Abstract
Aulacoseira granulata (Ehrenberg) Simonsen 1979 are considered as the eco-variable species which varies in density and diversity along with their morphological traits with the interference of environmental changes, so it is considered as one of the major ecological indicators of the water quality of lotic as well as lentic aquatic ecosystems. To assess major environmental factors which contribute to A. granulata bloom in the riverine system, a study was carried out from 2018 to 2019 comprising four different seasons at 11 sampling sites of river Ganga in the middle and lower stretch of river Ganga comprising freshwater and estuarine zones. For the analysis, different univariate, as well as multivariate, analytical tools such as principal component analysis (PCA) and water pollution index (WPI) were used. In the finding, it was observed that the average abundance of A. granulata was found maximum during the winter season. Among all the studied sites, the maximum average abundance was at Balagarh (71,576 cell l−1) and minimum at Diamond Harbour (68 cell l−1). The environmental factors such as dissolved oxygen, depth, and altitude showed a significant influence on the growth of A. granulata, while the water temperature negatively influenced the growth rate of A. granulata. The WPI showed a significantly negative correlation with cell length. Finally, the study concludes that the blooming of A. granulata is highly influenced by varied environmental conditions along the river Ganga, suggesting possible eutrophication. Therefore, a certain minimum flow and depth especially during the lean season have to be maintained for the sustenance of planktonic biota in the river Ganga.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Planktonic organisms are considered as a true biotic indicator of any sort of alterations in aquatic health. Being the nodal point of any aquatic food chain, it certainly depicts the condition through which an aquatic environment is passing by, as they are highly sensitive to any sort of abiotic abnormalities in the environment (Pham 2017). The planktonic bloom in several open water aquatic systems has now become a serious concern globally which is further coupled with increasing anthropogenic pollution (Nwankwegu et al. 2019; Hossain et al. 2012). Blooms are often a noticeable occurrence irrespective of the form of the aquatic ecosystem (lotic or lentic). Epiphytic plankton, mainly diatoms, are being used as ecological indicators for several decades (Rimet et al. 2015). In a lotic ecosystem such as rivers and streams, diatoms form one of the ubiquitous planktonic communities (Jia et al. 2009). These diatoms (Bacillariophyceae) have also been reported from the mighty Ganga river system in several past literatures (Hossain et al. 2012; Pandey et al. 2017; Srivastava et al. 2020). Diatom community representing the genus Aulacoseira (commonly known as pennate diatoms) belonging to the phylum Bacillariophyceae are widely available species in various inland freshwater systems (Chong et al. 2009). They are found mostly in colonial growth habitats. The growth rate of the diatoms is faster in contrast to other phytoplanktonic taxa (Wetz and Wheeler 2007). The species representing the genus Aulacoseira form a linear colony by linking together with spines (Cox and Cox 1996). They are very large, complex, oldest non-marine, and widely distributed diatoms (Ambwani et al. 2003) and best adapted to all inland water bodies like ponds, lakes, streams, and rivers. The genus Aulacoseira has been reported to have 144 species from all over the world (Guiry and Guiry 2021). It is also reported to indicate a eutrophic condition in rivers as they accumulate to form high biomass (Hötzel and Croome, 1996; Yang et al. 1997). Physical and chemical characteristics of an aquatic body like pH, temperature, altitude, etc. play a vital role in the diversification of diatoms (You et al. 2009). Though very widely spread, the diatom species Aulacoseira granulata is considered to be the most dominant in carbonate-rich eutrophic water bodies (Kilham and Kilham 1975; Margalef 1983). They are among the few bloom-forming diatom species (Aulacoseira sp., Cyclotella sp., Stephanodiscus sp., and Fragilaria sp.) in hyper-eutrophic freshwater environments (Ha et al. 2003). Earlier works have reflected dominancy of the phytoplankton group mainly by diatoms (Bacillariophyceae) on river Ganga contributing to 100.0, 56.0, and 50% in upper, middle, and lower zones, respectively (Pathak and Tyagi 2010). The formation of bloom, the impact of abundance, and growth of A. granulata have been documented in several lentic as well as lotic water bodies from all over the world (Wang et al. 2017; Wang et al. 2020), but the variation and quantified data on the abundance of A. granulata which are lacking in large flowing river systems are yet to be addressed. River Ganga is designated to be the largest river in India sprawling over 26% of the total Indian geographical area. The river gorges over three different eco-regions of India and thus sustaining a large number of multifarious in terms of several aquatic micro-flora and fauna (Sanghi 2013; Tripathi et al. 2017). In river Ganga, the diversity of diatoms was high during summer but density was high during winter due to A. granulata (Roshith et al. 2018). In this context, an attempt has been made to document the seasonal occurrence and pattern of A. granulata with the aim of assessing the onsets of blooming conditions, factors triggering its actuality in the river, and its impact on ecological aspects. The study will also quantify and evaluate A. granulata acting as a pollution indicator species in the large subtropical river of India.
Materials and methods
Study sites
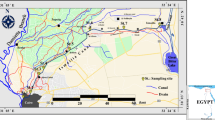
River Ganga is the largest in India and the fifth largest in the world. The river emerges out to be cool in the uplands in the north and warmer during its course of flow until it is finally confluence to form the biggest delta in the world before draining into the Bay of Bengal in the east. The sites covered were almost 1208 km and a total of 11 sampling stations comprising the Indian states of Bihar and West Bengal. The entire stretch has been divided into two zones based on their hydrological characteristics. Freshwater zones are mainly composed of Buxar, Patna, Bhagalpur, Farakka, Jangipur, and Berhampore. Likewise, the estuarine stretch covers the regions Balagarh, Tribeni, Godakhali, Diamond Harbour, and Fraserganj. All the sites have been selected based on their physical nature of water (tidal, etc.) and altitude gradient (Fig. 1).
Climatic condition
The entire Ganga basin specifically the middle and lower stretches experiences a typical monsoon climate extending from June to October. Although there are some differences in precipitation between the middle and lower Ganga, the annual amount of rainfall remains the same which is 1000 mm (Quincey 2017). Warm and dry seasons extend from March to May; however, winter starts from November and ends in February.
Sample collection and identification
Plankton samples were collected quarterly for 1 year (2018 to 2019) covering all four tropical seasons. Composite samples from the mid-channel of the river were collected manually against the water current using the typical country boat. To maintain uniformity, a total of 100 l of plankton samples were collected at first from the subsurface depth of 0.5–1.0 m using a water sampler from which 1 l of the mixed representative sample was taken out. Common conical tow net (mesh size 20 µm) was used to serve the purpose. One liter of plankton sample was also collected without any filtration, fixed in Lugol’s iodine solution, and analyzed based on sedimentation method to check if all representatives of the species are present. Plankton samples were collected in dry polyethylene containers using 4% buffered formalin as a preservative. The identification was done following the standard literature such as Moro (1991), Kulikovskiy et al. (2016), and the International Code of Biotic Nomenclature (ICBN). For valid and updated names, AlgaeBase was followed. Samples from rich eutrophic regions were diluted for analysis. For taxonomic identification and qualitative assessment, 0.5 ml of each mixed sample was analyzed under a microscope (Model No. Zeiss Scope A1). The counts were recorded in cell l−1. Cell size was represented by cell diameter and cell length; filament size was represented by filament width (cell diameter) and filament length (in terms of cell numbers per filament).
Water quality parameter
Water samples were collected between 0830 and 0930 h for determining various water quality parameters. In situ analysis of parameters like water temperature (℃), pH, conductivity (µS/cm), and turbidity (NTU) was carried out in the field by using YSI ProDSS multi-parameter probe, and velocity (m/sec) was measured using a digital flow meter. Depth was measured using an ultrasonic depth meter. Transparency (cm) was measured by a Secchi disk. Dissolved oxygen (ppm) was done by Winkler’s method in the field. Whereas for nutrient parameters such as nitrate (ppm), phosphate (ppm), sulfate (ppm), and silicate (ppm), water samples were taken to the laboratory at 4 °C. All the water quality parameters were analyzed following the APHA (2017).
Water pollution index (WPI)
For evaluation and characterization of the different sampling stations based on their chemical health, the water pollution index was calculated, which has been derived and modified from the nutrient pollution index developed in the USA by Dodds et al. (1998) and in South Korea by Lee and An (2009) and lastly modified by Atique and An (2019). For the analysis of the nutrient regime total nitrogen and total phosphorus, ratio of total nitrogen:total phosphorus was utilized. For the analysis of the organic matter, biochemical oxygen demand (BOD) was used. For ionic contents and solids, the total suspended solids and specific conductivity were used. Chlorophyll was used for the estimation of primary production (Wang et al. 2017). The calculated values were compared and graded based on scores, i.e., for excellent (31–35), good (25–29), fair (19–23), poor (13–17), and very poor (7–11).
Statistical analysis
A multivariate statistical analysis, principal component analysis (PCA), was followed using Past 4.03 software. So for the study, 29 different environmental variables, phytoplankton (PHYTO), A. granulata (AUL), zooplankton (ZOO), total plankton (TPLANK), water temperature (WT), depth (DEP), transparency (TRAN), velocity (VEL), turbidity (TUR), conductivity (COND), pH, dissolved oxygen (DO), biochemical oxygen demand (BOD), total alkalinity (TALK), free carbon dioxide (FCO2), calcium (CAL), total hardness (THAR), salinity (SAL), total nitrogen (TOTN), available nitrogen (AVN), total phosphorus (TOTP), silicate (SILI), total solid (TS), total dissolved solid (TDS), total suspended solid (TSS), chlorophyll A (CHA), chlorophyll B (CHB), chlorophyll C (CHC), and total chlorophyll (TCHL), were used, which were distributed at 11 different sampling stations (Fig. 4), i.e., Buxar, Patna, Bhagalpur, Farakka, Jangipur, Berhampore, Balagarh, Tribeni, Godakhali, Diamond Harbour, and Fraserganj.
Karl Pearson’s correlation was also performed to find out the influencing factors which determinedly affect the bloom and growth of A. granulata. Prior to analysis of the data, the Kolmogorov–Smirnov test for normality was also performed, and the data sets were found suitable for the analysis using SPSS software 20.1.
The Bray–Curtis cluster analysis was performed to know the similarity among the different stations based on total length, valve length, cell number, cell length, and density (Guinder et al. 2020).
Results and discussion
Morpho-taxonomic identification of A. granulata
Class: Coscinodiscophyceae.
Order: Aulacoseirales.
Family: Aulacoseiraceae.
A. granulata is a pinnate diatom belonging to the class Bacillariophyceae. The cells are tightly connected to form a long filament. The cell wall has rows of dots and a ring of spines, located at the end of the cell. The deep valve mantle forms a right-angled junction with the valve face. Valve is striate. Chloroplasts are small disk- or plate-like. The species is designated to be a nano-plankton (< 30 µm) comprising series of valves. The average total length was found to be 418 ± 2.89 µm while the mean valve length was recorded to be 12.45 ± 0.06 µm. The total length of the filament including spine length, valve length, total cell count, and cell length of a single filament varied significantly among all the stations. In the region (Berhampore, Balagarh, and Tribeni) where the density of A. granulata was more, the size range was observed lower (Table 1). Pictures of A. granulata are given in Fig. 2.
Seasonal abundance of A. granulata
It is noteworthy that the total volume of the plankton varies seasonally in all aquatic systems, and the same has been observed in the present study of river Ganga (Lakshminarayana 1965). During the pre-monsoon months (February to April), the density of A. granulata was observed to be 905 cell l−1. The count of A. granulata was recorded lowest (113 cell l−1) during the monsoon months (June to August). This can be attributed to the huge influx of water in the Ganga from adjacent tributaries during peak monsoon. This is characterized by high water velocity (avg. 0.6 m sec−1) and depth (av. 10.1 m) resulting in the breaking of the thermocline. During post-monsoon (September to October), the density of A. granulata was observed at 352 cell l−1. The riverine diatom, A. granulata, dominated during the winter season extending from December to January with the abundance of 52,970 cells l−1 throughout the entire river stretch (Fig. 3). This might be due to the sluggish nature of water velocity (avg. 0.4 m sec−1) and conducive environmental factors during the period.
Stretch-wise distribution of A. granulata abundance
The sudden prolific rise of diatom A. granulata has been recorded from all the stations with the highest value at Balagarh (285,863 cell l−1) followed by Tribeni (153,608 cell l−1) and Berhampore (20,942 cell l−1). The blooming conditions of A. granulata have been recorded to significantly peak during the winter months. During post-monsoon months, the highest abundance of A. granulata was found at Buxar and Tribeni (5652 cell l−1), while correspondingly the lowest abundance was recorded at the estuarine stretch (Godakhali. Diamond Harbour, and Fraserganj). All the stations showed the lowest dominance of A. granulata throughout monsoon. In this season, the highest dominance of the diatom was observed at Bhagalpur (780 cell l−1), while the least dominance was observed at Buxar and Diamond Harbour. The diatom A. granulata was recorded high at Bhagalpur (5652 cell l−1) and low at Berhampore during pre-monsoon. Stretch-wise seasonal variation of A. granulata is shown in Fig. 4.
Water pollution index
The water pollution index was calculated for the assessment of the different sampling stations (Table 2). The analysis revealed that all the sampling stations were in the fair category of the WPI, although the scores varied among the different stations between 19 and 23. A score of 19 was obtained from different stretches, viz., Buxar, Patna, Bhagalpur, Balagarh, and Fraserganj. A score value of 21 was obtained at Farakka, Tribeni, and Diamond Harbour, whereas comparatively higher scores of 23 were obtained at Jangipur, Berhampore, and Godakhali.
The correlation was done between WPI and several other morphological characteristics (total length of the filament, valve length, total cell count of one filament, cell length of one filament, and abundance) of A. granulata (Fig. 5). The same correlation was obtained between different structural parameters and a significantly negative correlation with WPI was obtained with cell length (r = − 0.663) (Fig. 5). Cell total length (r = − 0.587, p > 0.01), valve length (r = − 0.492, p > 0.01), cell number (r = − 0.518, p > 0.01), and cell density were found to negatively correlate with WPI but not significantly. It indicated when the value of WPI was high, the total length of the cell decreases.
Correlation between different water quality variables and A. granulata abundance
The Karl Pearson correlation was carried out in which the significant correlation (p < 0.05) was observed with different environmental constraints, i.e., water temperature (WT), depth (DEP), transparency (TRAN), velocity (VEL), turbidity (TUR), conductivity ( COND), pH, dissolved oxygen (DO), biochemical oxygen demand (BOD), total alkalinity (TALK), free carbon dioxide (FCO2), calcium (CAL), total hardness (THAR), salinity (SAL), total nitrogen (TOTN), available nitrogen (AN), total phosphorus (TOTP), silicate (SILI), total solid (TS), total dissolved solids (TDS), total suspended solids (TSS), chlorophyll A (CHA), chlorophyll B (CHB), chlorophyll C (CHC), and total chlorophyll (TCHL) (Table 3), which can be considered as influencing factors that determinedly affect the bloom and growth of A. granulata. Significantly positive correlation was observed with depth (r = 0.342), dissolved oxygen (r = 0.507), and chlorophyll A (r = 0.564). However, a significantly negative correlation was observed with water temperature (r = − 0.402).
Principal component analysis
In the study, 7 PCs were selected where the eigenvalue was > 1. The combined variance percentage for the 7 PCs was 78.84%. In the 1st PC where the variance percentage was 28.94%, a strong positive loading was observed with conductivity (r = 0.89), total hardness (r = 0.83), salinity (r = 0.91), and total suspended solids (r = 0.78), while moderate positive loading was observed with calcium (r = 0.74), chlorophyll B (r = 0.56), and chlorophyll C (r = 0.69). No strong negative loading was observed. Moderate negative loading was found with dissolved oxygen (r = − 0.65) and silicate (r = − 0.56). The 2nd PC having 13.27% variance has no strong positive loading. Moderate positive loading was observed with free carbon dioxide (r = 0.60) and chlorophyll C (r = 0.50), while there was no strong and moderate negative loading observed in the 2nd PC. In the 3rd PC, the variance percentage was 12.39% and moderate positive loading was observed with total alkalinity (r = 0.57) and total chlorophyll (r = 0.65). Moderate negative loading was observed with water temperature (r = − 0.50); in the 4th PC, a variance of 9.54% was observed. Moderate positive loading was observed with A. granulata (r = 0.65) and depth (r = 0.54), while moderate negative loading was observed with transparency (r = − 0.56) and total phosphorus (r = − 0.58). In 5th PC, the variance % was 5.49%, whereas no strong and moderate positive loading was observed. Moderate negative loading was found with biochemical oxygen demand (r = − 0.65). In the 6th PC, the variance % was 4.94%, and in this, no strong and moderate positive loading was observed. In the case of nutrient parameters, moderate negative loading was found with total nitrogen (r = − 0.55). In the 7th PC where the variance % was 4.30, there was no strong and moderate positive or negative loading observed in it. The results of PCA are given in Fig. 6 and Tables 4 and 5.
Cluster analysis
In the analysis, it was found that cophenetic correlation was 0.92 which shows the best suitability of the data for cluster analysis among the different stations. In the analysis, 2 paired groups were formed. In the first paired group, the similarity was found between Bhagalpur, Berhampore, Tribeni, and Balagarh. The 2nd group has 3 subgroups. Firstly, Patna and Buxar showed maximum similarity. Stations like Farakka and Godakhali showed a similar trend in the second subgroup. The same trend was followed in the third subgroup between Diamond Harbour and Fraserganj (Fig. 7).
WPI vis-à-vis morphological variation
For comparing the morphological traits by the WPI, we relied upon the Karl Pearson correlation methods. The data highlighted that all the morphological traits exhibited an inverse relationship with the WPI. Interestingly, the relationship between cell length and WPI indicated significant negative correlation (r = − 0.66, p > 0.01). The mean cell number per filament was also recorded relatively consistent with 10.94 ± 3.08. This can be attributed to that WPI influences the overall morpho characteristics of the diatom. The results outlined that the total cell length of the diatoms was observed to decrease significantly with the increased value of the WPI. Further, the findings suggest a significant variation in other diatom morphology with the chemical loadings. Total nitrogen showed a significant positive correlation with the valve length (r = 0.706, p > 0.05). Similarly, total phosphate revealed a significant positive relationship with valve length (r = 0.646, p > 0.05) and cell length (r = 0.687, p > 0.05), respectively. Silicate was also found to establish an intricate positive relationship with the morpho parameters like valve length (r = 0.711, p > 0.05) and cell length (r = 0.640, p > 0.05), respectively.
Discussion
Planktonic organisms are considered as the aquatic bioindicator in the open water system and especially in the riverine system (Parmar et al. 2016) and the blooming condition of any of the diatoms themself explain the eutrophic status of the water quality which signifies the lower water flow rate in the system (Yang et al. 2012). The study was conducted to monitor the blooming condition and distribution pattern of A. granulata in the Ganga river system.
Taxonomy
Bacillariophyceae is widely recognized as a diatom and is predominantly unicellular consisting of rigid box-shaped silicified cell walls recognized as frustules (Cupp 1943). The species is first reported by Simonsen in 1979 (Guiry and Guiry 2021) whose basionym is Gaillonella granulata (Ehrenberg) and the synonym is Melosira granulata (Ehrenberg) Ralfs (Mayama 1991). The morphological characteristics ensuring the confirmation of A. granulata consist of long linked cells with upright spines inserted on valves (Cox and Cox 1996). The variety is characterized by the presence of 1–4 separated pines (Bicudo et al. 2016); the average total length was 418 ± 2.89 µm; and the mean valve length was 12.45 ± 0.06-µm cell length (Cox and Cox 1996). The species A. granulata exhibits a diverse range of morphological variations (Kilham and Kilham 1975). The structural variation of A. granulata in the different aquatic ecosystems has been observed diversely (Wang et al. 2020).
Ecology and distribution: This species has a worldwide distribution (Guiry and Guiry 2014) and occurs in a wide range of trophic conditions but is mostly associated with eutrophic waters (Taylor et al. 2007).
Distribution and composition pattern
Many genera and species of phytoplankton communities belonging to Bacillariophyceae have the potential to enhance the degree of eutrophication in the river Ganga (Srivastava et al. 2016). Similarly, Roshith et al. (2018) and Parakkandi et al. (2021) have also observed that diatom, A. granulata, is a dominant species among the phytoplankton population in the summer and winter seasons. Lakshminarayana (1965) also reported that the diatoms had two peak points: one is summer season (March) and another one is winter (December) in the river Ganga. The present investigation also agreed with the findings that the species A. granulata has shown a higher degree of abundance during pre-monsoon (905 cell l−1) and winter season (53,188 cell l−1). The sudden plentiful rise of diatom A. granulata has been confronted from all the stations with the highest at Balagarh (285,863 cell l−1) followed by Tribeni (153,608 cell l−1) and Berhampore (20,942 cell l−1).
The Bray–Curtis cluster analysis was performed to know the similarity among the different stations based on total length, valve length, cell number, cell length, and density (Guinder et al. 2020). The present study indicated a similar trend of the population in all the freshwater and saline water zones. The introduction of saline water in the lower sites like Fraserganj and Diamond Harbour has also created alteration in the morphological structure. The results obtained were found in agreement with previous studies (Blum 1957; Kanaya and Kikuchi 2008).
Physicochemical properties
Among the different physicochemical parameters, the significantly influenceable parameters are water temperature (r = − 0.402), depth (r = 0.342), dissolved oxygen (r = 0.507), and chlorophyll A (r = 0.564), which were similar to the other studies of different water bodies such as Danjiangkou Reservoir, China (Zheng et al. 2020). Water temperature negatively affected the growth of A. granulata showing a higher growth rate during the winter season. The different nutrient parameters are dependent on water temperature (Thomas et al. 2017), which also affects the chlorophyll A composition and variation in the river system, by which the planktonic diversity and density are dependent (Moeller et al. 2019). Thus composition and variation of chlorophyll control the growth of plankton communities (Moeller et al. 2019). Similar observations, i.e., the influence of water temperature on plankton growth specifically freshwater diatoms, were found in several studies (Zhang et al. 2019). Similarly, the density has been positively influenced by depth during winter, and higher depths were observed in the river as similar to the Gharraf River of Iraq (Al-Husseini and Alsalman 2020). A significant positive correlation with dissolved oxygen was also observed; during daytime with sunlight, the photosynthetic activities of the phytoplankton get enhanced; and relatively more oxygen is released in the aquatic system when the numbers of phytoplankton is higher and is also depending on water temperature (Sekerci and Petrovskii 2018). In the present study, higher dissolved oxygen in the riverine system has also been reported during the post-monsoon and winter season. Chlorophyll A greatly influences the process of photosynthesis in aquatic plants (Tsai et al. 2019). Similar observations were also found in the present findings as a significantly positive correlation with chlorophyll A was found with A. granulata.
PCA bi-plot is a well-known statistical tool for the analysis of specific variable and their interdependency on the other environmental variables (Singh et al. 2019). The principal component analysis is a widely accepted multivariate statistical tool used for the analysis of the wide number of environmental factors affecting the diversity and density of the planktonic species (Pratiwi 2019; Herawati et al. 2020; Matta et al. 2020). In this finding, the 1st PC is having positive loading with conductivity, total hardness, salinity, total suspended solids, calcium, chlorophyll B, and chlorophyll C, while the negative loading was found with dissolved oxygen and silicate. The loading revealed the influence of the estuarine zone of the river. The same has been observed in other studies made in the estuarine stretch (Hooghly estuary) of river Ganga by Rakshit et al. (2017). The 2nd PC is having positive loading with free CO2, and chlorophyll C shows the impact of monsoon; due to cloudy weather, the free CO2 and higher chlorophyll content were observed in the riverine system; and the observations were the same as those of the Erai river of Maharashtra, India, and in the metropolitan city of Korea (Shende and Rathoure 2019; Hong et al. 2020). The 3rd PC is having positive loading with total alkalinity and total chlorophyll, while negative loading was observed with water temperature; the PC may also have denoted the contribution of organic matter loading and ion dissolution in the riverine system. The observations were similar to those of the upper stretch of river Ganga (Dimri et al. 2021). In the 4th PC, positive loading was observed with A. granulata and depth, and negative loading was found with transparency and total phosphorus. The PC signifies the importance and influence of river depth. The negative loading with water transparency signifies the relatively higher riverine velocity which causes turbidity in the river (Ayoade et al. 2009). The negative loading with total phosphorus also signifies the higher riverine flow in which the nutrients such as phosphorus and nitrogen get dissolved. Similar observations were also observed with the Mississippi River where the maximum blooms of cyanotoxin-producing plankton were observed (Bargu et al. 2011). In the 5th and 6th PC, negative loading was observed with biochemical oxygen demand and total nitrogen. This signifies the pollution status of the river and higher nutrient load which may cause eutrophication in the riverine system.
The use of the WPI for the zonation and chemical characterization of the riverine system has been widely used in the studies of the large river such as in the Shanchong River of China (Wang et al. 2015), Sava River, Serbia (Popovic et al. 2016), and Damodar River, India (Chakraborty et al. 2021). The score of the WPI was varied between 19 and 23, depicting fair water conditions throughout the stretch. The different WPI scores such as 19 were obtained from Buxar, Patna, Bhagalpur, Balagarh, and Fraserganj, while the score value of 21 was obtained at Farakka, Tribeni, and Diamond Harbour. However, comparatively higher scores of 23 were yielded at Jangipur, Berhampore, and Godakhali. The higher values signify the better quality of water and vice versa (Atique and An 2019, 2020). The structural differentiation of A. granulata has been used in many of the studies such as in the Pearl River of China (Wang et al. 2017, 2020) for the assessment of aquatic ecosystems. The correlation was done between the WPI and total length of the filament, valve length, total cell count of one filament, and cell length of one filament and abundance. A significantly negative correlation (r = − 0.663) with WPI was obtained with cell length. It is very much evident that the parameters related to the quantitative structure are very much influenced by the environmental changes (Bedoshvili et al. 2007; Poister et al. 2012). In the present study, the overall cell length varied significantly with the computed value of WPI. On the other hand, chemical parameters showed an intricate relationship with the morphological parameters also. Jewson (1992) has opined that these environmental parameters have a strong association with its growth.
As in the earlier studies, it was also found that the blooming condition of the diatoms like A. granulata signifies the deteriorated environmental condition of the riverine system which hinders the growth and survival of the native aquatic organisms (Joung et al. 2013; Poister et al. 2015) by adversely affecting their ecological niche. In the present study, also the deteriorated environment has been shown with many of the environmental variables and morphological traits of A. granulata which shows the significant correlation among each other..A. granulata is an indicator of water pollution because of its sustainability in a very stressful environmental condition (Grigoryeva et al. 2019).
Conclusion
From the analysis, it was found that the blooming condition of A. granulata was affected by water temperature, chlorophyll A, and dissolved oxygen. Maximum abundance was confronted during the winter season especially (December to January). The blooming condition of the plankton denotes the eutrophic status of the riverine system which may be corrected by increasing the riverine flow and reducing the anthropogenic activities in the vicinity of the river. Further studies in this direction would be of immense help in tracking the eco-health condition of the riverine system and beneficial for the greater perspective of stakeholders depending on this system.
Data availability
Not applicable.
References
Al-Husseini KH, Alsalman AIM (2020) Quarterly variation and their impact on phytoplankton dynamics in the Gharraf River environment in southern Iraq. Plant Arch 20:1354–1360
Ambwani K, Sahni A, Kar RK, Dutta D (2003) Oldest known non-marine diatoms (Aulacoseira) from the uppermost Cretaceous Deccan Intertrappean Beds and Lameta Formation of India. Rev Micropaléontologie 46:67–71
American Public Health Association (2017) Standard methods for the examination of water and wastewater. Washington, Dc, USA. Chapter 2000 pp, 1–105
Atique U, An K-G (2019) Reservoir water quality assessment based on chemical parameters and the chlorophyll dynamics in relation to nutrient regime. Polish J Environ Stud 28:
Atique U, An K-G (2020) Landscape heterogeneity impacts water chemistry, nutrient regime, organic matter and chlorophyll dynamics in agricultural reservoirs. Ecol Indic 110:105813
Ayoade AA, Agarwal NK, Chandola-Saklani A (2009) Changes in physicochemical features and plankton of two regulated high altitude rivers Garhwal Himalaya, India. Eur J Sci Res 27:77–92
Bargu S, White JR, Li C et al (2011) Effects of freshwater input on nutrient loading, phytoplankton biomass, and cyanotoxin production in an oligohaline estuarine lake. Hydrobiologia 661:377–389
Bedoshvili YD, Bondarenko NA, Sakirko M V, Likhoshvay E V (2007) The change in the length of colonies of the planktonic diatom Aulacoseira baicalensis in various stages of the annual cycle in Lake Baikal. Hydrobiol J 43:
Bicudo DC, Tremarin PI, Almeida PD et al (2016) Ecology and distribution of Aulacoseira species (Bacillariophyta) in tropical reservoirs from Brazil. Diatom Res 31:199–215
Blum JL (1957) An ecological study of the algae of the Saline River, Michigan. Hydrobiologia 9:361–408
Chakraborty B, Roy S, Bera A, et al (2021) Cleaning the river Damodar (India): impact of COVID-19 lockdown on water quality and future rejuvenation strategies. Environ Dev Sustain 1–15
Chong W, Huang Y, Shengbing H et al (2009) Variation of phytoplankton community before an induced cyanobacterial (Arthrospira platensis) bloom. J Environ Sci 21:1632–1638
Cox EJ, Cox EJ (1996) Identification of freshwater diatoms from live material. Chapman & Hall London
Cupp EE (1943) Marine plankton diatoms of the west coast of North America
Dimri D, Daverey A, Kumar A, Sharma A (2021) Monitoring water quality of River Ganga using multivariate techniques and WQI (water quality index) in Western Himalayan region of Uttarakhand, India. Environ Nanotechnology Monit Manag 15:100375
Dodds WK, Jones JR, Welch EB (1998) Suggested classification of stream trophic state: distributions of temperate stream types by chlorophyll, total nitrogen, and phosphorus. Water Res 32:1455–1462
Grigoryeva IL, Komissarov AB, Kuzovlev VV, Chekmareva EA (2019) Influence of thermal pollution on the ecological conditions in cooling reservoirs. Water Resour 46:S101–S109
Guinder VA, Malits A, Ferronato C et al (2020) Microbial plankton configuration in the epipelagic realm from the Beagle Channel to the Burdwood Bank, a marine protected area in sub-Antarctic waters. PLoS One 15:e0233156
Guiry MD, Guiry GM (2014) AlgaeBase.[Online]. National University of Ireland, Galway
Guiry MD, Guiry GM (2021) AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org. Accessed 3 Jul 2021
Ha K, Jang M-H, Joo G-J (2003) Winter Stephanodiscus bloom development in the Nakdong River regulated by an estuary dam and tributaries. Hydrobiologia 506:221–227
Herawati EY, Khasanah RI, Ambarwati M, Sofarini D (2020) The effect of hydro-oceanographic factors on the community structure of plankton in natural and artificial coral reefs in Paiton waters. Aquac Aquarium Conserv Legis 13:71–85
Hong J, Lee S, Lee K, Hong J (2020) Seasonal variations in the surface energy and CO2 flux over a high-rise, high-population, residential urban area in the East Asian monsoon region. Int J Climatol 40:4384–4407
Hossain MY, Jewel MAS, Fulanda B, et al (2012) Dynamics of cyanobacteria Planktothrix species (Oscillatoriales: Phormidiaceae) in earthen fish ponds, northwestern Bangladesh
Hötzel G, Croome R (1996) Population dynamics of Aulacoseira granulata (EHR.) Simonson (Bacillariophyceae, Centrales), the dominant alga in the Murray River, Australia. Arch für Hydrobiol 191–215
Jewson DH (1992) Size reduction, reproductive strategy and the life cycle of a centric diatom. Philos Trans R Soc London Ser B Biol Sci 336:191–213
Jia X-H, Jiang W-X, Li F-Q et al (2009) Impacts of large hydropower station on benthic algal communities. Ying yong sheng tai xue bao=. J Appl Ecol 20:1731–1738
Joung S-H, Park H-K, Lee H-J, Lee S-H (2013) Effect of climate change for diatom bloom at winter and spring season in Mulgeum Station of the Nakdong River, South Korea. J Korean Soc Water Environ 29:155–164
Kanaya G, Kikuchi E (2008) Spatial changes in a macrozoobenthic community along environmental gradients in a shallow brackish lagoon facing Sendai Bay, Japan. Estuar Coast Shelf Sci 78:674–684
Kilham SS, Kilham P (1975) Melosira granulata (Ehr.) Ralfs: morphology and ecology of a cosmopolitan freshwater diatom: with 2 figures and 1 table in the text. Int Vereinigung Für Theor Und Angew Limnol Verhandlungen 19:2716–2721
Kulikovskiy MS, Glushchenko AM, Genkal SI, Kuznetsova I V (2016) Identification book of diatoms from Russia. Filigran, Yarosl
Lakshminarayana JSS (1965) Studies on the phytoplankton of the River Ganges, Varanasi, India, Part II “The seasonal growth and succession of the plankton algae in the River Ganges.” Hydrobiologia 25:138–165
Lee H-J, An K-G (2009) The development and application of multi-metric water quality assessment model for reservoir managements in Korea. Korean J Ecol Environ 42:242–252
Margalef R (1983) Limnología. Omega Barcelona
Matta G, Kumar A, Nayak A et al (2020) Water quality and planktonic composition of river Henwal (India) using comprehensive pollution index and biotic-indices. Trans Indian Natl Acad Eng 5:541–553
Mayama S (1991) Taxonomic revisions to the differentiating diatom groups for water quality evaluation and some comments for taxa with new designations. Nov Hedwigia 52:
Moeller HV, Laufkötter C, Sweeney EM, Johnson MD (2019) Light-dependent grazing can drive formation and deepening of deep chlorophyll maxima. Nat Commun 10:1–8
Moro RS (1991) Morphology of Aulacoseira-granulata (EHR) Simonsen var australiensis (Grunow) nov-comb under light-microscopy. Arq Biol e Tecnol 34:353–359
Nwankwegu AS, Li Y, Huang Y et al (2019) Harmful algal blooms under changing climate and constantly increasing anthropogenic actions: the review of management implications. 3 Biotech 9:1–19
Pandey U, Pandey J, Singh A V, Mishra A (2017) Anthropogenic drivers shift diatom dominance–diversity relationships and transparent exopolymeric particles production in river Ganga: implication for natural cleaning of river water. Curr Sci 959–964
Parakkandi J, Saha A, Sarkar UK et al (2021) Spatial and temporal dynamics of phytoplankton in association with habitat parameters in a tropical reservoir, India. Arab J Geosci 14:1–15
Parmar TK, Rawtani D, Agrawal YK (2016) Bioindicators: the natural indicator of environmental pollution. Front Life Sci 9:110–118
Pathak V, Tyagi RK (2010) Riverine ecology and fisheries vis-a-vis hydrodynamic alterations. CIFRI Bull 35
Pham L (2017) Comparison between water quality index (WQI) and biological indices, based on planktonic diatom for water quality assessment in the Dong Nai River. Vietnam Pollution 3:311–323
Poister D, Kurth A, Farrell A, Gray S (2012) Seasonality of Aulacoseira ambigua abundance and filament length: biogeochemical implications. Plankt Benthos Res 7:55–63
Poister D, Schaefer A, Baert A et al (2015) Stimulated rejuvenation of dormant Aulacoseira granulata (Bacillariophyta) by Gloeocystis planctonica (Chlorophyta) in a eutrophic river. J Phycol 51:332–342
Popovic NZ, Duknic JA, Atlagic JZ et al (2016) Application of the water pollution index in the assessment of the ecological status of rivers: a case study of the Sava River, Serbia. Acta Zool Bulg 68:97–102
Pratiwi NTM (2019) Community structure of plankton at Central Kalimantan peat swamp area. In: IOP conference series: earth and environmental science. IOP Publishing, p 12003
Quincey DJ (2017) The Himalayan climate and water atlas. Mt Res Dev 37:155–156
Rakshit N, Banerjee A, Mukherjee J et al (2017) Comparative study of food webs from two different time periods of Hooghly Matla estuarine system, India through network analysis. Ecol Modell 356:25–37
Rimet F, Bouchez A, Montuelle B (2015) Benthic diatoms and phytoplankton to assess nutrients in a large lake: complementarity of their use in Lake Geneva (France–Switzerland). Ecol Indic 53:231–239
Roshith CM, Meena DK, Manna RK et al (2018) Phytoplankton community structure of the Gangetic (Hooghly-Matla) estuary: status and ecological implications in relation to eco-climatic variability. Flora 240:133–143
Sanghi R (2013) Our national river Ganga: lifeline of millions. Springer Science & Business Media
Sekerci Y, Petrovskii S (2018) Global warming can lead to depletion of oxygen by disrupting phytoplankton photosynthesis: a mathematical modelling approach. Geosciences 8:201
Shende S, Rathoure AK (2019) Physico-chemical properties of Erai River in monsoon near Chandrapur City (Maharashtra) India
Simonsen R (1979) The diatom system: ideas on phylogeny. Bacillaria 2:9–71
Singh KR, Dutta R, Kalamdhad AS, Kumar B (2019) Information entropy as a tool in surface water quality assessment. Environ Earth Sci 78:15
Srivastava K, Mishra S, Verma HO, et al (2020) Time scale changes of plankton in the river Ganga at Kanpur
Srivastava P, Verma J, Grover S, Sardar A (2016) On the importance of diatoms as ecological indicators in river ecosystems: a review. Indian J Plant Sci 5:70–86
Taylor JC, Harding WR, Archibald CGM (2007) An illustrated guide to some common diatom species from South Africa. Water Research Commission Pretoria
Thomas MK, Aranguren-Gassis M, Kremer CT et al (2017) Temperature–nutrient interactions exacerbate sensitivity to warming in phytoplankton. Glob Chang Biol 23:3269–3280
Tripathi S, Gopesh A, Dwivedi AC (2017) Fish and fisheries in the Ganga river: current assessment of the fish community, threats and restoration. J Exp Zool India 907–912
Tsai Y-C, Chen K-C, Cheng T-S et al (2019) Chlorophyll fluorescence analysis in diverse rice varieties reveals the positive correlation between the seedlings salt tolerance and photosynthetic efficiency. BMC Plant Biol 19:1–17
Wang C, Lek S, Lai Z, Tudesque L (2017) Morphology of Aulacoseira filaments as indicator of the aquatic environment in a large subtropical river: the Pearl River, China. Ecol Indic 81:325–332
Wang C, Wu N, Li W et al (2020) Curved filaments of Aulacoseira complex as ecological indicators in the Pearl River. China. Ecol Indic 118:106722
Wang Q, Wu X, Zhao B et al (2015) Combined multivariate statistical techniques, water pollution index (WPI) and Daniel trend test methods to evaluate temporal and spatial variations and trends of water quality at Shanchong River in the Northwest Basin of Lake Fuxian. China. PLoS One 10:e0118590
Wetz MS, Wheeler PA (2007) Release of dissolved organic matter by coastal diatoms. Limnol Oceanogr 52:798–807
Yang J-R, Basu BK, Hamilton PB, Pick FR (1997) The development of a true riverine phytoplankton assemblage along a lake-fed lowland river. Arch für Hydrobiol 243–260
Yang Q, Xie P, Shen H et al (2012) A novel flushing strategy for diatom bloom prevention in the lower-middle Hanjiang River. Water Res 46:2525–2534
You Q, Liu Y, Wang Y, Wang Q (2009) Taxonomy and distribution of diatoms in the genera Epithemia and Rhopalodia from the Xinjiang Uygur Autonomous Region, China. Nov Hedwigia 89:397
Zheng B-H, Chen Z-J, Li Y-Y et al (2020) Structural characteristics and driving factors of the planktonic eukaryotic community in the Danjiangkou Reservoir, China. Water 12:3499
Zhang Y, Peng C, Wang J et al (2019) Temperature and silicate are significant driving factors for the seasonal shift of dominant diatoms in a drinking water reservoir. J Oceanol Limnol 37:568–579
Acknowledgements
The authors acknowledge the National Mission for Clean Ganga (NMCG), Ministry of Water Resource, River Development and Ganga Rejuvenation, Government of India, for providing the financial support to conduct the study. Thanks are also due to all the local fishers who have given their cooperation in the sampling and study process
Funding
The funding for the mentioned research is made by the National Mission for Clean Ganga, Ministry of Jal Shakti, Government of India.
Author information
Authors and Affiliations
Contributions
TRM has done sampling and MS preparation; NKT has done water sample analysis, statistical analysis, and MS preparation; SK has helped in MS preparation; AR has helped statistical data interpretation and sampling; RKM has helped in MS correction; SB has helped in MS preparation; SR has helped in MS preparation; SDG has helped in riverine sampling; MB has prepared the GIS map; MHR has helped in MS preparation; HSS has helped in MS preparation; BKD has conceptualized and investigated the work. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The abovementioned work is carried out by following the ethical guidelines prescribed by the Institute Ethics Committee ICAR-CIFRI.
Consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Vitor Vasconcelos
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohanty, T.R., Tiwari, N.K., Kumari, S. et al. Variation of Aulacoseira granulata as an eco-pollution indicator in subtropical large river Ganga in India: a multivariate analytical approach. Environ Sci Pollut Res 29, 37498–37512 (2022). https://doi.org/10.1007/s11356-021-18096-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-18096-9