present study was carried out seasonally for a period of 3 years from July 2019 to June 2021. The Beledanga wetland is the ox-bow lake and a seasonally opened wetland situated in the lower Gangetic basin of North 24 Parganas district in West Bengal having a total area of 113.67 acres (46.0 ha). It is connected by a narrow channel with the river Ichhamati. The connection is established only during monsoon season. The wetland is mainly used for fishery purposes, jute retting, and irrigation of nearby agriculture farms. Three sampling stations have GPS coordinates (23°4′5.67″N 88°45′37.17″E, 23°4′45.33″N 88°45′21.53″E, 23°5′28.27″N 88°45′30.85″E).
The study area map was prepared with the help of QGIS 2.18 (Fig. 1). Three seasons were the pre-monsoon (January to April), monsoon (May to August), and post-monsoon (September to December).
Phytoplankton collection, preservation, and analysis
The samples were collected seasonally from three stations (88°75′96.68″E–23°07′08.03″N, 88°75′45.13″E–23°08′07.93″N, 88°75′ 60.1656″E–23°08′97.086″N) of the studied wetland. Sampling was done between 9 and 10 a.m. with the help of a wooden boat. The samples were taken in triplicates from each station. For the phytoplankton diversity and density study, 50 l sub-surface water was filtered through a 20-µm bolting silk mesh-sized plankton net (Gogoi et al. 2009). The filtered samples were shifted to HDPE plastic containers and preserved with 4% neutral buffered formalin. The identification was made by following some keynotes viz. Desikachary (1959), Prescott (1955), Bellinger and Sigee (2015), and Cox (1996). For valid and updated names, Algae Base (Guiry & Guiry, 2020) was followed. Taxonomic identification and quantitative assessment were done by the trinocular microscope (40 × and 60 × magnification; Model No.-Zeiss scopeA1). Quantitative analysis was done by the drop count method (Lackey, 1938), and the density was expressed as unit per liter.
Physico-chemical parameters
Water samples were collected seasonally from all the sampling sites in an acid-washed and autoclaved bottle. Several water variables like water temperature (WT), transparency (TRAN), pH, specific conductivity (SPC), alkalinity (ALK), total hardness (TH), turbidity (TUR), dissolved oxygen (DO), and total hardness and salinity (SAL) were analysed in the field. Dissolved oxygen, total alkalinity, and total hardness were analysed by titration method using standard methods in situ (APHA, 2017). pH, specific conductivity, water temperature, salinity, and TDS were measured by the digital probe (YSIPRO DSS). The transparency of the water body was measured with the help of a Secchi disk.
For the nutrients (silicate, sulfate, nitrate, total nitrogen, and phosphate), Ca++, and Mg++ analysis, the water samples were brought to the laboratory and preserved in cold conditions and analysed per APHA (2017).
Statistical analysis
The density and distribution of planktonic groups were analysed in excel. One-way analysis of variance (ANOVA) followed by Duncan’s test was performed in water variables and phytoplankton groups of the studied wetland of different seasons. Bray–Curtis similarity is based on hierarchical clusters of stations manifested through a dendrogram. Different indices (Shannon–Wiener index, Simpson’s index, and Pielou’s evenness index) were analysed using PAST (4.02) software. The canonical correspondence analysis (CCA) was used to know the water parameters that influence the phytoplankton community of the studied wetland in PAST (4.02) software.
Algal Genus Pollution Index
Palmer’s Algal Genus Pollution Index (Palmer, 1969) was used to evaluate the contamination status of the wetland. Twenty phytoplankton genera which were tolerant to organic pollution were used in the pollution index by assigning each genus with an index score in the range of 1 to 5. After the identification of all phytoplankton genera, the allocated factor of genera was calculated, and the value was known as the index value. An index value of more than 20 indicates high organic pollution, a value between 15 and 19 signifies probable organic pollution, and a value of smaller than 15 signifies low pollution.
Climatic variables
Time series grid data (annual) for temperature (1° × 1°) and rainfall (0.25° × 0.25°) for a period of 47 years 1975–2022 were obtained from the India Meteorological Department (Pai et al., 2014; Srivastava et al., 2009).
Results
Climate condition
The climatic parameter like rainfall and air temperature was taken from IMD sites to understand the effect of climate on phytoplankton. The variation of the air temperature and water temperature is given in Fig. 2. The air temperature was recorded as maximum during monsoon (30.25 ± 0.13) while minimum during pre-monsoon (22.73 ± 2.52). The analysis of monthly average temperature revealed a warming trend over the last few decades. Although the mean maximum temperature did not show a warming trend, the mean minimum and overall mean temperature showed warming trends indicating seasonal temperature changes. Rainfall was recorded as high during monsoon (10.36 ± 2.30) and lower during pre-monsoon (0.87 ± 0.56). The grid data of the annual average rainfall pattern over the last 4 decades is highly fluctuating and did not show any trend at the study site.
Physico-chemical factors
The physicochemical parameters of the wetland were analysed during three seasons and depicted in Fig. 3. One-way ANOVA and post hoc Duncan’s test of physicochemical parameters had shown significant seasonal differences (p < 0.05) for water temperature, transparency, specific conductivity, total alkalinity, DO, turbidity, TDS, Ca + + , phosphate, and silicate. The water temperature ranged from 21.41 ± 1.26 to 31.38 ± 0.21 °C, i.e., lower during pre-monsoon and higher during monsoon. Dissolve oxygen was found highest during post-monsoon (7.44 ± 0.16 ppm) and lowest during monsoon (6.75 ± 0.16 ppm). Transparency was recorded as maximum during pre-monsoon (165.89 ± 3.56 cm) and minimum during monsoon (83.29 ± 5.003 cm). Specific conductivity ranged from 332 ± 11.38 to 453 ± 12.67 µS, i.e., minimum during monsoon and maximum during pre-monsoon. Similarly, nutrients also varied significantly with seasons. Sulphate ranged from 0.23 ± 0.25 to 0.26 ± 0.25 ppm, i.e., lowest during pre-monsoon and highest during post-monsoon. Phosphate was recorded as maximum during pre-monsoon (0.13 ± 0.005 ppm) and minimum during post-monsoon (0.102 ± 0.01 ppm). Silicate was found high during pre-monsoon (15.07 ± 0.22 ppm) and low during monsoon (6.09 ± 0.44 ppm). The total nitrogen value was found high during the monsoon season (0.066 ± 0.05 ppm), while a lower value was found during the post-monsoon season (0.044 ± 0.03 ppm).
Nutrient dynamics
To identify the main factors that restrict the growth of different phytoplankton groups, the concentrations of three nutrients, such as total nitrogen, phosphate, and silicate fractions, were compared seasonally. As a result, there was less nitrogen available for phytoplankton development in the water than the Redfield ratio (16:1) in all seasons. Similarly, the Si:P ratio was noticed in the same range (15:1) during pre-monsoon (Brzezinski, 1985) but less in the other two seasons. It indicates silicate enrichment in the wetland during the pre-monsoon.
Phytoplankton composition and distribution
Overall 48 genera belonging to 10 algal taxonomic classes in Beledanga wetland were recorded during the study period. The algal groups were Bacillariophyceae (BAC), Mediophyceae (MED), Coscinodiscophyceae (COS), Chlorophyceae (CHL), Trebouxiophyceae (TRE), Ulvophyceae (ULV), Cyanophyceae (CYA), Zygnematophyceae (ZYG), Dinophyceae (DIO), and Euglenophyceae (EUG). The number of genera and percentage composition of algal groups were given in Figs. 4 and 5 respectively. The result showed that the maximum contribution in terms of density was by the group Bacillariophyceae (27%) followed by Cyanophyceae (16%) and Zygnematophyceae (14%). Species like Tabellaria spp., Meridion spp., Diatoma spp., Nitzschia spp., Gyrosigma spp., etc. were recorded under the group Bacillariophyceae, and the group was found to be dominant during pre-monsoon (1.55 × 103 unit l−1). Under the Coscinodiscophyceae, Coscinodiscus spp., Aulacoseira spp., and Urosolenia spp., Acanthoceros spp. were recorded, and the group was found to be maximum during pre-monsoon (3.31 × 102 unit l−1). Similarly under the group Mediophyceae, Cyclotella spp. and Centronella spp. were recorded and found to be high during pre-monsoon (4.37 × 102 unit l−1). Under the groups Ulvophyceae and Dinophyceae, only one genus each was recorded, i.e., Ulothrix spp. and Ceratium spp. respectively. Chlorophyceae was represented by Oedogonium spp., Scenedesmus spp., Tetraspora spp., Volvox spp., Pandorina spp., and Pediastrum spp. Treubaria spp. were recorded and found maximum during monsoon (5.82 × 102 unit l−1). The group Zygnematophyceae was found to be dominant during monsoon (8.39 × 102 unit l−1), and under the group, Closterium spp., Staurastrum spp., Cosmarium spp., and Spirogyra spp. were recorded. Euglenophyceae was chiefly dominated by Euglena spp., Trachelomonas spp. and Phacus spp. the maximum density of this group was observed maximum during monsoon (3.92 × 102 unit l−1). Under the group Cyanophyceae, many filamentous algae like Gloeotrichia spp., Anabaena spp., Nostoc spp., Oscillatoria spp., Lyngbya spp., and some colonial algae like Merismopedia spp. were recorded, and the abundance of the group was found maximum during monsoon (8.87 × 102 unit l−1). The total abundance of phytoplankton was found maximum during monsoon and minimum (4.081 × 103 unit l−1) during post-monsoon (3.316 × 103 unit l−1).
One-way ANOVA, post hoc, and Duncan’s test of phytoplankton genera had shown significant differences (p < 0.05) for some genera such as Tabellaria spp., Diatoma spp., Nitzschia spp., Pinnularia spp., Gyrosigma spp., Amphora spp., Fragilaria spp., Synedra spp., Surirella spp., Coscinodiscus spp., Urosolenia spp., Cyclotella spp., Treubaria spp., Acanthoceros spp., Centronella spp., Ceratium spp., Ulothrix spp., Tetraspora spp., Volvox spp., Pediastrum spp., Chlorella spp., Actinastrum spp., Trochiscia spp., Staurastrum spp., Cosmarium spp., Spirogyra spp., Desdium spp., Zygnema spp., Euglena spp., Trachelomonas spp., Gloeotrichia spp., Merismopedia spp., Spirulina spp., Anabaena spp., and Nostoc spp.
Phytoplankton diversity indices and richness
The diversity indices of wetlands in different seasons were calculated and depicted in Fig. 6. The Simpson index ranged between 0.966 and 0.975, i.e., minimum during monsoon and maximum during post-monsoon. The Shannon–Wiener diversity (H′) was found highest during post-monsoon (3.77) and minimum during monsoon (3.5). Margalef species richness index also showed similar observations, i.e., highest during post-monsoon (5.8) and minimum during monsoon (4.57). Pielou’s evenness index was found maximum during pre-monsoon (0.92) and minimum during monsoon (0.85). The highest number of taxa was found during post-monsoon (48 genera) followed by pre-monsoon (40 genera) and monsoon (39 genera).
Cluster analysis
The Bary–Curtis cluster analysis was carried out to recognize the similarity level of phytoplankton genus composition in three different stations of Beledanga wetland and given in Fig. 7. The cophenetic correlation was calculated as 0.766 indicating that the clustering was average. The analysis discovered that the highest of phytoplankton species was between Station-1 and Station-2, i.e., 90%. Similarly, a separate cluster was formed between Station-3 and Station-1 and Station-2 having a similarity of 87%.
Influence of physicochemical parameters on phytoplankton distribution
Canonical correspondence analysis
The CCA was done between the 48 genera of phytoplankton and 18 water variables (WT, TRAN, pH, DO, SPC, TDS, SAL, TUR, TH, FCO2, ALK, CA, MG, TN, AN, SILI, SUL, and PHOS). It was created taking account of all three seasons and depicted in Fig. 8. The length of the arrow denotes the significance of attributes with negative and positive correlation with axes 1 and 2. The Eigenvalue of axis 1 is 0.355 and 64.74% percentage of variance, while the Eigenvalue of axis 2 is 0.040 and 7.31% percentage of variance. Both the percentage of variance and Eigenvalue were found higher on axis 1 than the axis 2. Frustulia spp., Aulacoseira spp., Meridion spp., Nitzschia spp., Gyrosigma spp., Cymbella spp., Fragilaria spp., Centronella spp., and Scenedesmus spp. were positively influenced by phosphate, transparency, and free CO2. The variables DO, sulfate, TDS, salinity, total alkalinity, silicate, and specific conductivity had shown a close relationship with the phytoplankton genera such as Diatoma spp., Synedra spp., Surirella spp., Meloseira spp., Mastogloia spp., Amphora spp., Urosolenia spp., Cyclotella spp., Acantoceros spp., and Treubaria spp. Total nitrogen and nitrate positively influenced the Staurastrum spp., Ceratium spp., Closterium spp., Spirogyra spp., Cosmarium spp., Zygnema spp., and Pandorina spp. The species like Spirulina spp., Ulothrix spp., Anabaena spp., Phacus spp., Trachelomonas spp., Nostoc spp., Lyngbya spp., Volvox spp., Oscillatoria spp., Euglena spp., Chlorella spp., Actinastrum spp., Merismopedia spp., Trochiscia spp., Gloeotrichia spp., Spirogyra spp., and Euglena spp. were more gradient towards water temperature, Ca, Mg, pH, total hardness, and turbidity.
CCA analysis between different planktonic groups and environmental variables. WT water temperature, TRAN transparency, DO dissolved oxygen, SPC specific conductivity, TDS total dissolve solids, SAL salinity, TUR turbidity, TH total hardness, FCO2 free CO2, ALK total alkalinity, CA Ca, MG Mg, TN total nitrogen, AN nitrate, SILI silicate, SUL sulphate, PHOS total phosphate. Phytoplankton: 1—Tabellaria spp.; 2—Meridion spp.; 3—Diatoma spp.; 4—Nitzschia spp.; 5—Gyrosigma spp; 6—Frustulia spp.; 7—Pinnularia spp.; 8—Amphora spp.; 9—Cymbella spp.; 10—Fragilaria spp.; 11—Synedra spp.; 12—Surirella spp.; 13—Meloseira spp.; 14—Mastogloia spp.; 15—Coscinodiscus spp.; 16—Aulacoseira spp., 17—Urosolenia spp.; 18—Cyclotella spp.; 19—Treubaria spp.; 20—Acantoceros spp. 21—Centronella spp.; 22—Ceratium spp.; 23—Ulothrix spp., 24—Oedogonium spp., 25—Scenedesmus spp.; 26—Tetraspora spp., 27—Volvox spp., 28—Pandorina spp., 29—Pediastrum spp.; 30—Chlorella spp.; 31—Actinastrum spp., 32—Trochiscia spp.; 33—Closterium spp.; 34—Staurastrum spp.; 35—Cosmarium spp.; 36—Spirogyra spp.; 37—Desdium spp.; 38—Zygnema spp.; 39—Euglena spp.; 40—Phacus spp.; 41—Trachelomonas spp.; 42—Gloeotrichia spp., 43—Merismopedia spp.; 44—Spirulina spp.; 45—Anabaena spp.; 46—Nostoc spp.; 47—Oscillatoria spp.; 48—Lyngbya spp
Karl Pearson’s correlation
Karl Pearson’s coefficient of correlation was computed between all the algal groups and water variables to find out the influencing physic-chemical parameters on the phytoplankton group and depicted in Table 1 and Fig. 9. The study revealed that group Bacillariophyceae was significantly positively influenced by specific conductivity, transparency, alkalinity, phosphate, and silicate while negatively influenced by water temperature and turbidity. Coscinodiscophyceae had shown a significant positive relation with transparency, specific conductivity, alkalinity, phosphate, and salinity while a negative relation with turbidity. Cyanophyceae and Chlorophyceae were positively influenced by water temperature, turbidity, total hardness, Ca, Mg, and total nitrogen. Ulvophyceae, Trebouxiophyceae and Zygnematophyceae had shown a positive correlation with turbidity, water temperature, and Ca. The total phytoplankton density was positively influenced by DO, turbidity, total hardness, Ca, and total nitrogen.
Algal pollution index
A total of 11 genera of pollution-indicating algae were found during the three seasons and depicted in Table 1. The value of the index was found high during all three seasons (pre-monsoon, monsoon, and post-monsoon). This indicates a high organic pollution load throughout the study period.
Influence of climatic changes on the phytoplankton
The impact of climatic variation has been studied in the present study by observing the correlation as well as the linear trend line curve between the different phytoplankton groups with the temperature and rainfall (Figs. 10 and 11). From the Karl Pearson correlation (Fig. 12), it is evident that the majority of the phytoplankton groups have been influenced by the variation in the rainfall and temperature which has significantly influenced their distribution. The only phytoplankton group which does not show variation with temperature was Ulvophyceae while remaining all phytoplankton groups showed significant variation. The temperature positively influenced the groups like Chlorophyceae (r = 0.69, p > 0.5), Trebouxiophyceae (r = 0.80, p > 0.5), Zygnematophyceae (r = 0.77, p > 0.5), Euglenophyceae (r = 0.58, p > 0.5), Cyanophyceae (r = 0.65, p > 0.5) while negatively influenced Bacillariophyceae (r = − 0.81, p > 0.5), Coscinodiscophyceae (r = − 0.78, p > 0.5), and Mediophyceae (r = − 0.80, p > 0.5). Similarly, rainfall had a significant positive effect on the groups like Chlorophyceae (r = 0.80, p > 0.5), Trebouxiophyceae (r = 0.86, p > 0.5), Zygnematophyceae (r = 0.72, p > 0.5), Euglenophyceae (r = 0.71, p > 0.5), Cyanophyceae (r = 0.79, p > 0.5) while a positive effect on Bacillariophyceae (r = − 0.80, p > 0.5), Coscinodiscophyceae (r = − 0.67, p > 0.5), and Mediophyceae (r = − 0.78, p > 0.5).
From the linear regression curve, it is evident that the phytoplankton groups having R2 > 0.5 with temperature are Bacillariophyceae (R2 = 0.655), Coscinodiscophyceae (R2 = 0.617), Mediophyceae (R2 = 0.639), Dinophyceae (R2 = 0.545), CHL (R2 = 0.483), Trebouxiophyceae (R2 = 0.633), and Zygnematophyceae (R2 = 0.590). The linear regression curve phytoplankton groups have R2 > 0.5 with rainfall Bacillariophyceae (R2 = 0.639), Coscinodiscophyceae (R2 = 0.458), Mediophyceae (R2 = 0.610), Dinophyceae (R2 = 0.497), Ulvophyceae (R2 = 0.568), Chlorophyceae (R2 = 0.649), Trebouxiophyceae (R2 = 0.736), Zygnematophyceae (R2 = 0.512), Euglenophyceae (R2 = 0.489), and Cyanophyceae (R2 = 0.635). The linear regression curve for temperature and rainfall was given in Figs. 9 and 10 respectively.
Discussion
Plankton is the natural food for fish in aquatic ecosystems. However, the hypothesis that planktivorous fish play a role in the regulation of lower trophic levels in freshwater ecosystems has been the subject of intense debate for a long time, and several different approaches to interaction have been proposed (Mehner et al., 2002). Phytoplankton is the key indicator group for stagnant water, used for monitoring the wetland ecosystem.
Physicochemical parameters
The physicochemical profile of any aquatic ecosystem can influence the biotic community of the water body (Haque et al., 2021). The water temperature was found high during monsoon. A similar observation was found by many authors (Gogoi et al., 2019; Haque et al., 2021). The transparency was found minimal during monsoon; this might be due to the high rainfall, sediments, silts, and debris inorganic and organic particles were washing into the water body. Therefore transparency reduces during monsoons (Haque et al., 2021). The DO was maximum during post-monsoon which was similar to the study of Sharif et al. (2017). Similarly, the value of pH, alkalinity, hardness, and specific conductivity was found low during the monsoon season. A similar finding was reported by many researchers (Kumar et al., 2021; Mohanty et al., 2023; Venkateshwarlu et al., 2011).
Nutrient dynamics
Like other water variables, nutrients also varied with seasons. Total nitrogen was found low during post-monsoon. This could be a result of the photosynthetic plankton using total nitrogen (Gogoi et al., 2019). In pre-monsoon, phosphate levels were high, which may have been caused by low water levels. In post-monsoon, phosphate levels were low, maybe as a result of photoautotrophs consuming the phosphate and sediment acting as a buffer (Rajasegar, 2003). Silicate was found maximum during pre-monsoon and minimum during monsoon. The results showed that N:P was less than the Redfield ratio (16:1) in the studied wetland’s water level during all seasons, indicating that there was less nitrogen available for phytoplankton development. A similar observation was found in Kailash Khal of Sundarban by Gogoi et al. (2019). The concentration of silicate was found higher than other nutrients (phosphate and total nitrogen). The lower value of N:P ratio during all seasons might be due to the excess macrophyte plague in the wetland. As the macrophytes are autotrophic in nature, therefore for growth, they utilize phosphate and total nitrogen. Xu et al (2019) said that macrophytes absorb 49.85% and 18.35% of total nitrogen (TN) and total phosphorus (TP) from Taihu Lake, China. Another example was in Jiangsu Province, China. The macrophytes are used to reduce nitrogen (Zhao et al., 2020). Seasonal estimates of the N:P ratio, according to Choudhury and Bhadury (2015), mainly stayed below the Redfeld ratio of 16:1 at Sagar Island. This condition needs to be controlled for the development of this wetland. Otherwise, in future, the wetland will lose its phytoplankton groups which is the natural feed for almost all fishes.
Plankton composition and distribution
Overall 48 genera belonging to 10 algal taxonomic classes in the Beledanga wetland were recorded during the study period. Bacillariophyceae was found maximum during pre-monsoon, which might be due to high silica concentration, long photoperiod, and favourable environmental conditions. The minimum density was recorded during monsoon which might be due to an increase in depth due to high rainfall (Gogoi et al., 2021b). All the algal groups such as Chlorophyceae, Cyanophyceae, Zygnematophyceae, and Ulvophyceae were found maximum during monsoon, which might be due to rich nutrient concentration which comes from municipality waste and agricultural farming present around the wetland and high temperature (Gogoi et al., 2021a; Nowrouzi & Valavi, 2011). According to the current study, the significant prevalence of Coscinodiscophyceae during pre-monsoon may have been caused by an increase in salinity from post-monsoon to pre-monsoon. A similar observation was noticed by Gogoi et al. (2019) in the Kailash Khal wetland of Sundarban. One-way ANOVA and Duncan’s test of phytoplankton had shown significant differences (p < 0.05). The total phytoplankton density was found maximum during monsoon which might be due to the connectivity with the Icchamati river. The river connectivity has a great impact on the diversity and density of the phytoplankton community of wetland (Mohanty et al., 2023). The lowest abundance of phytoplankton abundance during post-monsoon is due to the considerable presence of macrophytes at that time (Gogoi et al., 2021c).
Diversity indices
Diversity indices were measured to recognize the status of ecological systems as they were used as indicators of the aquatic body (Cardoso et al., 2012). The Shannon–Wiener diversity (H′) was found highest during post-monsoon (3.77) and minimum during monsoon (3.5). A similar observation was found in the Karatoya River (KR) in Sirajgong District, Bangladesh by Haque et al. (2021). Both Shannon diversity index and Margalef’s species richness were noticed > 3 in Beledanga wetland water which signified quite rich in phytoplankton diversity and less pollution in the ecosystem (Gogoi et al., 2019; Parakkandi et al., 2021). The value of Pielou’s evenness index was found to be more than 0.92 during pre-monsoon during the study period, which signifies an even distribution of phytoplankton in the wetland. In contrast, minimum values of the diversity indices were shown during the monsoon season which might be due to the jute retting in the studied waterbody (Ghosh & Biswas, 2018).
Pollution indicator species
The present study revealed 11 genera of pollution indicating algae were recorded as listed by Palmer (1969). The value of the index was found high during all the three seasons such as pre-monsoon, monsoon, and post-monsoon. This indicates a high organic pollution load present during all the seasons of the study period. The Desmids and diatoms (Tabellaria and Cyclotella) were found to dominate in oligotrophic lakes (Reynolds, 1995). The species like Closterium spp., Staurastrum spp., and Cosmarium spp. are designated as good water quality indicators, and the absence of these species signifies the pollution condition in the waterbody (Hosmani & Mahadev, 2002).
Influence of physicochemical parameters on phytoplankton
Canonical corresponding analysis
The growth and reproduction of phytoplankton were regulated by water temperature (Munn et al., 1989). High water temperature helps in triggering the growth of Cyanobacteria (Gogoi et al., 2019; Li et al., 2013). Many of the phytoplankton such as Spirulina spp., Ulothrix spp., Anabaena spp., Phacus spp., Trachelomonas spp., Nostoc spp., Lyngbya spp., Volvox spp., Oscillatoria spp., Euglena spp., Chlorella spp., Actinastrum spp., Merismopedia spp., Trochiscia spp., Gloeotrichia spp., Spirogyra spp., and Euglena spp. had shown to be positively influenced by water temperature. According to Naskar et al. (2020), transparency, alkalinity, temperature, and total nitrogen were more influencing the phytoplankton genera. In our study, many phytoplankton genera were shown to be positively influenced by these physicochemical parameters. The growth of diatoms needs a high concentration of silica. In our study, diatoms such as Diatoma spp., Synedra spp., Surirella spp., Meloseira spp., Mastogloia spp., Amphora spp., Urosolenia spp., and Cyclotella spp. had shown to be positively influenced by silicate. Harris and Gurel (1986) opined that nutrients affected phytoplankton biomass. Thus, phosphate, total nitrogen, and sulfate had shown to be positively influenced many phytoplankton genera like Diatoma spp., Synedra spp., Surirella spp., Meloseira spp., Mastogloia spp., Amphora spp., Urosolenia spp., Cyclotella spp., Ceratium spp., Spirogyra spp., Zygnema spp., and Pandorina spp. The DO had a positive relationship with several phytoplankton genera such as Diatoma spp., Synedra spp., Surirella spp., Meloseira spp., Mastogloia spp., Amphora spp., Urosolenia spp., and Cyclotella spp. This is because of the photosynthetic activities of phytoplankton groups (Sekerci & Petrovskii, 2018). Specific conductivity had shown a positive effect on several phytoplankton genera (Potapova & Charles, 2003).
Karl Pearson’s correlation
In our study, Coscinodiscophyceae had shown a significant positive relation with salinity which was similar to the study by Gogoi et al. (2019). Ulvophyceae was positively influenced by total nitrogen; this might be due to the utilization of nutrients by photosynthetic plankton (Gogoi et al., 2019). The present study revealed that dissolved oxygen, turbidity, total hardness, Ca, and total nitrogen are positively related to total phytoplankton density.
Influence of climatic changes
In this study, it was compared with older NICRA papers showing general rainfall patterns in Bengal and North 24 Paraganas (Das et al., 2022; Sarkar et al., 2019, 2020). Air temperature has a significant effect on the growth of phytoplankton because the mean temperature showed a changing trend between 22.73 and 30.25, which seems favourable for the growth of phytoplankton. The present study revealed that temperature significantly influenced groups like Chlorophyceae, Trebouxiophyceae, Zygnematophyceae, Euglenophyceae, and Cyanophyceae positively. Several authors have reported that diatoms showed dominance during winter (Prants et al., 2015). In our study, a significant negative correlation was found with Bacillariophyceae, Coscinodiscophyceae, and Mediophyceae.
Conclusion
A total of 48 genera belonging to 10 algal taxonomic classes were recorded in the Beledanga wetland. The maximum contribution in terms of density is by the group Bacillariophyceae followed by Cyanophyceae and Zygnematophyceae. Both Shannon diversity index and Margalef’s species richness were noticed > 3 in Beledanga wetland which signified quite rich in phytoplankton diversity and less pollution in the ecosystem. The organic load pollution of the wetland was found high during all three seasons. This might be due to excessive jute retting and agricultural farming. The study again highlighted the most influencing physicochemical parameters (dissolved oxygen, turbidity, total hardness, Ca, and total nitrogen) on the growth of phytoplankton. Our study revealed N:P in studied wetlands less than the Redfield ratio (16:1) in all three seasons, while the Si:P ratio was noticed in the high range (15:1) during pre-monsoon. The current study enhanced knowledge of phytoplankton seasonal variation and the impact of physicochemical parameters on phytoplankton diversity and density, which may be applied to fisheries management and fish stocking for enhancement programmes.
Availability of data
Data will be provided on request of the corresponding author.
References
Ahmed, A., & Wanganeo, A. (2015). Phytoplankton succession in a tropical freshwater lake, Bhoj Wetland (Bhopal, India): Spatial and temporal perspective. Environmental Monitoring and Assessment,187, 1–12.
(APHA 2017) American Public Health Association, & American Water Works Association. (2017). Standard methods for the examination of water and wastewater. In Standard methods for the examination of water and wastewater (pp. 1000–1000).
Atique, U., & An, K.-G. (2020). Landscape heterogeneity impacts water chemistry, nutrient regime, organic matter and chlorophyll dynamics in agricultural reservoirs. Ecological Indicators,110, 105813.
Bellinger, E. G., & Sigee, D. C. (2015). Freshwater algae: Identification, enumeration and use as bioindicators. John Wiley & Sons.
Brzezinski, M. A. (1985). The Si: C: N ratio of marine diatoms: Interspecific variability and the effect of some environmental variables 1. Journal of Phycology,21, 347–357.
Cardoso, S. J., Roland, F., Loverde-Oliveira, S. M., & de Moraes Huszar, V. L. (2012). Phytoplankton abundance, biomass and diversity within and between Pantanal wetland habitats. Limnologica,42, 235–241.
Choudhury, A. K., & Bhadury, P. (2015). Relationship between N: P: Si ratio and phytoplankton community composition in a tropical estuarine mangrove ecosystem. Biogeosciences Discussions, 12(3), 2307–2355.
Cloern, J. E. (1996). Phytoplankton bloom dynamics in coastal ecosystems: A review with some general lessons from sustained investigation of San Francisco Bay, California. Reviews of Geophysics,34, 127–168.
Cox, E. J. (1996). Identification of freshwater diatoms from live material. Chapman & Hall London.
Das, B. K., Nandy, S. K., Gogoi, P., Sahoo, A. K., Naskar, M., & Kunui, A. (2022). Unraveling the phytoplankton altitudinal dynamics in relation to environmental variables in a Himalayan River, Teesta, India. Aquatic Sciences,84, 1–16.
Desikachary, T. V. (1959). Cyanophyta. Indian Council of Agricultural Research.
Gogoi, A., Buragohain, A. K., Choudhury, A., & Ahmed, G. A. (2009). Laboratory measurements of light scattering by tropical fresh water diatoms. Journal of Quantitative Spectroscopy and Radiative Transfer, 110(14–16), 1566–1578.
Gogoi, P., Das, S. K., Sarkar, S. D., Chanu, T. N., Manna, R. K., Sengupta, A., Raman, R. K., Samanta, S., & Das, B. K. (2021a). Environmental factors driving phytoplankton assemblage pattern and diversity: Insights from Sundarban eco-region, India. Ecohydrology & Hydrobiology,21, 354–367.
Gogoi, P., Kumari, S., Sarkar, U. K., Lianthuamluaia, L., Puthiyottil, M., Bhattacharjya, B. K., & Das, B. K. (2021b). Dynamics of phytoplankton community in seasonally open and closed wetlands in the Teesta-Torsa basin, India, and management implications for sustainable utilization. Environmental Monitoring and Assessment,193, 1–25.
Gogoi, P., Sinha, A., Sarkar, S. D., Chanu, T. N., Yadav, A. K., Koushlesh, S. K., Borah, S., Das, S. K., & Das, B. K. (2019). Seasonal influence of physicochemical parameters on phytoplankton diversity and assemblage pattern in Kailash Khal, a tropical wetland, Sundarbans, India. Applied Water Science,9, 1–13.
Gogoi, P., Sinha, A., Tayung, T., Naskar, M., Das Sarkar, S., Ramteke, M. H., Das, S. K., Kumar, K. L., Suresh, V. R., & Das, B. K. (2021c). Unravelling the structural changes of periphyton in relation to environmental variables in a semilotic environment in the Sundarban eco-region, India. Arabian Journal of Geosciences,14, 1–16.
Ghosh, D., & Biswas, J. K. (2018). Impact of jute retting on physicochemical profile of Chhariganga oxbow lake in Nadia district, West Bengal, India. Archives of Agriculture and Environmental Science, 3(1), 36–44.
Guiry, M. D., & Guiry, G. M. (2020). Algaebase.[online]. Worldwide electronic publication, National University of Ireland, Galway.
Haque, M., Jewel, M., Sayed, A., Akhi, M., Atique, U., Paul, A. K., Iqbal, S., Islam, M. S., Das, S. K., & Alam, M. M. (2021). Seasonal dynamics of phytoplankton community and functional groups in a tropical river. Environmental Monitoring and Assessment,193, 1–16.
Haque, S. A. (2006). Salinity problems and crop production in coastal regions of Bangladesh. Pakistan Journal of Botany,38, 1359–1365.
Harris, L., & Gurel, E. (1986). Price and volume effects associated with changes in the S&P 500 list: New evidence for the existence of price pressures. The Journal of Finance,41, 815–829.
Hosmani, S. P., & Mahadev, J. (2002). Scale forming and corrosive tendencies of water in 20 lakes of Mandya district and its relation to phytoplankton. Indian Journal of Environmental Protection,22, 852–857.
Jhingran, V. G., & Pullin, R. S. V. (1985). A hatchery manual for the common, Chinese, and Indian major carps. WorldFish.
Karnatak, G., Das, B. K., Sarkar, U. K., Borah, S., Roy, A., Parida, P., Lianthuamluaia, L., Das, A. K., Behera, B. K., & Pandit, A. (2022). Integration of pen aquaculture into ecosystem-based enhancement of small-scale fisheries in a macrophyte dominated floodplain wetland of India. Environmental Science and Pollution Research,29, 75431–75440.
Kumar, C., Ghosh, A., Debnath, M., & Bhadury, P. (2021). Seasonal dynamicity of environmental variables and water quality index in the lower stretch of the River Ganga. Environmental Research Communications,3(7), 075008.
Lackey, J. B. (1938). The manipulation and counting of river plankton and changes in some organisms due to formalin preservation. Public Health Reports (1896–1970), 2080–2093.
Li, F., Zhang, H., Zhu, Y., Xiao, Y., & Chen, L. (2013). Effect of flow velocity on phytoplankton biomass and composition in a freshwater lake. Science of the Total Environment,447, 64–71.
MEa, M. E. A. (2005). Ecosystems and human well-being: Wetlands and water synthesis.
Mehner, T., Benndorf, J., Kasprzak, P., & Koschel, R. (2002). Biomanipulation of lake ecosystems: Successful applications and expanding complexity in the underlying science. Freshwater Biology,47, 2453–2465.
Mohanty, T. R., Tiwari, N. K., Das, B. K., Swain, H. S., Jhonson, C., & Banerjee, T. (2023). Riverine connectivity influences the phytoplankton ecology in the open floodplain wetland of the lower river Ganga. Environmental Monitoring and Assessment,195(12), 1403.
Mohanty, T. R., Tiwari, N. K., Kumari, S., Ray, A., Manna, R. K., Bayen, S., Roy, S., Das Gupta, S., Ramteke, M. H., Swain, H. S., Bhor, M., & Das, B. K. (2022). Variation of Aulacoseira granulata as an eco-pollution indicator in subtropical large river Ganga in India: A multivariate analytical approach. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-021-18096-9
Munn, M. D., Osborne, L. L., & Wiley, M. J. (1989). Factors influencing periphyton growth in agricultural streams of central Illinois. Hydrobiologia,174, 89–97.
Nag, S. K., Das Ghosh, B., Nandy, S., Aftabuddin, M., Sarkar, U. K., & Das, B. K. (2023). Comparative assessment of carbon sequestration potential of different types of wetlands in lower Gangetic basin of West Bengal, India. Environmental Monitoring and Assessment,195, 154.
Naskar, M., Sarkar, S. D., Raman, R. K., Gogoi, P., Sahu, S. K., Chandra, G., & Bhor, M. (2020). Quantifying plankto-environmental interactions in a tropical river Narmada, India: An alternative model-based approach. Ecohydrology and Hydrobiology,20, 265–275.
Nowrouzi, S., & Valavi, H. (2011). Effects of environmental factors on phytoplankton abundance and diversity in Kaftar Lake. Journal of Fisheries and Aquatic Science,6, 130.
Pai, D. S., Rajeevan, M., Sreejith, O. P., Mukhopadhyay, B., & Satbha, N. S. (2014). Development of a new high spatial resolution (0.25× 0.25) long period (1901–2010) daily gridded rainfall data set over India and its comparison with existing data sets over the region. Mausam,65, 1–18.
Palmer, C. M. (1969). A composite rating of algae tolerating organic pollution 2. Journal of Phycology, 5(1), 78–82.
Parakkandi, J., Saha, A., Sarkar, U. K., Das, B. K., Puthiyottil, M., Muhammadali, S. A., Ramteke, M., Johnson, C., & Kumari, S. (2021). Spatial and temporal dynamics of phytoplankton in association with habitat parameters in a tropical reservoir, India. Arabian Journal of Geosciences,14, 1–15.
Potapova, M., & Charles, D. F. (2003). Distribution of benthic diatoms in US rivers in relation to conductivity and ionic composition. Freshwater Biology,48, 1311–1328.
Prants, S. V., Andreev, A. G., Budyansky, M. V., & Uleysky, M. Y. (2015). Impact of the Alaskan Stream flow on surface water dynamics, temperature, ice extent, plankton biomass, and walleye pollock stocks in the eastern Okhotsk Sea. Journal of Marine Systems,151, 47–56.
Prescott, G. W. (1955). Algae of the Panama Canal and its tributaries I. Flagellated organisms.
Rajasegar, M. (2003). Physico-chemical characteristics of the Vellar estuary in relation to shrimp farming. Journal of Environmental Biology,24, 95–101.
Reynolds, C. S. (1995). River plankton: The paradigm regained. The ecological basis for river management (pp. 161–174).
Sarkar, U. K., & Borah, B. C. (2018). Flood plain wetland fisheries of India: With special reference to impact of climate change. Wetlands Ecology and Management,26, 1–15.
Sarkar, U. K., Nag, S. K., Das, M. K., Karnatak, G., & Sudheesan, D. (2016). Conserving wetlands-An effective climate change adaptation in India.
Sarkar, S. D., Sahoo, A. K., Gogoi, P., Raman, R. K., Munivenkatappa, M. H., Kumari, K., Mohanty, B. P., & Das, B. K. (2019). Phytoplankton biomass in relation to flow dynamics: The case of a tropical river Mahanadi, India. Tropical Ecology,60, 485–494.
Sarkar, U. K., Mishal, P., Borah, S., Karnatak, G., Chandra, G., Kumari, S., Meena, D. K., Debnath, D., Yengkokpam, S., Das, P., DebRoy, P., Yadav, A. K., Aftabuddin, M., Gogoi, P., Pandit, A., Bhattacharjya, B. K., Tayung, T., Lianthuamluaia, L., & Das, B. K. (2020). Status, potential, prospects, and issues of floodplain wetland fisheries in India: Synthesis and review for sustainable management. Review in Fishers Science & Aquaculture,29, 1–32. https://doi.org/10.1080/23308249.2020.1779650
Sekerci, Y., & Petrovskii, S. (2018). Global warming can lead to depletion of oxygen by disrupting phytoplankton photosynthesis: A mathematical modelling approach. Geosciences,8, 201.
Sharif, A. S. M., Islam, M. S., Hoque, M. N., & Bhuyan, M. S. (2017). Spatial and temporal environmental effect of lower Meghna River & its estuary on phytoplankton. Bangladesh. System,8, 10–11.
Sommer, U., (1989). The role of competition for resources in phytoplankton succession. Plankton Ecology: Succession in Plankton Communities 57–106.
Srivastava, A. K., Rajeevan, M., & Kshirsagar, S. R. (2009). Development of a high resolution daily gridded temperature data set (1969–2005) for the Indian region. Atmospheric Science Letters,10, 249–254.
Thakur, R. K., Jindal, R., Singh, U. B., & Ahluwalia, A. S. (2013). Plankton diversity and water quality assessment of three freshwater lakes of Mandi (Himachal Pradesh, India) with special reference to planktonic indicators. Environmental Monitoring and Assessment,185, 8355–8373.
Ukaogo, P. O., Ewuzie, U., & Onwuka, C. V. (2020). Environmental pollution: Causes, effects, and the remedies. In Microorganisms for sustainable environment and health (pp. 419–429). Elsevier.
Venkateshwarlu, M., Shahnawaz, A., & Honneshappa, K. (2011). A study on plankton dynamics of two wetland systems in Shimoga District, Karnataka (India). Current Biotica,4(4), 461–468.
Winder, M., & Sommer, U. (2012). Phytoplankton response to a changing climate. Hydrobiologia,698, 5–16.
Xu, X., Zhou, Y., Han, R., Song, K., Zhou, X., Wang, G., & Wang, Q. (2019). Eutrophication triggers the shift of nutrient absorption pathway of submerged macrophytes: Implications for the phytoremediation of eutrophic waters. Journal of Environmental Management,239, 376–384.
Zhao, D., Chen, C., Lu, Q., Zhang, M., Zhang, H., & An, S. (2020). Combined use of cold-season and warm-season macrophytes in floating constructed wetlands to increase nitrogen removal in the early cold season. Journal of Cleaner Production,254, 120054.
Acknowledgements
The authors thank the director of ICAR-CIFRI for providing the research facilities to conduct the present study.
Funding
Authors are thankful to World Fish under W3 programme for providing the financial support to conduct the study.
Author information
Authors and Affiliations
Contributions
Basanta Kumar Das: Conceptualized, overall guidance and manuscript corrections; Trupti Rani Mohanty: Concept, manuscript preparation; Shreya Bhatachaya: manuscript editing; Kausik Mondal: Sample collection, taxonomic identification; Nitish Kumar Tiwari: Concept, statistical analysis, interpretation and manuscript preparation; Purna Chandra: Sample collection; Gunjan Karnatak, Lianthuamluia, Sangeeta Chakraborty, Pranaya Parida, Archan kanti Das, Aparna Roy; manuscript preparation and corrections.
Corresponding author
Ethics declarations
Ethical approval
All authors have read, understood, and complied as applicable with the statement on “Ethical Responsibilities of Authors” as found in the instructions for Authors.
The authors declare that they have strictly followed all the rules and principles of ethical and professional conduct while completing the research work. No specific permission was required to collect the phytoplankton samples at the study sites.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Das, B.K., Mohanty, T.R., Bhattachaya, S. et al. Seasonal influence of physicochemical and climatic parameters on phytoplankton diversity and abundance pattern in community managed semi-impacted floodplain wetland. Environ Monit Assess 196, 648 (2024). https://doi.org/10.1007/s10661-024-12760-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-024-12760-6