Abstract
Functional classification of phytoplankton could be a valuable tool in water quality monitoring in the eutrophic riverine ecosystems. This study is novel from the Bangladeshi perspective. In this study, phytoplankton cell density and diversity were studied with particular reference to the functional groups (FGs) approach during pre-monsoon, monsoon, and post-monsoon at four sampling stations in Karatoya River, Bangladesh. A total of 54 phytoplankton species were recorded under four classes, viz. Chlorophyceae (21 species) Cyanophyceae (16 species), Bacillariophyceae (15 species), and Euglenophyceae (2 species). A significantly higher total cell density of phytoplankton was detected during the pre-monsoon season (24.20 × 103 cells/l), while the lowest in monsoon (9.43 × 103 cells/l). The Shannon–Wiener diversity index varied significantly (F = 16.109, P = 000), with the highest value recorded during the post-monsoon season. Analysis of similarity (ANOSIM) identified significant variations among the three seasons (P < 0.0001, R = 0.9518). The similarity percentage (SIMPER) analysis pinpointed Ulothrix spp. (Melosira granulate and Cymbella spp.) as the most contributory species are causing such a noticeable difference. Fifty-four phytoplankton species recorded during the study period were classified into 20 functional groups, whereas D/J/M/MP/X1 was considered the most abundant FG in the Karatoya River. FGs of the Karatoya River were influenced mainly by the nutrients (PO4-P and NO3-N) enrichments. As a novel investigation on FGs of phytoplankton in Bangladesh, this study recommends additional surveys in other rivers and floodplains to improve our understanding of phytoplankton diversity and functional groups.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phytoplankton are remarkable aquatic organisms that are the primary producers in the aquatic food web. They constitute the rudimentary biological components of the aquatic ecosystem from where the energy is transferred to higher ranks via the food chain (Sharma et al., 2016). The diversity and abundance of phytoplankton in freshwater ecosystems are directly linked with the physicochemical water chemistry features of the waterbody (Ahmed & Wanganeo, 2015). Any changes in the physicochemical factors, particularly nutrients, elicit a quick response of the phytoplankton community (Atique & An, 2020; Haque et al., 2020). Therefore, water quality monitoring and management emerge as essential components of water’s physicochemical and biological features (Atique et al., 2020; Hara et al., 2020; Kim et al., 2021). The use of phytoplankton as bioindicator organisms can serve the purpose cost-effectively. Therefore, the presence and absence of phytoplankton in a water body can provide vital information and prevalent connections within an aquatic ecosystem (Thakur et al., 2013).
Using functional groups (FGs) is a relatively modern approach in exploring the role of phytoplankton communities in a waterbody. These FGs are comparatively more effective and efficient in the ecological depiction of a water body than the phylogenetic groups, where long lists of species or dominant taxonomical groups are used for several days (Kruk et al., 2002). These groups often share similar tolerance and sensitivity levels. According to their description, FGs are comprised of species with similar morphology, physiology, and ecology and frequently coexist (Dı́az & Cabido 2001; Mouillot et al., 2013; Salmaso et al., 2015; Qiu et al., 2016). They have identical ecological requirements but are not necessarily from the same phylogenetic group.
Investigations into the algal communities from a functional perspective can provide additional valuable information on the community-level response to environmental changes. Therefore, the role of FGs regarding their community characteristics can be determined and managed more precisely if the species are grouped into classes showing similar phenomena. FGs provide an easier way to examine and differentiate various seasonal variations in the phytoplankton community and help understand the effect of environmental fluctuations (Kruk et al., 2002; Naselli-Flores et al., 2003; Weithoff et al., 2001). Occasionally, examination of FGs could ratify the latest most quantitative method of describing the phytoplankton community (Kruk et al., 2002). Therefore, our understanding of FGs can improve the species’ selection dynamics in the aquatic environment (Bovo-Scomparin & Train, 2008; Sarmento et al., 2009). At present, 38 functional groups are described so far using alphanumerical codes (Padisák et al., 2009).
Although several studies have been conducted in different Bangladesh rivers, little is known about seasonality and ecology based on phytoplankton functional groups (Haque et al., 2019; Islam & Huda, 2016; Khondker & Abed, 2013). However, these groups have been discovered in lakes from several regions of the world (Celik & Ongun, 2008; Maraşlıoğlu & Gönülol, 2014; Soylu & Gönülol, 2010), rivers (Okogwu & Ugwumba, 2012; Devercelli & O'Farrell, 2013; Bortolini et al., 2014; Zanco et al., 2017; Huang et al., 2018), and artificial reservoirs (Becker et al., 2010; Borges et al., 2008).
Karatoya River is an essential river in Bangladesh that can be a suitable study area for studying the functional groups of phytoplankton. This historic waterbody has changed its course in the past and meanders into Bangladesh from Indian territory. This river is near to eutrophication due to reduced water flow and higher nutrient enrichment (Akhi et al., 2020). The continuous siltation process increasingly intensifies the threat of eutrophication. Furthermore, this river is contaminated by various metallic and nonmetallic chemicals originating from different industrial units, including textile, dying, pharmaceuticals, and leather (Zakir et al., 2012). Thus, determining the eutrophication level in this river using the phytoplankton FGs approach could help answer some critical questions.
Therefore, this study was designed to investigate the phytoplankton community of Karatoya River using the taxonomical and FGs approaches. The main objective of this study was to find out links between the present water quality condition and the persistent phytoplankton population. We also applied the FGs approach to investigate the phytoplankton species and finally provided a relationship between FGs and water quality parameters to describe the ecological status of the Karatoya River, which is also a representative of the tropical rivers in Bangladesh.
Materials and methods
Study area and duration
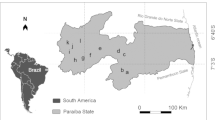
This study sampling was performed for 12 months from July 2016 to June 2017 at four study stations, viz. Nurina, Sontola, Garodoho, and Ghatina, in the Karatoya River (KR) at Sirajgong District, Bangladesh (Fig. 1). The 12 months’ samples were subdivided into three distinct seasons based on rainfall intensity. They were termed pre-monsoon (February to May), monsoon (June to September), and post-monsoon (October to January) seasons. We recorded all the major water quality and phytoplankton parameters during this study period.
Measurement of water quality parameters
A black-colored plastic bottle (capacity 2 l) was used for water sample collection. Approximately 500 ml of water sample from each sampling station was collected, and the measurements of water quality parameters were performed on the bank of the river between 9:00 AM and 12:00 PM. Water temperature was measured by a Celsius thermometer, while pH was determined with an electronic pH meter (Jenway 3020, UK). Transparency was measured with a blank and white color-coded Secchi disk. A HACH Kit Box (Model DR-2010, USA) was used to estimate the dissolved oxygen (DO), free carbon dioxide (CO2), alkalinity, and hardness. Phosphate-phosphorus (PO4-P) and nitrate-nitrogen (NO3-N) were measured using HACH Kit (DR-2020, USA) with high range chemicals (Phos. Ver. 3 Phosphate Reagent Powder Pillows for 25-ml sample for PO4-P analysis and Nitra Ver. 5 Nitrate Reagent Powder Pillows for 25-ml sample for NO3-N). Total dissolved solids (TDS) and electrical conductivity (EC) were measured by an Adwa AD31 waterproof EC/TDS tester.
Determination of phytoplankton species and functional groups (FGs)
Phytoplankton samples were collected from each study site during pre-monsoon, monsoon, and post-monsoon seasons. One hundred liters of water sample was filtered through a plankton net of 25-µm mesh size using a container of 10 l. Then, filtered samples were collected into a sample bottle and preserved immediately in 10% alcohol. The bottle was labeled and transferred to the laboratory for microscopic examination and identification. The concentrated sample vials were shaken to mix phytoplankton uniformly before microscopic examination. Each time, 1 ml of sample was drawn with the help of a dropper on a Sedge-wick Rafter Counting cell (S-R cell). The coverslips are placed, avoiding any air bubble formation (Zakir et al., 2012). Finally, the S-R counting cell was placed under the light microscope for identification and counting of phytoplankton. According to Cooke (1960), Needham and Needham (1962), and Mackenthun et al. (1964), the qualitative valuation of the phytoplankton was done. The quantitative cell density of phytoplankton was expressed as cells per liter of the water sample using the following formula (Stirling & Wilsey, 2001).
where N is the number of phytoplankton cells per liter of the original water, A is the total number of phytoplankton counted, C is the volume of the final concentration of the sample in ml, V is the volume of a field, F is the number of the field counted, and L is the volume of original water in liter.
All phytoplankton individuals, whether single cells, colonies, or filaments, were counted to estimate the phytoplankton cell density.
The Shannon–Wiener diversity index was calculated to understand the seasonal diversity of phytoplankton using the following formula (Shannon–wiener 1963):
H is the diversity index, n is the relative abundance (s/N), S is the number of individuals for each species, and N is the total number of individuals.
The functional groups (FGs) of Phytoplankton were described according to Padisák et al. (2009).
Statistical analyses
Seasonal variation in environmental variables was analyzed using one-way analysis of variance (ANOVA) at a 5% significance level using SPSS (Statistical Package for Social Sciences, version 20.0) software. The relationships between environmental variables and FGs were determined using Pearson’s correlation in SPSS 20.0. Canonical correspondence analysis (CCA) was also performed to evaluate the relationship between the environmental variables and phytoplankton classes. Before the examination, log10 (x + 1) transformation standardized environmental variables and phytoplankton cell density. One-way analysis of similarities (ANOSIM) was tested to evaluate the significant variations in temporal scale and visualized through non-metric multi-dimensional scaling (NMDS) analysis. Finally, similarity percentages (SIMPER) analyses (Clarke & Warwick, 1994) were performed to observe the percentage contribution and average dissimilarity among the three seasons. Similarity matrices were examined using the Bray–Curtis similarity index, which is used to quantify the differences in species composition. The Bray–Curtis index is always a number between 0 and 1. If 0, the samples share all the same species; if 1, they do not share any species. ANOSIM, SIMPER, and Shannon–Wiener diversity index were analyzed using Paleontological Statistics software version 3, while NMDS was performed using the vegan package in R software version 3.6.3.
Results
Environmental variables
Environmental variables varied significantly based on seasonal comparisons (Fig. 2). Temperature (Fig. 2A), transparency (Fig. 2B), DO (Fig. 2C), CO2 (Fig. 2D), alkalinity (Fig. 2F), hardness (Fig. 2G), TDS (Fig. 2J), and EC (Fig. 2K) showed evident seasonal variabilities at each study site (Table 1). Furthermore, significantly higher (P < 0.05) values of most water quality parameters were observed during the pre-monsoon period. Conversely, significantly higher (P < 0.05) nutrients, i.e., NO3-N (Fig. 2I) and PO4-P (Fig. 2L) as well as pH (Fig. 2E), were observed during the post-monsoon season. Most importantly, all the environmental variables displayed the lowest values during the monsoon season (Fig. 2).
Phytoplankton composition and assemblage structure
Fifty-four species of phytoplankton belonging to 4 classes were identified. Chlorophyceae, Cyanophyceae, Bacillariophyceae, and Euglenophyceae constituted 21, 16, 15, and 2 species with the percentage contribution of approximately 38.89%, 29.63%, 27.78%, and 3.70%, respectively. The total cell density of phytoplankton varied significantly (F = 67.475, P = 000) between the seasons. In contrast, the highest total cell density (24.20 × 103cells/l) was observed during the post-monsoon season and the lowest (9.43 × 103 cells/l) during monsoon (Fig. 3). Among the four phytoplankton groups, Chlorophyceae (5.76 × 103 cells/l) was the most abundant, while Euglenophyceae (0.45 × 103 cells/l) was the least abundant group. According to all seasons, sampling station 1 was dominated by Chlorophyceae and Cyanophyceae groups (Fig. 3A–B, E–F, I–J). On the other hand, Bacillariophyceae was the highest at station three during pre-monsoon (Fig. 3C) and monsoon (Fig. 3G). Station 4 showed a higher concentration of Euglenophyceae during pre-monsoon only (Fig. 3D). Phytoplankton, especially Euglenophyceae, significantly declined in monsoon (Fig. 3H).
The most abundant phytoplankton species recorded with a relative abundance > 10% was Volvox spp. (35.07%), followed by Melosira granulata (18.70%), Euglena spp. (18.54%), Oedogonium spp. (12.27%), Ulothrix spp. (11.56%), and Synedra ulna (10.10%) as shown in Table 2.
The Shannon–Wiener diversity index significantly varied (F = 16.109, P = 000) among the seasons. The highest diversity index value (3.90) was recorded during the post-monsoon season, while the lowest (3.77) was during the monsoon period (Fig. 4).
The phytoplankton assemblage significantly differed during the three seasons (ANOSIM, P = 0.0001, R = 0.9518). A two-dimensional NMDS based on Bray–Curtis’s similarity index divided the phytoplankton abundance during the pre-monsoon period from the monsoon and post-monsoon seasons (Fig. 5). SIMPER analysis exhibited an overall average dissimilarity of 23.10% among the seasons. The five species most significantly responsible for this difference in seasons are Melosira granulate (3.86%), Ulothrix spp. (3.65%), Oedogonium spp. (3.55%), Melosira varians (3.45%), and Amphora ovalis (3.39%). However, the average dissimilarity percentage between pre-monsoon to monsoon, pre-monsoon to post-monsoon, and monsoon to post-monsoon was 22.87%, 21.66%, and 24.77%, respectively. The most significant contributory species potentially responsible for this difference were Ulothrix spp., Melosira granulate, and Cymbella spp. The combined seasonal contributions were 5.21% (pre-monsoon to monsoon), 5.83% (pre-monsoon to post-monsoon), and 4.71% (monsoon to post-monsoon).
After considering seasonal segmentation and stations, the CCAs were performed to understand the complex relations of phytoplankton groups with environmental parameters (Fig. 6). The distance point of phytoplankton classes from environmental parameter directional tendencies indicated the perceptual ties between them. For instance, Cyanophyceae showed a closer association with DO, pH, and transparency during pre-monsoon (Fig. 6A). Conversely, Bacillariophyceae showed more relative links to alkalinity, phosphate, and conductivity during the post-monsoon (Fig. 6C). Considering average CCA, Cyanophyceae and Bacillariophyceae are affected closely by the associated environmental parameters at the sampling area (Fig. 6).
Phytoplankton functional group
The 54 phytoplankton species recorded during this investigation were classified into 20 functional groups (FGs), as presented in Table 3. Due to the absence of previous records as parts of the FGs, Calothrix spp., Nostoc spp., Rivularia spp., Euastrum binale, Microspora spp., Oedogonium spp., Spirogyra spp., Zygnema stellium, and Epithema spp. were excluded from functional groups classification. The seasonal dynamics observed by the relative abundance of the functional groups displayed a relative abundance of > 5% during the pre-monsoon season, while the representative FGs were D/J/M/MP/X1. However, during monsoon and post-monsoon periods, the dominant FGs were characterized as D/J/M/MP/S1/TB/X1 and D/J/M/MP/TB/X1/W1, respectively. Therefore, D/J/M/MP/X1 could be interpreted as the most abundant functional group in all the seasons (Table 3).
The correlation of water quality parameters and FGs showed a significantly strong relationship among and with the selected water quality parameters (Table 4). However, most dominant FGs (D/J/M/MP/X1) were negatively influenced by water temperature. At the same time, they showed a positive correlation with the ambient nutrients (PO4-P and NO3-N), and such conditions prevailed as favorable during the post-monsoon season (DO and pH).
Discussion
Seasonal environmental variations
The freshwater physicochemical status of an aquatic ecosystem significantly impacts the occurrence and distribution of the aquatic biota (Sharif et al., 2017). In this study, the water temperature fluctuated between 23.54 and 30.25 °C, with the lowest during the post-monsoon period that reached the maximum value during the monsoon season. Similar observations were reported by Bera et al. (2014), who also observed a seasonal water temperature variation during a comparable study period in an identical pattern. In addition, the intensive monsoonal rainfall triggered a higher amount of washing slits, sediments, debris, and organic and inorganic suspended particles into the river channel reducing the overall water transparency. The post-monsoon increase in DO reported in this study corroborated with the findings of Sharif et al. (2017).
Similarly, we observed the highest value of free carbon dioxide (CO2) in the pre-monsoon season. Such increased free CO2 could be attributed to the decomposition of the prevalent organic matter during this season at a higher rate. The seasonal impact was also apparent in the varying pH, alkalinity, hardness, PO4-P, NO3-N, TDS, and EC, as their values were recorded lower during the monsoon period. Various researchers have reported similar findings (Ali et al., 2020; Briola et al., 2010; Sayeed et al., 2015; Varol & Şen, 2018; Venkateshwarlu et al., 2011).
Seasonal patterns of phytoplankton assemblages
The total phytoplankton abundance in the Karotoya River demonstrated remarkable temporal variations, with a significantly higher (F = 67.475, P = 000) total cell density (24.20 × 103 cells/l) observed during the post-monsoon season, while the lowest (9.43 × 103 cells/l) during monsoon. The potential underlying reasons for the maximum total cell density recorded during the post-monsoon season could be their ability to flourish under weaker light availability, lower water temperature, and higher nutrient load, as Naik et al. (2009) have reported. Vajravelu et al. (2018) observed the post-monsoon nutrient enrichment was potentially accountable for the higher phytoplankton abundance in the Parangipettai coastal waters of the southeast Indian Coast. However, a lower abundance of phytoplankton has also been reported during the monsoon season characterized by heavy rainfall, decreased salinity, water temperature, pH, and higher turbidity which could be the leading causative factors for this phenomenon (Babu et al., 2013; Thillai et al., 2010).
Among the four phytoplankton groups, Chlorophyceae (5.76 × 103 cells/l) was the most abundant, while Euglenophyceae (0.45 × 103 cells/l) was the least abundant group. This river is characterized by reduced water flow and increasing siltation (Akhi et al., 2020). Therefore, stagnant or near to zero flow of the riverine water during the post-monsoon period triggered nutrient retention in the water column, increasing the nutrient load. This could be one of the principal reasons for a higher enrichment of the Chlorophyceae group during this study. Similarly, a higher density of Chlorophyceae was corroborated during low water flow by Flura et al. (2016) in Padma River, Bangladesh. The Padma River has displayed identical river flow characteristics to our studied river. The Chlorophyceae dominance has also been reported by Maraşlıoğlu and Gönülol (2014) in a nutrient-rich eutrophic Yedikır Dam Lake in Turkey.
Phytoplankton assemblages and diversity
Phytoplankton diversity indices are widely used in connection with water quality (Ahmed & Wanganeo, 2015). The Shannon–Wiener diversity index varied significantly (F = 16.109, P = 000) among the seasons, and the highest value (3.90) was recorded during the post-monsoon season, while the lowest (3.77) record was observed during monsoon. Therefore, the observed highest value during the post-monsoon period could be linked to the diverse species composition observed during this study. Moreover, the advantageous environmental circumstances with an elevated nutrient level in water could have explained another reason for the higher diversity indices during the post-monsoon season, as Dupuis and Hann (2009) reported.
On the contrary, a lower value of the diversity shown by the index during monsoon was potentially impacted by the higher and fluctuating water level augmented by the unfavorable environmental conditions. Roozen et al. (2003) also deemed flooding a disturbance factor that may cause water column instability, fluctuations in water level, and reduced water retention time (WRT). Thus, a strong flood sweeping away all the nutrients during the monsoon period and causing destabilized ecological settings could be one of the compelling factors for accelerated species loss and reduced number of plankton communities with a few individuals left (Wojciechowska et al., 2007).
The significantly differing phytoplankton community assemblage during the three seasons was due to the fluctuation in the available limiting nutrients in the riverine water (Danger et al., 2008). Furthermore, the phytoplankton community could have undergone a dilution effect during the monsoon season, potentially causing a lower phytoplankton cell density. As reported by Rahman and Huda (2012) and Ahmed and Alfasane (2004) in Padma River, higher water flow largely impacted the phytoplankton density. They have reported a lower phytoplankton cell density during the monsoon season. It is further supported by greater distances of phytoplankton from other environmental variables as illustrated in the CCA plot during monsoon (Fig. 6B). Typical river phytoplankton Melosira granulata, as reported by Reynolds (1988), was found as the most critical species responsible for higher dissimilarity among the seasons during this study. The same species has been reported as abundant in another study conducted by Ahmed and Alfasane (2004) in a Bangladeshi river. Other critical species identified through SIMPER analysis include Ulothrix spp., Oedogonium spp., Amphora ovalis, and Cymbella spp. cannot be discussed in further detail owing to the lack of species-specific studies conducted previously in Bangladesh. However, most of these species showed a relative abundance of > 10% during this investigation. Therefore, these species are critical in taking further in-depth monitoring of phytoplankton diversity and abundance.
Seasonal trends in the functional groups and water quality
The classification of the phytoplankton community into FGs and morphology-based functional groups (MBFGs) represents an essential tool for understanding the behavior and species dynamics in relation to environmental conditions (Salmaso & Padisák, 2007). The most dominant FGs (D/J/M/MP/X1) mainly abound at the low flow or stagnant water with higher nutrient enrichment of the water body. However, the abundance of these FGs was lower during the monsoon season and was primarily affected by turbulent floodwater currents. Thorp (2009) reported that stochastic processes are more intense in rivers, while the phytoplankton are influenced primarily by flow and flood pulse. Therefore, it is challenging to determine a compelling relationship between the environmental conditions and the phytoplankton distribution (Rodrigues et al., 2018).
Furthermore, the wash-out effect in a river during monsoon creates difficulty establishing the phytoplankton species in a river (Fraisse et al., 2013; Stanković et al., 2012). During the study period, the FGs (D/J/M/MP/X1) were sensitive to elevated temperature and benefited from increased nutrient loads in the water column during the post-monsoon season. The CCA plot of post-monsoon also supported this conclusion. Following heavy floods, the Karotoya River was enriched with nutrients during the post-monsoon season. However, during this season, water flow was significantly reduced. The river turned into a stagnant, nutrient-rich, and eutrophic waterbody, favoring an increased enrichment of FGs like D, L0, LM, M, MP, W1, and X1. Several researchers also reported similar observations (Padisák et al., 2009; Wu et al., 2011; Xiao et al., 2011).
The correlation analysis revealed consistency between FGs and water quality parameters in corroboration with the findings reported by Calijuri et al. (2002), Donald et al. (2013), and Fernández et al. (2014). These researchers have reported a negative influence of water temperature on the FGs D and a positive relationship with the nutrients (NO3-N). Furthermore, higher PO4-P was also found advantageous to the growth of FGs M, as was endorsed by the findings of Okogwu and Ugwumba (2012). Therefore, it was evident from the assessment of FGs in the Karatoya River that the periodic flooding triggering the seasonal hydrological alterations was the most critical factor influencing the phytoplankton community. Furthermore, the nutrient regime played a crucial role in shaping the phytoplankton community, similar to the other eutrophic water bodies across the globe (Fariñas et al., 2015).
Conclusion
This study was performed in a river primarily impacted by various factors, including intensive rainfall, rampant stagnations, higher nutrient enrichment, and increasing siltation. All these factors present an ecosystem conducive to specific phytoplankton communities and their functional groups. In conclusion, our findings illustrated hydrodynamics as the most responsible factor in determining the phytoplankton community formation in the Karatoya River. The phytoplankton diversity was the highest (3.90) during the post-monsoon season, while the lowest (3.77) during monsoon. Results also showed a significant difference in the phytoplankton community among the seasons brought by a typical river plankton Melosira granulate. Again, assessment of FGs in the Karatoya River and its correlation with environmental variables exposed that the periodic flooding was the most critical factor influencing the phytoplankton community.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on justifiable request.
References
Ahmed, A., & Alfasane, M. A. (2004). Ecological studies of the River Padma at Mawa Ghat, Munshiganj II. Primary productivity, phytoplankton standing crops and diversity. Pakistan Journal of Biological Sciences, 7(11), 1870–1875.
Ahmed, A., & Wanganeo, A. (2015). Phytoplankton succession in a tropical freshwater lake, Bhoj Wetland (Bhopal, India): Spatial and temporal perspective. Environmental Monitoring and Assessment, 187, 192.
Akhi, M. M., Jewel, M. A. S., Haque, M. A., et al. (2020). Multivariate approaches to determine the relationship between fish assemblage structure and environmental variables in Karatoya River, Bangladesh. Community Ecology, 21, 171–181.
Ali, M. M., Ali, M. L., Proshad, R., et al. (2020). Assessment of Trace Elements in the Demersal Fishes of a Coastal River in Bangladesh: A Public Health Concern. Thalass an International Journal of Marine Science, 36, 641–655.
Atique, U., & An, K. G. (2020). Landscape heterogeneity impacts water chemistry, nutrient regime, organic matter and chlorophyll dynamics in agricultural reservoirs. Ecological Indicators, 110(October 2019), 105813. https://doi.org/10.1016/j.ecolind.2019.105813
Atique, U., Kwon, S., & An, K. G. (2020). Linking weir imprints with riverine water chemistry, microhabitat alterations, fish assemblages, chlorophyll-nutrient dynamics, and ecological health assessments. Ecological Indicators, 117(June), 106652. https://doi.org/10.1016/j.ecolind.2020.106652
Babu, A., Varadharajan, D., Vengadesh, P. N., et al. (2013). Diversity of phytoplankton in different stations from Muthupettai, South east coast of India. Journal of Marine Science: Research & Development, 3, 128.
Becker, V., Caputo, L., Ordóñez, J., et al. (2010). Driving factors of the phytoplankton functional groups in a deep Mediterranean reservoir. Water Research, 44, 3345–3354.
Bera, A., Dutta, T. K., Patra, B. C., & Sar, U. K. (2014). Correlation study on zooplankton availability and physico-chemical parameters of Kangsabati Reservoir, West Bengal, India. International Research Journal of Environmental Science, 3, 28–32.
Borges, P. A. F., Train, S., & Rodrigues, L. C. (2008). Spatial and temporal variation of phytoplankton in two subtropical Brazilian reservoirs. Hydrobiologia, 607, 63–74.
Bortolini, J. C., Rodrigues, L. C., Jati, S., & Train, S. (2014). Phytoplankton functional and morphological groups as indicators of environmental variability in a lateral channel of the Upper Paraná River floodplain. Acta Limnologica Brasiliensia, 26, 98–108.
Bovo-Scomparin, V. M., & Train, S. (2008). Long-term variability of the phytoplankton community in an isolated floodplain lake of the Ivinhema River State Park, Brazil. Hydrobiologia, 610, 331–344.
Briola, M., Guevaram M., Menzies, D., et al. (2010). The Effects of pH on the Abundance of Phytoplankton for Mariculture. Ketchikan High School, 2610 Fourth AvenueKetchikan, Alaska 99901.
Calijuri, M., & do C, Dos Santos ACA, Jati S, . (2002). Temporal changes in the phytoplankton community structure in a tropical and eutrophic reservoir (Barra Bonita, SP—Brazil). Journal of Plankton Research, 24, 617–634.
Celik, K., & Ongun, T. (2008). Spatial and temporal dynamics of the steady-state phytoplankton assemblages in a temperate shallow hypertrophic lake (Lake Manyas, Turkey). Limnology, 9, 115–123.
Clarke, K. R., & Warwick, R. M. (1994). Similarity-based testing for community pattern: The two-way layout with no replication. Marine Biology, 118, 167–176.
Cooke, W. B. (1960). The Systematics of Fresh Water Biology
Danger, M., Daufresne, T., Lucas, F., et al. (2008). Does Liebig’s law of the minimum scale up from species to communities? Oikos, 117, 1741–1751.
Devercelli, M., & O’Farrell, I. (2013). Factors affecting the structure and maintenance of phytoplankton functional groups in a nutrient rich lowland river. Limnologica, 43, 67–78.
Dı́az S, Cabido M, . (2001). Vive la différence: Plant functional diversity matters to ecosystem processes. Trends in Ecology and Evolution, 16, 646–655.
Donald, D. B., Bogard, M. J., Finlay, K., et al. (2013). Phytoplankton-specific response to enrichment of phosphorus-rich surface waters with ammonium, nitrate, and urea. PLoS One, 8, e53277.
Dupuis, A. P., & Hann, B. J. (2009). Climate change, diapause termination and zooplankton population dynamics: An experimental and modelling approach. Freshwater Biology, 54, 221–235.
Fariñas, T. H., Bacher, C., Soudant, D., et al. (2015). Assessing phytoplankton realized niches using a French national phytoplankton monitoring network. Estuarine, Coastal and Shelf Science, 159, 15–27.
Fernández, C., Cáceres, E. J., & Parodi, E. R. (2014). Phytoplankton Development in a highly eutrophic man-made lake from the Pampa plain of Argentina-a functional approach. International Journal of Environmental Research, 8(2), 1–14.
Flura, A. M. A., Hossain, M. R. A., Rubel, A., et al. (2016). Assessment of physicochemical conditions and plankton populations of the river Padma, Bangladesh. Asian Australas Journal of Bioscience and Biotechnology, 1, 86–94.
Fraisse, S., Bormans, M., & Lagadeuc, Y. (2013). Morphofunctional traits reflect differences in phytoplankton community between rivers of contrasting flow regime. Aquatic Ecology, 47, 315–327.
Haque, M. A., Jewel, M. A. S., Zinat, A., et al. (2019). Phytoplankton community structure and environmental variables as indicators of organic pollution in Padma River, Bangladesh. International Journal of Ecology and Environmental Science, 45, 19–29.
Haque, M. A., Jewel, M. A. S., Atique, U., Paul, A. K., & Iqbal, S. (2020). Seasonal and spatial variation of flagellate communities in a tropical river. Limnologica, 85, 125824. https://doi.org/10.1016/j.limno.2020.125824
HaRa, J., Atique, U., & An, K.-G. (2020). Multiyear Links between Water Chemistry, Algal Chlorophyll, Drought-Flood Regime, and Nutrient Enrichment in a Morphologically Complex Reservoir. International Journal of Environmental Research and Public Health, 17(9), 3139. https://doi.org/10.3390/ijerph17093139
Huang, G., Li, Q., Wang, X., et al. (2018). Responses of phytoplankton functional groups to environmental factors in the Maixi River, southwest China. Journal of Limnology, 77, 88–99.
Islam, S. M. D., & Huda, M. E. (2016). Water pollution by industrial effluent and phytoplankton diversity of Shitalakhya River, Bangladesh. Journal of Scientific Research, 8, 191–198.
Khondker, M., & Abed, S. G. (2013). Seasonality of phytoplankton productivity of the river turag of Dhaka in relation to its water quality. Bangladesh Journal of Botany, 42, 287–294.
Kim, J. Y., Atique, U., Mamun, M., & An, K.-G. (2021). Long-Term Interannual and Seasonal Links between the Nutrient Regime, Sestonic Chlorophyll and Dominant Bluegreen Algae under the Varying Intensity of Monsoon Precipitation in a Drinking Water Reservoir. International Journal of Environmental Research and Public Health, 18(6), 2871. https://doi.org/10.3390/ijerph18062871
Kruk, C., Mazzeo, N., Lacerot, G., & Reynolds, C. S. (2002). Classification schemes for phytoplankton: A local validation of a functional approach to the analysis of species temporal replacement. Journal of Plankton Research, 24, 901–912.
Mackenthun, K. M., Ingram, W. M., & Porges, R. (1964). Limnological aspects of recreational lakes. US Department of Health, Education, and Welfare, Public Health Service.
Maraşlıoğlu, F., & Gönülol, A. (2014). Phytoplankton community, functional classification and trophic state indices of Yedikır Dam Lake (Amasya). Journal of Biology and Environmental Science, 8, 133–141.
Mouillot, D., Graham, N. A. J., Villéger, S., et al. (2013). A functional approach reveals community responses to disturbances. Trends in Ecology and Evolution, 28, 167–177.
Naik, S., Acharya, B. C., & Mohapatra, A. (2009). Seasonal variations of phytoplankton in Mahanadi estuary, east coast of India. Intenational Journal of Geo-Marine Science, 38(2), 184–190.
Naselli-Flores, L., Padisák, J., Dokulil, M. T., Chorus, I. (2003). Equilibrium/steady-state concept in phytoplankton ecology. In: Phytoplankton and Equilibrium Concept: The Ecology of Steady-State Assemblages. Springer, pp 395–403.
Needham, J. G., & Needham, P. R. (1962). A guide to the study of freshwater biology. Holdenday. Inc San Fransisco
Okogwu, O. I., & Ugwumba, A. O. (2012). Response of phytoplankton functional groups to fluctuating water level in two shallow floodplain lakes in Cross River, Nigeria. Inland Waters, 2, 37–46.
Padisák, J., Crossetti, L. O., & Naselli-Flores, L. (2009). Use and misuse in the application of the phytoplankton functional classification: A critical review with updates. Hydrobiologia, 621, 1–19.
Qiu, X., Huang, T., & Zeng, M. (2016). Differences in phytoplankton dynamics and community structure between a wet year and dry year in the Zhoucun Reservoir. Journal of Freshwater Ecology, 31, 377–391.
Rahman, M. A., & Huda, M. E. (2012). Study of the seasonal variations in physicochemical and biological aspects of the Padma River at Paturia Ghat, Manikganj. Jahangirnagar Univ Environmental Bulletin, 1, 55–66.
Reynolds, C. S. (1988). Functional morphology and the adaptive strategies of freshwater phytoplankton. In C. D. Sandgren (Ed.), Growth and reproductive strategies of freshwater phytoplankton (pp. 388–433). Cambridge University Press.
Rodrigues, L. C., Pivato, B. M., Vieira, L. C. G., et al. (2018). Use of phytoplankton functional groups as a model of spatial and temporal patterns in reservoirs: A case study in a reservoir of central Brazil. Hydrobiologia, 805, 147–161.
Roozen, F. C. J. M., Van Geest, G. J., Ibelings, B. W., et al. (2003). Lake age and water level affect the turbidity of floodplain lakes along the lower Rhine. Freshwater Biology, 48, 519–531.
Salmaso, N., Naselli-Flores, L., & Padisak, J. (2015). Functional classifications and their application in phytoplankton ecology. Freshwater Biology, 60, 603–619.
Salmaso, N., & Padisák, J. (2007). Morpho-functional groups and phytoplankton development in two deep lakes (Lake Garda, Italy and Lake Stechlin, Germany). Hydrobiologia, 578, 97–112.
Sarmento, H., Isumbisho, M., Stenuite, S., et al. (2009). Phytoplankton ecology of Lake Kivu (eastern Africa): Biomass, production and elemental ratios. Int Vereinigung Für Theor Und Angew Limnol Verhandlungen, 30, 709–713.
Sayeed, M. A., Hossain, M. A. R., Wahab, M. A., et al. (2015). Water and sediment quality parameters in the Chalan Beel, the largest wetland of Bangladesh. Chinese Journal of Oceanology and Limnology, 33, 895–904.
Sharif, A. S. M., Islam, M. S., Hoque, M. N., & Bhuyan, M. S. (2017). Spatial and temporal environmental effect of lower Meghna River & its estuary on phytoplankton. Bangladesh. System, 8, 10–11.
Sharma, R. C., Singh, N., & Chauhan, A. (2016). The influence of physico-chemical parameters on phytoplankton distribution in a head water stream of Garhwal Himalayas: A case study. Egyptian Journal of Aquatic Research, 42, 11–21.
Soylu, E. N., & Gönülol, A. (2010). Functional classification and composition of phytoplankton in Liman Lake. Turkish Journal of Fisheries and Aquatic Science, 10, 53–60.
Stanković, I., Vlahović, T., Udovič, M. G., et al. (2012). Phytoplankton functional and morpho-functional approach in large floodplain rivers. In: Phytoplankton responses to human impacts at different scales. Springer, pp 217–231
Stirling, G., & Wilsey, B. (2001). Empirical relationships between species richness, evenness, and proportional diversity. The American Naturalist, 158, 286–299.
Thakur, R. K., Jindal, R., Singh, U. B., & Ahluwalia, A. S. (2013). Plankton diversity and water quality assessment of three freshwater lakes of Mandi (Himachal Pradesh, India) with special reference to planktonic indicators. Environmental Monitoring and Assessment, 185, 8355–8373.
Thillai, R. K., Rajkumar, M., Sun, J., et al. (2010). Seasonal variations of phytoplankton diversity in the Coleroon coastal waters, southeast coast of India. Acta Oceanologia Sinica, 29, 97–108.
Thorp, J. H. (2009). Models of ecological processes in riverine ecosystems. In: G.E. LIKENS, ed. River ecosystem ecology. San Diego: Academic Press, 2010, pp. 212–219.
Vajravelu, M., Martin, Y., Ayyappan, S., & Mayakrishnan, M. (2018). Seasonal influence of physico-chemical parameters on phytoplankton diversity, community structure and abundance at Parangipettai coastal waters, Bay of Bengal, South East Coast of India. Oceanologia, 60, 114–127.
Varol, M., & Şen, B. (2018). Abiotic factors controlling the seasonal and spatial patterns of phytoplankton community in the Tigris River, Turkey. River Research and Applications, 34, 13–23.
Venkateshwarlu, M., Shahnawaz, A., & Honneshappa, K. (2011). A study on plankton dynamics of two wetland systems in Shimoga District, Karnataka (India). Current Biotechnology, 4, 461–468.
Weithoff, G., Walz, N., & Gaedke, U. (2001). The intermediate disturbance hypothesis—species diversity or functional diversity? Journal of Plankton Research, 23, 1147–1155.
Wojciechowska, W., Pasztaleniec, A., & Solis, M. (2007). Diversity and dynamics of phytoplankton in floodplain lakes [Bug River, Eastern Poland]. Oceanological and Hydrobiological Studies, 36, 199–208.
Wu, N., Schmalz, B., & Fohrer, N. (2011). Distribution of phytoplankton in a German lowland river in relation to environmental factors. Journal of Plankton Research, 33, 807–820.
Xiao, L. J., Wang, T., Hu, R., et al. (2011). Succession of phytoplankton functional groups regulated by monsoonal hydrology in a large canyon-shaped reservoir. Water Research, 45, 5099–5109.
Zakir, H. M., Rahman, M. M., Rahman, A., et al. (2012). Heavy metals and major ionic pollution assessment in waters of midstream of the river Karatoa in Bangladesh. Journal of Environmental Science and Natural Resources, 5, 149–160.
Zanco, B. F., Pineda, A., Bortolini, J. C., et al. (2017). Phytoplankton functional groups indicators of environmental conditions in floodplain rivers and lakes of the Paraná Basin. Acta Limnologica Brasiliensia, 29, e119.
Acknowledgements
The authors would like to thank the Government of the People’s Republic of Bangladesh for providing available funding through the National Science and Technology (NST) fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Haque, M.A., Jewel, M.A.S., Akhi, M.M. et al. Seasonal dynamics of phytoplankton community and functional groups in a tropical river. Environ Monit Assess 193, 704 (2021). https://doi.org/10.1007/s10661-021-09500-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-021-09500-5