Abstract
The study presented the occurrence of antibiotics in 16 different hospital effluents, the removal of antibiotics in urban wastewater treatment plant (WWTP), and the potential ecotoxicological risks of the effluent discharge on the aquatic ecosystem. The total concentration of antibiotics in hospital effluents was ranged from 21.2 ± 0.13 to 4886 ± 3.80 ng/L in summer and from 497 ± 3.66 to 322,735 ± 4.58 ng/L in winter. Azithromycin, clarithromycin, and ciprofloxacin were detected the highest concentrations among the investigated antibiotics. The total antibiotic load to the influent of the WWTP from hospitals was 3.46 g/day in summer and 303.2 g/day in winter. The total antibiotic contribution of hospitals to the influent of the WWTP was determined as 13% in summer and 28% in winter. The remaining 87% in summer and 72% in winter stems from the households. The total antibiotic removal by conventional physical and biological treatment processes was determined as 79% in summer, whereas it decreased to 36% in winter. When the environmental risk assessment was performed, azithromycin and clarithromycin in the effluent from the treatment plant in winter posed a high risk (RQ > 10) for the aquatic organisms (algae and fish) in the receiving environment. According to these results, the removal efficiency of antibiotics at the WWTP is inadequate and plant should be improved to remove antibiotics by advanced treatment processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
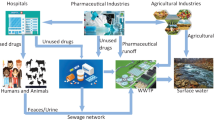
In recent years, pharmaceuticals (PhCs) are extensively used in human therapy and animal husbandry. PhCs are potentially hazardous, persistent, and ubiquitous in the environment. PhCs have become a major concern for human health and the environment in nowadays. Because, PhCs have biologic activity and they may cause undesired effects in non-target organisms in environment media (Mendoza et al. 2015). Also, these compounds are often resistant to biodegradation in aquatic and terrestrial ecosystems. Urban wastewater is the most important source of PhCs in an aquatic environment. After PhCs are consumed, they are excreted into the sewerage system in the form of parent compound at the rate of 30–90% as active compounds (Lyons 2014). Depending on the chemical properties of the compound, 5 to 90% of the antibiotics are excreted from the body as parent compounds (Kümmerer 2009). In hospitals, large quantities of the PhCs are consumed every day. Hospital wastewaters are generally discharged directly into the public sewerage system without any pre-treatment (Perrodin et al. 2013; Verlicchi et al. 2012; Carraro et al. 2016). Hospital wastewaters contribute to the load of the PhCs in influent of a wastewater treatment plant (WWTP). It is not known how much this contribution is (Kümmerer 2008). To date, very limited data has been reported on the percentage contribution of hospital effluents towards the load of pharmaceuticals in WWTPs in the literature. In addition, unused or expired drugs are at the disposal to the sewerage system (Kümmerer 2001; Götz and Keil 2007). Conventional WWTP do not provide for the removal of PhCs efficiently (Alygizakis et al. 2016; Feng et al. 2013). Therefore, PhCs have been detected in many environmental media such as surface water (Vystavna et al. 2012), ground water (Fram and Belitz 2011), drinking water (Focazio et al. 2008), wastewater (Pedrouzo et al. 2011), and in sludge (Zhou et al. 2011) in many countries at concentrations generally in the ng/L to μg/L range.
Since 1940, antibiotics are extensively used for the treatment of infectious diseases (Wang et al. 2018). They are used as growth promotion in fish farms and livestock (Zhou et al. 2013). Antibiotics were also generated from biological activity and process in microorganisms, plants, and animals (Hu et al. 2018; Kümmerer 2008). Antibiotics are pharmaceutical compounds commonly used in hospitals. It is reported that antibiotics are one of the highest load groups from the hospitals and widely detected in hospital effluents (Verlicchi et al. 2010; Santos et al. 2013; Mendoza et al. 2015). Antibiotics should be particularly taken into account for their role in the entry of antibiotics into multi-resistant microorganisms in domestic wastewaters (Brown et al. 2006). Of the Turkey drug consumption, 18.1% is composed of antibiotics, 8.4% of respiratory system drugs, 6.3% of cardiovascular system drugs, 5.2% of metabolism and digestive drugs and 3.7% of nervous system drugs. The antibiotic consumption in Turkey is 2–3 times more than the annual consumption in European countries. Although getting antibiotics without prescription was possible in Turkey before 2016, the Ministry of Health forbade the sale of antibiotics without prescription in that year. There is no legal control mechanism on the concentrations and/or discharges of antibiotics in the environment in Turkey yet.
People are exposed to pharmaceutical residues in many ways (milk, meat, contaminated fertilizer and products exposed to sewage sludge, fish, and drinking water, etc.). Many pharmaceutical compounds are detected at low concentrations in the environment, but the long-term effects of being exposed to one or more low-level pollutants are unknown. In particular, the excessive and inappropriate use of antibiotics contributes to the spread of antibiotic resistance in the environment. In the Water Framework Directive of the European Union in 2012, the limit value was defined for three pharmaceuticals including diclofenac, 17α-ethinyl estradiol, and 17β-estradiol in the monitoring list of priority pollutants. In 2015, four pharmaceuticals including azithromycin, clarithromycin, erythromycin, and estrone were also added to the list. Today, there are approximately 3000 licensed pharmaceutical products in the market. More pharmaceutical compounds should be included in this list, and they should be monitored within the water quality standards. Also, legal procedures should be developed to monitor pollutants in effluent of WWTP. More effective studies for measurement and monitoring of pharmaceuticals in the environment should be carried out.
The purpose of this study is (i) to determine the concentrations and distributions of antibiotics in summer and winter in the effluents of 16 hospitals in different sizes, in the influents and effluents of urban WWTP, (ii) to determine the antibiotic contribution of the hospitals to the urban wastewater, (iii) to determine the removal rate for each antibiotic compound with the conventional WWTP, and (iv) to evaluate the potential ecotoxicological risks for aquatic organisms in the receiving environment. The results of the study will be useful in taking necessary precautions before discharging hospital wastewaters to the sewerage system, updating the existing urban WWTP, and developing environmental sustainable policies concerning pharmaceuticals.
Material and methods
Chemicals and equipment
All chemicals were of analytical reagent grade. Erythromycin (ERY) and sulfamethazine (SMZ) were obtained from Sigma (Switzerland), while azithromycin (AZI), sulfamethoxazole (SMX), trimethoprim (TMP), chlortetracycline (CTC), ciprofloxacin (CIPRO), clarithromycin (CLAR), oxytetracycline (OXY), and doxycycline (DOXY) were obtained from Fluka (Switzerland). CIPRO was dissolved in a methanol/acetonitrile (17.5/7.5, v/v) containing 0.2% HCl in order to obtain stock solutions, while TMP and CTC were dissolved in a methanol/acetonitrile (1/1, v/v). The other remaining compounds were dissolved in methanol. Prepared stock solutions were stored in dark environments at − 20 °C in amber vials until used. Methanol, acetonitrile, hydrochloric acid (37%), formic acid (98%), and Na2EDTA (ethylenediaminetetraacetic acid disodium salt solution) were obtained from Merck Co (Darmstadt, Germany). All solvents were of HPLC grade. Glass fiber filter (1.2 μm pore size) were acquired from Whatman (USA); naylon (0.45 μm pore size) and PTFE syringe filters (0.22 μm pore size) were acquired from Sartorius (Göttingen, Germany). The Oasis HLB (hydrophilic-lipophilic) (60 mg, 3 mL) cartridges were purchased from Waters Corporation. The high-purity nitrogen gas was obtained from the nitrogen generator (Peak Scientific). Deionized water was purified with Millipore Milli-Q Plus water purification system (Millipore, USA).
Wastewater samples
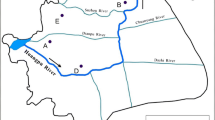
Hospital wastewaters: Samples were taken from effluents of 16 hospitals. Three of the hospitals are university hospitals, one is pediatric hospital, and the others are general hospitals. University hospitals are large hospitals with between 903 and 1298 beds. Pediatric hospital is a medium-size hospital with 363 beds. Four general hospitals are medium-sized hospitals with between 194 and 600 beds, while eight hospitals are small-sized hospitals with between 27 and 103 beds. Effluents of all the hospitals are directly discharged without any treatment process into the combined sewerage systems reaching the urban WWTP. The contribution of the hospitals to the total flow entering the WWTP is approximately 3.5%. Two experimental periods were carried out in August 2015 (summer) and in January 2016 (winter). Portable automatic composite micro-sampler (Durko, Turkey) was used to collect wastewater samples from each hospital. Every day, 2-hcomposite samples were collected at 8 a.m., 4 p.m., and 8 p.m. and then samples were combined. Samples were transferred to amber glass bottles and stored at 4 °C until the analysis was performed.
Urban WWTP and samples: WWTP receives urban wastewaters including domestic and hospital wastewaters. It was designed for 1,600,000 population equivalent, and influent flow rate is on 300,000 m3/day in 2030. WWTP performs primary treatment (screening, grit removal, and preliminary sedimentation), a biological treatment including nitrogen and phosphorus removal, secondary sedimentation, and disinfection system with ultraviolet. Treated wastewaters are discharged into Lake Tuz through main discharge channel. Twenty-four-hour composite wastewater samples were taken from the influent and effluent of WWTP. The samples were collected on the same day with the hospital effluents. Wastewater samples were transferred in amber glass bottles and stored at 4 °C until the analysis was performed.
Analytical method
For analysis of antibiotics, wastewater samples were vacuum filtered through 1.2-μm glass fiber filter followed by 0.45-μm nylon membrane filter. Na2EDTA reduces the binding of pharmaceutical compounds to cations in water and thus improves the extraction recovery of some pharmaceutical compounds (López-Serna et al. 2011). Na2EDTA (0.1 M) was added to achieve a final Na2EDTA concentration of 0.1% in the wastewaters. Aliquots of 200 mL wastewaters were preconcentrated solid-phase extraction. A lipophilic-hydrophilic balanced Oasis HLB (60 mg, 3 mL) cartridge was conditioned at 5 mL of methanol and 5 mL of deionized water. After sample preconcentration, cartridge was rinsed with 5 mL of deionized water and dried under vacuum for 10 min. The elution of the antibiotics in the cartridge was carried out with 2 × 4 mL of methanol. The extract was evaporated to dryness under a gentle nitrogen stream. Finally, it was re-dissolved in 200 μL of methanol/water (10/90, v/v). Each sample was analyzed in duplicate. In order to control quality, the spike sample extraction was performed regularly together with the analyses of real samples. Also, procedural blanks were carried out for potential contamination problems.
Analyses of antibiotics were performed using liquid chromatography (Agilent 1260 HPLC, USA), equipped with a 6460 jet stream Triple Quadrupole mass spectrophotometer (MS). Chromatographic separation was carried out with Agilent Poroshell 120 EC-C18 (100 mm × 3 mm, particle size 2.7 μm) column. MS detection was performed with electrospray ionization (ESI) at the positive ion mode. Analyses were performed using eluent A (deionized water containing 0.5% formic acid and 2 mM ammonium formate) and eluent B (methanol) at a flow rate of 0.5 mL/min. The column temperature was 35 °C and the injection volume was 2 μL. Analytical method validation parameters including m/z, limit of detection (LOD), limit of quantification (LOQ), linearity range, linearity (R2), repeatability, and recoveries obtained for target antibiotics in different matrices (WWTP influent and WWTP effluent) are given in Table 1. The calibration curve was prepared by using at least seven standard solutions in the linear range given in Table 1. R2 values were higher than 0.992 for all antibiotics. The LODs (signal-to-noise = 3) were determined in the range 0.004 and 0.867 pg/L for target analytes while the LOQs (signal-to-noise = 10) were determined in the range 0.012 and 2.890 pg/L for target analytes. Relative standard deviations (RSDs) were below 9.12% for all compounds. Recoveries of the antibiotics were determined in the range of 83 ± 5 and 102 ± 4% in WWTP influent and 85 ± 0 and 100 ± 5% in WWTP effluent by analyzing fortified wastewaters spiked to 2000 ng/L.
Physicochemical analysis of wastewater samples
Generally, hospital wastewater is considered as domestic wastewater and it is discharged in the municipal sewerage system without any pre-treatment. Parameters such as pH, electrical conductivity (EC), total suspended solid (TSS), and chemical oxygen demand (COD) order by legislation for assessing the quality of a common wastewater. Therefore, pH, EC, TSS, and COD analyses of the wastewaters were carried out in the study. pH and EC measurements were carried out after taking samples of the wastewater using portable pH and EC meter (Hach brand). The measurement of TSS was performed according to Standard Methods (APHA 1992). The analysis of COD was performed by using commercial kits with WTW brand spectrophotometer.
Ecotoxicological risk assessment
Risk quotients (RQs) were used to evaluate the potential ecotoxicological risks of antibiotics on the aquatic ecosystem. RQs for each antibiotics were calculated as the quotient between their measured environmental concentration (MEC) and the predicted non-effect environmental concentration (PNEC) of the substance (European Commission 2003). The maximum concentration determined for each antibiotics in the wastewaters was taken as MEC value. PNEC values used in the calculation of environmental risk for three different trophic levels (fish, Daphnia magna and algae) are given in Table 2. RQ was evaluated according to commonly used risk ranking criterion. RQ < 0.1 is considered insignificant risk (no adverse effect expected), 0.1 < RQ < 1 is considered low risk (potential adverse effects), 1 < RQ < 10 is considered moderate risk (probable adverse effect), and RQ > 10 is considered high risk to aquatic organisms (adverse effect) (Verlicchi et al. 2012; Deblonde and Hartemann 2013).
Results and discussion
Results of physicochemical analysis hospital effluents, inlet and outlet wastewaters in WWTP
Table 3 represents physicochemical analysis data of hospital effluents and influents and effluents of urban WWTP. The pH values of the hospital effluents were in the range of discharge limit values determined by KOSKI. The effluents of the WWTP meet the discharge standards of the receiving media. The TSS values for the hospital wastewaters range from 18 to 1124 mg/L in summer and from 92 to 1218 mg/L in winter. TSS values of the hospitals were found to vary very much both in summer and winter samplings. Six out of the 16 hospitals in summer and 7 out of the 16 hospitals in winter exceeded the discharge limit value. The effluent of WWTP meets the receiving media discharge standards for summer but do not meet the standard values for winter. It is seen in Table 3 that both summer and winter samplings of the hospital wastewaters meet the limits of discharge to sewage system in terms of COD parameter. The effluent of the WWTP meets the receiving media discharge standards for summer sampling but do not meet the standard values for winter sampling. While there is not much change in pH, TSS and COD values in effluents of some hospitals are determined more higher than in influents in WWTP. Table 3 also represents conventional parameter concentrations determined by effluents in different-size hospitals and urban wastewater in the literature. While the pH and COD values are similar to those detected in hospital effluents, TSS values in the literature are lower than our results. Also, as can be seen in Table 3, TSS and COD values in effluents of hospitals were two or three times higher than in influents of WWTP. In the literature, correlation analyses were carried out to evaluate the possible relationship between the concentrations of the pharmaceuticals and the conventional parameter in WWTP influent wastewaters, and their removal efficiencies in WWTP. Santos et al. (2009) determined positive correlations between the concentration of the some pharmaceutical compounds (caffeine, ibuprofen, ketoprofen and naproxen) and some of the influent characterization parameters (TSS, BOD, COD, TP, and Oil). The removal rates of the pharmaceutical compounds were found to be positively or negatively correlated with the removal of the wastewater characterization parameters. Sari et al. (2014) observed higher correlation between diclofenac and TSS concentrations in the WWTP influent wastewaters. Also, the removal rate of diclofenac was found to be correlated with nitrogen removal efficiency in WWTP.
Occurrence of antibiotics in hospital effluents and inlet and outlet wastewaters in WWTP
The range of concentration and the mean concentration calculated for each antibiotics in hospital effluents during summer and winter are given in Table 4. It also represents the antibiotic values detected in the influent and effluent waters of the WWTP. While the total antibiotic concentration in hospital effluents was 21.2 ± 0.13–4886 ± 3.80 ng/L in summer, it was determined as 497 ± 3.66–322,735 ± 4.58 ng/L in winter. The total antibiotic concentrations in WWTP influent (from 166 ± 1.68 to 6735 ± 3.42 ng/L) and in WWTP effluent (from 34.7 ± 0.80 to 4315 ± 2.98 ng/L) were relatively different from the hospital effluent. All the studied antibiotics were detected in all samples. Only AZI, SMX, TMP, and SMZ in some hospital effluents were determined below the method quantification limit value. AZI, CLAR, and CIPRO were detected at highest concentrations in hospital effluents during both summer and winter. The lowest average concentration were found for CTC, SMZ, and OXY in summer and SMX and SMZ in winter. The most prevalent compounds among the antibiotics were AZI, CIPRO, and CLAR in influent and effluent samples in WWTP. AZI, DOXY, SMX, and SMZ in effluent samples in WWTP were determined below the detection limit value. The total antibiotic concentrations detected in WWTP inlet wastewater in winter were higher than the detected values in summer. It can be considered that the concentration determined in wastewater increased with increase in the use of antibiotics in winter.
Table 5 shows the antibiotic concentrations reported in literature in hospital effluents and inlet and outlet wastewaters of WWTP. Several studies have been reported CIPRO among the most detected in hospital effluents. CIPRO is a fluoroquinolone group antibiotic and over 70% excreted as parent compound through urine (Marx et al. 2015). The concentration of CIPRO detected in the study were similar with those reported by the hospital wastewaters in Spain (Gros et al. 2013), France (Dinh et al. 2017), China (Chang et al. 2010), and USA (Brown et al. 2006). Otherwise, higher CIPRO concentrations were detected in Indian (up to 236.6 μg/L), Sweden (up to 101 μg/), Norway (up to 41.752 μg/L), and Portugal (up to 38.689 μg/L). SMX, TMP, OXY, SMZ, and DOXY concentrations detected in hospital effluents in summer and winter were lower than data reported in literature in Table 5. AZI and CLAR concentrations were found reaching up to 162.5 ± 0.67 and 159.73 ± 0.97 μg/L in winter, respectively. These concentrations were higher than data reported for AZI and CLAR in the literature. Among the antibiotic compounds, CIPRO was also predominantly detected in the influent and effluent of the WWTP. After that, CLAR and SMX were detected intensively. While AZI, CLAR, and CIPRO in the influent and effluent of the treatment plant were lower than data detected in Canada, they were generally found to be higher than those detected in Spain, in Italy, and in Portugal. SMX and SMZ were not detected in the influent and effluent of the WWTP, but they were detected at high concentrations reported in the other countries. There could be several reasons for different determinations of antibiotics in hospital effluents, influent, and effluent of WWTP. For example, number of the beds, number and types of wards and units, average water consumption, the season conducted of the study, used analytical method, number of general service (kitchen, laundry, etc.), management policies, and cultural and geographic factors.
Contribution of hospital loads to urban wastewater
The daily water consumption of hospitals for different purposes and services is very high. In the literature, the amount of wastewater per person in hospitals is 660–1500 L/day, and 1000 L/day is used as a typical value (Metcalf and Eddy 2003). In order to calculate the load of antibiotics consumed in hospitals, the wastewater flow generated per bed was accepted as 1000 L/day.bed. The flow rate of WWTP at the time of sampling is 159,800 m3/day. The load of antibiotics discharged into the WWTP from households was determined as 26.52 g/day in summer and 1076 g/day in winter. The antibiotic contributions to the influent of the WWTP from the hospitals are given in Table 6. The contribution of each of the hospital to the urban wastewater was in the range of 0.011–3.57% in summer and 0.003–11.4% in winter. The contribution rates of the hospitals do not vary depending on the number of bed or flow in hospital. Higher antibiotic levels were determined in domestic and hospital wastewaters during winter related to the higher consumption during cold season. While the total antibiotic contribution of the hospitals was determined as 13.07% in summer, it was determined as 28.19% in winter. The remaining 86.93% in summer and 71.82% in winter stems from the households. Hospital contribution to the load of the antibiotics into urban wastewaters has no great impact. The total antibiotic load to the influent of the WWTP from hospitals was 3.46 g/day in summer and 303.2 g/day in winter. Santos et al. (2013) determined that approximately 40 g/day (41%) contributed to the influent of a treatment plant of 11 antibiotics generated from 4 different hospitals in Portugal in February and May 2011. Verlicchi et al. (2012) conducted a study on the presence of total 73 compounds in 12 different therapeutic classes in effluent of two different hospitals and in influent and effluent of a treatment plant in Italy, and as a result of their study, they found that the highest contribution was obtained for antibiotics (such as ofloxacin, AZI, and CLAR) and that the reason for this was they are consumed in hospitals in large amounts and they become stable after they are excreted from the body. While the amount of pharmaceuticals discharged from household totals to 62% of the total pharmaceutical load in the WWTP, the remaining 38% stems from the hospital. Dinh et al. (2017) assessed the concentration of 23 antibiotics discharged from hospitals and urban wastewater to a treatment plant in France. In the study, the studied site was equipped with a separate sewerage system and wastewater treated in WWTP included in 60% domestic and 40% hospital effluents; the mean flow rate was 425 m3/day in WWTP. The antibiotic concentrations detected at the effluent of the hospital wastewater (0.04–17.9 μg/L) were 10 times higher than those detected in urban wastewater (0.03–1.75 μg/L). In addition, the antibiotic contribution to the WWTP was determined to be 90%. The total antibiotic load to the influent of the WWTP was determined as 1.1 and 5.3 mg/day. In our study, sewerage system was equipped with combined sewerage system. The portion of the total wastewater from the 16 hospitals to the urban wastewater is approximately 3.5%. The different antibiotic contribution to the WWTP might be explained by sewerage system, flow rate, size and bed capacity of the hospital in the catchment, and hospital characteristics.
Potential environmental risks
The RQ value was calculated by considering the worst possible scenario according to the European Guidelines (2003), i.e., by assuming the highest level detected in the wastewater as MEC. The RQ values calculated for antibiotics in hospital effluent, WWTP influent, and WWTP effluent in summer and winter are given in Table 7. RQs of AZI and CLAR were obtained higher than 10 for fish and algae test organisms, which means high risk to aquatic organisms. The RQs of ERY and CLAR in summer and of AZI in winter for Daphnia magna were determined between 1 and 10. Also, the RQ values of ERY and CIPRO for algae and of CLAR for fish and Daphnia were obtained between 1 and 10 meaning a moderate risk for aquatic organisms. The RQs of some antibiotics including AZI (for fish and algae), ERY (for Daphnia and algae), SMX (for algae), CIPRO (for fish, Daphnia and algae), CLAR (for Daphnia and algae), and OXY (for algae) were determined between 0.1 and 1, which indicates potential risk for aquatic organisms. The RQ values for the other compounds were determined to be lower than 0.1 which means no negative effect is expected in the receiving medium. When the results are evaluated in terms of test organisms, WWTP effluents in winter exhibit a high risk for fish and algae in the receiving environment. According to these results, the existing conventional treatment system is not sufficient to reduce the potential environmental risk. Very low antibiotic concentrations are able to select antibiotic-resistant bacteria and also mobile genetic elements carrying antibiotic resistance genes (Gullberg et al. 2011; Andersson and Hughes 2014; Baquero and Coque 2014). Thus, a number of antibiotic input sources to the environment that might promote the selection of antibiotic-resistant genes and bacterial strains are highlighted. Indeed, antibiotic-resistant pathogens have emerged and were disseminated among human and animal populations worldwide. Pathogens such as methicillin-resistant Staphylococcus aureus and beta lactam-resistant Enterobacteriaceae have become a global concern (Rizzo et al. 2013). Vancomycin-resistant Enterococci, a leading cause of nosocomial infections, were detected in wastewater (Rosenberg Goldstein et al. 2014). Similar results were achieved in previous studies carried out in the literature. Kosma et al. (2014) determined RQ values higher than 10 for TMP and SMX in the effluents of the treatment plant in Greece, which means high risk to aquatic organisms. Santos et al. (2013) also determined that CIPRO, SMX, AZI, CLAR, and ofloxacin antibiotics pose a risk for algae in the receiving environment in terms of their concentrations in effluent and that the antibiotic removal efficiency of the treatment plant is inadequate. There is a potential ecotoxicological risk for the receiving environment in terms of CLAR, ERY, SMX, and ofloxacin compounds, detected in the effluents of a treatment plant in Italy (Verlicchi et al. 2012). As a result of the risk assessment carried out for fluoroquinolone antibiotics in the wastewater of a hospital in Pakistan, the RQ value of CIPRO was determined to be 1750 for algae (Ashfaq et al. 2017). The results show that the receiving media, where the effluents of the treatment plant are discharged, should be given close attention to because antibiotics have been discharged into the environment at concentrations that can threaten the aquatic ecosystem. Estimation of RQ is usually made for each compound in studies and can be performed for a limited number of compounds. Considering the environmental risks that can occur for about 3000 pharmaceuticals that are likely to reach the surrounding environments, it is clear that the situation is much more serious. In addition, with the industrial development, not only pharmaceuticals but also many chemicals (such as pesticides, PCBs, and PBDEs) are used in everyday life and they mix with the wastewater with various flows. Since there are no specific treatment plants to remove these compounds, these pollutants are also discharged to the receiving environment. In addition to pharmaceuticals, the cumulative risks to be generated by these compounds should be taken into consideration and necessary precautions should be taken.
Removal of antibiotics in WWTP
Removal rate (%) of antibiotics in WWTP was calculated using Eq. (1). minfluent and minfluent are the load of antibiotics in WWTP influent and effluent, respectively.
Figure 1 shows the removal rates of antibiotics in summer and winter in an urban WWTP containing a physical and a biological treatment unit with activated sludge process. While the removal efficiency varied between 0.93 ± 0.10% (CTC) and 100 ± 0% (AZI and DOXY) in summer, it varied between 0% (SMX and SMZ) and 44 ± 3% (TMP) in winter. The total antibiotic removal was 79% in summer, whereas it decreased to 36% in winter. The removal efficiency of tetracycline group antibiotics (CTC, OXY, DOXY) was found to be lower. It has been reported in China (Xu et al. 2007) and in Finland (Vieno et al. 2007) that temperature affects the process and causes lower biodegradation due to lower water temperature in winter. Depending on the chemical property and structure of the compound, the processes such as sorption and biotic or abiotic transformation may affect the fate and transportation of the antibiotics in the environment. Solid-liquid partition coefficient (Kd) is an important parameter that plays a role in the removal of pharmaceuticals in wastewater treatment, and compounds with Log Kd < 2.7 show weak sorption on the sludge (Ternes et al. 2004). Results indicate that AZI, ERY, SMX, CIPRO, CLAR, and SMZ having Log Kd < 2.7 values do not constitute an important sorption on the sludge and that the treatment is achieved through biodegradation. TMP, CTC, and OXY may be thought to be accumulated mostly in the sludge in terms of LogKd value. When the removal rates of antibiotic compounds in the WWTP containing conventional activated sludge systems in different countries are examined, the removal efficiencies obtained for AZI, TMP, CIPRO, CLAR, DOXY, and SMZ in summer are generally high. For CTC and OXY compounds, the removal efficiencies obtained both in summer and winter are generally lower. Also, it is seen in Table 8 that negative removal efficiency was obtained for many compounds. In New Mexico, the presence and removal of 11 antibiotics in the influent and effluent of 6 WWTPs were examined and SMX, TMP, CIPRO, and ofloxacin were detected at high concentrations in hospital wastewaters and the influent of the treatment plant. SMZ, TMP, and ofloxacin were detected in the WWTP effluent between the ranges of 110–470 ng/L. The removal rate of the compounds in the treatment plant was determined between 20 and 77% (Brown et al. 2006). When the literature studies are reviewed, it is observed that the existing treatment processes usually involve conventional primary and secondary treatments and that these processes are inadequate for the removal of many antibiotic compounds, and the removal efficiency generally ranges from 10 to 100% (Santos et al. 2007; Luo et al. 2014; Verlicchi et al. 2010). Even in plants containing the same processes, different removal efficiencies can be determined for the same compounds. The removal efficiency can vary depending on the biodegradability and physicochemical properties of the compound in the water (solubility, evaporation tendency, adsorption tendency on to activated sludge), concentration of the compound concentration, treatment process, process operating parameters, precipitation rate, and geographical characteristics.
Conclusions
The total concentration of antibiotics in the 16 hospital wastewaters was determined as 21.2 ± 0.13–4886 ± 3.80 ng/L (mean 630 ± 1.64 ng/L, median 183 ± 1.64) in summer and 497 ± 3.66–322,735 ± 4.80 ng/L (mean 42,014 ± 4.80 ng/L, median 3802 ± 2.68 ng/L) in winter. While the total antibiotic concentration in the influents of the treatment plant was determined as 166 ± 1.68 ng/L in summer and,6375 ± 3.42 ng/L in winter, it was determined in effluents of the WWTP as 34.7 ± 0.80 ng/L in summer and 4315 ± 3.42 ng/L in winter. AZI, CLAR, and CIPRO were the antibiotic compounds found at the highest concentration. The concentrations of antibiotics detected in wastewater in winter were determined to be higher than detected in summer. The total antibiotic contribution of 16 different hospitals to the urban wastewater was determined as 13% for summer and 28% for winter. This means that approximately 87% of antibiotic load in summer and 72% in winter reaches the WWTP through domestic wastewater. Therefore, the contribution of the general consumers to antibiotic load was higher than that of hospitals. Removal efficiencies of antibiotics in WWTP by conventional physical and biological treatment processes were determined as 79% in summer and 36% in winter. AZI and CLAR in the effluent of the hospital and the influent and effluent of the WWTP in winter pose a high risk (HQ > 10) for the aquatic organisms (algae and fish) in the receiving environment. These antibiotics might produce alterations in the gram-positive bacterial organisms, as well as in the frequency of anaerobes (Hecht 2004). Therefore, instead of applying pre-treatment at the hospitals before discharging the hospital wastewater into the sewerage, it is very critical for the existing domestic WWTP to be modified with advanced treatment technologies to remove antibiotics and even other pharmaceutical compounds from the wastewater. Although there have been new technologies such as activated carbon, UV treatment, or advanced oxidation processes for the removal of pharmaceuticals in the wastewaters, the best option should be found for different situations. This is important both for ecological impact and for reducing the risk to human health.
Environmental quality standards for priority pharmaceuticals should be determined as soon as possible. Investigations conducted on ecotoxicology, risk characterization, and water treatment should be encouraged. Alternative drugs that are less harmful to the environment should be used. On all these matters, the local administration, pharmaceutical and chemical industry, health organizations, water management authorities, and the public should work together. The pharmaceutical industry must also keep its ends up for pollution control measurement and monitoring. The development of “green by design” medicines should be initiated as in all areas. Non-hazardous medicines that are better adsorbed throughout the treatment and that are less persistent in the environment should be designed. Effective recall and disposal methods should be established for unused pharmaceuticals. People should be informed about how to recycle antibiotics that have not been used or that have expired. Doctors should be encouraged to write prescriptions more carefully. The public should be informed about the pharmaceutical problem in the environment and alternative medications/medicine.
References
Alygizakis NA, Gago-Ferrero P, Borova VL, Pavlidou A, Hatzianestis I, Thomaidis NS (2016) Occurrence and spatial distribution of 158 pharmaceuticals, drugs of abuse and related metabolites in offshore seawater. Sci Total Environ 541:1097–1105
Andersson DI, Hughes D (2014) Microbiological effects of sublethal levels of antibiotics. Nat Rev Microbiol 12:465–478
APHA (1992) Standard methods for the examination of water and waste water, 17th edn. American Public Health Association, Washington, DC
Ashfaq M, Noor N, Rehman MSU, Sun Q, Mustafa G, Nazar MF, Yu CP (2017) Determination of commonly used pharmaceuticals in hospital waste of Pakistan and evaluation of their ecological risk assessment. Clean Soil Air Water 45:1–10
Baquero F, Coque TM (2014) Widening the spaces of selection: evolution along sublethal antimicrobial gradients. MBio 5:1–3
Behera SK, Kim HW, Oh JE, Park HS (2011) Occurrence and removal of antibiotics, hormones and several other pharmaceuticals in wastewater treatment plants of the largest industrial city of Korea. Sci Total Environ 409:4351–4360
Blair B, Nikolaus A, Hedman C, Klaper R, Grundl T (2015) Evaluating the degradation, sorption, and negative mass balances of pharmaceuticals and personal care products during wastewater treatment. Chemosphere 134:395–401
Brown K, Kulis J, Thomson B, Chapman T, Mavhinney D (2006) Occurrence of antibiotics in hospital, residental and dairy effluent, municipal wastewater and the Rio Grande in New Mexico. Sci Total Environ 366:772–783
Carraro E, Bonetta S, Bertino C, Lorenzi E, Bonetta S, Gilli G (2016) Hospital effluents management: chemical, physical, microbiological risks and legislation in different countries. J Environ Manag 168:185–199
Chagas TP, Seki LM, Cury JC, Oliveira JA, Dávila AM, Silva DM, Asensi MD (2011) Multiresistance, beta-lactamase-encoding genes and bacterial diversity in hospital wastewater in Rio de Janeiro, Brazil. J Appl Microbiol 111:572–581
Chang X, Meyer M, Liu X, Zhao Q, Chen H, Chen J, Qui Z, Yang L, Cao J, Shu W (2010) Determination of antibiotics in sewage from hospitals, nursery and slaughter house, wastewater treatment plant and source water in changing region of Three Gorge Reservoir in China. Environ Pollut 158:1444–1450
Collado N, Rodriguez-Mozaz S, Gros M, Rubirola A, Barceló D, Comas J, Rodriguez-Roda I, Buttiglieri G (2014) Pharmaceuticals occurrence in a WWTP with significant industrial contribution and its input into the river system. Environ Pollut 185:202–212
Commission of the European Communities (2003) Technical guidance document in support of commission directive 93/67/ EEC on risk assessment for new notified substances. Part II, Environmental Risk Assessment. Office for official publication of the European Communities, Luxembourg
Cunningham VL, Buzby M, Huthcinson T, Mastrocco F, Parke N, Roden N (2006) Effects of human pharmaceuticals on aquatic life: next steps. Environ Sci Technol 40:3456–3462
Deblonde T, Hartemann P (2013) Environmental impact of medical prescriptions: assessing the risks and hazards of persistence, bioaccumulation and toxicity of pharmaceuticals. Public Health 127:312–317
Dinh QT, Moreau-Guigon E, Labadie P, Alliot F, Teil MJ, Blanchard M, Eurin J, Chevreuil M (2017) Fate of antibiotics from hospital and domestic sources in a sewage network. Sci Total Environ 575:758–766
Diwan VJ, Tamhankar A, Aggarwal M, Stålsby Lundborg C (2009) Detection of antibiotics in hospital effluents in India. Curr Sci 97:1752–1755
Feng L, Hullebuscha ED, Rodrigo MA, Esposito G, Oturan MA (2013) Removal of residual anti-inflammatory and analgesic pharmaceuticals from aqueous systems by electrochemical advanced oxidation processes a review. Chem Eng J 228:944–964
Focazio MJ, Kolpin DW, Barnes KK, Furlong ET, Meyer MT, Zaugg SD, Barbere LB, Thurman ME (2008) A national reconnaissance for pharmaceuticals and other organic wastewater contaminants in the United States—II, untreated drinking water sources. Sci Total Environ 402:192–200
Fram MS, Belitz K (2011) Occurrence and concentrations of pharmaceutical compounds in groundwater used for public drinking-water supply in California. Sci Total Environ 409:3409–3417
Galletti A (2011) Pharmaceutical compounds in waters. Investigations on hospital effluents as a source of environmental contamination and on their treatability. In: PhD thesis in science of engineering, University of Ferrara, Italy
Göbel A, McArdell CS, Joss A, Siegrist H, Giger W (2007) Fate of sulfonamides, macrolides, and trimethoprim in different wastewater treatment technologies. Sci Total Environ 372:361–371
Götz K, Keil F (2007) Drug disposal in private households: does the disposal of pharmaceuticals via domestic sanitary devices contribute to water contamination? Umweltwiss Schadst Forsch 19:180–188
Gros M, Petrovic M, Ginebreda A, Barceló D (2010) Removal of pharmaceuticals during wastewater treatment and environmental risk assessment using hazard indexes. Environ Int 36:15–26
Gros M, Rodriguez-Mozaz S, Barcelo D (2013) Rapid analysis of multiclass antibiotic residues and some of their metabolites in hospital, urban wastewater and river water by ultra-high-performance liquid chromatography coupled to quadrupole-linear ion trap tandem mass spectrometry. J Chromatogr A 1292:173–188
Guerra P, Kim M, Shah A, Alaee M, Smyth SA (2014) Occurrence and fate of antibiotic, analgesic/anti-inflammatory, and antifungal compounds in five wastewater treatment processes. Sci Total Environ 473:235–243
Gullberg E, Cao S, Berg OG, Ilbäck C, Sandegren L, Hughes D, Andersson DI (2011) Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathog 7:1–9
Halling-Sørensen B, Holten-Lützhøft HC, Andersen HR, Ingerslev F (2000) Environmental risk assessment of antibiotics: comparison of mecillinam, trimethoprim and ciprofloxacin. J Antimicrob Chemother 46:53–58
Harada A, Komori K, Nakada N, Kitamura K, Suzuki Y (2008) Biological effects of PPCPs on aquatic lives and evaluation of river waters affected by different wastewater treatment levels. Water Sci Technol 58:1541–1546
Hecht DW (2004) Prevalence of antibiotic resistance in anaerobic bacteria: worrisome developments. Clin Infect Dis 39:92–97
Holten-Lützhøft HC, Halling-Sørensen B, Jobrgensen SE (1999) Algal toxicity of antibacterial agents in Danish fish farming. Arch Environ Contam Toxicol 36:1–6
Hu J, Zhou J, Zhou S, Wu P, Tsang YF (2018) Occurrence and fate of antibiotics in a wastewater treatment plant and their biological effects on receiving waters in Guizhou. Process Saf Environ Prot 113:483–490
Kim Y, Choi K, Jung J, Park S, Kim PG, Park J (2007) Aquatic toxicity of acetaminophen, carbamazepine, cimetidine, diltiazem and six major sulfonamides, and their potential ecological risks in Korea. Environ Int 33:370–375
Kosma CI, Lambropoulou DA, Albanisa TA (2014) Investigation of PPCPs in wastewater treatment plants in Greece: occurrence, removal and environmental risk assessment. Sci Total Environ 466:421–438
Kümmerer K (2001) Drugs in the environment: emission of drugs, diagnostic aids and disinfectants into wastewater by hospitals in relation to other sources—a review. Chemosphere 45:957–969
Kümmerer K (2008) Pharmaceuticals in the environment; sources, fate, effects and risks. Freiburg, Germany
Kümmerer K (2009) Antibiotics in the aquatic environment, a review, part I. Chemosphere 75:417–434
Li B, Zhang T (2011) Mass flows and removal of antibiotics in two municipal wastewater treatment plants. Chemosphere 83:1284–1289
Liang SX (2007) Applications of MBR in hospital wastewater treatment. Water Sav Irrig J 1:59–60
Lindberg R, Jarnheimer PA, Olsen B, Johansson M, Tysklind M (2004) Determination of antibiotic substances in hospital sewage water using solid phase extraction and liquid chromatography/mass spectrometry and group analogue internal standards. Chemosphere 57:1479–1488
Lindberg RH, Wennberg P, Johansson MI, Tysklind M, Andersson BAV (2005) Screening of human antibiotic substances and determination of weekly mass flows in five sewage treatment plants in Sweden. Environ Sci Technol 39:3421–3429
López-Serna R, Petrović M, Barceló D (2011) Development of a fast instrumental method for the analysis of pharmaceuticals in environmental and wastewaters based on ultrahigh performance liquid chromatography (UHPLC)-tandem mass spectrometry (MS/MS). Chemosphere 85:1390–1399
Luo Y, Guo W, Hao Ngo H, Nghiem LD, Hai FI, Zhang J, Liang S, Wang XC (2014) A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci Total Environ 473:619–641
Lyons G (2014) Pharmaceuticals in the environment: a growing threat to our tap water and wildlife. A CHEM Trust report. http://www.chemtrust.org/wp-content/uploads/CHEM-Trust-Pharma-Dec14.pdf
Marx C, Mühlbauer V, Schubert S, Oertel R, Ahnert M, Krebs P, Kuehn V (2015) Representative input load of antibiotics to WWTPs: predictive accuracy and determination of a required sampling quantity. Water Res 76:19–32
Mendoza A, Aceña J, Pérez S, López de Alda M, Barceló D, Gil A, Valcárce Y (2015) Pharmaceuticals and iodinated contrast media in a hospital wastewater: a case study to analyse their presence and characterise their environmental risk and hazard. Environ Res 140:225–241
Metcalf and Eddy (2003) Burton F, Tchobanoglous G, Stensel HD (eds) Wastewater engineering: treatment and reuse, 4th edn. Metcalf & Eddy, Inc., Tata McGraw-Hill Publishing Company Limited, New Delhi
Muela A, Orruno M, Alonso ML, Pazos M, Arana I, Alonso RM, Jimenez RM, Garaizabal I, Maguregui MI, Barcina I (2011) Microbiological parameters as an additional tool to improve wastewater treatment plant monitoring. Ecol Indic 11:431–437
Mungray AK, Patel K (2011) Coliforms removal in two UASB ASP based systems. Int Biodeter Biodegr 65:23–28
Nafo I (2012) Full-scale plant for the elimination of pharmaceuticals in hospital wastewater-comparison of advanced treatment technologies, 16th International EWA Symposium, IFAT, 8th-9 th May, Munich
NOAA (2006) United States National Oceanic and Atmospheric Administration, pharmaceuticals in the environment. http://www.chbr.noaa.gov/peiar/
Park S, Choi K (2008) Hazard assessment of commonly used agricultural antibiotics on aquatic ecosystems. Ecotoxicology 17:526–538
Pedrouzo M, Borrull F, Pocurull E, Maria Marcé R (2011) Presence of pharmaceuticals and hormones in waters from sewage treatment plants. Water Air Soil Pollut 217:267–281
Pena A, Paulo M, Silva LJG, Seifrtová M, Lino CM, Solich P (2010) Tetracycline antibiotics in hospital and municipal wastewaters: a pilot study in Portugal. Anal Bioanal Chem 396:2929–2936
Periasamy D, Sundaram A (2013) A novel approach for pathogen reduction in wastewater treatment. J Environ Health Sci Eng 11:1–9
Perrodin Y, Christine B, Sylvie B, Alain D, Jean-Luc BK, Cécile CO, Audrey R, Elodie B (2013) A priori assessment of ecotoxicological risks linked to building a hospital. Chemosphere 90:1037–1046
Prado T, Silva DM, Guilayn WC, Rose TL, Gaspar AMC, Miagostovich MP (2011) Quantification and molecular characterization of enteric viruses detected in effluents from two hospital wastewater treatment plants. Water Res 45:1287–1297
Prayitno Z, Kusuma B, Yanuwiadi B, Laksmono RW (2013) Study of hospital wastewater characteristic in Malang city. Int J Eng Sci 2:13–16
Rizzo L, Manaia C, Merlin C, Schwartz T, Dagot C, Ploy MC, Michael I, Fatta-Kassinos D (2013) Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: a review. Sci Total Environ 447:345–360
Rosenberg Goldstein RE, Micallef SA, Gibbs SG, He X, George A, Sapkota A, Joseph SW, Sapkota AR (2014) Occupational exposure to Staphylococcus aureus and Enterococcus spp. among spray irrigation workers using reclaimed water. Int J Environ Res Public Health 11:4340–4355
Sanderson H, Thomsen M (2009) Comparative analysis of pharmaceuticals versus industrial chemicals acute aquatic toxicity classification according to the United Nations classification system for chemicals. Assessment of the (Q)SAR predictability of pharmaceuticals acute aquatic toxicity and their predominant acute toxic mode-of-action. Toxicol Lett 187:84–93
Sanderson H, Johnson DJ, Wilson CJ, Brain RA, Solomon KR (2003) Probabilistic hazard assessment of environmentally occurring pharmaceuticals toxicity to fish, daphnids and algae by ECOSAR screening. Toxicol Lett 144:383–395
Santos JL, Aparicio I, Alonso E (2007) Occurrence and risk assessment of pharmaceutically active compounds in wastewater treatment plants. A case study: Seville city (Spain). Environ Int 33:596–601
Santos JL, Aparicio I, Callejón M, Alonso E (2009) Occurrence of pharmaceutically active compounds during 1-year period in wastewaters from four wastewater treatment plants in Seville (Spain). J Hazard Mater 164:1509–1516
Santos L, Gros M, Rodriguez-Mozaz S, Delerue-Matos C, Pena A, Barceló D, Montenegro MC (2013) Contribution of hospital effluents to the load of pharmaceuticals in urban wastewaters: identification of ecologically relevant pharmaceuticals. Sci Total Environ 461:302–316
Sarafraz S, Khani M, Yaghmaeian K (2007) Quality and quantity survey of hospital wastewater in hormozgan province. Iranian J Environ Health Sci Eng 41:43–50
Sari S, Ozdemir G, Yangin-Gomec C, Zengin GE, Topuz E, Aydin E, Pehlivanoglu-Mantas E, Okutman-Tas D (2014) Seasonal variation of diclofenac concentration and its relation with wastewater characteristics at two municipal wastewater treatment plants in Turkey. J Hazard Mater 272:155–164
Suarez S, Lema JM, Omil F (2009) Pre-treatment of hospital wastewater by coagulatio flocculation and flotation. Bioresour Technol 100:2138–2146
Tahiri EM, Benaabidate L, Nejjari C, Benbrahim KF (2012) Assessment of physicochemical and biological parameters of Al Ghassani hospital wastewaters, Fez-Morocco. J Mater Environ Sci 3:115–120
Ternes TA, Herrmann N, Bonerz M, Knacker T, Siegrist H, Joss A (2004) A rapid method to measure the solid-water distribution coefficient (Kd) for pharmaceuticals and musk fragrances in sewage sludge. Water Res 38:4075–4084
Thomas KV, Dye C, Schlabach M, Langford KH (2007) Source to sink tracking of selected human pharmaceuticals from two Oslo city hospitals and a wastewater treatment works. J Environ Monit 9:1410–1418
Tousova Z, Oswald P, Slobodnik J, Blaha L, Muz M, Hu M, Brack W, Krauss M, Di Paolo C, Tarcai Z, Seiler TB, Hollert H, Koprivica S, Ahel M, Schollée JE, Hollender J, Suter MJ, Hidasi AO, Schirmer K, Sonavane M, Ait-Aissa S, Creusot N, Brion F, Froment J, Almeida AC, Thomas K, Tollefsen KE, Tufi S, Ouyang X, Leonards P, Lamoree M, Torrens VO, Kolkman A, Schriks M, Spirhanzlova P, Tindall A, Schulze T (2017) European demonstration program on the effect-based and chemical identification and monitoring of organic pollutants in European surface waters. Sci Total Environ 601:1849–1868
Tran NH, Chen H, Reinhard M, Mao F, Yew-Hoong Gin K (2016) Occurrence and removal of multiple classes of antibiotics and antimicrobial agents in biological wastewater treatment processes. Water Res 104:461–472
Varela AR, André S, Nunes OC, Manaia CM (2014) Insights into the relationship between antimicrobial residues and bacterial popula- tions in a hospital-urban wastewater treatment plant system. Water Res 54:327–336
Verlicchi P, Galletti A, Petrovic M, Barceló D (2010) Hospital effluents as a source of emerging pollutants: an overview of micropollutants and sustainable treatment options. J Hydrol 389:416–428
Verlicchi P, Al Aukidy M, Galletti A, Petrovic M, Barceló D (2012) Hospital effluent: investigation of the concentrations and distribution of pharmaceuticals and environmental risk assessment. Sci Total Environ 430:109–118
Veterinary Medicines Directorate (2015) United Kingdom Veterinary Medicines Directorate Woodham Lane New Haw Addlestone Surrey KT15 3LS, Doxylin 433 mg/g, powder for use in drinking water for chickens and Turkeys, UK/V/0522/001/DC
Vieno NM, Harkki H, Tuhkanen T, Kronberg L (2007) Occurrence of pharmaceuticals in river water and their elimination a pilot-scale drinking water treatment plant. Environ Sci Technol 41:5077–5084
Vystavna Y, Huneau F, Grynenko V, Vergeles Y, Celle-Jeanton H, Tapie N (2012) Pharmaceuticals in rivers of two regions with contrasted socio-economic conditions: occurrence, accumulation, and comparison for Ukraine and France. Water Air Soil Pollut 223:2111–2124
Wang Q, Wang P, Yang Q (2018) Occurrence and diversity of antibiotic resistance in untreated hospital wastewater. Sci Total Environ 621:990–999
Watkinson A, Murby E, Costanzo S (2007) Removal of antibiotics in conventional and advanced wastewater treatment: implications for environmental discharge and wastewater recycling. Water Res 41:4164–4176
Xu W, Zhang G, Li X, Zou S, Li P, Hu Z, Li J (2007) Occurrence and elimination of antibiotics at four sewage treatment plants in the Pearl River Delta (PRD), South China. Water Res 41:4526–4534
Yamashita N, Yasojima M, Nakada N, Miyajima K, Komori K, Suzuki Y, Tanaka H (2006) Effects of antibacterial agents, levofloxacin and clarithromycin, on aquatic organisms. Water Sci Technol 53:65–72
Zhou LJ, Ying GG, Zhao JL, Yang JF, Wang L, Yang B, Liu S (2011) Trends in the occurrence of human and veterinary antibiotics in the sediments of the Yellow River, Hai River and Liao River in northern China. Environ Pollut 159:1877–1885
Zhou JL, Maskaoui K, Lufadeju A (2012) Optimization of antibiotic analysis in water by solid-phase extraction and high performance liquid chromatography-mass spectrometry/mass spectrometry. Anal Chim Acta 731:32–39
Zhou LJ, Ying GG, Liu S, Zhang RQ, Lai HJ, Chen ZF, Pan CG (2013) Excretion masses and environmental occurrence of antibiotics in typical swine and dairy cattle farms in China. Sci Total Environ 444:183–195
Zuccato E, Castiglioni S, Bagnati R, Melis M, Fanelli R (2010) Source, occurrence and fate of antibiotics in the Italian aquatic environment. J Hazard Mater 179:1042–1048
Funding
This work was supported by Turkish Academia of Sciences Awards for Outstanding Young Scientists (TÜBA-GEBIP).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Ester Heath
Rights and permissions
About this article
Cite this article
Aydin, S., Aydin, M.E., Ulvi, A. et al. Antibiotics in hospital effluents: occurrence, contribution to urban wastewater, removal in a wastewater treatment plant, and environmental risk assessment. Environ Sci Pollut Res 26, 544–558 (2019). https://doi.org/10.1007/s11356-018-3563-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-3563-0