Abstract
The continued frequent detection of pharmaceuticals in the environment is of major concern due to potential human and ecological risks. This study evaluated 30 antibiotics from 8 classes: sulphonamides (SAs), penicillins (PNs), fluoroquinolones (FQs), macrolides (MLs), lincosamides (LINs), nitroimidazoles (NIs), diaminopyrimidines (DAPs), salfones and 4 anthelmintics benzimidazoles (BZs) in surface water and sediments from River Sosiani in Eldoret, Kenya. Samples were collected during the wet and dry seasons and subjected to solid phase extraction using HLB cartridges. A liquid chromatography tandem mass spectrometry (LC–MS/MS) method was used for the simultaneous quantification of the compounds. Chromatographic separation was on a reversed-phase Zorkax Eclipse Plus C18 column eluted in a gradient program and compounds detected by mass spectrometer operated in a positive electrospray ionization (+ ESI) mode. Twenty-eight antibiotics were detected in water where 22 had a 100% detection frequency and the remaining 4 showed detection frequencies ranging from 5 to 47%. Three BZs had a 100% detection frequency. Detectable concentrations of the pharmaceuticals in water ranged between 0.1 and 247 ng L−1 and 0.01 and 974 µg kg−1 in the sediments. The sulfonamide, sulfamethoxazole, had the highest concentration in water (247 ng L−1), whereas penicillin G showed the highest concentrations in sediments (414–974 µg kg−1). Quantified pharmaceuticals decreased in the order SAs > DAPs > FQs > ATs > PNs ≈ MCs ≈ LNs > NIs in water, and followed the order PNs > BZs > FQs > MLs > DAPs ≈ LNs > NIs > SAs in sediments. Risk quotients (RQw) showed that sulfamethoxazole and ciprofloxacin were of high ecological risk in the surface water (RQw values of 1.11 and 3.24, respectively), whereas penicillin V, ampicillin, penicillin G, norfloxacin, enrofloxacin, erythromycin, tylosin, and lincomycin were of medium ecological risk in the aquatic system. The findings show high prevalence of pharmaceuticals in surface water and sediments and are therefore potential ecological hazards. Such information is vital when devising mitigation strategies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antibiotics are natural, synthetic, and semi-synthetic compounds which show antimicrobial activities on bacteria or other single-celled microorganisms (Catteau et al., 2018). Antibiotics have revolutionized medicine in many respects, and countless lives have been saved; their discovery was a turning point in human history (Davies & Davies, 2010). They represent one of the largest therapeutic categories used in the treatment of infectious diseases caused by bacteria (Schmieder & Edwards, 2012). Antibiotic drugs are predominantly used to treat bacterial diseases in human therapy and as veterinary medicines to prevent and treat bacterial diseases in animal husbandry (Kümmerer, 2009). Besides these fundamental applications of antibiotics in treatment of certain types of diseases in animals and humans, a significant portion is used in animal feed as supplements to promote animal growth (Chattopadhyay, 2014; Economou & Gousia, 2015; Poole & Sheffield, 2013), more so in developing countries where it is harder to successfully regulate the use of antibiotics (Eagar et al., 2012; Van et al., 2020). This broadness of applications has caused an increase in the consumption of antibiotics (Kraemer et al., 2019; Pokharel et al., 2020).
The residual antibiotics from human and animal use can enter the environment via various pathways, including wastewater effluent discharge, runoff from land to which agricultural or human waste has been applied, and leaching (Bottoni et al., 2010; Faleye et al., 2018; Polianciuc et al., 2020; Sodhi et al., 2021). Antibiotics might also be added to the environment through improper disposal of unused and expired antibiotics, where they end up in wastewater, landfills, water supplies, and drains (Anwar et al., 2020; Kotwani et al., 2021). Domestic effluents are considered a major contributor of antibiotic contamination of surface and wastewaters (Zheng et al., 2012), but effluents from pharmaceutical industries (Kotwani et al., 2021) and hospitals’ effluents are also of great concern (Bansal, 2019; Brown et al., 2006; Ngigi et al., 2020). Furthermore, the intensive practice of aquaculture for food production can lead to inadvertent introduction of antibiotics into surrounding surface waters (Hoa et al., 2011; Zheng et al., 2012).
Antibiotics are often partially metabolized after administration, and a significant portion of the antibiotic can be excreted as the parent compound or in conjugated forms that can be converted back to the parent antibiotic. For example, fluoroquinolones, whose therapeutic prescription in human medicine is between 300 and 600 mg per day, are almost all excreted as unchanged compounds in urine and are, consequently, discharged into hospital sewage or municipal wastewater (Lindberg et al., 2004). This ultimately contributes to residual antibiotics in recipient waters. Thus, sewage sludge and manure, used as fertilizer in agricultural land, are often contaminated with antibiotics (Thiele-Bruhn, 2003). Subsequent runoffs from such contaminated agricultural land transfer some of the residues into environmental water systems such as surface water.
Therefore, the continued intensive use of antibiotics in livestock production and human medication has led to their frequent detection in different environmental matrices. Antibiotics have been reported in hospital wastewater effluents (Anwar et al., 2020; Aydin et al., 2019; Bansal, 2019; Diwan et al., 2009; Lindberg et al., 2004; Ngigi et al., 2020; Yao et al., 2021), wastewater treatment plant (WWTP) effluents (Golchin et al., 2021; Kortesmäki et al., 2020; Ngigi et al., 2020; Rodriguez-Mozaz et al., 2020), WWTP biosolids, soil, surface waters, groundwater (Burke et al., 2016; Clarke & Smith, 2011; Hu et al., 2018; Li et al., 2021; Martín et al., 2015; Yang & Carlson, 2003), sediments, biota and drinking water (Hanna et al., 2018; Li et al., 2014; Meng et al., 2021; Wang et al., 2016), and in different forms of natural waters systems (Litynska et al., 2021) at ng L−1 to low μg L−1 levels. It is therefore important to continuously monitor the residual antibiotics in WWTPs and in surface water. There are few studies on surveillance of antibiotic residues in the environment in Kenya. For river surface water, antibiotics from different classes were reported at varying concentrations including the sulphonamides sulfamethoxazole, sulfamethazine, and sulfadiazine at concentrations of up to 142.6, 0.47, and 0.84 μg L−1, respectively; β-lactam (penicillins) including amoxicillin (up to 9 μg L−1), ampicillin (up to 0.9 μg L−1), and penicillin G, dicloxacillin, and nafcillin (< 1.0 μg L−1); the fluoroquinolones ciprofloxacin and norfloxacin (up to 4.9 and 2.8 μg L−1); and trimethoprim (up to 9.5 μg L−1) (K’oreje et al., 2012; Kairigo et al., 2020a, b; Kimosop et al., 2016; Ngigi et al., 2020; Opanga, 2018). Other environmental matrices studied include river sediments (Kairigo et al., 2020b; Kandie et al., 2020; Kimosop et al., 2016), hospital wastewater, and lagoons and WWTPs (Kimosop et al., 2016; Ngigi et al., 2020). Establishing residual antibiotics in water sediments, surface and hospital wastewater is important for baseline information, generation of knowledge, bridging existing knowledge gaps, and in policy formulations. This study investigated the occurrence of 4 anthelmintics benzimidazoles (BZs) and 28 antibiotics from the classes: sulphonamides (SAs), β-lactams-penicillins (PNs), Fluoroquinolones (FQs), macrolides (MLs), lincosamides (LINs), nitroimidazoles (NIs), and two other antibiotics dapsone (a sulfone) and trimethoprim (a Diaminopyrimidine) in sediments and surface water from River Sosiani in Eldoret Municipality. Kenya, as one of the developing countries, has few reports on the status of pharmaceuticals contamination of surface and wastewaters. Environmental risks can only be assessed when the background contamination levels have been established.
Materials and methods
Chemicals and reagents
Standard pharmaceutical reference compounds (> 98% purity) and high-performance liquid chromatography-grade water (LC water) were obtained from Merck through Scientific Laboratory Supplies Ltd. (Kenya Branch). Table 1 gives the list of the compounds and abbreviations, while table S1 (supplementary material) gives the physicochemical properties of the compounds used in this study. High-performance liquid chromatography-grade methanol (MeOH) and acetonitrile (ACN); formic acid (HCOOH) and ammonium formate (NH4HCO2) were purchased from Kobian Scientific Ltd. (Nairobi, Kenya). Oasis hydrophilic–lipophilic balance cartridges (HLB, 200 mg/6 mL; 60 mg, 3 mL) and glass microfiber filters (0.7, 0.45, 0.22 µm) were obtained from Milford, MA (Waters, USA). All other reagents were of analytical grade and were purchased from Kobian Scientific Ltd. Individual standard stock solutions were prepared at a concentration of 1 g/L, in either methanol or acetonitrile and stored at 4 ºC.
Study area and sampling
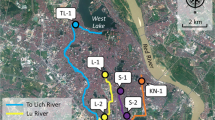
The study was conducted in Uasin Gishu County, within Eldoret municipality (Fig. 1) in Kenya. Surface water samples and water sediments were collected from River Sosiani (which traverses through the town) in Eldoret Municipality during the wet season (June, 2020) and the dry season (February, 2021). A judgemental random sampling method was used whereby 10 sampling points were identified (Table 2) in the suburban and the urban sections of the river in close proximity to urban settlements, hospitals, and the city’s sewer system or where wastewater discharges occur followed by random sampling from each selected site. Water samples were collected in 2.5 L amber glass bottles that had been pre-cleaned successively with saturated MeOH-EDTA solution, rinsed with distilled water, and then dried in an oven at 100 °C. Prior to sample collection, the bottles were pre-rinsed three times with river water. Five 0.5-L grab water samples were collected randomly at each sampling point (away from the edges of the river) and combined to form a composite 2.5 L sample (in duplicate). The temperature and pH were recorded in situ. The pH of the water ranged from 7.0 to 8.0, with majority of the samples being neutral. Sodium azide (0.5 g L−1) was added into each sample bottle immediately after sampling to inhibit potential biodegradation. Samples were placed in cooler box during transportation, acidified with HCl to a pH of 3 and stored at 4 °C in a cold room prior to extraction. Ten grab sediment samples (approximately 200 g) were also collected (in duplicate), coded as SP1 to SP10, and transported together with the water samples, where they were stored at – 20 °C in a cold room prior to extraction.
Sample preparation
One-liter subsamples (from the composite samples) were filtered sequentially using 0.7 µm and 0.45 µm Millipore filter prior to SPE clean-up. The Oasis hydrophilic-lipophilic balance (HLB) cartridges (200 mg, 6 mL) were preconditioned with 6 mL MeOH followed by 6 mL LC-water. Each 1-L water sample (in triplicate) was then passed through the cartridge at a flow rate of 8 mL/min using an SPE vacuum Manifold (MilliporeSigma™ Supelco™ Visiprep, Thermo Fisher Scientific), washed with 10% MeOH in LC-water and air-dried for 10 min. The cartridges were eluted with 3 mL of 70: 30 v/v MeOH-ACN solution and reduced to a volume of 100 µL under a gentle flow of nitrogen. The sample was reconstituted to 1 mL with a 2: 1 (v/v) LC-Water-ACN solution containing 5 mM NH4HCO2 (acidified with 0.1% HCOOH), filtered through a 0.22-µm glass membrane filter and stored at 4 °C prior to LC–MS/MS measurement.
For the sediment samples, a 2-g aliquot of sample was weighed into a 50-mL centrifuge tube. Six milliliter of MeOH: ACN: H2O: water (60: 20: 20) solvent mixture was added to the tube followed by vortexing for one minute. The mixture was then sonicated for 20 min and centrifuged at 4500 rpm for 10 min, after which the supernatant was collected in a 15-mL glass test tube. A repeat extraction using 6 mL of 100% methanol was done and extracts were pooled in the 15-mL tube. The pooled extract was concentrated using a rotary evaporator to approximately 1 mL and reconstituted to 10 mL using LC-water. Extracts were passed through 0.45-µm glass microfilters and cleaned-up by SPE method using Oasis (HLB) cartridges (60 mg, 3 mL). The SPE procedure was same as that of the water samples, using 3 mL of preconditioning, washing, and eluting solutions.
LC–MS/MS analysis
Samples were analyzed using Agilent LC-1290 infinity II system (Germany) coupled to an API 6460c triple Quad Mass Spectrometer (Applied Biosystems/MDS Sciex Instruments, Toronto, Canada) system equipped with a Zorbax Eclipse Plus C18 RRHD, 50 mm × 2.1 mm, 1.8 µm column and a guard column (3.0 × 4 mm) of the same material. The binary solvent gradient elution program (Solvent A: 0.1% aqueous HCOOH, 5 mM ammonium formate and Solvent B: 0.1% HCOOH acetonitrile) of 23 min was set up as follows: 0 min, 0% B; 1 min, 5% B; 3 min, 10% B; 7 min, 20% B; 11 min, 30% B; 15 min, 40% B; 17 min, 50% B; 19 min, 70% B; 21 min, 80% B; and 23 min, 5% B. The oven temperature was set at 35 °C, a flow rate of 0.30 mL min−1, and an injection volume of 10 µL. A post-time of 3 min was set between sample runs for the column re-equilibration. Mass spectrometer (MS) was operated in positive electrospray ionization (+ ESI) mode with the following source parameters: gas temperature 325 °C, gas flow 1 L min−1, and nebulizer gas 45 psi capillary voltage of 4000 V. Precursor ion and two or three product ions were used for compound identification (Supplementary Table S2).
Method evaluation and validation
The SPE clean-up method was evaluated by spiking mixed standards to 1-L blank water samples at two concentrations (0.01 and 0.5 µg L−1) and subjecting the samples to the same SPE procedure as the samples. One-gram triplicate sediment samples were also spiked to a final concentration of 0.1 and 0.5 µg kg−1 and subjected to similar extraction and SPE clean-up procedure as the sediment samples. Recoveries (%) were determined for each of the analyte compound as the ratio of quantified amount to that of the spiked amount multiplied by 100%. Repeat blank sample spike experiments (in water) were conducted in different days to evaluate the intra-day and inter-day method precision and accuracy of recoveries for representative test pharmaceuticals from different classes (Table S3, supplementary material).
Reference standard compounds of concentrations 0.1–2 µg mL−1 in 2: 1 (v/v) LC-Water-ACN solution containing 5 mM NH4HCO2 (acidified with 0.1% HCOOH) were prepared and injected into the LC–MS/MS to determine linearity of the quantification method and as external calibration standards. A set of six replicates of lowest calibration mixed standard were injected into LC–MS/MS instrument and used to determine the limit of detection (LOD) and limit of quantification (LOQ). LOD and LOQ were evaluated as; LOD = (3.3 × σ/s), while LOQ = (10 × σ/s), where σ is standard deviation of the replicates and s is the slope. There was good precision among the replicates for each compound, and this reproducibility confirmed ruggedness of method.
The determined method performance parameters including the linearity, accuracy, and repeatability expressed as the correlation coefficient (R2) of calibration curves,% recoveries, LOD, LOQ, and standard deviation for replicate measurements for water samples, and spiked samples are presented in Tables 3 and S3 (supplementary material). Linearity was good (R2 > 0.93) for all test compounds. The% recoveries ranged from 83 to 114% for water samples and 80–108% for the sediments. The LOD of the adopted method ranged from 0.003 to 0.317 ng L−1 whereas the LOQ range was 0.010–0.908 ng L−1. All values reported were above the LOQ for each corresponding analyte compound. The method was therefore robust enough for the study.
Ecological risk assessment
The potential ecological risk of the pharmaceutical residues in water was evaluated using risk quotient RQw, which was evaluated based on maximum measured environmental concentration (MEC) to the predicted no-effect concentration (PNEC, ng L−1) using Eq. (1) (Yin, 2021).
PNECw (maximum drug concentration with no adverse effect on the microorganisms or the ecosystem in the environment) was determined using the evaluation (or assessment) factor, AF (Eq. 2).
where EC50 is the half-maximum effect concentration (ng L−1). EC50 and NOEC50 values (for the most sensitive species) were obtained from literature (Chen et al., 2021a; Oh et al., 2004; Sharma et al., 2021). NOEC50 values were used in cases where EC50 could not be obtained (Table S7, supplementary material). An AF value of 1,000 was used when acute toxicity (EC50) data was used, and 100 when chronic toxicity data, NOEC50 was used (Jiang et al., 2014). From the commonly used criteria of ranking the RQ values, RQ range of 0.01 to 0.1 is considered as low risk, 0.1 > RQ < 1 medium risk, and RQ > 1 as high risk, and this criterion was adopted in this study (Yan et al., 2013).
Data analysis
Descriptive and other data analysis were done using Microsoft Excel 2016 (Apple Inc., USA) computer software. Pearson’s correlation (r, ρ = 0.05) was used for the comparison of pharmaceutical residues in river surface water and in sediments. Statistical comparison of the intra- and inter-day recoveries was done to evaluate the precision and accuracy (% coefficient of variation, CV, and% accuracy) of the method. Pseudo-partitioning coefficients (Kp,s) of test compounds between water and sediments were used to estimate the sorption capacity of these compounds and were calculated using the equilibrium: Kp,s = Cs/Cw, where Cw is the mean concentration of the target compound in the water sample (μg L−1), and Cs is the mean concentration of the target compound in the sediment sample (ng g−1) (Chen et al., 2021b).
Results and discussion
Residual pharmaceuticals in water
Water samples were randomly obtained from 10 selected sites in Sosiani river (Eldoret) and analyzed for antibiotic residues. From a total of 34 compounds that were investigated, varied amounts of residues were detected for 28 compounds is surface water, representing 79% of analytes. The six compounds that were not detectable included sulfaguanidine, sulfamonomethoxine, sulfachlorpyridazine, sulfaquinoxaline, dapsone, and flubendazole. Detectable amounts of SAs ranged from 0.08 to 247.0, PNs from 0.19 to 10.37, QNs from 13.68 to 56.02, MCs 3.32 to 9.08, LNs (LIC) from 3.67 to 7.77, NIs (MET) from 3.11 to 5.56 and TMP ranged from 0.17 to 67.32 ng L−1. The amount of determined BZs (OFX, MEB, and ALB) ranged from 1.97 to 21.39 ng L−1. Therefore, the concentrations (based on maximum amount) decreased in the order SAs > TMP > FQs > ATs > PNs ≈ MCs ≈ LNs > NIs (Tables 4, 5 and 6). Though the highest detected amount was that of a sulfonamide compound (SMXZ), the FQs were generally present in higher amounts than the other classes of compounds determined (Tables 4, S4a and b). The three FQs were detected at all sampling points, presenting a 100% frequency. AMP was detected at only one sampling point, SP4 (0.2 ng L−1), a similar observation to that of STZ. PEG was detected at four sampling points (2% frequency), whereas the other two PNs (AMX and CLX) were detected at all sampling points. For all other detected compounds (other than SAs, FQs, and PNs), 100% detection frequency was observed. There was no observed trend in concentrations of detected compounds downstream. This suggests that dilution was not an influencing factor, and point source pollution may have played a major role, taking into account that the sampling area has several hospitals, residential areas (discharge of wastewater) and, to a small extent, some agricultural activities (Table 2). Sampling point SP4 recorded higher concentrations than other sites for most of the compounds, which strongly supports the idea of point source pollution as the site may be a recipient of large amounts of wastewater from a nearby hospital. Surface runoffs, from poorly managed domestic and hospital wastes and effluents, might have contributed to the pharmaceuticals in the investigated surface water.
Of the 10 SAs present in detectable amounts, SMXZ had the highest concentration of 247.0 ng L−1 followed by SMZ with 23.3 ng L−1 (Tables 4 and S4a-supplemantary material). The average concentration increased in the order SMPZ < SDZ < SMTZ < SDMT < SDX < SXZ < SPD < SMZ < SMXZ. This observed trend did not follow the order of solubility of the compounds in water, and was not significantly correlated (r = 0.39, ρ = 0.05) to the octanol–water coefficient (log Kow). STZ was detected at only one sampling point (SP4) in the wet season at a concentration of 7.4 ng L−1 (Table 4). The frequency of detection of SAs varied among determined compounds and ranged from 0.05 to 100%. Considering the average amount for each compound at all sampling points, there was no discernable trend in the concentrations of SAs while comparing amounts in dry and wet seasons (supplementary Fig. S1a). This indicated contributions from point sources as well as surface runoffs. In general, SMTZ, SXZ, SDMT, and SDX recorded higher amounts in wet season (difference not significant), SPD and SXZ had higher amounts in dry season (difference significant for SPD, and significant for most sites for SXZ) whereas the concentrations of SMXZ and SMPZ were variable for the two seasons. It was noted that point sources contributed more to the concentrations of SDZ, SMTZ, SMZ, SMPZ, SPD, SMXZ, and SXZ during the dry season for points SP4 and SP6, and a similar observation for SDZ, SMZ, SMPZ, SPD, SMXZ, and SXZ for the points SP5, SP7, SP8, and SP9. Compared to other sampling points, SP4 had the highest concentrations of SDZ, SMTZ, SMZ, SMPZ, SMXZ, and SXZ in the dry season. Hospital and domestic effluents were the major sources of SAs in the sampled surface water (Table 2), going by the description of the study area.
Globally, reported concentrations of various SAs in surface water vary from non-detectable (nd) levels to 5320 ng L−1 for SMXZ (Chen & Zhou, 2014; Dinh et al., 2017; Kuang et al., 2020; Matongo et al., 2015; Ncube et al., 2021; Zhang et al., 2012), and up to 6192, 112.5, and 530.1 ng L−1 for SMZ, SDZ, and SPD, respectively (Chen & Zhou, 2014; Díaz-Cruz et al., 2008; Dinh et al., 2017; Yao et al., 2017). Also, a reported amount of up to 66.0 and 6.0 ng L−1 for SMM and SFQ, respectively, (Chen et al., 2018), and up to 47.5 and 15.6 ng L−1 STZ and SXZ, respectively (Li et al., 2021). However, high levels of SMXZ of 19.4 and 142.6 μg L−1 were reported for upstream and downstream of Mitheu river water in Kenya (Kairigo et al., 2020a). Other reported SA contaminations in surface water (Nairobi, Chania, Sagana, Kanyulu, and Mwania rivers) in Kenya are concentrations of up to 23.35, 0.47, and 0.84 μg L−1 of SMXZ, SMZ, and SDZ, respectively (K’oreje et al., 2012; Kairigo et al., 2020b; Ngigi et al., 2020), whereas values of < 0.08 μg L−1 were determined for SDX and SCP (Ngigi et al., 2020).
Concentrations of the seven PNs ranged from 0.19 to 10.37 ng L−1, with average amounts increasing in the order AMP < AMX < PEG < CLX < PEV < DCX < NAF (Tables 5 and S4b). A 100% frequency of detection was observed for five of the compounds except AMP (detected at sampling point SP4 during dry season only) and PEG (21% frequency). Also, notable observation was that the maximum concentrations of PEV, CLX, and DCX were obtained from sampling point SP4 during the wet season. Generally, concentrations of PEV, AMX, DCX, NAF, and CLX were high during the wet seasons (supplementary Fig. S1b), though the differences were not significant (ρ = 0.05) except AMX at SP9, DCX at SP4, and CLX at SP8 where the amounts were higher in dry the season). Among the PNs, AMP is among the most frequently detected antibiotic in aquatic systems, having a reported concentration of up to 13,800 ng L−1in surface water (Dinh et al., 2017; Ncube et al., 2021). Others include AMX, PEG, and PEV at concentrations 200, 250, and 10 ng L−1, respectively, in surface water (Dinh et al., 2017; Watkinson et al., 2009). In Kenya, the reported penicillin’s in water include AMX at 900 ng L−1 in Mitheu river (Kairigo et al., 2020b), AMP, PEG, DCX, NAF, and oxacillin at a range of < 120–500 ng L−1 (Ngigi et al., 2020) in Nairobi river.
Concentrations of the three FQs (100% frequency detection) ranged from 13.68 to 56.02 ng L−1, which increased (average concentration) in the order CIP < ENR < NOR (Tables 5 and S4b). The highest amount of CIP and NOR of 56.02 and 52.19 ng L−1, respectively, were recorded from sampling SP4 during the dry season. The concentrations of the three FQs was variable, and no seasonal trend could be drawn (supplementary Fig. S1b). FQs, like SAs are also commonly found in aquatic systems. In surface waters, they have been reported at varied concentrations, for example, reported CIP concentrations ranged from non-detectable (nd) to 5,015,000 ng L−1 in Leça river in Portugal, Yamuna river in India, and Charmoise river and Arc river in France (Dinh et al., 2017; Feitosa-Felizzola & Chiron, 2009; Fernandes et al., 2020; Mutiyar & Mittal, 2014), the highest concentration having been reported in the Ganges river in India (Sharma et al., 2019). Enrofloxacin, ENR, has similarly been studied in surface water with reported concentrations of up to 181,609 ng L−1 (Sharma et al., 2019; Yao et al., 2017). Likewise, NOR has reported ranges of 9.57–1261 ng L−1 in Leça river, Portugal and Jianghan Plain, Central China (Dinh et al., 2017; Yao et al., 2017), and a high concentration of 16,148–251,137 ng L−1 in Ganges River in India (Sharma et al., 2019). Ofloxacin, though not determined in this study, is also a commonly reported pollutant in surface water at concentrations of up to 11,700 ng L−1 (Bhagat et al., 2020). Among the reported concentrations of FQs in surface water in Kenya include NOR at levels of up to 4900 ng L−1 and CIP of up to 2800 ng L−1 (Kairigo et al., 2020a, b).
Of the two MCs, TLY was detected at higher levels in the range 5.46–9.08 ng L−1 and 3.32–6.73 ng L−1 for ERY (Tables 6 and S4c). ERY amounts were higher during the dry season (difference not significant) whereas the concentration of TLY varied for the two seasons (supplementary Fig. S1c). ERY has been previously reported in surface water at a range of 32.3–2910 ng L−1 in China (Yao et al., 2017), nd–240 ng L−1 in South Africa (Matongo et al., 2015), and TLY at 9.8–74.2 ng L−1 in Italy (Zuccato et al., 2005). Concentrations of up to1.9 μg L−1 of ERY were reported in Nairobi river water in Kenya (Ngigi et al., 2020). Similar to ERY, the concentration of LIN (lincosamide) ranged from 3.67 to 7.77 ng L−1 and was significantly higher during the dry season (supplementary Fig. S1c). There a few studies reporting the occurrence of LIN in surface water, for example, a concentration range of 1.9–248.9 ng L−1 in surface water (rivers, lake, and ponds) (Bhagat et al., 2020; Castiglioni et al., 2005; Feitosa-Felizzola & Chiron, 2009). Amount of MET, a nitroimidazole, was generally low and ranged from 3.11 to 5.56 ng L−1. However, K’oreje et al. (2012) reported a concentration of over 500 ng L−1 in their study within Nairobi river basin, Kenya. Concentration of TRM, a diaminopyrimidine, ranged from 0.17 to 67.32 ng L−1, which was higher during the dry season except at sampling point SP2. Notably, is the highest amount of TRM, which was obtained from site SP4 in the dry season. Studies have reported TRM concentrations of up 13,680 ng L−1 in surface water (Archundia et al., 2017; Dinh et al., 2017; Fernandes et al., 2020; Zhang et al., 2012, 2020). Concentrations of up to 9480 ng L−1 were reported in Kenya for TRM in surface water (K’oreje et al., 2012; Kairigo et al., 2020b; Ngigi et al., 2020) from Nairobi and Mitheu rivers.
Four anthelmintics, ATs (commonly used to treat parasitic worms) from benzimidazole class, were determined in surface water and were obtained in the range 1.97–21.39 ng L−1. FLB was not detected at all sampling points, whereas the other three had a 100% occurrence frequency and their concentrations increased in the order OXF < MEB < ALB (Tables 6 and S4c). The concentrations of MET, OFX, and MEB were similar during the wet and dry season and variable without any obvious tend for ALB. A detection frequency of 77 to 100% was reported for 19 ATs in river water (Chen et al., 2021b), whereas the same study reported a concentration range of nd–61.12 ng L−1 for seven BZs, namely, ALB, OXF, MEB, ricobendazole (RIC), fenbendazole (FEN), flubendazole (FLU), and thiabendazole (THI). Other studies have reported up to 197 ng L−1 anthelmintics in surface water (Kumirska et al., 2015; Zrnčić et al., 2014); however, very few studies have been done on these pollutants in aquatic environments.
The differences in detected amounts of the antibiotics and benzimidazole anthelmintics in the surface water may be attributed to several factors including physicochemical characteristics of the compounds, usage patterns in the environs, anthropogenic activities, biogeochemical processes, surface runoffs, in addition to environmental fate processes such as biodegradation, photo-degradation, and adsorption (Chen et al., 2018; Kümmerer, 2009; Tang et al., 2015). As a result, the order of detections in different studies may differ. For example, in a study by Duong et al. (2021), the average concentrations (ng L−1) of five classes of antibiotics decreased in the order SAs (117.9) > β-lactams (31.28) > quinolones (20.19) > MLs (17.74) > TMP (8.93). Though, it is notable that the study ranked SAs as the highest and this agrees with the observation in this study. For the detected compounds, there was a negative correlation between the obtained concentrations and log Kow (r = − 0.2). However, a positive correlation existed between the concentrations and solubility (r = 0.71) (Table S6a, b in supplementary material).
Largely, all the antibiotics and benzimidazoles determined in this study had concentrations within globally reported values. As a primary source of antibiotics in aquatic systems, final effluents from WWTPs (both hospital and domestic) contain a variety antibiotic residues (Rodriguez-Mozaz et al., 2020) as antibiotics are not fully eliminated (Abuin et al., 2006), which was one of the major contributing factors in the studied river water system, judging from the location. The behavior and fate of antibiotics in the aquatic environment are influenced by the physical and chemical characteristics of the compounds (molecular structure, size, shape, solubility, hydrophobicity, and sorption potentials), environmental factors (pH), and the climatic conditions (temperature and precipitation), as well as biological factors (microbial degradation). Thus, the detection of antibiotics in aquatic systems is greatly affected by their sorption potentials (Kd) in soil particles (among other factors), where surface runoffs and leaching are contributory pathways. Those with low Kd (< 5 L kg−1) and half-life of less than 21 days are easily transported to aquatic systems, whereas those with high Kd (> 5 L kg−1) tend to accumulate and persist in the soil matrix. For example, SAs have relatively low Kd of 0.2 to 2.0 L kg−1 and organic carbon normalized partition coefficient (Koc), which indicates their low sorption affinity to soil and sediment particles (Thiele-Bruhn, 2003) compared to FQs (Kd of 70 to 5000 L kg−1) (Sarmah et al., 2006; Van Dijk & Keukens, 2000), and are therefore likely to be mobile in the aqueous runoff component following application in soil. However, Kd values vary widely in different types of soils. Moreover, SAs are extensively used to treat human and animal diseases, hence they are ubiquitous in aquatic environments. For SMXZ, it is often used in combination with TRM (Cotrim) because this enhances the effectiveness of the sulfonamide. This combination is moderately mobile and hydrophilic (log Kow values ≤ 0.91), hence easily transferable into the aquatic environment (Viana et al., 2021), and this may be one of the factors that led to the detection of this compound at higher levels compared to other SAs.
Some antibiotics form conjugated metabolites that may be converted back to parent compounds after excretion. An example is the formation of acetylated metabolites such as N4-acetylsulfamethoxazole or N4-acetylsulfamethazine (in SAs), which can be converted back to the parent form (Viana et al., 2021). Such metabolites have been detected in WWTPs, implying that they form part of residual pollutants that enter recipient surface waters from this source. For most antibiotics, the deconjugation of conjugated active and inactive metabolites into the active parent molecules in WWTPs via biological transformation by microbial enzymes such as glucuronidases and sulfatases, can lead to negative removals of such compounds (example, ERY), hence their discharge into receiving surface waters (Brown & Wong, 2018; Mirzaei et al., 2018).
FQs are considered relatively stable in the environment than other antibiotics, which allows them to persist for longer periods, spreading further and hence accumulating in water and sediments. The fate of these FQs in aquatic systems is mainly dominated by adsorption (log Koc ≥ 4.2, strong sorption) and photodegradation reactions and therefore rapidly move from water to soil/sediments and onto organic particles solution, hence the occurrence in surface water, although adsorption is more critical (Cardoza et al., 2005; Viana et al., 2021). The fact that FQs are easily adsorbed by sediments, accounts for their detection in low concentrations in surface water as compared to SAs (Li et al., 2021). CIP and ENR are among the most frequently detected FQs. Presence of ENR can contribute to increased amounts of CIP as it is the primary degradation product of enrofloxacin (about 13–60% of ENR is metabolized into ciprofloxacin) (Cardoza et al., 2005; Viana et al., 2021). Lincomycin is moderately mobile and therefore detected in surface water, having been mobilized from primary sources such as domestic and wastewater treatment effluents (Zhu et al., 2020) and from animal manure or animal production (Kuchta & Cessna, 2009).
Benzimidazole anthelmintics are widely used for helminthic infections and are utilized in animal husbandry, agriculture, aquaculture, and human health (Zajíčková et al., 2020). Their detection in the environment, like antibiotics, is through anthropogenic activities, including sewage discharge, agricultural irrigation, and livestock breeding (Sim et al., 2013). BZs were reported to be the dominant class in river water among six other ATs due to their heavy use (Chen et al., 2021b). With respect to the study area, a combination of these sources is possible as the location has several hospitals, residential houses, and some agricultural activities.
Pharmaceuticals in sediments
Sediments were collected at each corresponding water sampling point from Sosiani River and tested for presence of the selected pharmaceuticals using described methods. Residual pharmaceuticals varied for individual compounds in the range of undetected to 26.4 µg kg−1 (dry weight) for most of the compounds (Fig. 2a–c). However, for the two compounds PEG (PNs) and ALB (BZs), the concentration ranged from 414 to 974 µg kg−1 and 3–125 µg kg−1, respectively (Fig. 2d). The frequency of occurrence was 100% for all detected compounds except for two SAs, SDZ and SMZ, with 40 and 20% occurrence, respectively. Residual concentrations in sediments were significantly higher than in surface water (in orders of 10 to 1000 times). Of the eight classes detected in sediments, the amounts followed the decreasing order (based on maximum amounts); PNs > BZs > FQs > MLs > DAPs ≈ LNs > NIs > SAs. Out of the 16 SAs analyzed, 8 were detected in the sediments. The quantified amounts for each class ranged as follows: PNs 0.83–973.87, BZs 1.95–124.56, FQs 13.22–26.35, MLs 3.97–11.64, LNs 4.27–6.55, NIs 1.98–5.10, DAPs (TMP) 0.08–6.55, and SAs 0.01–4.13 µg kg−1. It was also observed that the point SPD 4 had relatively higher amounts compared to other sampling points. Seven compounds were not detected in sediments, which included STZ, SMM, SCP, SFQ, DPS, AMP, and FLB. All pharmaceuticals that were not detected in water were also not detectable in the sediments except for the sulfonamide STZ that was quantifiable in water but not in sediments.
Investigations on occurrence of pharmaceuticals in sediments have been conducted globally and present varied results for antibiotics and other compounds. A report by Chen et al. (2018) placed the average detected amounts of five categories of target antibiotics in sediments in decreasing order, tetracyclines (88.8 ± 34.2 µg kg−1) > fluoroquinolones (53.7 ± 33.6 µg kg−1) > β-lactamases (33.7 ± 23.4 µg kg−1) > sulfonamides (18.1 ± 12.8 µg kg−1) > others (11.5 ± 9.8 µg kg−1). Of these, SDZ, NOR, CLX, oxcytetracycline, and LIN showed a 100% detection frequency. Among the reported FQs in sediments are CIP, NOR, ENR, and ofloxacin with values of up to 569, 225, 8.19, and 1560 µg kg−1, respectively (Chen & Zhou, 2014; Dinh et al., 2017; Li et al., 2021; Yang et al., 2010). A concentration range from 1700 to 3500 ng g−1 dry weight was reported for a number of antibiotics in river sediments, among which were four FQs (CIP, NOR, ofloxacin, and enoxacin) (Dinh et al., 2017). Though SMXZ was not detected in this study, high concentrations of this compound of up to 344 µg kg−1 have been reported elsewhere (Dinh et al., 2017; Wei et al., 2013). Other reported SAs include SMZ, SPD, SDZ, sulfaquinoxaline, and sulfathiazole, at 19.7, 9.1, 2.07, 0.08–0.9, and 0.6 µg kg−1, respectively (Chen & Zhou, 2014). The macrolide, ERY, has been detected at a range of 1.5–24.6 µg kg−1 (Chen & Zhou, 2014) and a range of nd–87.55 µg kg−1 for TRM in sediments (Matongo et al., 2015). ALB and ricobendazole were reported as the most dominant BZs in sediments, with concentration ranges of nd–596.06 and nd–68.63 ng g−1 for the two compounds, respectively (Chen et al., 2021b). Metronidazole was detected at a concentration of up to 1253.5 ng g−1 in sediments from a dam in Msunduzi river catchment, South Africa (Matongo et al., 2015).
There are only two studies (to the best of our knowledge) reporting occurrence of antibiotics (up to this moment) in river sediments in Kenya of amounts of up to 474, 26.6, 13.3, 94, 85, and 94 µg kg−1 CIP, NOR, TRM, AMP, SMXZ, and chloramphenicol, respectively (Kairigo et al., 2020b; Kimosop et al., 2016). Environmental concentration of antibiotics in sediments ranges from a few ng kg−1 to few hundreds µg kg−1 (Chen et al., 2018), hence supporting observed results in this study where the quantified amount ranged from 0.01 to 974 µg kg−1. Sediments are important sinks for contaminants, hence concentrating antibiotics and other pollutants. Additionally, sediments are in an anaerobic environment that inhibits the degradation of antibiotics and limits exposure to light, hence the concentrations of some antibiotics in sediments are greater than in water (Li et al., 2021). As earlier noted, FQs are easily adsorbed onto sediments (Li et al., 2021), hence their higher concentrations compared to SAs and MLs. Likewise, BZs can be stably adsorbed in sediments under the conditions of suitable pH and appropriate proportion of organic matter (Pavlović et al., 2018).
Comparison of pharmaceutical residues in river surface water and in sediments
Pearson’s correlation was used to establish if there was any relationship between concentrations found in surface water and sediments for those compounds that were detected in both compartments. Additionally, pseudo-partitioning coefficients (Kp,s) of the target compounds between water and sediment were also evaluated. In aquatic systems, the sorption is not in equilibrium; hence, Kp,s is not always a valuable parameter but is however useful for estimating the sorption capacity of concerned compounds. From Pearson’s correlations (ρ = 0.05), concentrations of the compounds in water were positively and significantly correlated to solubility (r = 0.71), whereas the concentration in sediments was negatively correlated (not significant) to the solubility (r = −0.02) (Tables S6a, b, supplementary material). Further, the concentrations of the compounds in water had no significant correlation to the concentrations in the sediments (r = −0.09). The Kp,s values ranged from 52 to 943, 797 to 322,190, 870 to 919, 1171 to 1262, and 1111 to 7372 L Kg−1 for SAs, PNs, FQs, MLs, and BZs, respectively (Table S5, supplementary material). SAs were among the compounds with low Kp,s values, indicating their low adsorption in sediments. Kp,s values of over 2000 L kg−1 correspond to highly adsorbed compounds. Hence, the BZs, ALB, and the penicillin PEG were strongly adsorbed in sediments, with potential of accumulation. Other compounds were moderately adsorbed (FQs, MLs, LIN, and MET). However, for such Kp,s values, data should be obtained for a considerable period of time for fair representation of sorption equilibrium with relation to sources of the pollutants.
Generally, as observed in this study, concentrations of the determined pharmaceuticals from seven different classes varied in surface water and river sediments. Pharmaceuticals are ubiquitous and are among the major anthropogenic pollutants in the environment. Previous studies have reported presence of antibiotics and other pharmaceuticals in water and river sediments worldwide. Concentrations in surface water range from a few ng L−1 to a few µg L−1 (Bottoni et al., 2010; Chen et al., 2021a; Kuang et al., 2020; Wang et al., 2016). Detected amounts of antibiotics from the classes SAs, FQs, PNs, MLs, βLs, and TRM in surface water were within reported values (Chen et al., 2018; Kuang et al., 2020; Wang et al., 2016). Though not as widely studied in environment as the antibiotics, ATS have also been reported in surface water and sediments within the same range as antibiotics (Chen et al., 2021b; Kumirska et al., 2015), similar to findings in this study.
Ecological risk analysis
From the RQw values, 50% of the compounds (9 out of 18) were of medium ecological risk in aquatic system, whereas 39% presented low risk. These included the PNs (PEV, AMP, and PEG), FQs (NOR and ENR), the macrolides (ERY and TLY), and LIN (Tables 7 and S7-supplementary material). The sulfonamide, sulfamethoxazole (SMXZ), and the fluoroquinolone, ciprofloxacin (CIP), posed a high risk to aquatic life, having RQw values of 1.11 and 3.24, respectively. The rest of the evaluated compounds posed no risk. Many pharmaceuticals pose ecological risk because of their continuous usage (some in large consumption) and strong environmental persistence. Previous ecological risk assessment reported SMXZ as having potential to cause medium damage to Daphnia in the aquatic ecosystem (Chen & Zhou, 2014) and was the only SA posing a risk to algae in effluent water, with an risk quotient > 7 in a report by García-Galán et al. (2011). High RQs of 335.5 were reported for sulfachloropyridazine to green algae and 152 to Daphnia magna in ditch water, and the ecological and human health risks caused by sulfonamide mixtures were larger than the individual risks (Qin et al., 2020). In a review by Duan et al. (2022), the sulfonamides SDZ, SMXZ, and SMZ reportedly posed a great risk to the aquatic system. Results by Tang et al. (2015) suggested that SMXZ, ofloxacin, CIP, and ENR in the surface water of Lake Chaohu and inflowing rivers might pose a high risk to algae and plants. Enoxacin, CIP, and SMXZ posed high ecological risks (RQ > 1) to the aquatic organisms Vibrio fischeri, Microcystis aeruginosa, and Synechococcus leopoliensis, respectively, in aquatic environments (Zhang et al., 2012). For the benzimidazole ALB, its occurrence was classified as of medium risk to selected organisms, in the river and water source of Tuojiang River in Sichuan, China (Chen et al., 2021b). It is evident from ecological risk assessments that pharmaceuticals at certain concentrations pose a threat to aquatic environments. Further studies are necessary to fully understand the hazards that these pollutants present, more so considering the human health risks posed by ARB and ARGs present in aquatic environments (Khan et al., 2019).
Conclusion
In this study, 28 antibiotics and 4 benzimidazoles were investigated in surface water and sediments of an urban and suburban river. Their occurrence differed widely according to classes of the pharmaceuticals and was mainly influenced by anthropogenic activities within the sampled location. SAs had the highest contribution in the surface water, and SMXZ had the highest concentration of 247.0 ng L−1. The concentrations in surface water decreased in the order SAs > TMP > FQs > ATs > PNs ≈ MCs ≈ LNs > NIs. Residual pharmaceuticals in the sediments varied from nd to 974 µg kg−1. PEG and ALB represented the highest concentration in sediments in the range 414–974 µg kg−1 and 3–125 µg kg−1, respectively, while the order of detection was PNs > BZs > FQs > MLs > DAPs ≈ LNs > NIs > SAs. Ecological risk assessments showed that SMXZ and CIP were of high risk in the surface water, whereas PEV, AMP, PEG NOR, ENR, ERY, TLY, and LIN were of medium ecological risk in the aquatic system. Continuous monitoring of these pollutants is necessary as researcher’s endeavor to understand more on their fate in the environment, human, and ecological risks posed by these compounds, development of policies for interventions, and sustainable mitigation strategies.
Data availability
All data for the manuscript is available on request.
References
Abuin, S., Codony, R., Compañó, R., Granados, M., & Prat, M. D. (2006). Analysis of macrolide antibiotics in river water by solid-phase extraction and liquid chromatography-mass spectrometry. Journal of Chromatography A, 1114(1), 73–81. https://doi.org/10.1016/J.CHROMA.2006.02.032
Anwar, M., Iqbal, Q., & Saleem, F. (2020). Improper Disposal of Unused Antibiotics: An Often Overlooked Driver of Antimicrobial Resistance. https://doi.org/10.1080/14787210.2020.1754797,18(8),697-699.10(1080/14787210),pp.1754797,2020
Archundia, D., Duwig, C., Lehembre, F., Chiron, S., Morel, M. C., Prado, B., et al. (2017). Antibiotic pollution in the Katari subcatchment of the Titicaca Lake: Major transformation products and occurrence of resistance genes. The Science of the Total Environment, 576, 671–682. https://doi.org/10.1016/J.SCITOTENV.2016.10.129
Aydin, S., Aydin, M. E., Ulvi, A., & Kilic, H. (2019). Antibiotics in hospital effluents: Occurrence, contribution to urban wastewater, removal in a wastewater treatment plant, and environmental risk assessment. Environmental Science and Pollution Research, 26(1), 544–558. https://doi.org/10.1007/S11356-018-3563-0
Bansal, O. P. (2019). Antibiotics in hospital effluents and their impact on the antibiotics resistant bacteria and remediation of the antibiotics: A review. Network Pharmacology, 4(4), 6–30. Retrieved April 15, 2022 from, www.iaees.org/
Bhagat, C., Kumar, M., Tyagi, V. K., & Mohapatra, P. K. (2020). Proclivities for prevalence and treatment of antibiotics in the ambient water: A review. npj Clean Water 2020 3:1, 3(1), 1–18. https://doi.org/10.1038/s41545-020-00087-x
Bottoni, P., Caroli, S., & Caracciolo, A. B. (2010). Pharmaceuticals as Priority Water Contaminants. https://doi.org/10.1080/02772241003614320,92(3),549-565.10.1080/02772241003614320
Brown, K. D., Kulis, J., Thomson, B., Chapman, T. H., & Mawhinney, D. B. (2006). Occurrence of antibiotics in hospital, residential, and dairy effluent, municipal wastewater, and the Rio Grande in New Mexico. Science of the Total Environment, 366(2–3), 772–783. https://doi.org/10.1016/J.SCITOTENV.2005.10.007
Brown, A. K., & Wong, C. S. (2018). Distribution and fate of pharmaceuticals and their metabolite conjugates in a municipal wastewater treatment plant. Water Research, 144, 774–783. https://doi.org/10.1016/J.WATRES.2018.08.034
Burke, V., Richter, D., Greskowiak, J., Mehrtens, A., Schulz, L., & Massmann, G. (2016). Occurrence of antibiotics in surface and groundwater of a drinking water catchment area in Germany. Water Environment Research, 88(7), 652–659. https://doi.org/10.2175/106143016X14609975746604
Cardoza, L. A., Knapp, C. W., Larive, C. K., Belden, J. B., Lydy, M., & Graham, D. W. (2005). Factors affecting the fate of ciprofloxacin in aquatic field systems. Water, Air, and Soil Pollution, 161(1–4), 383–398. https://doi.org/10.1007/S11270-005-5550-6
Castiglioni, S., Bagnati, R., Calamari, D., Fanelli, R., & Zuccato, E. (2005). A multiresidue analytical method using solid-phase extraction and high-pressure liquid chromatography tandem mass spectrometry to measure pharmaceuticals of different therapeutic classes in urban wastewaters. Journal of Chromatography. A, 1092(2), 206–215. https://doi.org/10.1016/J.CHROMA.2005.07.012
Catteau, L., Zhu, L., Van Bambeke, F., & Quetin-Leclercq, J. (2018). Natural and hemi-synthetic pentacyclic triterpenes as antimicrobials and resistance modifying agents against Staphylococcus aureus: A review. Phytochemistry Reviews 2018 17:5, I7(5), 1129–1163. https://doi.org/10.1007/S11101-018-9564-2
Chattopadhyay, M. K. (2014). Use of antibiotics as feed additives: A burning question. Frontiers in Microbiology, 5, 334. https://doi.org/10.3389/FMICB.2014.00334/BIBTEX
Chen, Y., Chen, H., Zhang, L., Jiang, Y., Gin, K. Y. H., & He, Y. (2018). Occurrence, Distribution, and risk assessment of antibiotics in a subtropical river-reservoir system. Water 2018, 10(2), 104. https://doi.org/10.3390/W10020104
Chen, S., Gan, Z., Li, Z., Li, Y., Ma, X., Chen, M., et al. (2021b). Occurrence and risk assessment of anthelmintics in Tuojiang River in Sichuan, China. Ecotoxicology and Environmental Safety, 220, 112360. https://doi.org/10.1016/J.ECOENV.2021.112360
Chen, H., Zheng, W., Shen, X., Zhang, F., Zhou, X., Shen, J., & Lu, M. (2021a). Occurrence, Distribution, and ecological risk assessment of antibiotics in different environmental media in Anqing, Anhui Province, China. International Journal of Environmental Research and Public Health 2021, 18(15), 8112. https://doi.org/10.3390/IJERPH18158112
Chen, K., & Zhou, J. L. (2014). Occurrence and behavior of antibiotics in water and sediments from the Huangpu River, Shanghai, China. Chemosphere, 95, 604–612. https://doi.org/10.1016/J.CHEMOSPHERE.2013.09.119
Clarke, B. O., & Smith, S. R. (2011). Review of ‘emerging’ organic contaminants in biosolids and assessment of international research priorities for the agricultural use of biosolids. Environment International, 37(1), 226–247. https://doi.org/10.1016/J.ENVINT.2010.06.004
Davies, J., & Davies, D. (2010). Origins and evolution of antibiotic resistance. Microbiology and Molecular Biology Reviews: MMBR, 74(3), 417. https://doi.org/10.1128/MMBR.00016-10
Díaz-Cruz, M. S., García-Galán, M. J., & Barceló, D. (2008). Highly sensitive simultaneous determination of sulfonamide antibiotics and one metabolite in environmental waters by liquid chromatography-quadrupole linear ion trap-mass spectrometry. Journal of Chromatography. A, 1193(1–2), 50–59. https://doi.org/10.1016/J.CHROMA.2008.03.029
Dinh, Q. T., Moreau-Guigon, E., Labadie, P., Alliot, F., Teil, M. J., Blanchard, M., & Chevreuil, M. (2017). Occurrence of antibiotics in rural catchments. Chemosphere, 168, 483–490. https://doi.org/10.1016/J.CHEMOSPHERE.2016.10.106
Diwan, V., Tamhankar, A. J., Aggarwal, M., Sen, S., Khandal, R. K., Lundborg, C. S., et al. (2009). Detection of antibiotics in hospital effluents in India. Current Science, 97(12). Retrieved April 15, 2022, from http://www.biomedcentral.com/1471-2458/10/414
Duan, W., Cui, H., Jia, X., & Huang, X. (2022). Occurrence and ecotoxicity of sulfonamides in the aquatic environment: A review. Science of The Total Environment, 820, 153178. https://doi.org/10.1016/J.SCITOTENV.2022.153178
Duong, H. A., Phung, T. V., Nguyen, T. N., Phan Thi, L. A., & Pham, H. V. (2021). Occurrence, distribution, and ecological risk assessment of antibiotics in selected urban lakes of Hanoi, Vietnam. Journal of Analytical Methods in Chemistry, 2021. https://doi.org/10.1155/2021/6631797
Eagar, H., Swan, G., & Van Vuuren, M. (2012). A survey of antimicrobial usage in animals in South Africa with specific reference to food animals. Journal of the South African Veterinary Association, 83(1), 8. https://doi.org/10.4102/JSAVA.V83I1.16
Economou, V., & Gousia, P. (2015). Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infection and Drug Resistance, 8, 49–61. https://doi.org/10.2147/IDR.S55778
Faleye, A. C., Adegoke, A. A., Ramluckan, K., Bux, F., & Stenström, T. A. (2018). Antibiotic residue in the aquatic environment: Status in Africa. Open Chemistry, 16(1), 890–903. https://doi.org/10.1515/CHEM-2018-0099/ASSET/GRAPHIC/J_CHEM-2018-0099_FIG_003.JPG
Feitosa-Felizzola, J., & Chiron, S. (2009). Occurrence and distribution of selected antibiotics in a small Mediterranean stream (Arc River, Southern France). Journal of Hydrology, 364(1–2), 50–57. https://doi.org/10.1016/J.JHYDROL.2008.10.006
Fernandes, M. J., Paíga, P., Silva, A., Llaguno, C. P., Carvalho, M., Vázquez, F. M., & Delerue-Matos, C. (2020). Antibiotics and antidepressants occurrence in surface waters and sediments collected in the north of Portugal. Chemosphere, 239, 124729. https://doi.org/10.1016/J.CHEMOSPHERE.2019.124729
García-Galán, M. J., Díaz-Cruz, M. S., & Barceló, D. (2011). Occurrence of sulfonamide residues along the Ebro river basin: Removal in wastewater treatment plants and environmental impact assessment. Environment International, 37(2), 462–473. https://doi.org/10.1016/J.ENVINT.2010.11.011
Golchin, M., Khani, M., Sadani, M., Sadeghi, M., Jahangiri, M., Golchin, M., et al. (2021). Occurrence and fate of amoxicillin and penicillin G antibiotics in hospital wastewater treatment plants: A case study - Gonbad Kavous. Iran. South African Journal of Chemistry, 75, 98–105. https://doi.org/10.17159/0379-4350/2021/V75A11
Hanna, N., Sun, P., Sun, Q., Li, X., Yang, X., Ji, X., et al. (2018). Presence of antibiotic residues in various environmental compartments of Shandong province in eastern China: Its potential for resistance development and ecological and human risk. Environment International, 114, 131–142. https://doi.org/10.1016/J.ENVINT.2018.02.003
Hoa, P. T. P., Managaki, S., Nakada, N., Takada, H., Shimizu, A., Anh, D. H., et al. (2011). Antibiotic contamination and occurrence of antibiotic-resistant bacteria in aquatic environments of northern Vietnam. The Science of the Total Environment, 409(15), 2894–2901. https://doi.org/10.1016/J.SCITOTENV.2011.04.030
Hu, Y., Yan, X., Shen, Y., Di, M., & Wang, J. (2018). Antibiotics in surface water and sediments from Hanjiang River, Central China: Occurrence, behavior and risk assessment. Ecotoxicology and Environmental Safety, 157, 150–158. https://doi.org/10.1016/J.ECOENV.2018.03.083
Jiang, Y., Li, M., Guo, C., An, D., Xu, J., Zhang, Y., & Xi, B. (2014). Distribution and ecological risk of antibiotics in a typical effluent–receiving river (Wangyang River) in north China. Chemosphere, 112, 267–274. https://doi.org/10.1016/J.CHEMOSPHERE.2014.04.075
K’oreje, K. O., Demeestere, K., De Wispelaere, P., Vergeynst, L., Dewulf, J., & Van Langenhove, H. (2012). From multi-residue screening to target analysis of pharmaceuticals in water: Development of a new approach based on magnetic sector mass spectrometry and application in the Nairobi River basin, Kenya. Science of the Total Environment, 437, 153–164. https://doi.org/10.1016/J.SCITOTENV.2012.07.052
Kairigo, P., Ngumba, E., Sundberg, L. R., Gachanja, A., & Tuhkanen, T. (2020a). Contamination of surface water and river sediments by antibiotic and antiretroviral drug cocktails in low and middle-income countries: Occurrence, risk and mitigation strategies. Water (switzerland), 12(5), 1376. https://doi.org/10.3390/W12051376
Kairigo, P., Ngumba, E., Sundberg, L. R., Gachanja, A., & Tuhkanen, T. (2020b). Occurrence of antibiotics and risk of antibiotic resistance evolution in selected Kenyan wastewaters, surface waters and sediments. Science of The Total Environment, 720, 137580. https://doi.org/10.1016/J.SCITOTENV.2020.137580
Kandie, F. J., Krauss, M., Massei, R., Ganatra, A., Fillinger, U., Becker, J., et al. (2020). Multi-compartment chemical characterization and risk assessment of chemicals of emerging concern in freshwater systems of western Kenya. Environmental Sciences Europe, 32(1), 1–12. https://doi.org/10.1186/S12302-020-00392-9/FIGURES/6
Khan, F. A., Söderquist, B., & Jass, J. (2019). Prevalence and diversity of antibiotic resistance genes in Swedish aquatic environments impacted by household and hospital wastewater. Frontiers in Microbiology, 10(APR), 688. https://doi.org/10.3389/FMICB.2019.00688/BIBTEX
Kimosop, S. J., Getenga, Z. M., Orata, F., Okello, V. A., & Cheruiyot, J. K. (2016). Residue levels and discharge loads of antibiotics in wastewater treatment plants (WWTPs), hospital lagoons, and rivers within Lake Victoria Basin. Kenya. Environmental Monitoring and Assessment, 188(9), 1–9. https://doi.org/10.1007/S10661-016-5534-6/METRICS
Kortesmäki, E., Östman, J. R., Meierjohann, A., Brozinski, J. M., Eklund, P., & Kronberg, L. (2020). Occurrence of antibiotics in influent and effluent from 3 major wastewater-treatment plants in Finland. Environmental Toxicology and Chemistry, 39(9), 1774–1789. https://doi.org/10.1002/ETC.4805
Kotwani, A., Joshi, J., & Kaloni, D. (2021). Pharmaceutical effluent: A critical link in the interconnected ecosystem promoting antimicrobial resistance. Environmental Science and Pollution Research, 28(25), 32111–32124. https://doi.org/10.1007/S11356-021-14178-W/TABLES/1
Kraemer, S. A., Ramachandran, A., & Perron, G. G. (2019). Antibiotic pollution in the environment: from microbial ecology to public policy. Microorganisms, 7(6). https://doi.org/10.3390/MICROORGANISMS7060180
Kuang, Y., Guo, X., Hu, J., Li, S., Zhang, R., Gao, Q., et al. (2020). Occurrence and risks of antibiotics in an urban river in northeastern Tibetan Plateau. Scientific Reports 2020 10:1, 10(1), 1–11. https://doi.org/10.1038/s41598-020-77152-5
Kuchta, S. L., & Cessna, A. J. (2009). Lincomycin and spectinomycin concentrations in liquid swine manure and their persistence during simulated manure storage. Archives of Environmental Contamination and Toxicology, 57(1), 1–10. https://doi.org/10.1007/S00244-008-9229-Z
Kumirska, J., Wagil, M., Stolte, S., Maksymiuk, M., Puckowski, A., Maszkowska, J., et al. (2015). Anthelmintics in the aquatic environment: A new analytical approach. Current Analytical Chemistry, 12(3), 227–236. https://doi.org/10.2174/1573411012666151009193940
Kümmerer, K. (2009). Antibiotics in the aquatic environment – A review – Part I. Chemosphere, 75(4), 417–434. https://doi.org/10.1016/J.CHEMOSPHERE.2008.11.086
Li, D., Shao, H., Huo, Z., Xie, N., Gu, J., & Xu, G. (2021). Typical antibiotics in the receiving rivers of direct-discharge sources of sewage across Shanghai: Occurrence and source analysis. RSC Advances, 11(35), 21579–21587. https://doi.org/10.1039/D1RA02510D
Li, X. W., Xie, Y. F., Li, C. L., Zhao, H. N., Zhao, H., Wang, N., & Wang, J. F. (2014). Investigation of residual fluoroquinolones in a soil–vegetable system in an intensive vegetable cultivation area in Northern China. Science of the Total Environment, 468–469, 258–264. https://doi.org/10.1016/J.SCITOTENV.2013.08.057
Lindberg, R., Jarnheimer, P. Å., Olsen, B., Johansson, M., & Tysklind, M. (2004). Determination of antibiotic substances in hospital sewage water using solid phase extraction and liquid chromatography/mass spectrometry and group analogue internal standards. Chemosphere, 57(10), 1479–1488. https://doi.org/10.1016/J.CHEMOSPHERE.2004.09.015
Litynska, M., Kyrii, S., Nosovska, O., & Ryzhenko, N. (2021). Problem of antibiotics in natural water: A review. Water And Water Purification Technologies. Scientific And Technical News, 31(3), 26–34. https://doi.org/10.20535/2218-930032021247159
Martín, J., Santos, J. L., Aparicio, I., & Alonso, E. (2015). Pharmaceutically active compounds in sludge stabilization treatments: Anaerobic and aerobic digestion, wastewater stabilization ponds and composting. The Science of the Total Environment, 503–504, 97–104. https://doi.org/10.1016/J.SCITOTENV.2014.05.089
Matongo, S., Birungi, G., Moodley, B., & Ndungu, P. (2015). Pharmaceutical residues in water and sediment of Msunduzi River, KwaZulu-Natal, South Africa. Chemosphere, 134, 133–140. https://doi.org/10.1016/J.CHEMOSPHERE.2015.03.093
Meng, T., Cheng, W., Wan, T., Wang, M., Ren, J., Li, Y., & Huang, C. (2021). Occurrence of antibiotics in rural drinking water and related human health risk assessment. Environmental Technology, 42(5), 671–681. https://doi.org/10.1080/09593330.2019.1642390
Mirzaei, R., Yunesian, M., Nasseri, S., Gholami, M., Jalilzadeh, E., Shoeibi, S., & Mesdaghinia, A. (2018). Occurrence and fate of most prescribed antibiotics in different water environments of Tehran, Iran. The Science of the Total Environment, 619–620, 446–459. https://doi.org/10.1016/J.SCITOTENV.2017.07.272
Mutiyar, P. K., & Mittal, A. K. (2014). Occurrences and fate of selected human antibiotics in influents and effluents of sewage treatment plant and effluent-receiving river Yamuna in Delhi (India). Environmental Monitoring and Assessment, 186(1), 541–557. https://doi.org/10.1007/S10661-013-3398-6
Ncube, S., Nuapia, Y. B., Chimuka, L., Madikizela, L. M., & Etale, A. (2021). Trace detection and quantitation of antibiotics in a South African stream receiving wastewater effluents and municipal dumpsite leachates. Frontiers in Environmental Science, 9, 365. https://doi.org/10.3389/FENVS.2021.733065/BIBTEX
Ngigi, A. N., Magu, M. M., & Muendo, B. M. (2020). Occurrence of antibiotics residues in hospital wastewater, wastewater treatment plant, and in surface water in Nairobi County, Kenya. Environmental Monitoring and Assessment, 192(1), 1–16. https://doi.org/10.1007/s10661-019-7952-8
Oh, S.-G., Kim, J.-K., Park, S.-Y., Lee, M.-J., & Choi, K.-H. (2004). Aquatic toxicities of major antimicrobial and anthelmintic veterinary pharmaceuticals and their potential ecological risks -Proceedings of the Korean Environmental Health Society Conference (pp. 173–177). Korea: Korean Society of Environmental Health (한국환경보건학회). Retrieved May 24, 2022 from, https://www.koreascience.or.kr/article/CFKO200436036094227.jsp-k1ff8j=SSMHB4&py=2012&vnc=v27n6&sp=588
Opanga, M. A. (2018). Assessment of selected antibiotics in ngong river and their removal using activated carbon. February 23, 2023 from, http://erepository.uonbi.ac.ke/bitstream/handle/11295/104925/OPANGA.pdf?isAllowed=y&sequence=1
Pavlović, D. M., Glavač, A., Gluhak, M., & Runje, M. (2018). Sorption of albendazole in sediments and soils: Isotherms and kinetics. Chemosphere, 193, 635–644. https://doi.org/10.1016/J.CHEMOSPHERE.2017.11.025
Pokharel, S., Shrestha, P., & Adhikari, B. (2020). Antimicrobial use in food animals and human health: Time to implement ‘One Health’ approach. Antimicrobial Resistance and Infection Control, 9(1), 1–5. https://doi.org/10.1186/S13756-020-00847-X/METRICS
Polianciuc, S. I., Gurzău, A. E., Kiss, B., Georgia Ștefan, M., & Loghin, F. (2020). Antibiotics in the environment: Causes and consequences. Medicine and Pharmacy Reports, 93(3), 231. https://doi.org/10.15386/MPR-1742
Poole, T., & Sheffield, C. (2013). Use and misuse of antimicrobial drugs in poultry and livestock: Mechanisms of antimicrobial resistance - PubAg. Pakistan Vterinary Journal, 33(3), 266–271. Retrieved April 14, 2023 from, https://pubag.nal.usda.gov/catalog/58191
Qin, L. T., Pang, X. R., Zeng, H. H., Liang, Y. P., Mo, L. Y., Wang, D. Q., & Dai, J. F. (2020). Ecological and human health risk of sulfonamides in surface water and groundwater of Huixian karst wetland in Guilin, China. The Science of the Total Environment, 708. https://doi.org/10.1016/J.SCITOTENV.2019.134552
Rodriguez-Mozaz, S., Vaz-Moreira, I., Varela Della Giustina, S., Llorca, M., Barceló, D., Schubert, S., et al. (2020). Antibiotic residues in final effluents of European wastewater treatment plants and their impact on the aquatic environment. Environment International, 140, 105733. https://doi.org/10.1016/j.envint.2020.105733
Sarmah, A. K., Meyer, M. T., & Boxall, A. B. A. (2006, October). A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere. https://doi.org/10.1016/j.chemosphere.2006.03.026
Schmieder, R., & Edwards, R. (2012). Insights into antibiotic resistance through metagenomic approaches. Future Microbiology, 7(1), 73–89. https://doi.org/10.2217/FMB.11.135
Sharma, B. M., Bečanová, J., Scheringer, M., Sharma, A., Bharat, G. K., Whitehead, P. G., et al. (2019). Health and ecological risk assessment of emerging contaminants (pharmaceuticals, personal care products, and artificial sweeteners) in surface and groundwater (drinking water) in the Ganges River Basin, India. Science of the Total Environment, 646, 1459–1467. https://doi.org/10.1016/J.SCITOTENV.2018.07.235
Sharma, L., Siedlewicz, G., & Pazdro, K. (2021). The toxic effects of antibiotics on freshwater and marine photosynthetic microorganisms: State of the art. Plants, 10(3), 1–15. https://doi.org/10.3390/PLANTS10030591
Sim, W. J., Kim, H. Y., Choi, S. D., Kwon, J. H., & Oh, J. E. (2013). Evaluation of pharmaceuticals and personal care products with emphasis on anthelmintics in human sanitary waste, sewage, hospital wastewater, livestock wastewater and receiving water. Journal of Hazardous Materials, 248–249(1), 219–227. https://doi.org/10.1016/J.JHAZMAT.2013.01.007
Sodhi, K. K., Kumar, M., Balan, B., Dhaulaniya, A. S., Shree, P., Sharma, N., & Singh, D. K. (2021). Perspectives on the antibiotic contamination, resistance, metabolomics, and systemic remediation. SN Applied Sciences, 3(2), 1–25. https://doi.org/10.1007/S42452-020-04003-3/FIGURES/2
Tang, J., Shi, T., Wu, X., Cao, H., Li, X., Hua, R., et al. (2015). The occurrence and distribution of antibiotics in Lake Chaohu, China: Seasonal variation, potential source and risk assessment. Chemosphere, 122, 154–161. https://doi.org/10.1016/J.CHEMOSPHERE.2014.11.032
Thiele-Bruhn, S. (2003). Pharmaceutical antibiotic compounds in soils – A review. Journal of Plant Nutrition and Soil Science, 166(2), 145–167. https://doi.org/10.1002/JPLN.200390023
Van Dijk, J., & Keukens, H.J. (2000). The Stability of Some Veterinary Drugs and Coccidiostats during Composting and Storage of Laying Hen and Broiler Faeces. In Ginkel, L.A. and Ruiter, A., Eds., Residues of Veterinary Drugs in Food, Proceedings of the EuroResidue IV Conference, Veldhoven, 356–360
Van, T. T. H., Yidana, Z., Smooker, P. M., & Coloe, P. J. (2020). Antibiotic use in food animals worldwide, with a focus on Africa: Pluses and minuses. Journal of Global Antimicrobial Resistance, 20, 170–177. https://doi.org/10.1016/J.JGAR.2019.07.031
Viana, P., Meisel, L., Lopes, A., De Jesus, R., Sarmento, G., Duarte, S., et al. (2021). Identification of antibiotics in surface-groundwater. A tool towards the ecopharmacovigilance approach: A Portuguese case-study. Antibiotics, 10(8). https://doi.org/10.3390/ANTIBIOTICS10080888/S1
Wang, H., Wang, N., Wang, B., Zhao, Q., Fang, H., Fu, C., et al. (2016). Antibiotics in drinking water in Shanghai and their contribution to antibiotic exposure of school children. Environmental Science and Technology, 50(5), 2692–2699. https://doi.org/10.1021/ACS.EST.5B05749/SUPPL_FILE/ES5B05749_SI_001.PDF
Watkinson, A. J., Murby, E. J., Kolpin, D. W., & Costanzo, S. D. (2009). The occurrence of antibiotics in an urban watershed: From wastewater to drinking water. Science of the Total Environment, 407(8), 2711–2723. https://doi.org/10.1016/J.SCITOTENV.2008.11.059
Wei, Y., Zhang, Y., Xu, J., Guo, C., Li, L., & Fan, W. (2013). Simultaneous quantification of several classes of antibiotics in water, sediments, and fish muscles by liquid chromatography-tandem mass spectrometry. Frontiers of Environmental Science & Engineering 2013 8:3, 8(3), 357–371. https://doi.org/10.1007/S11783-013-0580-6
Yan, C., Yang, Y., Zhou, J., Liu, M., Nie, M., Shi, H., & Gu, L. (2013). Antibiotics in the surface water of the Yangtze Estuary: Occurrence, distribution and risk assessment. Environmental pollution (Barking, Essex : 1987), 175, 22–29. https://doi.org/10.1016/J.ENVPOL.2012.12.008
Yang, S., & Carlson, K. (2003). Evolution of antibiotic occurrence in a river through pristine, urban and agricultural landscapes. Water Research, 37(19), 4645–4656. https://doi.org/10.1016/S0043-1354(03)00399-3
Yang, J. F., Ying, G. G., Zhao, J. L., Tao, R., Su, H. C., & Chen, F. (2010). Simultaneous determination of four classes of antibiotics in sediments of the Pearl Rivers using RRLC-MS/MS. Science of the Total Environment, 408(16), 3424–3432. https://doi.org/10.1016/J.SCITOTENV.2010.03.049
Yao, L., Wang, Y., Tong, L., Deng, Y., Li, Y., Gan, Y., et al. (2017). Occurrence and risk assessment of antibiotics in surface water and groundwater from different depths of aquifers: A case study at Jianghan Plain, central China. Ecotoxicology and Environmental Safety, 135, 236–242. https://doi.org/10.1016/J.ECOENV.2016.10.006
Yao, S., Ye, J., Yang, Q., Hu, Y., Zhang, T., Jiang, L., et al. (2021). Occurrence and removal of antibiotics, antibiotic resistance genes, and bacterial communities in hospital wastewater. Environmental Science and Pollution Research, 28(40), 57321–57333. https://doi.org/10.1007/S11356-021-14735-3/FIGURES/5
Yin, Z. (2021). Distribution and ecological risk assessment of typical antibiotics in the surface waters of seven major rivers. China. Environmental Science: Processes & Impacts, 23(8), 1088–1100. https://doi.org/10.1039/D1EM00079A
Zajíčková, M., Nguyen, L. T., Skálová, L., Raisová Stuchlíková, L., & Matoušková, P. (2020). Anthelmintics in the future: Current trends in the discovery and development of new drugs against gastrointestinal nematodes. Drug Discovery Today, 25(2), 430–437. https://doi.org/10.1016/J.DRUDIS.2019.12.007
Zhang, G., Lu, S., Wang, Y., Liu, X., Liu, Y., Xu, J., et al. (2020). Occurrence of antibiotics and antibiotic resistance genes and their correlations in lower Yangtze River, China. Environmental pollution (Barking, Essex : 1987), 257. https://doi.org/10.1016/J.ENVPOL.2019.113365
Zhang, R., Zhang, G., Zheng, Q., Tang, J., Chen, Y., Xu, W., et al. (2012). Occurrence and risks of antibiotics in the Laizhou Bay, China: Impacts of river discharge. Ecotoxicology and Environmental Safety, Complete(80), 208–215. https://doi.org/10.1016/J.ECOENV.2012.03.002
Zheng, Q., Zhang, R., Wang, Y., Pan, X., Tang, J., & Zhang, G. (2012). Occurrence and distribution of antibiotics in the Beibu Gulf, China: Impacts of river discharge and aquaculture activities. Marine Environmental Research, 78, 26–33. https://doi.org/10.1016/J.MARENVRES.2012.03.007
Zhu, X., Wang, X., Wang, L., Fan, X., Li, X., & Jiang, Y. (2020). Biodegradation of lincomycin in wastewater by two-level bio-treatment using chloroperoxidase and activated sludge: Degradation route and eco-toxicity evaluation. Environmental Technology and Innovation, 20. https://doi.org/10.1016/J.ETI.2020.101114
Zrnčić, M., Gros, M., Babić, S., Kaštelan-Macan, M., Barcelo, D., & Petrović, M. (2014). Analysis of anthelmintics in surface water by ultra high performance liquid chromatography coupled to quadrupole linear ion trap tandem mass spectrometry. Chemosphere, 99, 224–232. https://doi.org/10.1016/J.CHEMOSPHERE.2013.10.091
Zuccato, E., Castiglioni, S., & Fanelli, R. (2005). Identification of the pharmaceuticals for human use contaminating the Italian aquatic environment. Journal of Hazardous Materials, 122(3), 205–209. https://doi.org/10.1016/J.JHAZMAT.2005.03.001
Funding
The authors are thankful to Africa Centre of Excellence in Phytochemicals, Textile, and Renewable Energy (ACE II-PTRE) for the financial support, National Research Fund (NRF) research grant No. NRF/2/MMC/666 for provision of reference standards and meeting cost of LC–MS/MS analysis.
Author information
Authors and Affiliations
Contributions
Catherine Chemtai conducted the sampling, did sample preparations, obtained funding from Africa Centre of Excellence in Phytochemicals, Textile, and Renewable Energy (ACE II-PTRE) for her studies, and wrote the initial manuscript draft. Fredrick O. Kengara and Anastasiah N. Ngigi obtained funds, did project conception and design, provided supervision, and contributed to the editing of the manuscript. Anastasiah N. Ngigi was involved in project administration, method validation, and optimization and carried out data analysis and interpretation. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chemtai, C., Kengara, F.O. & Ngigi, A.N. Levels and ecological risk of pharmaceuticals in River Sosiani, Kenya. Environ Monit Assess 195, 431 (2023). https://doi.org/10.1007/s10661-023-11022-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-11022-1