Abstract
Antibiotics have attracted global attention due to the ecological risks to environment. In this paper, solid-phase extraction and ultra-performance liquid chromatography triple quadrupole mass spectrometry (LC–MS/MS) were utilized to analyze the fugitive characteristics of 10 antibiotics of sulfonamides (sulfadiazine, sulfamethazine, sulfadimidine, sulfathiazole, sulfapyridine, sulfamethoxazole) and tetracyclines (tetracycline, oxytetracycline, chlortetracycline and doxycycline) in the coastal waters and surfece sediments of the Yangtze River Estuary and the ecological risks of antibiotics in water were estimated using ecological risk assessment method. The results have showed that 7 of the 10 antibiotics were detected in the water, with total concentrations ranging from 0.652 to 434.47 ng/L. 8 antibiotics were detected in the sediment, with total concentrations ranging from 0.091 to 499.23 ng/g. The main antibiotic species detected in the sediment and water varied seasonally. Higher concentrations in spatially distributed areas where rivers meet and where human activities have a more significant impact. The ecological risks were found to be higher in spring and autumn than those in winter and summer. Spatial variation in individual microbial communities was not evident in the sediments. The relationship between antibiotics and microorganisms in the environment was predominantly positive. Physical and chemical factors were significantly correlated for both antibiotics and microbial communities. This study can provide research ideas for other types of antibiotics and provide a basis for the prevention of antimicrobial resistance (AMR).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antibiotics have been widely used in medicine and animal husbandry (Zhang et al. 2015). It can alter cell growth processes, treat diseases and promote the production of agricultural farming (Zhang et al. 2014). China is one of the largest producers and consumers of antibiotics in the world (Li et al. 2019). During the utilization, some of the antibiotics cannot be fully absorbed into the human or animal bodies, but discharged into the environment, leading to a series of environmental pollution challenges. Antibiotics can induce bacteria to develop drug resistance and trigger the problem of resistance gene pollution (Pruden et al. 2012). Currently, antibiotics have been detected in both waters and sediments of rivers around the world with high concentrations, and their persistence in the environment poses a safety risk to human health (Liu et al. 2018a; Liu et al. 2019). Numerous studies (Liu et al. 2019, 2020; Feng et al. 2019; Yao et al. 2017) have shown the presence of five classes of antibiotics in water environment, named as tetracyclines (TCs), sulfonamides (SAs), macrolides (MLs), quinolones (FQs), and chloramphenicol (CPs). Phan et al. (2011) found that sulfamethoxazole was the major pollutant in urban canals in northern Vietnam with concentrations of 612–4330 ng/L, while macrolide antibiotics were detected with high frequency in urban canals. Kolpin et al. (2002) analyzed the water quality of 139 rivers in more than 30 states in the United States and 21 antibiotics were detected in the waters. Zhou et al. (2017) analyzed 41 antibiotics in the waters of Guangzhou section of the Pearl River and 9 antibiotics were detected in surface water during the flood season with the main antibiotic of ofloxacin. In the Yangtze River Estuary, higher concentrations of sulfonamides and chloramphenicol were found in surface waters (Zhang et al. 2015). The distributions of antibiotics in the Yangtze River Estuary are extremely complex due to the influence of human activities and hydro-sediment dynamics.
Therefore, 10 common antibiotics including sulfonamides (sulfadiazine, sulfamethazine, sulfadimidine, sulfathiazole, sulfapyridine, sulfamethoxazole) and tetracyclines (tetracycline, oxytetracycline, chlortetracycline and doxycycline) were selected as target antibiotics in this study to analyze the antibiotic occurrence in the Yangtze River Estuary. The seasonal variations and spatial distributions of sulfonamide and tetracycline antibiotics in water and sediments were analyzed and their ecological risks were evaluated. The microbial community were characterized using 16S amplicon sequencing and the mechanism of antibiotic effects on the microbial community was identified. Results in this study can provide important information and scientific basis for the pollution control and treatment in the Yangtze River Estuary and similar regions.
Carbapenem antibiotics are a class of atypical ß-lactamases antibiotics developed in the 1970s with a novel structure that distinguishes them from penicillins and cephalosporins (Huang and Mu 2007). The antibacterial mechanism of carbapenem antibiotics is to impede the synthesis of cell wall mucopeptides by inhibiting cell wall mucopeptide synthetase, which kills bacteria by defecting the bacterial cell wall (Peaper et al. 2013). Carbapenems have a broader antibacterial spectrum (Mohammed et al. 2017). Antibacterial activity is stronger, and highly stable to the ultra-broad-spectrum ß-lactamases produced by most bacteria; lower toxicity and almost no cross-resistance with other ß-lactamases antibiotics, to ensure the safety of the basis of several multidrug-resistant pathogens also have a better antimicrobial effect, and is currently used in clinical practice for the treatment of multi-drug resistant gram-negative bacterial infections (Zhou et al. 2010). There are four main mechanisms by which bacteria fight against carbapenems: production of carbapenemases by bacteria (Yang 1990), deletion, mutation, and decreased expression of outer membrane proteins (Qi et al. 2010), overexpression of active efflux pump proteins (Shen et al. 2011), and alteration of the target site of drug action. The current rapid spread of carbapenem-resistant bacteria is mainly associated with mechanisms such as the horizontal spread of plasmids carrying resistance genes and the vertical spread of clonal strains (Wang et al. 2017). Antimicrobials are critical to the management of animal and human health. However, with repeated exposure to antibiotics, bacteria can evolve different mechanisms for drug resistance. In turn, such mechanisms can spread between bacteria, subsequently creating superbugs that are resistant to multiple antibiotics. The study of sulfonamide and tetracycline antibiotics can provide research ideas for other types of antibiotics and provide a basis for preventing antimicrobial resistance (AMR).
The pattern of change of sulfonamide and tetracycline antibiotics in water and sediment, ecological risk assessment and microbial community characterization were analyzed for four seasons. It aims to obtain the distribution characteristics, ecological risk, and the correlation between microbial communities and antibiotics for these two classes of antibiotics.
Materials and methods
Sample collection
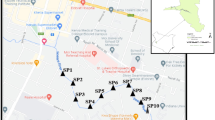
In this study, the coastal areas of the Yangtze River Estuary were selected as the study region. Water and sediment samples were collected from eight locations, as shown in Fig. 1 and Table 1. Four times of sampling were conducted in Spring (7, May), Summer (14, July), Autumn (16, October) of 2021 and Winter (15, January) of 2022. Water and sediment samples were collected using water collector and sediment grab, respectively.
Both water samples and surface sediment samples (0-5 cm) were collected from eight locations. Brown glass bottles were used to store water samples which were kept in darkness with low temperatures to prevent the decomposition of antibiotics. Two liters of water samples were taken at each sampling site, of which 1L was for antibiotic analysis and 1L was for microbial community characterization. The physicochemical parameters of the water including temperature, acidity and alkalinity, salinity, redox potential, dissolved oxygen and turbidity were also measured on site using a portable water quality meter (AP-5000, England, Aquaread).
LHK (S1) is the mouth of the Liu River into the Yangtze River, the confluence of two rivers; SDK (S2) is near the urban sewage treatment plant; WSK (S3) is also the confluence of rivers and the mouth of the Huangpu River into the Yangtze River; ZY (S4) is also near the urban sewage treatment plant; SJG (S5) is located downstream of the Jiuduansha wetland; CYNC (S6) is near a large amount of agriculture and farming, and It is an area where the impact of antibiotics is more serious; DZH (S7) is also a confluence of rivers and is the mouth of the Dazhi River into the Yangtze River; NH (S8) is located in a wetland reserve.
Instruments and reagents
A total of six antibiotics from the sulphonamide group: sulfadiazine(SD), sulfamethazine (SM), sulfadimidine (SMT), sulfathiazole (ST), sulfapyridine (SP), sulfamethoxazole (SMX), and four antibiotics from the tetracycline group: tetracycline (TC), oxytetracycline (OTC), chlortetracycline (CTC) and doxycycline (DXC) were selected as the target antibiotics. The antibiotics were detected by solid-phase extraction and ultra-performance liquid chromatography triple quadrupole mass spectrometry (LC–MS/MS). All samples were sent to Hangzhou Research Interest Information Technology Co. for antibiotic testing. Calibration curve with good linearity and correlation coefficient of 0.95 or more. The precision of the sample was investigated and the RSD% was below 13.2, which was in accordance with the requirements. The recoveries of the spiked samples ranged from 84.6% to 102.5% with the relative standard deviations (RSDs) of 1.4% ~ 4.0%. In the spiked recovery experiment, three concentration levels of onefold limit of quantification, fivefold limit of quantification and tenfold limit of quantification were selected for spiking, and six parallel samples were made for each concentration level, and the results met the requirements. 16S rRNA gene was amplified to characterize the microbial community in the samples, and the amplification products were sent to Shanghai Weihuan Biotechnology Co. In the present study, the Illumina HiSEq sequencing platform was used to construct small fragment profiles for paired-end sequencing. On the basic of this analysis, the top twenty microorganisms in terms of high relative abundance were selected for further study. The sequence with the highest frequency occurring in the OTU was selected as the representative sequence which was used on the classification annotation, and the classification level was defined as kingdom, phylum, class, order, family, genus, and species.
Ecological risk assessment methods
-
(1) Environmental risk assessment of antibiotics
In this study, the risk quotient (RQ) values was used to evaluate the ecological risk of antibiotics in water samples in the Yangtze River Estuary (Feng et al. 2019; Li et al. 2020; Liu et al. 2020). According to the European Union (EU) environmental risk analysis guidance (Yao et al. 2017), the risk quotient (RQ) was calculated as:
$$\mathrm{RQ}=\mathrm{MEC}/\mathrm{PNEC}$$(1)of which the MEC is the measured environmental concentration of antibiotics in ng/L; PNEC refers to the predicted no-effect concentration of antibiotics in ng/L, which was usually obtained from literature toxicology experiments or ECOSAR data model, as shown in Table 2.
The ecological risk level was determined based on the RQ values, when RQ was greater than or equal to 1, it means high risk; when RQ was greater than or equal to 0.1 and less than 1, it is medium risk; when RQ was greater than or equal to 0.01 and less than 0.1, it is low risk; and when RQ was less than 0.01, there is no risk.
Several studies (Syberg et al. 2009; Yang et al. 2008) have shown that when multiple antibiotics exist in water, the toxic effects will be increased. Therefore, the accumulated risk quotient (Syberg et al. 2009; Yang et al. 2008) method (RQsum) is used to represent the ecological risk of multiple antibiotics to aquatic ecosystems, which is calculated as:
$${\mathrm{RQ}}_{\mathrm{sum}}=\mathrm{\Sigma RQ}$$(2)of which the ΣRQ is the sum of RQ values. when RQsum was greater than or equal to 1, it means high risk; when RQsum was greater than or equal to 0.1 and less than 1, it is medium risk; when RQsum was greater than or equal to 0.01 and less than 0.1, it is low risk; and when RQsum was less than 0.01, there is no risk.
There are no values for all antibiotics, which are also kind of described in other articles (Ding et al 2021; Tran et al 2019; Xu et al 2019).
-
(2) Health risk assessment of antibiotics
Environmental health risk is used to evaluate the risk of person who exposed to a contaminated environment (Cui et al. 2018). It jointly considers the environmental pollution and human health. The environmental health risk of antibiotics is calculated by Eq. 3:
$$RQ=\frac{MEC\times {K}_{T}}{DWEL}$$(3)$$DWEL=\frac{ADI\times BW}{DWI\times AB\times FOE}$$(4)where KT is the proportion of antibiotics remaining in the water after treatment in range of 0.3–0.6, the maximum value 0.6 was taken in present paper (Gaffney et al. 2015); DWEL is the drinking water equivalent value in μg/L; ADI is the average daily acceptable intake [μg/(kg.d)]; BW is the average body weight (kg), which is taken as 75 kg for adults and 29.3 kg for children (Gaffney et al. 2015); DWI is the average daily water intake (L/d), which is taken as 2.96 L/d for adults and 1.32 L/d for children (Gaffney et al. 2015); AB is the gastrointestinal absorption rate, which is taken as 1 (Gaffney et al. 2015); FOE is the frequency of exposure (350d/a), which is taken as 0.96 for calculation (Gaffney et al. 2015). RQ is greater than or equal to 1 indicating a potential health risk to the exposed population. The specific values are shown in Table 3.
The ADI needs to be determined by specialized experiments, so the ADI for some antibiotics is not available. In previous studies, the authors used several of the available ADI to calculate the health risks of antibiotics (Jin et al 2016; Shi 2018; Zhu et al 2014).
Methods of analysis
In this study, Excel software was used for data processing and summarization, and GraphPad Prism 9 was used for the production of bar charts and correlation heat maps. The Chao1 index was used to express the abundance of bacterial communities, and the Shannon and Simpson indices were used to express the homogeneity of bacterial communities. Correlation analysis was used to analyze the relationships between antibiotics and microbial communities, antibiotics and physicochemical factors, and physicochemical factors and microbial communities. 16SrRNA sequencing was adopted to reveal the microbial community characteristics and the correlation between microbial community and antibiotics in spring and summer.
Results
Characterizations of antibiotic concentrations in waters
7 antibiotics (sulfadiazine, sulfamethazine, sulfadimidine, sulfapyridine, sulfamethoxazole, oxytetracycline, doxycycline) among 10 target antibiotics were detected in the water samples, and the total concentration of antibiotics ranged from 0.652 to 434.47 ng/L. The detection rate and concentrations such as maximum value, minimum value, average value and median value are shown in Table 4 and Table 5. 5 antibiotics (sulfadiazine, sulfamethazine, sulfadimidine, sulfapyridine, sulfamethoxazole) were detected in spring, with the concentrations ranging from nd to 434.47 ng/L. 7 antibiotics (sulfadiazine, sulfamethazine, sulfadimidine, sulfapyridine, sulfamethoxazole, oxytetracycline, doxycycline) were detected in summer with concentrations ranging from nd to 25.8 ng/L. 5 antibiotics (sulfadiazine, sulfadimidine, sulfapyridine, sulfamethoxazole, doxycycline) were detected in autumn with the concentrations ranging from nd to 231.78 ng/L. 4 antibiotics (sulfadiazine, sulfadimidine, sulfapyridine, sulfamethoxazole) were detected in winter with the concentrations ranging from nd to 36.2 ng/L. Among the sulfonamide antibiotics, only sulfathiazole was not detected in all samples, while the total detection rate of sulfadiazine, sulfadimidine and sulfapyridine all reached more than 50%. Among the tetracycline antibiotics, tetracycline and chlortetracycline were not detected in all samples. Antibiotics with relatively higher detection rates were sulfapyridine, sulfadiazine, sulfamethoxazole, sulfadimethoxazole, sulfadimidine and sulfamethazine. In water, the total detection rate of sulfonamide antibiotics was 70.83%, 77.01%, 52.08%, and 47.92% in spring, summer, fall, and winter, respectively; and the total detection rate of tetracycline antibiotics was 0, 25%, 15.63%, and 0 in spring, summer, fall, and winter, respectively. In general, sulfonamide antibiotics were the main antibiotics detected in the coastal areas of Yangtze River Estuary, and the detection rate was much higher than that of tetracyclines. Sulfonamides antibiotics are mainly used in medical and farming applications with high chemical structural stability. They can easily migrate and be commonly detected in the water environment in China (Gaffney et al. 2015). Recently, the utilization of sulfonamides antibiotics has been reduced due to the significant side effects. Meanwhile, sulfonamides antibiotics are easily hydrolyzed, photolyzed, and adsorbed into sediments (Feng et al. 2019), which leads to the low detection rate in water column. The mean concentrations of antibiotics in water samples in winter (January) were as follows: sulfamethazine (250.81 ng/L) > sulfadiazine (20.46 ng/L) > sulfapyridine (18.47 ng/L) > sulfamethoxazole (14.09 ng/L) > sulfadimidine (10.08 ng/L) in spring (May); sulfamethazine (14.80 ng/L) > sulfadiazine (11.54 ng/L) > sulfamethoxazole (4.04 ng/L) > sulfapyridine (3.88 ng/L) > sulfadimidine (2.42 ng/L) > oxytetracycline (1.80 ng/L) > doxycycline (1.39 ng/L) in summer (July); doxycycline (59.92 ng/L) > sulfapyridine (2.20 ng/L) > sulfadimidine (0.97 ng/L) > sulfamethoxazole (0.77 ng/L) > sulfadiazine (0.54 ng/L) in autumn (October); sulfadimidine (10.76 ng/L) > sulfapyridine (4.66 ng/L) > sulfadiazine (1.51 ng/L) > sulfamethoxazole (0.45 ng/L).
Comparing with antibiotic concentrations in other waters in China, it was found that, sulfadiazine concentrations were higher than those in Taihu Lake (Ding et al. 2021) (nd-30 ng/L) and the Bohai Rim Basin (Zhao et al. 2022) (nd-1.74 ng/L) and lower than those in the Guangzhou section of the Pearl River (7–410 ng/L) (Zhou et al. 2017). Sulfamethoxazole concentrations were lower than those in Taihu Lake (Ding et al. 2021) (nd ~ 23.3 ng/L) and the Guangzhou section of the Pearl River (Zhou et al. 2017) (2.66 ~ 210 ng/L), and higher than those in the Nanjing section of the Yangtze River (Li et al. 2020) (nd ~ 9.67 ng/L) and the Bohai Sea basin (Zhao et al. 2022) (nd ~ 8.87 ng/L). The concentration of oxytetracycline was lower than that in the Taihu Lake basin (Ding et al. 2021) (nd ~ 15.7 ng/L), the Bohai Sea basin (Zhao et al. 2022) (nd ~ 23.9 ng/L) and the Guangzhou section of the Pearl River (Zhou et al. 2017) (nd ~ 349 ng/L). The Guangzhou section of the Pearl River (7 ~ 410 ng/L) > sulfadiazine > Taihu Lake (0 ~ 30 ng/L) > the Bohai Sea basin (0 ~ 1.74 ng/L). The Guangzhou section of the Pearl River (2.66 ~ 210 ng/L) > Taihu Lake(0 ~ 23.3 ng/L) > sulfamethoxazole > the Nanjing section of the Yangtze River (nd ~ 9.67 ng/L) > the Bohai Sea basin (0 ~ 8.87 ng/L). The Guangzhou section of the Pearl River (0 ~ 349 ng/L) > the Bohai Sea basin (0 ~ 23.9 ng/L) > Taihu Lake (0 ~ 15.7 ng/L) > oxytetracycline. Sulfamethazine is mainly used in medical treatment and aquaculture. From comparing the results of various regions, the use of sulfadiazine in the southern region of China is greater than that in the northern region, and the rate of use decreases as we move further north (Liu et al 2019).
Temporal and spatial distributions of antibiotics in waters
The seasonal variations of antibiotics in the coastal areas of the Yangtze River Estuary is shown in Figs. 2 and 3. It was shown that the highest concentration of antibiotics occurred in spring (May), which was followed by autumn (October). While the relatively lower concentrations were found in winter (January) and summer (July). The dominate antibiotics were mainly sulfonamides in spring and summer, and tetracyclines in autumn, which may be related to local medication habits. Influenza is highly occurred in spring, resulting in the increasing of antibiotics. However, the temperature is higher in spring (May) with longer light time. Antibiotics are rapidly photolyzed under natural conditions with greater biological activity, which causes the consumption of antibiotics (Liao et al 2020).
The antibiotic concentration was 5 ~ 6 times lower in summer than that in spring, which is the similar trends as in most waters such as rivers, lakes and reservoirs of China (Liu et al 2019; Zhang et al 2014). In summer, the larger runoff has dilution effect on antibiotics, and the higher temperature enhanced the photolysis of antibiotics. The antibiotic concentrations in present paper were about 3–6 times higher in spring than those in summer in a reservoir of Shanghai (Jin et al. 2016). Liao et al. (2020) indicated the similar results according to the investigations of antibiotics in Lianhua Reservoir of Xiamen. The amounts of local antibiotic utilization also have great influence on antibiotics distributions in waters (Zhu et al 2014). The concentration of doxycycline at study region was higher in summer at several sampling sites than other antibiotic concentrations. Tetracycline antibiotics were used much more than sulfonamide antibiotics in most countries (Xu et al. 2021). It was shown that the use of tetracycline antibiotics in China is 71,900t and the use of sulfonamide antibiotics is 3,900t, which were 3,230t and 18.7t respectively in the US (Kim et al. 2011; Sarmah et al. 2006). In winter, the concentrations of sulfonamide antibiotics were greater than those of tetracycline antibiotics.
Spatially, the concentration of antibiotics in waters from the largest to the smallest is shown as follows: W2 > W7 > W4 > W5 > W3 > W6 > W1 > W8 (W1, W2, W3, W4, W5, W6, W7 and W8 represent the water samples at S1, S2, S3, S4, S5, S6, S7 and S8, respectively.). S2 and S4 were located around the sewage treatment plant and the higher concentrations of antibiotics were related with the discharge of the sewage water. W7 was located near the confluence of the Daji River and the Yangtze River. The pollutants from upstream carried by runoffs were discharged into the waters around W7, leading to a high concentration of antibiotics. W5 and W3 were all affected by the human activities through the discharge of wastewater from nearby domestic sewage, industry and hospital, resulting higher antibiotic concentration in the waters.
Characterizations of antibiotic concentrations in sediments
8 antibiotics (sulfadiazine, sulfamethazine, sulfadimidine, sulfapyridine, sulfamethoxazole, oxytetracycline, chlortetracycline, doxycycline) were detected in sediments of the coastal areas of the Yangtze River Estuary, and the total concentration of antibiotics ranged from 0.091 to 499.23 ng/g. The detection rates and characteristic concentrations such as maximum value, minimum value, mean value and median value are shown in Table 6 and Table 7. 5 antibiotics (sulfamethazine, sulfapyridine, oxytetracycline, chlortetracycline, doxycycline) were detected in spring, with concentrations ranging from nd to 499.23 ng/g. 4 antibiotics (sulfadiazine, sulfamethazine, oxytetracycline, doxycyclin) were detected in summer with concentrations ranging from nd to 15.7 ng/g. 4 antibiotics (sulfapyridine, sulfadimidine, sulfamethoxazole, doxycycline) were detected in autumn with concentrations ranging from nd to 43.31 ng/g. 3 antibiotics (sulfapyridine, sulfamethoxazole, doxycycline) were detected in winter with concentrations ranging from nd to 16.3 ng/g. Among the sulfonamide antibiotics, sulfathiazole was not detected in all samples. Among the tetracycline antibiotics, tetracycline was not detected in all samples. Antibiotics with relatively higher detection rates were doxycycline, sulfadiazine, sulfapyridine, oxytetracycline and sulfadiazine. In sediments, the total detection rate of sulfonamide antibiotics was 16.67%, 20.83%, 12.50%, and 14.58% in spring, summer, fall, and winter, respectively; and the total detection rate of tetracycline antibiotics was 34.38%, 31.25%, 21.88%, and 6.25% in spring, summer, fall, and winter, respectively. In general, tetracyclines antibiotics were the main antibiotics detected in sediments of the coastal areas of Yangtze River Estuary, and the detection rate was higher than that of sulfonamides. Sulfonamide antibiotics were weakly adsorbed by sediments, while tetracycline antibiotics were easily adsorbed by the sediments (Zhou et al. 2017), leading to higher detection rate of tetracycline antibiotics in the sediments. The mean concentrations of antibiotics in sediments of different samples were as follows: oxytetracycline (85.72 ng/g) > doxycycline (32.67 ng/g) > chlortetracycline (26.18 ng/g) > sulfamethazine (5.96 ng/g) > sulfapyridine (0.75 ng/g) in spring (may); oxytetracycline (6.83 ng/g) > sulfamethazine (2.29 ng/g) > sulfadiazine (1.89 ng/g) > doxycycline (1.55 ng/g) in summer (july); doxycycline (8.75 ng/g) > sulfapyridine (0.06 ng/g) = sulfamethoxazole (0.06 ng/g) > sulfadimidine (0.01 ng/g) in autumn (october); sulfamethoxazole (2.45 ng/g) > doxycycline (1.42 ng/g) > sulfapyridine (0.04 ng/g) in winter (January).
Comparing antibiotic concentrations in sediments in present study with those in other areas in China, it was found that sulfamethazine and sulfamethoxazole concentrations were both higher than those in the Guangzhou section of the Pearl River (nd ~ 4.6 ng/g, nd ~ 1.96 ng/g) (Zhou et al. 2017). Doxycycline and oxytetracycline concentrations were higher than those in the Three Gorges Reservoir (nd ~ 6.4 ng/g, 1.63 ~ 10.76 ng/g) (Yan et al. 2018), while oxytetracycline concentrations were lower than those in the Guangzhou section of the Pearl River (nd ~ 3433 ng/g) (Zhou et al. 2017). The concentrations of chlortetracycline were higher than those in Three Gorges Reservoir (3.6 ~ 32.34 ng/g) (Yan et al. 2018) and were also higher than those in the Guangzhou section of the Pearl River (nd ~ 181 ng/g) (Zhou et al. 2017). Sulfamethazine > the Guangzhou section of the Pearl River (nd ~ 4.6 ng/g); Sulfamethoxazole > the Guangzhou section of the Pearl River (nd ~ 1.96 ng/g); Doxycycline > the Three Gorges Reservoir (nd ~ 6.4 ng/g); The Guangzhou section of the Pearl River (nd ~ 3433 ng/g) > oxytetracycline > the Three Gorges Reservoir (1.63 ~ 10.76 ng/g); Chlortetracycline > the Guangzhou section of the Pearl River (nd ~ 181 ng/g) > in the Three Gorges Reservoir (3.6 ~ 32.34 ng/g).
Temporal and spatial distribution patterns of antibiotics in sediments
The seasonal variations of antibiotics in the coastal areas of the Yangtze River Estuary are shown in Figs. 4 and 5. It was shown that the seasonal variations was strong and similar to the trend of antibiotics in waters, and the highest concentration of antibiotics occurred in spring (May), which was followed by summer (July). While the relatively lower concentrations were found in autumn (October) and winter (January). Comparing to the concentrations of antibiotics in waters, the concentrations of tetracycline antibiotics were greater than those of sulfonamide antibiotics. The dominate antibiotics were mainly doxycycline, chlortetracycline and oxytetracycline, with fewer sulfonamide antibiotics in spring. Sulfonamide antibiotics are more likely to migrate from the sediment to the water (Qiu et al 2018). Sediment is weak to both adsorption and desorption of sulfonamide antibiotics, and tetracycline antibiotics are readily decomposed and readily adsorbed into sediment (Liu et al. 2018b), so they are detected at higher rates in sediment.
The antibiotic concentration in spring was 10 times higher than that in other seasons. The detected antibiotic species in spring did not differ much from other seasons. The detection rates of sulfonamide antibiotics in sediments were significantly smaller than those in waters. The detection rates of sulfonamide antibiotics in sediments were below 50%, because sulfonamide antibiotics were more easily migrated from sediment to water (Gaffney et al 2015). In summer, the concentrations of antibiotics in sediment were lower. Because the rainfall is high and the flow of the Yangtze River and other runoff is high, the riverbed sediment is more disturbed, and some of the substrates are re-suspended and enter the water. In spring, the runoff flow is relatively low. Therefore, the estuarine sediment environment is more stable and has a stronger sorption capacity for antibiotics. The production and use of antibiotics will have an impact on the seasonal variations of antibiotics, including the seasonal variations of production of pharmaceutical manufacturers, the seasonal variation of human infectious diseases and the use of antibiotics in the farming industry. For example, spring is the high season of influenza, and the use of antibiotics will increase a lot, which leads to the expansion of antibiotic production by antibiotic manufacturers.
Spatially, antibiotic concentrations in sediments was listed in order from largest to smallest as SS6 > SS3 > SS2 > SS5 > SS4 > SS1 > SS8 > SS7 (SS1, SS2, SS3, SS4, SS5, SS6, SS7 and SS8 represent the sediment of S1, S2, S3, S4, S5, S6, S7 and S8, respectively). Located in the tidal flats with greater accumulation of pollutants, S6 has higher concentration of antibiotics in sediments. S3、S2、S5 and S4 have similar antibiotic concentrations, indicating the same level of antibiotic contamination at these sampling sites. S7 and S1 were located in the areas of river confluences, where antibiotics concentrations were low due to the strong suspension of sediment to water caused by runoff disturbance. S8 has lower antibiotics concentrations, because it was far away from the urban center with less influenced of human activities.
Ecological risks assessment
The results of ecological risks of antibiotics in waters using Eq. (1) are shown in Fig. 6. The ecological risks quotient of seven antibiotics ranged from 0–0.79 in spring, 0–0.23 in summer, 0–0.73 in autumn, and 0–0.05 in winter. It was indicated that the ecological risk was higher in spring and autumn than that in winter and summer. In spring, the ecological risk was mainly caused by sulfamethoxazole and sulfadiazine. For sulfamethoxazole, the values of ecological risks were greater than 0.1 at all 8 sites, which belonged to moderate ecological risk. For sulfadiazine, the values were greater than 0.1 in W2, W3, W4, and W7, while they were less than 0.1 at other sites, indicating the low ecological risk.
In summer, the spatial distributions of risk values were similar as that in spring, with only lower values. The ecological risk was also mainly caused by sulfamethoxazole and sulfadiazine in summer, with a maximum value of 0.23. In autumn, the ecological risk of doxycycline was the highest. In winter, the ecological risk was close to 0, which was lower than that in other seasons. In general, higher ecological risk in the coastal areas of the Yangtze River Estuary were caused by sulfamethoxazole, sulfadiazine and doxycycline.
The accumulated ecological risk results are shown in Fig. 7. For the accumulated risk quotient, the ecological risk was greater in spring than in the other three seasons. The values of ecological risk were listed from largest to smallest as W2 > W7 > W3 > W4 > W5 > W6 > W1 > W8. High ecological risks occurred at W2 and W7.
The cumulative ecological risks of antibiotics in waters. (a) Health risk values of sulfadiazine in water for adults and children, (b) Health risk values of sulfamethazine in water for adults and children, (c) Health risk values of sulfapyridine in water for adults and children, (d) Health risk values of sulfamethoxazole in water for adults and children, (e) Health risk values of oxytetracycline in water for adults and children
The environmental health risk calculated by Eq. (3) are shown in Fig. 8. Generally, the health risks to adults and children through the drinking water route caused by 5 selected antibiotics (sulfadiazine, sulfamethazine, sulfapyridine, sulfamethoxazole, oxytetracycline) were between 0 and 0.23. The main health risk factors in the study region were sulfamethazine, sulfadiazine, sulfapyridine, sulfamethoxazole, and oxytetracycline. The risk values of 4 antibiotics (sulfadiazine, sulfamethazine, sulfapyridine, sulfamethoxazole) were the highest in spring followed by summer.
Microbial community characteristics
Microbial community diversity analysis
The microbial community diversity characteristics in spring and summer based on 16SrRNA sequencing are shown in Fig. 9. The Chao1 indices of sediment samples were greater than those in water samples, indicating that the bacterial community richness was higher in sediment than in water of study region. The Chao1 and Shannon indices of microorganisms were smaller in spring than those in summer, which indicates that temperature plays an important role in the growth and reproduction of microorganisms (Zhang 2012). The seasonal variation of Simpson index was relatively small, indicating the close homogeneity of microbial communities in spring and summer.
Spatially, the Chao1 index of water samples was the highest at S4 and that of sediment samples was higher at both S4 and S3, indicating that effluent discharge around S4 promoted the bacterial colonization and increased the diversity of the microbial community in the environment. Both Chao1 and Shannon indices of water samples were lower at S3, where the microbial community diversity and evenness were lower.
Classification of microbial community diversity
The microbial community structure in study region was analyzed and classified at the phylum level to discuss the bacterial community composition, relative abundance, and spatial and temporal differences in the coastal areas of the Yangtze River Estuary.
According to Fig. 10, Proteobacteria, Actinobacteriota, Bacteroidota, Planctomycetes, Verrucomicrobia, Cyanobacteria, Acidobacteria and Chloroflexi were the main groups of bacteria in water samples in spring, while Proteobacteria, Bacteroidetes, Cyanobacteria, Verrucomicrobia, Nitrospirae, Acidobacteria, Chloroflexi and Firmicutes were the main groups in sediment samples in spring. In summer, Proteobacteria, Cyanobacteria, Bacteroidetes, Actinobacteriota, Verrucomicrobia, Planctomycetes, Acidobacteria and Firmicutes were the main groups in water samples, and Proteobacteria, Bacteroidota, Verrucomicrobia, Acidobacteria, Chloroflexi, Planctomycetes, Desulfobacteria and Cyanobacteria were the main groups in sediment samples.
In present paper, higher percentage of Proteobacteria was found in both water and sediment samples, which was consistent with results in previous studies (Qiu et al. 2018; Song et al. 2011). Proteobacteria is the largest bacterial phylum including many common pathogenic bacteria. Some studies have shown that E. coli, which belongs to the Proteobacteria, in the aqueous environment carry a large number of antibiotic resistance genes (Divya and Hatha 2019). The relative abundance of Cyanobacteria in water samples was greater in summer than that in spring. Cyanobacteria are associated with eutrophication in water and are also the hosts of antibiotic resistance genes (Wang et al 2020; Dias et al. 2019). The abundance of Actinobacteriota in water samples was greater in spring than that in summer.
In sediment samples, the relative abundance of Chloroflexi was greater than in water samples, with small seasonal variation. It was consistent with results of the Bolta River (Wang 2020). Chloroflexi is related to photosynthesis. The light intensity was less in spring than that in summer, leading to greater relative abundance in summer than in spring. Actinobacteriota is a source of natural antibiotics and a range of drugs (Demain and Sanchez 2009), which are widely used in clinical practice. More drugs are used in spring than in summer. Therefore, the relative abundance of Actinobacteriota in sediment samples was greater in spring than that in summer.
Spatially, the relative abundance of Proteobacteria in water samples was greater at W3 and W8 than that at other locations. The lowest relative abundance of Proteobacteria in water samples occurred at W6. Higher abundance of Proteobacteria at W3 and W8 was related to the upstream sewage treatment plant (Luo et al 2019), In sediment samples, the spatial variation of individual microbial communities was not significant.
Correlation analysis between microbial community and antibiotics
To investigate the interaction between antibiotics and microorganisms, Spearman correlation analysis was performed with antibiotics at the Phylum level of the sample microorganisms in different seasons, and the results are shown in Fig. 11.
From the results of the correlation analysis, it can be seen that the correlations between antibiotics and microorganisms were greater in water samples than those in sediment samples. The values of correlation coefficient p were higher in spring than those in summer. In water samples, sulfamethoxazole related with the bacteriophage the strongest in spring. In sediment samples, sulfapyridine, sulfamethazine, sulfamethazine and doxycycline all strongly related with the bacteriophage in spring.
In summer, stronger relationship were found between soxytetracycline and phylum, and between doxycycline and phylum in water samples, and between oxytetracycline and phylum in sediment samples. In water samples, Proteobacteria, Actinobacteriota, Patescibacteria, Dependentiae, Planctomycetes and Firmicutes were positively correlated with the detected antibiotics, while Armatimonadetes, Verrucomicrobia, Dadabacteria, Cyanobacteria and Desulfobacterota were negatively correlated with the detected antibiotics. In sediments samples, Spirochaetes, Omnitrophicaeota and Verrucomicrobiota were positively correlated with the detected antibiotics, while Cyanobacteria, Actinobacteriota, Epsilonbacteraeota and Nitrospinae were negatively correlated with the antibiotics detected.
The existence of antibiotics have an inhibitory effect on the growth of microorganisms. Most of the current antibiotics originate from the synthesis of microorganisms, and the biodegradation of antibiotics is also closely related to microorganisms. Doxycycline and oxytetracycline had negative correlations with most of the phyla, indicating that these two antibiotics have an inhibitory effect on the growth of microorganisms. Sulfapyridine, sulfamethazine, and sulfamethoxazole had positive correlations with most of phyla, suggesting that these antibiotics may originate from the synthesis of microorganisms.
Correlation analysis between microbial community and physical and chemical factors
The spring and summer physicochemical factors for water are shown in Table 8.
In this study, the correlation between microbial communities and physical and chemical factors at the phylum level in spring and summer water was selected and the results are shown in Fig. 12 and 13.
The Nitrospirae and Dadabacteria showed strong positive correlations with conductivity, turbidity and salinity in the spring water, while the Dependentiae showed a negative correlation with pH. Overall most of the phyla showed negative correlations with conductivity. Actinobacteriota, Verrucomicrobia and Cyanobacteria were showing high negative correlations with conductivity. Turbidity showed a negative correlation with most phyla. Actinobacteriota and Cyanobacteria were showing a high negative correlation with turbidity. Redox potential was not strongly correlated with most of the phyla. Temperature was positively correlated with most phyla, with strong correlations for Planctomycetes and Chloroflexi. Dissolved oxygen had a strong positive correlation with Bacteroidetes and Verrucomicrobia. Epsilonbacteraeota had a strong positive correlation with pH. Salinity was negatively correlated with most of the phyla, with Actinobacteriota, Verrucomicrobia, and Cyanobacteria having a strong negative correlation with salinity.
Conductivity in summer waters showed a strong positive correlation with Actinobacteriota and a strong negative correlation with Campilobacterota. Turbidity was negatively correlated with most phyla, with strong correlations with Cyanobacteria, Bacteroidetes, Actinobacteriota. Redox potential was positively correlated with most phyla, with positive correlations with Planctomycetes, Bdellovibrionota, Gemmatimonadota. Gemmatimonadota had a strong positive correlation with redox potential and Firmicutes had a strong negative correlation with redox potential. Temperature was negatively correlated with most of the phyla, with high summer temperatures being detrimental to microbial survival. pH was negatively correlated with most of the phyla. Firmicutes had a strong positive correlation with pH. Dissolved oxygen and salinity were negatively correlated with most phyla, Bacteroidetes was more positively correlated with dissolved oxygen, Proteobacteria and Campilobacterota were more positively correlated with salinity.
In general, Nitrospirae, Dadabacteria, Dependentiae were more strongly correlated with physical and chemical factors in spring. In summer, Proteobacteria, Cyanobacteria, Bacteroidetes, Actinobacteriota, Firmicutes, Bdellovibrionota, Campilobacterota were more strongly correlated with physical and chemical factors, indicating that high temperature microbial activity in summer.
Correlation analysis between antibiotics and physical and chemical factors
In order to study the interaction between antibiotics and physical and chemical factors in different seasons, the correlation analysis between antibiotics and physical and chemical factors in water in spring and summer was selected for spearman's correlation analysis, and the results are shown in Fig. 14.
The results of the correlation analysis showed that in spring, temperature had a negative correlation with SD and SMX. pH had a strong negative correlation with SD and a weak positive correlation with SP. Redox potential correlated weakly with with antibiotics. Dissolved oxygen had a strong negative correlation with SMT and SMX. Conductivity and salinity had a strong negative correlation with SD and a weak correlation with other antibiotics. Turbidity had a negative correlation with antibiotics and a strong correlation with SD.
In summer temperature had a positive correlation with most antibiotics. pH had a strong negative correlation with SMX, pH had a negative correlation with sulphonamide antibiotics and a positive correlation with tetracycline antibiotics. Redox potential correlated strongly with SM positively. Dissolved oxygen was negatively correlated with most antibiotics, and SM and SMX were more negatively correlated with dissolved oxygen. Conductivity was more negatively correlated with SMX and conductivity was more positively correlated with DXC. Salinity was negatively correlated with most of the antibiotics, SM and SMX were more negatively correlated with salinity. Turbidity was positively correlated with most antibiotics and SD was more positively correlated with turbidity.
In general, the correlation between physicochemical factors and antibiotics was less in spring than in summer. Antibiotics were more negatively correlated with physicochemical factors in the spring. Turbidity was negatively correlated in the spring and mostly positively correlated in the summer.
Conclusions
In this study,10 antibiotics were analyzed in water and sediment samples collected from the coastal areas of the Yangtze River Estuary in Shanghai, China. The total antibiotic concentrations ranged from 0.652 to 434.47 ng/L in water samples and from 0.091 to 499.23 ng/g in sediment samples. Sulfonamide antibiotics were the major antibiotics in water samples and it was tetracycline antibiotics in sediment samples. Obvious seasonal differences in antibiotics concentrations were found in the study region, with higher concentrations in spring and lower concentrations in winter and summer. Seasonal variation of antibiotics in sediments were similar to that in waters. The antibiotics concentrations in water samples were higher near river confluences and sewage treatment plants, and lower at the areas far from urban centers. The antibiotics concentrations in sediment samples were lower at river confluences and away from urban locations. Ecological risk was higher in spring and autumn than in winter and summer. The cumulative risk quotient in spring was greater than other seasons. According to the environmental health risk, antibiotics generally have higher risk in spring and summer. Sulfadiazine, sulfamethazine, sulfapyridine and sulfamethoxazole had higher risk values in spring than in summer.
In addition, the relative abundances of microbial communities in water and sediment samples were revealed by metagenomics analysis. The significant correlations were identified between antibiotics and microorganisms. Notably, the Proteobacteria was the most abundant microorganism. Moreover, doxycycline and oxytetracycline showed negative correlations in most phylas, indicating that these two antibiotics inhibited microbials growth. While sulfapyridine, sulfamethazine, and sulfamethoxazole showed positive correlations in most phylas, indicating that these antibiotics originate from microbial synthesis. Generally, positive relationship were found between antibiotics and microorganisms in the study region. The presence of antibiotics in the environment does not only inhibit or kill bacteria singularly but also plays a regulatory role in microbial communities. Nitrospirae, Dadabacteria, Dependentiae were more strongly correlated with physical and chemical factors in spring. In summer, Proteobacteria, Cyanobacteria, Bacteroidetes, Actinobacteriota, Firmicutes, Bdellovibrionota, Campilobacterota were more strongly correlated with physical and chemical factors, indicating that high temperature microbial activity in summer. The correlation between physicochemical factors and antibiotics was less in spring than in summer. Antibiotics were more negatively correlated with physicochemical factors in the spring. Turbidity was negatively correlated in the spring and mostly positively correlated in the summer. The data obtained in this study can provide scientific information for antibiotic contamination control in coastal environment.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Białk-Bielińska A, Stolte S, Arning J, Uebers U, Böschenb A, Stepnowski P, Marianne M (2011) Ecotoxicity evaluation of selected sulfonamides. Chemosphere 85(6):928–933. https://doi.org/10.1016/j.chemosphere.2011.06.058
Cui CZ, Han Q, Jiang L, Ma L, Jin L, Zhang D, Lin KF, Zhang TY (2018) Occurrence, distribution, and seasonal variation of antibiotics in an artificial water source reservoir in the Yangtze River delta, East China. Environ Sci Pollut Res Int 25:1–10. https://doi.org/10.1007/s11356-018-2124-x
Demain AL, Sanchez S (2009) Microbial drug discovery: 80 years of progress. J Antibiot 62:5–16. https://doi.org/10.1038/ja.2008.16
Dias E, Oliveira M, Manageiro V, Vasconcelos V, Canica M (2019) Deciphering the role of cyanobacteria in water resistance: Hypothesis justifying the antibiotic resistance (phenotype and genotype) in Planktothrix genus. Sci Total Environ 652:447–454. https://doi.org/10.1016/j.scitotenv.2018.10.167
Ding JN, Liu SJ, Zou JM, Shi JZ, Zou H, Shi HX (2021) Spatiotemporal Distributions and Ecological Risk Assessments of Typical Antibiotics in Surface Water of Taihu Lake. Environ Sci 42(4):1811–1819. https://doi.org/10.13227/j.hjkx.202009082
Divya SP, Hatha AAM (2019) Screening of tropical estuarine water in south-west coast of India reveals emergence of ARGs-harboring hypervirulent Escherichia coli of global significance. Int J Hyg Environ Health 222(2):235–248. https://doi.org/10.1016/j.ijheh.2018.11.002
Feng MJ, Zhang Q, Song NH, Bu YQ, Yang ZB (2019) Occurrence Characteristics and Risk Assessment of Antibiotics in Source Water of the Nanjing Reach of the Yangtze River. Environ Sci 40(12):5286–5293. https://doi.org/10.13227/j.hjkx.201905139
Gaffney VJ, Almeida CMM, Rodrigues A, Ferreira E, Benoliel MJ, Cardoso VV (2015) Occurrence of pharmaceuticals in a water supply system and related human health risk assessment. Water Res 72:199–208. https://doi.org/10.1016/j.watres.2014.10.027
Huang JZ, Mu LJ (2007) Research overview of carbapenem antibiotics[J]. Overseas Med (antibiotics) 2007(04):145–154
Jin L, Jiang L, Han Q, Xue JY, Ye H, Cao GM (2016) Distribution Characteristics and Health Risk Assessment of Thirteen Sulfonamides Antibiotics in a Drinking Water Source in East China. Environ Sci 37(7):2515–2521. https://doi.org/10.13227/j.hjkx.2016.07.013
Kim KR, Owens G, Kwon SI (2011) Occurrence and environmental fate of veterinary antibiotics in the terrestrial environment. J Water, Air Soil Pollut 214(1–4):163–174. https://doi.org/10.1007/s11270-012-1316-0
Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Buxton HT (2002) Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: A national reconnaissance. Environ Sci Technol 36(6):1202–1211. https://doi.org/10.1021/es011055j
Li Y, Zhang LY, Liu XS, Ding J (2019) Ranking and prioritizing pharmaceuticals in the aquatic environment of China. Sci Total Environ 658:333–342. https://doi.org/10.1016/j.scitotenv.2018.12.048
Li H, Chen Y, Feng MJ, Wang B, Bu YQ (2020) Pollution characteristics and risk assessment of antibiotics in Nanjing drinking water sources. Acta Scientiae Circumstantiae. 40(4):1269–1277. https://doi.org/10.13671/j.hjkxxb.2019.0451
Liao J, Wei XQ, Xiao YQ, Li QS, Fan HY, Liu XJ, Zhang MZ, Liu CX (2020) Pollution Characteristics and Risk Assessment of Antibiotics in Lianhua Reservoir. Environ Sci 41(09):4081–4087. https://doi.org/10.13227/j.hjkx.202002084
Liu XH, Lu SY (2018) Occurrence and ecological risk of typical antibiotics in surface water of the Datong Lake, China. China Environ Sci 38(1):320–329. https://doi.org/10.19674/j.cnki.issn1000-6923.2018.0038
Liu X, Lu S, Wei G, Xi BD, Wang WL (2018a) Antibiotics in the aquatic environments: A review of lakes, China. Sci Total Environ 627:1195–1208. https://doi.org/10.1016/j.scitotenv.2018.01.271
Liu YX, Zhou ZH, Qu H, Wei XD, Zhao JL, Liu YS (2018) Pollution characteristics of typical antibiotics in sediments of Guangzhou River section of the Pearl River. J South China Normal Univ (Natural Science Edition) 50(04):48–54
Liu X, Wang Z, Wang XL, Yang C, Li EH, Wei HM (2019) Status of Antibiotic Contamination and Ecological Risks Assessment of Several Typical Chinese Surface-Water Environments. Environ Sci 7(5):2094–2100. https://doi.org/10.13227/j.hjkx.201808105
Liu YH, Feng MJ, Wang B, Zhao X, Guo RX, Bu YQ, Zhang SH, Chen JQ (2020) Distribution and potential risk assessment of antibiotic pollution in the main drinking water sources of Nanjing, China. Environ Sci Pollut Res 27(17):21429–21441. https://doi.org/10.1007/s11356-020-08516-7
Luo X, Zhang WL, Yuan LX, Xu M, He L, Jiang YF, Zhong WZ, Zhang Y (2019) Correlation between resistance genes and microbial community in polluted rivers. China Environ Sci 39(6):2606–2613. https://doi.org/10.19674/j.cnki.issn1000-6923.2019.0310
Mohammed H, Aladdin et al (2017) Recent updates of carbapenem antibiotics. Eur J Med Chem Chimie Therapeutique 131(5):185–195
Peaper DR, Kulkarni MV, Tichy AN et al (2013) Rapid detection of carbapenemase activity through monitoring ertapenem hydrolysis in Enterobacteriaceae with LC-MS/MS. Bioanalysis 5(2):147–157. https://doi.org/10.4155/bio.12.310
Phan TPH, Managaki S, Nakada N, Takada H, Shimizu A, Anh DH, Viet PH, Suzuki S (2011) Antibiotic contamination and occurrence of antibiotic-resistant bacteria in aquatic environments of northern Vietnam. Sci Total Environ 409(15):2894–2901. https://doi.org/10.1016/j.scitotenv.2011.04.030
Pruden A, Arabi M, Storteboom HN (2012) Correlation between upstream human activities and riverine antibiotic resistance genes. Environ Sci Technol 46(21):11541–11549. https://doi.org/10.1021/es302657r
Qi WY, Hui W, Hong LS et al (2010) Phenotypic and genotypic characterization of Enterobacteriaceae with decreased susceptibility to carbapenems: results from large hospital-based surveillance studies in China. Antimicrob Agents Chemother 54(1):573–577. https://doi.org/10.1128/AAC.01099-09
Qiu WH, Sun J, Fang MJ, Luo SS, Tian YQ, Dong PY, Xu BT, Zheng CM (2018) Occurrence of antibiotics in the main rivers of Shenzhen, China: Association with antibiotic resistance genes and microbial community. Sci Total Environ 653:334–341. https://doi.org/10.1016/j.scitotenv.2018.10.398
Sarmah AK, Meyer MT, Boxall ABA (2006) A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics in the environmental. Chemosphere 65:725–759. https://doi.org/10.1016/j.chemosphere.2006.03.026
Shen JL, Zhu DM, Wu WH (2011) Carbapenem antibiotic-resistant Pseudomonas aeruginosa outer membrane pore protein OprD_2. Chin J Infect Chemother 11(04):281–286. https://doi.org/10.16718/j.1009-7708.2011.04.004
Shi YZ (2018) Characterization of antibiotic pollution in Danjiangkou Reservoir and assessment of ecological health risks. Peking University, Beijing
Song H, Li Z, Du B, Wang G, Ding Y (2011) Bacterial communities in sediments of the shallow Lake Dongping in China. J Appl Microbiol 112:79–89. https://doi.org/10.1111/j.1365-2672.2011.05187.x
Syberg K, Jensen TS, Cedergreen N, Rank J (2009) On the use of mixture toxicity assessment in reach and the water framework directive: a review. Hum Ecol Risk Assess Int J 15(6):1257–1272. https://doi.org/10.1080/10807030903304922
Tran NH, Hoang L, Nghiem LD, Nguyen NMH, Ngo HH, Guo WS, Trinh QH, Mai NH, Chen HT, Nguyen DD, Ta TT, Gin KY (2019) Occurrence and risk assessment of multiple classes of antibiotics in urban canals and lakes in Hanoi, Vietnam. Sci Total Environ 692:157–174. https://doi.org/10.1016/j.scitotenv.2019.07.092
Wang YQ (2020) Distribution characteristics of typical antibiotics, antibiotic resistance genes and microbial community in Ebinur Lake Basin. Jinan: Shandong Normal University. https://doi.org/10.27280/d.cnki.gsdsu.2020.001969
Wang Y, Zhang RM, Li JY, Wu ZW, et al (2017) Comprehensive resistome analysis reveals the prevalence of NDM and mcr-1 in Chinese poultry production. Journal of Laboratory and Precision Medicine, 2(6). https://doi.org/10.1038/nmicrobiol.2016.260
Wang ZY, Chen QW, Zhang JY, Guan TS, Chen YC, Shi WQ (2020) Critical roles of cyanobacteria as reservoir and source for antibiotic resistance genes. Environ Int 144:106034. https://doi.org/10.1016/j.envint.2020.106034
Xu MJ, Huang HT, Li N, Li F, Wang DH, Luo Q (2019) Occurrence and ecological risk of pharmaceuticals and personal care products (PPCPs) and pesticides in typical surface watersheds, China. Ecotoxicol Environ Saf 175:289–298. https://doi.org/10.1016/j.ecoenv.2019.01.131
Xu LY, Zhang H, Xiong P, Zhu QQ, Liao CY, Jiang GB (2021) Occurrence, fate, and risk assessment of typical tetracycline antibiotics in the aquatic environment: A review. Sci Total Environ 753:141975. https://doi.org/10.1016/j.scitotenv.2020.141975
Yan MT, Xu C, Huang YM, Nie HY, Wang J (2018) Tetracyclines, sulfonamides and quinolones and their corresponding resistance genes in the Three Gorges Reservoir, China. Sci Total Environ 03:840–848. https://doi.org/10.1016/j.scitotenv.2018.03.085
Yang Y (1990) Biochemical characterization of a β-lactamase that hydrolyzes penems and carbapenems from two Serratia marcescens isolates. Antimicrob Agents Chemother 34:755–758
Yang LH, Ying GG, Su HC, Stauber LS, Adams MS, Binet MT (2008) Growth-inhibiting effects of 12 antibacterial agents and their mixtures on the freshwater microalga pseudokirchneriella subcapitata. Environ Toxicol Chem 27(5):1201–1208. https://doi.org/10.1897/07-471.1
Yao LL, Wang YX, Tong L, Deng YM, Li YG, Guo W, Dong CJ, Duan YH, Zhao K (2017) Occurrence and risk assessment of antibiotics in surface water and groundwater from different depths of aquifers: A case study at Jianghan Plain, central China. Ecotoxicol Environ Saf 135:236–242. https://doi.org/10.1016/j.ecoenv.2016.10.006
Zhang Y (2012) Study on microbial diversity and anammox bacteria identification in lake sediments. Huazhong Agricultural University, Wuhan
Zhang Q, Xin Q, Zhu JM, Cheng JP (2014) The antibiotic contaminations in the main water bodies in China and the associated environmental and human health impacts. Environ Chem 33(7):1075–1083. https://doi.org/10.7524/j.issn.0254-6108.2014.07.001
Zhang QQ, Ying GG, Pan CG, Liu YS, Zhao L (2015) Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ Sci Technol 49(11):6772–6782. https://doi.org/10.1021/acs.est.5b00729
Zhao FQ, Gao H, Li RJ, Jin SC, Zhang HB, Li SS, Zhang KY, Shu Q, Na GS (2022) Occurrences and risk assessment of antibiotics in water bodies of major rivers in Bohai Rim Basin. China Environ Sci 42(1):109–118. https://doi.org/10.19674/j.cnki.issn1000-6923.20211012.010
Zhou P, Sun YY, Sun Y (2010) Advances in safety and bacterial resistance of carbapenems. Pharm J 29(06):750–754
Zhou ZH, Zhao JL, Wei XD, Liu MS (2017) Co-occurrence and ecological risk of antibiotics in surface water of Guangzhou section of Pearl River. Ecol Environ Sci 26(6):1034–1041. https://doi.org/10.16258/j.cnki.1674-5906.2017.06.017
Zhu TT, Song ZF, Yin KH, Peng SH (2014) Study on current situation and health risk of antibiotics residue in source water of Xili Reservoir in Shenzhen. Environ Pollut Control 36(5):49–53. https://doi.org/10.15985/j.cnki.1001-3865.2014.05.013
Acknowledgements
This study was funded by the Shanghai Innovation Action Plans (20230742500, 22ZR1464200, 22230712900), National Natural Science Foundation of China (51961145106, 42072281), the Fundamental Research Funds for the Central Universities (22120210576), and the Top Discipline Plan of Shanghai Universities-Class I (2022-3-YB-03).
Funding
This study was funded by the Shanghai Innovation Action Plans (20230742500, 22ZR1464200, 22230712900), National Natural Science Foundation of China (51961145106, 42072281), the Fundamental Research Funds for the Central Universities (22120210576), and the Top Discipline Plan of Shanghai Universities-Class I (2022–3-YB-03).
Author information
Authors and Affiliations
Contributions
Shuguang Liu: Conceptualization, Methodology, Software. Xin Li: Data curation, Writing-Original Draft. Sha Lou: Methodology, Writing-review & editing. Qiuhong Xu: Investigation. Yuchen Jin: Writing-review & editing. Radnaeva Larisa Dorzhievna: Investigation. Nikitina Elena: Investigation. Makhinov Aleksei Nikolavich: Methodology, Writing- Reviewing & Editing. Araruna José Tavares: Writing-Reviewing & Editing. Fedorova Irina Viktorovna: Writing-Reviewing & Editing.
Corresponding author
Ethics declarations
Ethical approval
This study does not involve any ethical issues.
Consent to participate
All authors have read and approved this version of the article, and due care has been taken to ensure the integrity of the work.
Consent to publish
All authors have agreed to publish.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Responsible Editor: Ester Heath
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, S., Li, X., Lou, S. et al. Occurrence of sulfonamides and tetracyclines in the coastal areas of the Yangtze River (China) Estuary. Environ Sci Pollut Res 30, 118567–118587 (2023). https://doi.org/10.1007/s11356-023-30698-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-30698-z