Abstract
The river Ganga has several floodplain wetlands that support its ecology and ecosystem. Phytoplankton is an important component of the aquatic ecosystem, which plays an important role as a bioindicator for the assessment of aquatic health. The present study was conducted between 2018 and 2019 to understand the seasonal variation in the phytoplankton diversity of the Charaganga wetland and, parallelly, in the river Ganga in Nabadweep, India. The study explains how riverine connectivity affects the structure of the algal community in the wetland ecosystem. In the study, it has been observed that in the wetland, maximum mean phytoplankton density was noticed during pre-monsoon, i.e., 4079 unit l−1 followed by post-monsoon 3812 unit l−1 and monsoon 550 unit l−1, respectively. In the river system, the phytoplankton density varied from 78 unit l−1 to 653 unit l−1 seasonally, i.e., highest during monsoon and lowest during pre-monsoon. In both the ecosystems, i.e., wetland and river, the supreme influential group was Cyanophyceae followed by diatoms. One-way ANOVA showed a significant variation (p > 0.05) of three algal groups of phytoplankton (Bacillariophyceae, Coscinodiscophyceae, Chlorophyceae) in the river, while in the wetland, no significant variation (p > 0.05) was found among the other algal groups. The observed higher Shannon and Margalef’s species richness value in the wetland was observed than in the river defines the significance and importance of the wetland ecosystem, which may support the growth and conservation of various aquatic organisms as well. The study highlighted that the influencing abiotic factors like water temperature, dissolved oxygen, pH, and nutrients have affected the phytoplankton community in both the water bodies, i.e., wetland and river. We concluded that river connectivity is required to restore the biotic flora of the wetland ecosystem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Floodplain wetlands are the life providers for the millions of relict aquatic organisms. It supports their ecological niche and is considered the priority habitat for the majority of aquatic organisms (Chaparro et al., 2019). Wetlands are well known for their ability to generate higher productivity than coral reefs or rainforests (Bijoy Nandan et al., 2014). Wetland also provides favourable habitats to vast number of aquatic organisms. The majority of fish depend on the wetland ecosystem for their food and breeding grounds (Frank et al., 2022). Many studies also illustrate that it plays a significant role in the conservation of fish, as the fish organisms move either in search of a breeding ground or to hatch their young in relatively less flowing water (Sarkar et al., 2020). Wetland provides several ecological services as well as a source of income for nearby residents (Kataki et al., 2021). It is also known as the “kidney of the Earth” as it helps to reduce pollution status by filtering it (Rai et al., 2013). It is the transitional zone between terrestrial and aquatic points that provides distinct hydrological conditions (Mitsch et al., 2013).
Plankton is well known for its role in the monitoring of any water body (Meena et al., 2019). The role of plankton as an ecological indicator for the assessment of the environmental health of aquatic ecosystems by means of their community structure, diversity, distribution, and species richness is well known (Mohanty et al., 2022). Phytoplankton occupies the base position in the ecological pyramid due to its autotrophic mode of nutrition (Tas & Gonulol, 2007; Saravanakumar et al., 2008). A healthy aquatic ecosystem’s wealth is considered phytoplankton (Boyd & Tucker, 1998), as their abundance and composition are required to form a healthy food chain (Sridhar et al., 2006). The productivity of any water body (lotic and lentic) is chiefly influenced by multiple environmental constraints (Ye et al., 2019). So, a swift change in either their composition or abundance can make effective changes in their productivity (Hammer & Bastian, 2020). As a result, regular monitoring of natural wetlands is required, with a focus on the relationship between phytoplankton and its surroundings at a minimum level of information. Like fishes’ floodplain, wetlands also support the growth of a vast number of planktonic groups (Kahsay et al., 2022). As plankton does not have specialized movement organs, they flow with the help of flowing riverine water (Shen et al., 2021). The riverine flow may have also influenced the plankton assemblage and distribution pattern.
The biodiversity of the riverine water body is also very rich, but due to its flowing nature, it cannot stay on one side. The mighty River Ganga is believed to be the most sacred and holy River in India. It originates from the Himalayas and flows down to the Gangetic plain before draining into the Bay of Bengal, forming the world’s largest delta, the Sundarban (Tiwari et al., 2022a, b). It supports a large variety of aquatic flora and fauna because it covers several types of ecoregions (freshwater, brackish water, and estuarine water). The river Ganga is the largest river system in India. It is the ecological niche for a vast number of ecologically important biological organisms (Tiwari et al., 2022a, b).
Charaganga is an open floodplain wetland and oxbow lake situated in West Bengal (Nabadweep), India, connected with the river Ganga through a narrow channel throughout the year (Ghosh & Biswas, 2015). The wetland sustains a large amount of multifariousness in terms of several aquatic microflora and fauna. The wetland is used for many purposes, i.e., fishery, jute retting, irrigation, etc. by the local people.
There are several studies available on seasonal variation of phytoplankton community in wetland and river (Li et al. 2022; Huang et al. 2023). Li et al. (2022) investigated the seasonal variation of plankton in Zhalong wetland of China and reported that the density of plankton was noticed high during summer season. Gao et al. (2022) carried out the seasonal variation study at the Xijiang River of China, and seasonal variation has also been observed. But the study of influence of river connectivity with open wetland was scanty. However, there are some studies available like in the Rhoe river of France where the phytoplankton density as well as diversity is affected by the riverine connectivity in wetland, as the wetland is greatly impacted by the influx of water which has impacted the plankton dynamics in the wetland ecosystem, by varying water quality measures. The study exhibited by (Gogoi et al., 2021a, b) also explained that riverine connectivity in two of the wetland ecosystems, i.e., open and closed wetlands where the relatively higher planktonic distribution was observed in the open wetland where higher plankton abundance was observed. The present study evaluates the impact of riverine connectivity in the wetland ecosystem. The impact was evaluated by using the “plankton” which are essential aquatic organisms and are used as the indicators for the assessment of the ecological habitat. As it is well known that the influx of river water affects the wetland ecosystem by affecting the ecological niche for the majority of aquatic organisms, which acts as home for them, the study will be helpful in terms management and conservation aspects of wetlands and will give a better result for stakeholders. As wetlands are important fish-breeding grounds, the study will also help the policymakers to make helpful strategies for the conservation of wetland ecosystem and entire food web for which plankton have an important part.
Materials and methods
Study area
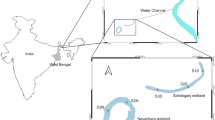
The present investigation was conducted for a period of 2 years from January 2018 to December 2019 in the river Ganga at Nabadweep as well as in the open floodplain wetland “Charaganga” (23°25′56.9316″ N, 88°21′ 55.3392″ E) wetland of Nadia district, West Bengal, India (Fig. 1). The studied wetland is an open oxbow-shaped wetland receiving water from the river Ganga with continuous inflow and outflow through a narrow channel at the northeast corner of a loop of the river (Ghosh et al. 2018). Five sampling stations having GPS coordinates (23°27′34.53″ N, 88°21′07.50″ E), (23°27′44.14″ N, 88°20′58.18″ E), (23°26′50.08″ N, 88°19′41.80″ E), (23°25′56.95″ N, 88°21′50.34″ E), and (23°26′09.71″ N, 88°21′39.39″ E) were selected covering different sites of wetland along with connectivity region to cover all diversity and composition patterns of Charaganga wetland. For river sampling, the Nabadweep sampling station was selected at river Ganga, where the wetland is connected with the river (23°26′2.86″ N, 88°22′3.97″ E). The study area map was prepared with the help of ArcGIS (Fig. 1).
Seasonal samplings were carried out by following the categorization of the Indian Meteorological Department (IMD), which were the pre-monsoon (March to June), monsoon (July to October), and post-monsoon (November to February).
Phyto-plankton collection, preservation, and analysis
Monthly sampling was performed between 7 AM and 8 AM using a traditional small wooden boat. The collection of the phytoplankton was carried out by following the standard protocols used by Wetzel and Likens (1991), APHA (2017), and Kumar et al. (2020). During the sampling, 50 l of water from each sampling site of wetland and river was filtered through a typical plankton net of a conical shape, fitted with a stainless-steel ring of very fine mesh size (20 μm). Plankton samples were collected in HDPE plastic containers using 4% neutral buffered formalin as a preservative. The identification was made by following various keynotes, viz., Desikachary (1959), Prescott (1982), Bellinger and Sigee (2015), and Cox and Cox (1996). For validation of updated names, algae base (Guiry and Guiry 2014) was followed. Taxonomic identification and quantitative assessment were done by taking 0.5 ml of each mixed sample. The samples were analyzed under the trinocular microscope (40× and 60× magnifications; Model-Zeiss scope A1). Quantitative analysis was done by the drop count method (Lackey 1938), and the density was expressed as unit per liter.
Physico-chemical parameter analysis
Throughout the study period, sampling was carried out at five different sampling sites of wetland and parallelly from the river. In situ analysis of various parameters such as water temperature (°C), conductivity (μS/cm), pH, and turbidity (NTU) was carried out in the field by using YSI-Pro DSS multi-parameter probe. Depth was measured using an ultrasonic depth sounder (Hondex Bx-7, Japan). Transparency (cm) was measured with the help of the Secchi disc. Dissolved oxygen, free CO2, total alkalinity (carbonate and bicarbonate), and total hardness were measured by following APHA (2017). For the analysis of nutrient parameters such as nitrate (ppm), phosphate (ppm), silicate (ppm), calcium (Ca2+), and magnesium (Mg2+), water samples were taken in an acid-washed HDPE bottle (1 l) and were analyzed following APHA (2017). For chlorophyll analysis, the water sample was filtered on-field by using magnesium carbonate with the help of Whatman Filter paper (45 μm). Then, the collected residue for chlorophyll analysis was taken to the laboratory in cold conditions (4 °C) and analyzed with the help of the acetone-extraction method (APHA 2017).
Statistical analysis
To evaluate the density and distribution of planktonic groups, the descriptive analysis was carried out in MS Excel. Water variables were subjected to a one-way analysis of variance (ANOVA) to understand the significant variations of water variables among seasons using SPSS (22) software. In addition to one-way ANOVA, the post hoc test (Duncan’s) was performed in the phytoplankton groups of the wetland of different seasons. Bray−Curtis similarity is based on hierarchical clusters of stations manifested through a dendrogram. Different indices (Simpson’s index, Shannon-wiener index, and Pielou’s evenness index) were analyzed using PAST (4.02) software. The CCA was done to know the influencing water parameter that affects the phytoplanktonic community of the studied wetland and river in PAST (4.02) software.
Algal Genus Pollution Index (Palmer, 1969)
Palmer’s Algal Genus Pollution Index is used to identify the pollution status of an aquatic body by using certain plankton genera as a pollution indicator. A pollution index factor of 1 to 5 is being allocated to twenty phytoplankton genera that are tolerant to organic pollution. After identification of the phytoplankton, the genera-allocated factor was given and the index factor is then calculated. When the value of the index was more than 20, indicates high organic pollution, a value between 15 and 19 indicates probable organic pollution, and a value of less than 15 indicates low pollution. The Algal Pollution Index is calculated for wetlands and rivers during the 3 seasons.
Trophic State Index (TSI)
TSI is used to determine the algal biomass in the production of the aquatic ecosystem. The range of the index varies from 0 to 100. The range of TSI for lakes and reservoirs varies between 30 and 50 indicating oligotrophic to mesotrophic trophic status, while the index value between 50 and 60 indicates mesotrophic to eutrophic trophic status (Carlson, 1977). It has been calculated using multiple variables such as chlorophyll-a, Secchi depth (transparency), and total phosphorus. Where chlorophyll-a is a better predictor of algal biomass than either of the other indices (Carlson and Simpson, 1996), Secchi depth provides the transparency value of the aquatic system, while total phosphorus is the major nutrient contributing to the production status of the aquatic system.
Results
Analysis of physico-chemical parameters of wetland
The average physico-chemical parameters of the wetland were analyzed and given in Fig. 2. Among the 17 water variables, six parameters (water temperature, pH, specific conductivity, total alkalinity, nitrate, phosphate) varied significantly (p ≤ 0.05) during different seasons. The mean water temperature varied between (24.86 ± 0.4 °C) and (30.78 ± 0.93 °C). The maximum temperature was recorded during pre-monsoon, and the minimum was during post-monsoon. The highest value of pH was recorded during post-monsoon (8.31 ± 0.27 ppm), and the lowest was observed during monsoon (7.44 ± 0.29 ppm). Total alkalinity was noticed high during post-monsoon (149 ± 7.4 ppm) and lowest during monsoon (103.6 ± 3.2 ppm). The mean value of specific conductivity fluctuated between (205.8 ± 1.8 μS−1) and (310.4 ± 16.6 μS−1) (maximum during pre-monsoon and minimum during monsoon). Among the nutrients, total phosphate was found to be low as compared to other nutrients. Total phosphate was noticed high during post-monsoon (0.05 ± 0.02 ppm) and lowest during monsoon (0.02 ± 0.01 PPM). Nitrite was found to be in the optimum range having higher values during pre-monsoon (0.83 ± 0.23 ppm), while relatively lower values were found during monsoon and (0.18 ± 0.03 ppm).
Analysis of physico-chemical parameters of river
The mean physico-chemical parameters of the river are given in Fig. 3. Among the 17 water variables, seven parameters (water temperature, dissolved oxygen, transparency, specific conductivity, hardness, total alkalinity, and calcium) varied significantly (p ≤ 0.05) among seasons. Water temperature ranged from (24.55 ± 0.35 °C) to (31.92 °C ± 0.17 °C); the maximum temperature was recorded during monsoon and the minimum during post-monsoon. The highest value of dissolved oxygen was recorded during the post-monsoon (8.55 ± 0.45 ppm) and the lowest during monsoon (4.86 ± 0.26 ppm). Transparency was found highest during post-monsoon (38.25 ± 2.75 cm) and lowest during monsoon (28.62 ± 0.02 cm). The mean total alkalinity was found higher during pre-monsoon (161.5 ± 0.5 ppm), while a relatively lower value was observed during the monsoon (125 ± 7 ppm). Specific conductivity was found high during pre-monsoon (369.5 ± 0.5 μS−1) and lowest during monsoon (254.95 ± 2.4 μS−1). The mean total hardness of the water body varied between (119 ± 0.7 ppm) and (161 ± 0.5 ppm).
Algal composition of studied wetland
Overall, a total of 55 genera of 11 distinct taxonomic classes, Bacillariophyceae (11 genera), Coscinodiscophyceae (2 genera), Mediophyceae (1 genus), Dinophyceae (1 genus), Xanthophyceae (2 genera), Ulvophyceae (2 genera), Chlorophyceae (13 genera), Trebouxiophyceae (4 genera), Zygnematophyceae (5 genera), Euglenophyceae (3 genera), and Cyanophyceae (12genera), were recorded from the studied wetland. The percentage density of different algal groups in 2 years is shown in Fig. 4; the highest abundance was contributed by Cyanophyceae (33%), followed by Bacillariophyceae (21%), Dinophyceae (16%), and Zygnematophyceae (12%). In different seasons, average abundance value of phytoplankton was observed highest during pre-monsoon (4049 unit l−1) and the least dominance was observed during monsoon (548 unit l−1). The percentage of algal contribution of the studied wetland is illustrated in Fig. 4.
Among diatom, the dominating genera were Aulacoseira sp., Navicula sp., Nitzschia sp., and Fragilaria sp. Under Cyanophyceae, dominating genera were Microcystis sp. Chroococcus sp., Oscillatoria sp., and Phormidium sp. In the group, Chlorophyceae Oedogonium sp. and Eudorina sp. were found to be dominated under the group Ulvophyceae Ulothrix sp. and Cladophora sp. were found. In Group Xanthophyceae, the recorded genera were Centitractus sp. and Tribonema sp. Under the group, Dinophyceae and Mediophyceae single genera were found, i.e., Ceratium sp. and Cyclotella sp., respectively. In the group Zygnematophyceae, Spirogyra sp., Mougeotia sp., Cosmarium sp., etc. were found. Finally, in the class Euglenophyceae, Trachelomonas sp., Euglena sp., and Phacus sp. were found. ANOVA followed by post hoc test portrayed no significant variation between seasons (p > 0.05) for the phytoplankton groups.
During the post-monsoon, highest densities of diatom (483 unit l−1), Cyanophyceae (169 unit l−1), and Zygnematophyceae (185 unit l−1) were found at St-4; Chlorophyceae (54 unit l−1) and Euglenophyceae (17 unit l−1) were in St-3; and Dinophyceae (643 unit l−1) and Xanthophyceae (17 unit l−1) were in St-1 and St-2, respectively. During the monsoon season, distribution and dominance patterns of algal groups were followed in this manner: Bacillariophyceae (60 unit l−1) and Cyanophyceae (28 unit l−1) were found in the highest density in St-3, Chlorophyceae (31 unit l−1) was in St-2, Euglenophyceae (49 unit l−1) was in St-1, and Conjugatophyceae was recorded dominant in St-2. During the pre-monsoon group, Chlorophyceae (188 unit l−1) and Cyanophyceae (607 unit l−1) were found to be the dominating group in St-1, and Dinophyceae (29 unit l−1) and Xanthophyceae (16 unit l−1) were at St-3. Bacillariophyceae (251 unit l−1) was found to be dominant at St-2.
Algal composition of River Ganga
A total of 29 genera belonging to 8 algal classes Bacillariophyceae (5 genera), Coscinodiscophyceae (2 genera), Mediophyceae (1 genus), Zygnematophyceae (3 genera), Trebouxiophyceae (4 genera), Chlorophyceae (5 genera), Cyanophyceae (6 genera), and Euglenophyceae (2 genera) were recorded from the studied site of river Ganga. The contribution pattern of the different groups contributed differently as the group Cyanophyceae (74%) contributed the highest density followed by Coscinodiscophyceae (16 %). During the different seasons, the density of phytoplankton was maximum during monsoon 653 unit l−1 and minimum during pre-monsoon 77 unit l−1. Among Coscinodiscophyceae, Aulacoseira sp., Navicula sp., Fragilaria sp., Cyclotella sp., etc. were found. In the group Chlorophyceae, the recorded genera were Pediastrum sp., Volvox sp., Eudorina sp., etc. In the group, Zygnematophyceae, Closterium sp., Spirogyra sp., and Mougeotia sp. were recorded. Cyanobacteria are represented by the genera Oscillatoria sp., Microcystis sp., Phormidium sp., Dolichospermum sp., and Merismopedia sp. Three groups of phytoplankton (Bacillariophyceae, Coscinodiscophyceae, Chlorophyceae) had shown significant pre-monsoon and monsoon seasonal variation (p > 0.05). The percentage of algal contribution of the studied site of river Ganga is in Fig. 5.
Species richness and diversity
In the case of season, the expected number of species during different seasons was in the order of pre-monsoon (42) > monsoon (41) > post-monsoon (36). Among the different sampling stations, the expected number of species was in the order of St-3 (42) > St-4, St-1, St-2 (39) > St-5 (36). Diversity indices varied seasonally in the Charaganga wetland, and details are shown in Fig. 6. The Shannon–Weiner index (H′) was high during monsoon (3.13) and lowest during post-monsoon (2.23). Margalef species richness was also found to be high during monsoon (6.35) and low during post-monsoon (4.33). The result of the index value showed that the Shannon–Wiener diversity (H′) ranged between 2.66 and 2.93 and was highest at station St-3 while lowest at station St-1. Margalef species richness had shown similar observations, highest at station St-3 (4.28) and lowest at station St-1 (4.89).
Season-wise diversity indices were calculated in the river Ganga and shown in Fig. 7. All the indices like Simpson, Shannon, Evenness, and Margalef index were found high during the post-monsoon (0.80, 2.21, 0.48, and 3.92) and lowest during the monsoon (0.21, 0.49, 0.16, and 1.39). Both Margalef’s species richness and Shannon diversity index were found comparatively high in the wetland depicting moderately rich phytoplankton diversity in the system than in the river.
Bray–Curti’s hierarchical cluster
Station-wise Bary–Curti’s cluster analysis (Fig. 8) was done to know the degree of similarity of the algal group’s distribution among the sampling stations of Charaganga wetland and river Ganga. The cophenetic correlation was calculated as 0.9194 indicating that the clustering result was good and shown by the dendrogram. The highest similarity of algal groups was found between St-5 and river, followed by St-3 and St-4. Another group was formed between St-3 and St-4 and St-5 and river. Later on, St-2 and St-1 formed a separate group.
Algal Genus Pollution Index
A total of 20 phytoplankton genera are scheduled as pollution indicators by Palmer (1969); in the present study, out of which, twelve genera were recorded from the wetland and ten genera were recorded from the river during all three seasons. The recorded algal pollution indicates genera, and the index value is given in Table 1. The index value is noticed to be more than 20 in wetland during the three-season indicating high organic load, while in the river, the calculated value is less than 15 during pre-monsoon and monsoon indicating low pollution load in the riverine water.
Tropic State Index (TSI)
The TSI value of wetland was recorded highest during pre-monsoon (37.61) and comparatively lower during monsoon (34.35), while in the riverine water, the index is found maximum during monsoon (41.71) and minimum during post-monsoon. The Tropic State Index of both river and wetland in three different seasons is given in Fig. 9.
Effect of different physico-chemical parameters on phytoplankton communities
After considering the seasonal variation of an algal community of both wetland and river, the CCA was performed to recognize the relation between phytoplankton groups and environmental parameters.
Canonical correspondence analysis of wetland
Canonical correspondence analysis (CCA) was done (Fig. 10) to find the relationship and distribution of 17 different environmental attributes like water temperature, DO, pH, turbidity, transparency, specific conductivity, free CO2, depth, total alkalinity, total hardness, Chl-a, calcium, magnesium, and nutrients (nitrate, nitrate, phosphate, silicate) along with 11 different planktonic groups. CCA biplot was formed, taking into consideration all three seasons, i.e., pre-monsoon, monsoon, and post-monsoon at five different sampling stations. The length of the arrow displays the negative and positive correlation on axis 1 and axis 2. The calculated eigenvalues were found higher on axis 1 than on axis 2, 3, and 4 having an eigenvalue of 0.34 elucidating 70.8 % correlation, and axis 2 exhibits a 0.14 eigenvalue having 29.2% value between different algal groups and water parameters. The analysis depicted that Euglenophyceae, Coscinodiscophyceae, Mediophyceae, and Chlorophyceae were positively correlated with water temperature, depth, nitrate, and total nitrogen. These groups were found to be dominant during monsoon. The class Bacillariophyceae and Trebouxiophyceae were negatively correlated with the majority of the parameters like water temperature, depth, nitrate, and total nitrogen. Zygnematophyceae and Dinophyceae were positively correlated with transparency, free CO2, specific conductivity, total hardness, total alkalinity, pH, phosphate, and magnesium. The densities of these recorded groups were found high during post-monsoon. The group Xanthophyceae, Ulvophyceae, and Cyanophyceae were positively influenced by turbidity, DO, silicate, and Chl-a. These groups were found to be dominating during pre-monsoon.
Canonical correspondence analysis for river
CCA was done between 17 different environmental attributes like water temperature, DO, pH, turbidity, transparency, specific conductivity, free CO2, depth, total alkalinity, total hardness, Chl-a, calcium, magnesium, and nutrients (total N, nitrate, phosphate, silicate) along with 8 different planktonic groups (Fig. 11). CCA biplot was formed taking into consideration all three seasons, i.e., pre-monsoon, monsoon, and post-monsoon at the river. The calculated eigenvalues were found higher on axis 1 than on axis 2, having an eigenvalue of 0.43 elucidating 84.15% correlation, and axis 2 exhibits a 0.08 eigenvalue having a 15.85% value. From the analysis, it was observed that Coscinodiscophyceae, Chlorophyceae, and Zygnematophyceae were positively influenced by total alkalinity, total hardness, specific conductivity, magnesium, and calcium during pre-monsoon. Similarly, algal groups like Bacillariophyceae, Mediophyceae, and Euglenophyceae were influenced by dissolved oxygen, transparency, phosphate, and chlorophyll-a during post-monsoon. Trebouxiophyceae and Cyanophyceae were influenced by turbidity, depth, water temperature, silicate, and pH.
Discussion
Wetland is the most enriched ecosystem of the Earth, which endeavors a large variety of aquatic flora and fauna including livelihood to the resident fishers (Singha et al. 2023; Sandilyan et al. 2022). But with the change in the climatic condition and the global warming, the riverine ecology as well as wetland ecology changed a lot over the years across the globe. Significant global warming changes have been observed in many of the wetlands such as Nenjiang river basin. In India, also the impact of global warming in relation to climatic variation has been addressed in many of the wetland ecosystem (Nag et al. 2023). The present study evaluated the impact of riverine connectivity on the wetland ecosystem, by taking plankton as the important bioindicator tool. In the wetland ecosystem, it is also important that seasonal influence and variation of the planktonic genera are assessed carefully, so that their dependency on the other aquatic organisms can be monitored, which might have affected the entire aquatic food web. Additionally, wetlands serve also as a connecting link between the flowing and stagnant water system, acting as a kidney for the conservation of the aquatic biota. The impact of the wetland ecosystem as well as riverine water quality affects the plankton which are the important bioindicators in any of the aquatic environment.
Seasonal variation in the environmental parameters
In the freshwater ecosystem, the physico-chemical parameters play a vital role in regulating the biotic community present in the water body (Sharif et al. 2017). In the present study, the wetland water temperature ranged between 24.86 and 30.78 °C; the maximum temperature was observed during pre-monsoon and minimum during post-monsoon which may be due to climatic changes and solar radiation (Palit and Mukherjee 2010). The result was observed similar in line with the studies made by Gogoi et al. (2019) in the Kailash Khal wetland of Sunderban region, India. The level of total alkalinity reached a peak during post-monsoon might be due to the faster decomposition of plants and other organic wastes in the wetland and the slower dilution of riverine water in the system (Chaurasia and Pandey 2007). The minimum value of nutrients (phosphate and nitrate) was recorded during the monsoon which might be due to the higher rate of dilution of rainwater and riverine water in the wetland, which might have diluted the wetland’s water as has been observed in the wetlands of Punjab, India (Singh et al., 2022). So, from the above discussion, it may be noted that significant effect of river water as well as spatio-temporal variations has been observed in the entire wetland ecological condition.
Similar to wetland, the monitoring of the different water quality parameters has been made for the river system. In the river, the water temperature was recorded minimum during post-monsoon and maximum during monsoon, which signifies the impact of climatic variability in the river system. Bera et al. (2022) and Haque et al. (2021) observed similar water temperature variations during their study period in a tropical river such as Kangsabati river of India. Similarly, transparency was recorded at a minimum during the monsoon which might be due to heavy rainfall washing off sediments and silts, incorporating organic and inorganic particles from the surrounding into the river which helps in reducing the transparency at that time. Sharif et al. (2017) and Haque et al. (2021) reported an increase in dissolve oxygen which was similar to our findings. Other water parameters, like specific conductivity, hardness, and total alkalinity, were recorded lower during monsoon. Various workers gave similar findings (Sayeed et al. 2015; Varol and Şen 2018; Venkateshwarlu et al. 2011).
The impact of climatic as well as seasonal variations has been observed in both of the ecosystems, i.e., wetland as well as river ecosystem, which might have affected the wetland as well as riverine ecology illustrating to change in the plankton ecology. The change in the plankton composition can alter the entire wetland as well as riverine food web of the dependent aquatic organisms (Balzer et al. 2023).
Phytoplankton abundance
In wetland ecosystem
The present study recorded 58 genera belonging to 7 distinct taxonomic groups, i.e., Bacillariophyceae, Chlorophyceae, Cyanophyceae, Dinophyceae, Xanthophyceae, Conjugatophyceae, and Euglenophyceae, in the wetland ecosystem. The numbers of observed genera were comparatively higher than the previous findings in the same wetland carried out by (Ghosh and Biswas, 2015), during which the impact of jute rating was observed in the study.
The study had shown differentiation in the algal groups in different seasons at all five sampling stations of the wetland. The study was similar to the other studies which were made in the different wetland ecosystems for the diversity, density, and assemblage pattern of the plankton that vary seasonally and site-wise (Lakshminarayana 1965; Gogoi et al. 2019). Considering the average data of phytoplankton for 2 years, it had been observed that the highest abundance of phytoplankton was recorded during pre-monsoon which might be due to stable evaporation and transpiration of water that enhances the nutrient loading of water (Harris and Gurel 1986). The lowest abundance has been recorded during monsoon which might be due to the dilution of water due to rainfall as well as from the river which promotes a sudden change in physico-chemical parameters of water like decreased water temperature, pH, and higher turbidity (Sahu et al. 2012; Gogoi et al. 2019) and jute retting (Ghosh and Biswas 2015). The group Cyanophyceae was found to be a dominating group among all algal groups which may be due to the influence of influxes of river water in the wetland ecosystem and have influenced the wetland’s ecology. The similar observations have been made in the Zhalong wetland (Li et al., 2022). The study reported many of the filamentous genera (Lyngbya sp., Oscillatoria sp., Nostoc sp., etc.) of this group were noticed high during pre-monsoon, which may be due to relatively higher temperature and their mechanism of buoyancy (Ernst et al. 2006; Visser et al. 2016), along with nitrogen-fixing capability (Chan et al. 2004; Issa et al. 2014). Next to all these, the Cyanophyceae and diatoms were found to be a dominating group. Among the diatoms, Navicula sp., Fragilaria sp., Aulacoseira sp., and Pinnularia sp. were found to be dominating species. A high density of diatom was found during the post-monsoon (Mitbavkar and Anil 2008). The considerable presence of filamentous algae (Cyanophyceae, Chlorophyceae,) during pre-monsoon, can be explained due to sufficient amount of light availability (Das Sarkar et al. 2019; Gogoi et al. 2021a, b), and the nutrient load comes from the local area and through the river channel. Post hoc analysis reveals there was no significant variation observed in the algal community among the three seasons in the wetland. This may be possible due to the continuous inflow and outflow of river water which regulates the phytoplankton community.
In riverine ecosystem
In the river, high density of the algal community was found high during the monsoon which may be due to the high nutrient load by runoff water from the city area. The growing industries on the bank of the river and the several wastewater outflows in the river mainstream are one of the major challenges affecting the river water quality. For instance, on the bank of Nabadweep, there are large cities and it is a tourist place. Indeed, the moderately purified municipal wastes elevated the nutrients downstream, which influences the phytoplankton species richness significantly. The maximum abundance of Cyanobacteria during the monsoon was related to high nutrient load (phosphate and nitrate) and specific conductivity (Dixit et al. 2017) carried by influx of fertilizers by nearby farmlands and efflux of effluents from the jute rating. Similar to the results of wetland, the river also has chief abundance of diatoms which occupy the second position in the case of density. The density and diversity of the algal community in the wetland was observed comparatively very high than the river, representing a unique observation, as very few studies are available which describe the similarity or differences of plankton in the river as well as wetland system (Xiao et al., 2020). The changes in the plankton dynamics are due to influx as well as efflux of river and wetland water in two of the different symbiotic ecological habitats. Post hoc analysis reveals there was significant variation in Bacillariophyceae, Coscinodiscophyceae, and Chlorophyceae among the three seasons in the river, which was similar to the studies made in the other rivers like Yellow River of China (Liu et al. 2023).
Phytoplankton assemblages and diversity
The condition of an aquatic ecosystem can be measured by diversity which is regarded as a true biotic indicator (Cardoso et al. 2012). Shannon–Weiner index and Margalef index were found high during monsoons; it might be due to the high-water inflow towards the wetland through the narrow connection that enhances the nutrient condition. The Margalef’s species richness and Shannon diversity index were found >2.7 in the Charaganga wetland during all the seasons which indicated moderately rich phytoplankton diversity in the system which coincides with the observation by Arumugam et al. (2016).
All the diversity index of phytoplankton in the river was recorded as high during post-monsoon (0.80, 2.21, 0.48, and 3.92), while the lowest record was observed during monsoon (0.21, 0.49, 0.16, and 1.39). High index value during post-monsoon might be due to elevated nutrient levels in a water body as Dupuis and Hann (2009) have reported. The relatively lower value of diversity during monsoon might be due to higher and fluctuating water level that causes unfavorable condition for the phytoplankton community (Haque et al. 2021). The significant seasonal variation of the phytoplankton communities in riverine water was due to fluctuation in the available limiting nutrients.
Cluster analysis is the multivariate statistical tool used for the classification of different study sites based on their similarities between them (Alam et al. 2021). Bray–Curtis cluster analysis is the authenticated cluster analysis tool used for the classification based on different similarities. In our study, the highest similarity of algal groups had shown between St-5 of wetland and river. It was confirmed that the riverine connection has a significant effect on the algal community of the wetland.
The algal genius pollution index is used to identify the status of aquatic ecosystems (Parakkandi et al. 2021). The calculated pollution index of wetlands was comparatively higher than that of the river, which might be due to stagnant water in the ecosystem. In the wetland, high organic load was recorded during monsoon and pre-monsoon. During monsoons, intensive rainfall triggers high organic and inorganic suspended particles (Bera et al., 2014).
The Trophic State Index of the wetland was noticed at a minimum during monsoon; then, the value increases in post-monsoon and reaches the maximum during the pre-monsoon. In the case of the river, the index was highest during the monsoon. This indicates the algal biomass of the wetland is regulated due to riverine connectivity.
Effect of different physico-chemical parameters on phytoplankton communities
Canonical correspondence analysis of wetland
Water temperature has a significant effect on algal growth and its proliferation as it regulates entire physiological mechanism of plankton and dependent aquatic organisms (Smith 1950; Munn et al. 1989). In our study, Euglenophyceae, Coscinodiscophyceae, Mediophyceae, and Chlorophyceae were positively correlated with water temperature. Bacillariophyceae had shown a negative correlation with water temperature. It indicates diatoms favor low temperatures for growth (Prants et al. 2015). The photosynthetic activities of phytoplankton are intensified during the day, and when the number of phytoplankton is higher, more oxygen is produced in the aquatic ecosystem (Sekerci and Petrovskii 2018). Thus, dissolved oxygen (DO) had a positive correlation with total phytoplankton abundance. It should be noted that the density of phytoplankton is dependent on nutrient levels as reported by Harris (1986). Many algal communities had shown positively regulated by phosphate, nitrate, nitrite, and silicate. Zygnematophyceae and Dinophyceae were positively correlated with total alkalinity and pH. Photosynthesis affects the carbon equilibrium which tends to increase pH (Lopez-Archilla et al. 2004). Similarly, alkaline water could act as a trap for carbon dioxide, and thereby, photosynthetic groups are benefited (Imhoff et al. 1979). Therefore, highly alkaline water leads to positive relationships with phytoplankton (Zhao et al. 2017).
Canonical correspondence analysis of river
Coscinodiscophyceae, Chlorophyceae, and Zygnematophyceae were positively influenced by total alkalinity, specific conductivity, magnesium, and calcium. A similar observation was given by Potapova and Charles (2003). In the river also, many algal groups, due to photosynthetic activities, correlated with DO. The production and abundance of phytoplankton are regulated by depth and water transparency (Reynolds 1995). In our study, Trebouxiophyceae and Cyanophyceae were influenced by depth while Bacillariophyceae, Mediophyceae, and Euglenophyceae were influenced by transparency. Higher temperatures resulted in higher fractions of cyanobacteria in phytoplankton communities (Naskar et al. 2020). In the present study, Cyanophyceae were positively influenced by water temperature as similar observations were also recorded in the different studies by Seeta et al. (2018).
Climatic uncertainties like rise in air temperature and rainfall irregularities also play an important role in altering the physico-chemical condition of waterbody. Due to global warming, some wetland reduces its size and became dryland. This is leading to loss of biodiversity in future (Desta et al. 2012). To maintain the ecological stability of wetland the riverine connection is mandatory. It will help in maintaining the rich diversity of the wetland as well as the nutritional security, income, and livelihood of the surrounding people.
Conclusion
Nowadays, the assessment of the aquatic environment can be best carried by the help of the resident aquatic organisms, in which plankton plays a vital role as the ecological indicator. The present study reports the relatively higher pollution indicating planktonic genera in the wetland ecosystem in all the seasons. However, the riverine connectivity encourages the purification of the wetland water by the help of dilution. Many of the organisms have uniqueness due to their ecological niche, which can be illustrated by the presence of Ceratium sp. which illustrates its relatively higher suitability for growth and proliferation in the wetland ecosystem. The different ecological variables in the form of water quality parameters have also influenced the plankton assemblage pattern. The present investigation highlighted the significance of common influencing factors like water temperature, dissolved oxygen, pH, turbidity, transparency, specific conductivity, free CO2, depth, total alkalinity, total hardness, Chl-a, calcium, magnesium, and nutrients (total nitrate, nitrate, phosphate, silicate) in influencing the algal community of both water bodies, i.e., wetland and river. Among the biodiversity indices, the observed higher Shannon and Margalef’s species richness value in wetland also describes its ecological importance. The present work also explains how riverine connectivity is essential for the restoration of biotic flora of wetland. The observed higher density of plankton in wetland also illustrates its favorability for the breeding ground and food availability of the aquatic organisms such as fish. The study will be helpful for the policymakers, researchers, and environmentalists to make the future research and planning strategies for the monitoring and conservation of riverine as well as wetland’s biodiversity.
Data availability
Experimental data will be provided by the corresponding authors on reasonable request.
Code availability
Not applicable.
References
Alam, R., Ahmed, Z., Seefat, S. M., & Nahin, K. T. K. (2021). Assessment of surface water quality around a landfill using multivariate statistical method, Sylhet, Bangladesh. Environmental Nanotechnology, Monitoring & Management, 15, 100422.
APHA. (2017). Standard methods for the examination of water and wastewater, (23).
Arumugam, S., Sigamani, S., Samikannu, M., & Perumal, M. (2016). Assemblages of phytoplankton diversity in different zonation of Muthupet mangroves. Regional Studies in Marine Science, 3, 234–241.
Balzer, M. J., Hitchcock, J. N., Hadwen, W. L., Kobayashi, T., Westhorpe, D. P., Boys, C., & Mitrovic, S. M. (2023). Experimental additions of allochthonous dissolved organic matter reveal multiple trophic pathways to stimulate planktonic food webs. Freshwater Biology, 68(5), 821–836.
Bellinger, E. G., & Sigee, D. C. (2015). Freshwater algae: Identification, enumeration and use as bioindicators. John Wiley & Sons.
Bera, A., Dutta, T. K., Bhattacharya, M., Patra, B. C., & Kumar Sar, U. (2022). Anthropogenic stress on river health: With special reference to Kangsabati River, West Bengal, India. In River Health and Ecology in South Asia (pp. 39–61). Springer.
Bera, K. (2014). Geo-informatics approach to demarcate ground water potential zone in semi-arid region of Kansai-Tangai interfluves area. International Multidisciplinary e-Journal, 3(9), 1–9.
Bijoy Nandan, S., Jayachandran, P. R., & Sreedevi, O. K. (2014). Spatio-temporal pattern of primary production in a tropical coastal Wetland (Kodungallur-Azhikode Estuary), South West Coast of India. Journal of Coastal Development, 17, 392.
Boyd, C. E., & Tucker, C. S. (1998). Ecology of aquaculture ponds. In Pond aquaculture water quality management (pp. 8–86). Springer.
Cardoso, S. J., Roland, F., Loverde-Oliveira, S. M., & de MoraesHuszar, V. L. (2012). Phytoplankton abundance, biomass and diversity within and between Pantanal wetland habitats. Limnologica, 42(3), 235–241.
Carlson, R. E. (1977). A trophic state index for lakes 1. Limnology and Oceanography, 22(2), 361–369.
Carlson, R. E., & Simpson, J. (1996). A coordinator’s guide to volunteer lake monitoring methods. North American Lake Management Society, 96, 305.
Chan, F., Pace, M. L., Howarth, R. W., & Marino, R. M. (2004). Bloom formation in heterocystic nitrogen-fixing cyanobacteria: The dependence on colony size and zooplankton grazing. Limnology and Oceanography, 49(6), 2171–2178.
Chaparro, G., O’Farrell, I., & Hein, T. (2019). Multi-scale analysis of functional plankton diversity in floodplain wetlands: Effects of river regulation. Science of the Total Environment, 667, 338–347.
Chaurasia, M., & Pandey, G. C. (2007). Study of physico-chemical characteristics of some water ponds of Ayodhya-Faizabad. Indian Journal of Environmental Protection, 27(11), 1019.
Cox, E. J., & Cox, E. J. (1996). Identification of freshwater diatoms from live material (Vol. 158). Chapman & Hall.
Desikachary, T. V. (1959). Cyanophyta. Indian Council of Agricultural Research.
Desta, H., Lemma, B., & Fetene, A. (2012). Aspects of climate change and its associated impacts on wetland ecosystem functions: A review. Journal of American Science, 8(10), 582–596.
Dixit, R. B., Patel, A. K., Toppo, K., & Nayaka, S. (2017). Emergence of toxic cyanobacterial species in the Ganga River, India, due to excessive nutrient loading. Ecological Indicators, 72, 420–427. https://doi.org/10.1016/j.ecolind.2016.08.038
Dupuis, A. P., & Hann, B. J. (2009). Climate change, diapause termination and zooplankton population dynamics: An experimental and modelling approach. Freshwater Biology, 54(2), 221–235.
Ernst, B., Neser, S., O’Brien, E., Hoeger, S. J., & Dietrich, D. R. (2006). Determination of the filamentous cyanobacteria Planktothrix rubescens in environmental water samples using an image processing system. Harmful Algae, 5(3), 281–289.
Frank, S. J. D., Gopi, G. V., Lakshminarayanan, N., & Pandav, B. (2022). Factors influencing occurrence and species richness of heronries in the wetlands of Tamil Nadu, India. Wetlands, 42(1), 1–10.
Gao, J., Shen, L., Nie, Z., Zhu, H., Cao, L., Du, J., & Xu, G. (2022). Microbial and planktonic community characteristics of Eriocheir sinensis culture ponds experiencing harmful algal blooms. Fishes, 7(4), 180.
Ghosh, D., & Biswas, J. K. (2015). Impact of jute retting on phytoplankton diversity and aquatic health: Biomonitoring in a tropical oxbow lake. Journal of. Ecological Engineering, 16(5), https://doi.org/10.12911/22998993/60449
Ghosh, D., & Biswas, J. K. (2018). Impact of jute retting on physicochemical profile of Chhariganga oxbow lake in Nadia district, West Bengal, India. Archives of Agriculture and Environmental Science, 3(1), 36–44.
Gogoi, P., Das, S. K., Sarkar, S. D., Chanu, T. N., Manna, R. K., Sengupta, A., et al. (2021a). Environmental factors driving phytoplankton assemblage pattern and diversity: Insights from Sundarban eco-region, India. Ecohydrology & Hydrobiology, 21(2), 354–367.
Gogoi, P., Kumari, S., Sarkar, U. K., Lianthuamluaia, L., Puthiyottil, M., Bhattacharjya, B. K., & Das, B. K. (2021b). Dynamics of phytoplankton community in seasonally open and closed wetlands in the Teesta–Torsa basin, India, and management implications for sustainable utilization. Environmental Monitoring and Assessment, 193, 1–25.
Gogoi, P., Sinha, A., Sarkar, S. D., Chanu, T. N., Yadav, A. K., Koushlesh, S. K., et al. (2019). Seasonal influence of physicochemical parameters on phytoplankton diversity and assemblage pattern in Kailash Khal, a tropical wetland, Sundarbans, India. Applied Water Science, 9(7), 1–13.
Guiry, M. D., & Guiry, G. M. (2014). AlgaeBase. National University of Ireland.
Hammer, D. A., & Bastian, R. K. (2020). Wetlands ecosystems: Natural water purifiers? In Constructed wetlands for wastewater treatment (pp. 5–19). CRC Press.
Haque, M., Jewel, M., Sayed, A., Akhi, M., Atique, U., Paul, A. K., et al. (2021). Seasonal dynamics of phytoplankton community and functional groups in a tropical river. Environmental Monitoring and Assessment, 193(11), 1–16.
Harris, L., & Gurel, E. (1986). Price and volume effects associated with changes in the S&P 500 list: New evidence for the existence of price pressures. The Journal of Finance, 41(4), 815–829.
Huang, Z., Pan, B., Soininen, J., Liu, X., Hou, Y., & Liu, X. (2023). Seasonal variation of phytoplankton community assembly processes in Tibetan Plateau floodplain. Frontiers in Microbiology, 14, 1122838.
Imhoff, J. F., Sahl, H. G., Soliman, G. S. H., & Trüper, H. G. (1979). The Wadi Natrun: Chemical composition and microbial mass developments in alkaline brines of eutrophic desert lakes. Geomicrobiology Journal, 1(3), 219–234.
Issa, A. A., Abd-Alla, M. H., & Ohyama, T. (2014). Nitrogen fixing cyanobacteria: Future prospect. Advances in Biology and Ecology of Nitrogen Fixation, 2, 24–48.
Kahsay, A., Lemmens, P., Triest, L., De Meester, L., Kibret, M., Verleyen, E., et al. (2022). Plankton diversity in tropical wetlands under different hydrological conditions (Lake Tana, Ethiopia). Frontiers in Environmental Science, 189.
Kataki, S., Chatterjee, S., Vairale, M. G., Sharma, S., Dwivedi, S. K., & Gupta, D. K. (2021). Constructed wetland, an eco-technology for wastewater treatment: A review on various aspects of microbial fuel cell integration, low temperature strategies and life cycle impact of the technology. Renewable and Sustainable Energy Reviews, 148, 111261.
Kumar, J., Alam, A., Sarkar, U. K., Das, B. K., Kumar, V., & Srivastava, S. K. (2020). Assessing the phytoplankton community and diversity in relation to physico-chemical parameters in a tropical reservoir of the River Ganga basin, India. Sustainable Water Resources Management, 6, 1–15.
Lackey, J. B. (1938). The manipulation and counting of river plankton and changes in some organisms due to formalin preservation. Public Health Reports (1896-1970), 2080–2093.
Lakshminarayana, J. S. S. (1965). Studies on the phytoplankton of the River Ganges, Varanasi, India, Part II “The seasonal growth and succession of the plankton algae in the River Ganges”. Hydrobiologia, 25(1), 138–165.
Li, X., Zhao, Y., Chai, F., Yu, H., Sun, X., & Liu, D. (2022). Phytoplankton community structure dynamics in relation to water environmental factors in Zhalong Wetland. International Journal of Environmental Research and Public Health, 19(22), 14996.
Liu, X., Song, J., Ren, Y., Zhan, D., Liu, T., Liu, K., et al. (2023). Spatio-temporal patterns of zooplankton community in the Yellow River estuary: Effects of seasonal variability and water-sediment regulation. Marine Environmental Research, 106060. https://doi.org/10.1016/j.marenvres.2023.106060
Lopez-Archilla, A. I., Moreira, D., López-García, P., & Guerrero, C. (2004). Phytoplankton diversity and cyanobacterial dominance in a hypereutrophic shallow lake with biologically produced alkaline pH. Extremophiles, 8(2), 109–115.
Meena, D. K., Lianthuamluaia, L., Mishal, P., Swain, H. S., Naskar, B. K., Saha, S., et al. (2019). Assemblage patterns and community structure of macro-zoobenthos and temporal dynamics of eco-physiological indices of two wetlands, in lower Gangetic plains under varying ecological regimes: A tool for wetland management. Ecological Engineering, 130, 1–10.
Mitbavkar, S., & Anil, A. C. (2008). Seasonal variations in the fouling diatom community structure from a monsoon influenced tropical estuary. Biofouling, 24(6), 415–426.
Mitsch, W. J., Bernal, B., Nahlik, A. M., Mander, Ü., Zhang, L., Anderson, C. J., Jørgensen, S. E., & Brix, H. (2013). Wetlands carbon, and climate change. Landscape Ecology, 28, 583–597. https://doi.org/10.1007/s10980-012-9758-8
Mohanty, T. R., Tiwari, N. K., Kumari, S., Ray, A., Manna, R. K., Bayen, S., et al. (2022). Variation of Aulacoseira granulata as an eco-pollution indicator in subtropical large river Ganga in India: A multivariate analytical approach. Environmental Science and Pollution Research. https://doi.org/10.1007/s11356-021-18096-9
Munn, M. D., Osborne, L. L., & Wiley, M. J. (1989). Factors influencing periphyton growth in agricultural streams of central Illinois. Hydrobiologia, 174(2), 89–97.
Nag, S. K., Das Ghosh, B., Nandy, S., Aftabuddin, M., Sarkar, U. K., & Das, B. K. (2023). Comparative assessment of carbon sequestration potential of different types of wetlands in lower Gangetic basin of West Bengal, India. Environmental Monitoring and Assessment, 195(1), 154.
Naskar, M., Sarkar, S. D., Raman, R. K., Gogoi, P., Sahu, S. K., Chandra, G., & Bhor, M. (2020). Quantifying plankto-environmental interactions in a tropical river Narmada, India: An alternative model-based approach. Ecohydrology & Hydrobiology, 20(2), 265–275.
Palit, D., & Mukherjee, A. (2010). Characterization of physico-chemical properties of water and soil in Lalbandha fresh water wetland in Birbhum District West Bengal. Ecology, Environment and Conservation Journal, 15(4), 239–245.
Palmer, C. M. (1969). A composite rating of algae tolerating organic pollution 2. Journal of phycology, 5(1), 78–82.
Parakkandi, J., Saha, A., Sarkar, U. K., Das, B. K., Puthiyottil, M., Muhammadali, S. A., et al. (2021). Spatial and temporal dynamics of phytoplankton in association with habitat parameters in a tropical reservoir, India. Arabian Journal of Geosciences, 14(10), 1–15.
Potapova, M., & Charles, D. F. (2003). Distribution of benthic diatoms in US rivers in relation to conductivity and ionic composition. Freshwater Biology, 48(8), 1311–1328.
Prants, S. V., Andreev, A. G., Budyansky, M. V., & Uleysky, M. Y. (2015). Impact of the Alaskan Stream flow on surface water dynamics, temperature, ice extent, plankton biomass, and walleye pollock stocks in the eastern Okhotsk Sea. Journal of Marine Systems, 151, 47–56.
Prescott, G. W. (1982). Algae of the Western Great Lakes Area (pp. 1–977). Otto Koeltz Science Pub.
Rai, U. N., Tripathi, R. D., Singh, N. K., Upadhyay, A. K., Dwivedi, S., Shukla, M. K., et al. (2013). Constructed wetland as an ecotechnological tool for pollution treatment for conservation of Ganga river. Bioresource Technology, 148, 535–541.
Reynolds, C. S., (1995) River plankton – The paradigm regained. In: D. Harper and A. J. D. Ferguson (eds.), Ecological Basis for River Management. Wiley, New York, pp. 161–174.
Sahu, G., Satpathy, K. K., Mohanty, A. K., & Sarkar, S. K. (2012). Variations in community structure of phytoplankton in relation to physicochemical properties of coastal waters, southeast coast of India.
Sandilyan, S. (2022). Alien fish species in Indian inland wetlands: Current status and future challenges. Wetlands Ecology and Management, 30(2), 423–437.
Saravanakumar, A., Rajkumar, M., Thivakaran, G. A., & Serebiah, J. S. (2008). Abundance and seasonal variations of phytoplankton in the creek waters of western mangrove of Kachchh-Gujarat. Journal of Environmental Biology, 29(2), 271.
Sarkar, S. D., Sahoo, A. K., Gogoi, P., Raman, R. K., Munivenkatappa, M. H., Kumari, K., et al. (2019). Phytoplankton biomass in relation to flow dynamics: The case of a tropical river Mahanadi, India. Tropical Ecology, 60(4), 485–494.
Sarkar, U. K., Mishal, P., Borah, S., Karnatak, G., Chandra, G., Kumari, S., et al. (2020). Status, potential, prospects, and issues of floodplain wetland fisheries in India: Synthesis and review for sustainable management. Reviews in Fisheries Science and Aquaculture, 29(1), 1–32. https://doi.org/10.1080/23308249.2020.1779650
Sayeed, M., Hossain, M. A. R., Wahab, M., Hasan, M., Simon, K. D., & Mazumder, S. K. (2015). Water and sediment quality parameters in the Chalan Beel, the largest wetland of Bangladesh. Chinese Journal of Oceanology and Limnology, 33(4), 895–904.
Seeta, Y., & Reddy, P. M. (2018). Ecological studies of the River Krishna near Gadwal, Telangana with reference to water quality. G-Journal of Environmental Science and Technology, 5(4), 37–39.
Sekerci, Y., & Petrovskii, S. (2018). Global warming can lead to depletion of oxygen by disrupting phytoplankton photosynthesis: A mathematical modelling approach. Geosciences, 8(6), 201.
Sharif, A. S. M., Islam, M. S., Hoque, M. N., & Bhuyan, M. S. (2017). Spatial and temporal environmental effect of lower Meghna River & its estuary on phytoplankton, Bangladesh. System, 8(9), 10–11.
Shen, J., Qin, G., Yu, R., Zhao, Y., Yang, J., An, S., et al. (2021). Urbanization has changed the distribution pattern of zooplankton species diversity and the structure of functional groups. Ecological Indicators, 120, 106944.
Singh, Y., Singh, G., Khattar, J. S., Barinova, S., Kaur, J., Kumar, S., & Singh, D. P. (2022). Assessment of water quality condition and spatiotemporal patterns in selected wetlands of Punjab, India. Environmental Science and Pollution Research, 29(2), 2493–2509.
Singha, P., & Pal, S. (2023). Wetland transformation and its impact on the livelihood of the fishing community in a flood plain river basin of India. Science of The Total Environment, 858, 159547.
Smith, G. M. (1950). Fresh-water algae of the United States.
Sridhar, R., Thangaradjou, T., Kumar, S. S., & Kannan, L. (2006). Water quality and phytoplankton characteristics in the Palk Bay, southeast coast of India. Journal of environmental biology, 27(3), 561–566.
Tas, B., & Gonulol, A. (2007). An ecologic and taxonomic study on phytoplankton of a shallow lake, Turkey. Journal of Environmental Biology, 28(2), 439.
Tiwari, N. K., das Gupta, S., Swain, H. S., Jha, D. N., Samanta, S., Manna, R. K., et al. (2022a). Water quality assessment in the ecologically stressed lower and estuarine stretches of river Ganga using multivariate statistical tool. Environmental Monitoring and Assessment, 194(7), 1–26.
Tiwari, N. K., Mohanty, T. R., Swain, H. S., Manna, R. K., Samanta, S., & Das, B. K. (2022b). Multidecadal assessment of environmental variables in the river Ganga for pollution monitoring and sustainable management. Environmental Monitoring and Assessment, 194(8), 1–33.
Varol, M., & Şen, B. (2018). Abiotic factors controlling the seasonal and spatial patterns of phytoplankton community in the Tigris River, Turkey. River Research and Applications, 34(1), 13–23.
Venkateshwarlu, M., Shahnawaz, A., & Honneshappa, K. (2011). A study on plankton dynamics of two wetland systems in Shimoga District, Karnataka (India). Current Biotica, 4(4), 461–468.
Visser, P. M., Ibelings, B. W., Bormans, M., & Huisman, J. (2016). Artificial mixing to control cyanobacterial blooms: A review. Aquatic Ecology, 50(3), 423–441.
Wetzel, R. G., Likens, G. E., Wetzel, R. G., & Likens, G. E. (1991). Inorganic nutrients: nitrogen, phosphorus, and other nutrients. Limnological Analyses, 81–105.
Xiao, R., Wang, Q., Zhang, M., Pan, W., & Wang, J. J. (2020). Plankton distribution patterns and the relationship with environmental gradients and hydrological connectivity of wetlands in the Yellow River Delta. Ecohydrology & Hydrobiology, 20(4), 584–596.
Ye, X., Meng, Y., Xu, L., & Xu, C. (2019). Net primary productivity dynamics and associated hydrological driving factors in the floodplain wetland of China’s largest freshwater lake. Science of the Total Environment, 659, 302–313.
Zhao, W., Li, Y., Jiao, Y., Zhou, B., Vogt, R. D., Liu, H., et al. (2017). Spatial and temporal variations in environmental variables in relation to phytoplankton community structure in a eutrophic river-type reservoir. Water, 9(10), 754.
Funding
Funding for the abovementioned work is provided by the National Mission for Clean Ganga, Ministry of Jal Shakti, Government of India.
Author information
Authors and Affiliations
Contributions
Trupti Rani Mohanty: plankton analysis and MS preparation; Nitish Kumar Tiwari: field sampling, water quality analysis, statistical analysis, and MS preparation; Basanta Kumar Das: conceptualization, fund acquisition, and MS review; Himanshu Sekhar Swain: MS writing; Canciyal Jhonson: MS writing; Tanushree Banarjee: MS writing. All the author’s reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical responsibilities of authors
All authors have read, understood, and have complied as applicable with the statement on “Ethical responsibilities of Authors” as found in the Instructions for Authors and are aware that with minor exceptions, no changes can be made to authorship once the paper is submitted.
Ethical approval
All the abovementioned work has been carried out as per the approval of the Institute’s ethical committee, ICAR-CIFRI.
Consent to participate
Not applicable
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mohanty, T.R., Tiwari, N.K., Das, B.K. et al. Riverine connectivity influences the phytoplankton ecology in the open floodplain wetland of the lower river Ganga. Environ Monit Assess 195, 1403 (2023). https://doi.org/10.1007/s10661-023-11983-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-11983-3