Abstract
In the present work, the potential of Cephalosporium strain in degrading the pre-treated (ultraviolet irradiation followed by nitric acid treatment) low-density polyethylene and high-density polyethylene films was investigated. Our observations revealed a significant weight reduction of 24.53 ± 0.73% and 18.22 ± 0.31% in pre-treated low-density polyethylene and high-density polyethylene films respectively, after 56 days of incubation with the Cephalosporium strain. Changes in the physicochemical properties of the mineral salt medium (MSM) were studied to assess the extent of biodegradation. The pH of the MSM decreased gradually during the incubation period, whereas its total dissolved solids and conductivity values increased steadily. Fourier transform infrared spectroscopy (FTIR) indicated the formation of hydroxyl and C = C groups in biodegraded low-density polyethylene films, while in the case of biodegraded high-density polyethylene films it indicated the \(-\)CH2 stretching. Furthermore, the thermogravimetric analysis (TGA) revealed an enhancement in the thermal stabilities of both the LDPE and HDPE films post the biodegradation. Modifications in the polymer surface morphologies after UV irradiation, chemical treatment, and biodegradation steps were visualized via scanning electron microscopy (SEM) analysis. All our observations confirm the ability of the Cephalosporium strain in biodegrading the pre-treated LDPE and HDPE films.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plastics are manmade polymers that have found their way into various aspects of our lives since their invention. By the virtue of their versatile properties, these synthetic polymers have earned myriad applications in the fields of transportation, construction, telecommunication, and medicine. A huge amount of non-biodegradable thermoplastics are fabricated and utilized every year for packaging due to their better properties over paper based products (Muhonja et al., 2018). Properties such as durability, low cost, and lightweight are associated with the versatile applications of these plastics. Most familiar thermoplastics include polyvinyl chloride (PVC), polystyrene (PS), polypropylene (PP), and polyethylene (PE). Consequently, a huge amount of non-biodegradable thermoplastic waste is also generated every year. The proportion of polyethylene in this plastic debris is considerably high, as it is the most readily available single-use plastic. Low-density polyethylene (LDPE) and high-density polyethylene (HDPE) are the two most widely consumed polyethylene types, each having its unique applications. HDPE is relatively more linear and contains less branching, rendering it with a higher packing density (Li et al., 2019). HDPE is utilized as the crude material in the manufacturing of various utilities such as food containers, flexible pipes, waterproof fabrics, household plastic ware, packaging bags, and agricultural films. In India, in 2021, approximately 12.14 million tons of plastics are manufactured, of which 15.73% (1.91 million tons) is HDPE, making it the third most manufactured plastic in the country (Chemical and Petrochemical Statistics at a Glance—2021, 2021). Due to its xenobiotic origin and recalcitrant nature, several million tons of discarded polyethylene plastics are accumulated in the environment. These accumulated plastics are slowly intoxicating the marine and terrestrial ecosystems, endangering the lives of many aquatic and onshore animals. Remediation of this accumulated plastic waste has become crucial to restore the ecological balance (Sojobi & Zayed, 2022).
Three different approaches, landfilling, incineration, and recycling are commonly used to eliminate the accumulated waste plastics. The landfilling technique suffers from restrictions such as the unavailability of land and decreased soil fertility of the landfilled area (Singh & Pant, 2016; Sojobi & Owamah, 2014). On the other hand, the incineration technique generates toxic fumes which are carcinogenic that lead to air pollution (Sojobi et al., 2016). Incineration of polyethylene releases toxic emissions such as dioxin, hydrogen chloride, fine particulate matter, and cadmium (Awasthi et al., 2017b). Lastly, the recycling technique is limited by the higher costs associated with the recycling of polyethylene (Farzi et al., 2019). To overcome the limitations of landfilling, incineration, and recycling processes, biodegradation is found to be a potential approach to overcome the plastic wastes accumulated in the environment (Shabani et al., 2015). Biodegradation of polymers occurs through three steps, biodeterioration, biofragmentation, and assimilation. In the biodeterioration step, microorganisms come in contact with polymers and during the biofragmentation step, the enzymes secreted by the microbes depolymerize the large heavy polymers into smaller and lighter polymers (oligomers, dimmers, and monomers). In the assimilation step, these smaller molecules infiltrate the outer semi-permeable membrane of microorganisms, and the microorganisms utilize these smaller carbon molecules as a source of energy (Muhonja et al., 2018).
Polyethylene is mainly considered non-biodegradable because of its hydrophobic nature which restrains the diffusion of possible reactive molecules generated by the microorganisms. The use of anti-oxidants during the manufacturing process and the high molecular weights of the polymer chain are a few other factors that account for its recalcitrant nature (Jamil et al., 2017; Koutny et al., 2006). Polyethylene persists in the soil and marine ecosystems for several decades due to the absence of necessary functional groups for microbial degradation (Tribedi & Sil, 2013). Polyethylene sheet showed only partial degradation with negligible weight loss when buried underground for 12–32 years (Ghatge et al., 2020). As only a little degradation is possible in case of the virgin polyethylene, pre-treatments such as ultraviolet irradiation, surfactant-induced oxidation, thermal, acid, gamma irradiation, etc. are necessary to accelerate the biodegradation (Awasthi et al., 2017a; Chaudhary et al., 2021; Mukherjee et al., 2017; Sheik et al., 2015). These pre-treatment methods deteriorate the structural integrity and insert the necessary functional groups in the polyethylene samples thereby making them susceptible to the enzymatic attack of the microbes. Furthermore, treatments such as acid and ultraviolet irradiation incorporate physicochemical changes in the polymer matrix thus transforming the polymer’s nature from hydrophobic to partially hydrophilic. Awasthi et al. (2017a) subjected LDPE films to thermal pre-treatment for 10 days and achieved an enhanced biodegradation weight loss of 8.4 ± 3% using Rhizopus oryzae NS5 in 1 month. Rajandas et al. (2012) performed the FTIR-ATR (Fourier transform infrared-attenuated total reflectance) analysis to determine the biodegradation extent in the LDPE samples which were pre-treated with nitric acid for 10 days. Tribedi and Dey (2017) exposed the pre-treated samples of LDPE to microorganisms available in the soil and found a decrease in the terminal double bond and the carbonyl bond index after 28 days. Ojha et al. (2017a, b) also reported a similar reduction in the carbonyl bond index of the LDPE samples which were studied for biodegradation under the exposure of potential fungal strains for 90 days. This decrease in the carbonyl bond index was interpreted as the consumption of carbonyl residues by soil microorganisms.
Brown et al. (1974) showed that the Cephalosporium strain can survive on waste plastics. Also, the capability of the Cephalosporium strain in deteriorating nitric acid–treated LDPE and HDPE films were reported in the literature (Chaudhary & Vijayakumar, 2020a; Chaudhary et al., 2022). However, the biodegradation of ultraviolet irradiated and nitric acid–treated LDPE and HDPE films by Cephalosporium strain is not reported elsewhere. The present study was carried out to understand the synergic effect of ultraviolet irradiation and nitric acid treatment on the biodegradation of LDPE and HDPE samples when exposed to the Cephalosporium strain. The pre-treated LDPE and HDPE films were incubated with Cephalosporium strain for 56 days and the extent of biodegradation was analyzed in detail by weight loss (%), FTIR, TGA, and SEM.
Materials and methods
Materials
Cephalosporium strain (NCIM 1251) was procured from the National Collection of Industrial Microorganism (NCIM), NCL, Pune, India. The Cephalosporium culture was nurtured on potato dextrose agar (PDA) at 28 °C and was kept at 4 °C. All the required chemicals were purchased from Sigma-Aldrich Chemicals Pvt. Ltd. LDPE film of 69 µm and HDPE film of 65 µm thickness with densities of 0.92 and 0.95 g/cm3 respectively were used for biodegradation study.
Methods
Pre-treatments of polyethylene films
The pre-treatment and biodegradation methodology is shown in Fig. 1. HDPE and LDPE films were scissored into 4 × 4-cm-sized strips. These strips were irradiated under ultraviolet light (15 W, 50 Hz) over 1 week in a laminar air-flow chamber. After the UV irradiation, the films were further separately exposed to 50 ml nitric acid (69%) solution for 7 days. Then, these films were rinsed and cleaned with deionized water and ethanol and were further dried at 60 °C in a vacuum oven for 6 h to eliminate the moisture. After this, the films were preserved in a laminar air-flow chamber to avoid any sort of microbial contamination.
In vitro biodegradation study
Mineral salt medium (MSM) for the incubation of the Cephalosporium strain with polymer films was prepared using a similar procedure as reported by Chaudhary and Vijayakumar (2020b). Positive and negative controls were prepared for each of the LDPE and HDPE samples, for a clearer examination of the biodegradation process. A 100 ml of MSM, pre-treated LDPE (or) HDPE, and Cephalosporium strain make up the positive control. On the other hand, 100 ml of MSM, pre-treated LDPE (or) HDPE films make up the negative control. These flasks were then kept in a rotary shaker at 28 °C at 120 rpm for 56 days.
Analysis of biodegradation
Adherence of microbes to the polymer film surfaces and the subsequent assimilation of these polymer molecules as a carbon source resulted in the weight loss in the polymer films after the incubation period of 56 days. This weight loss was calculated by the following formula,
where L1 represents the initial weight of the pre-treated LDPE (or) HDPE films and L2 represents the final weight of the pre-treated LDPE (or) HDPE films after the microbial attack.
Variations in the characteristic polymer properties such as the presence of functional groups, the surface morphology, and the thermal stability were analyzed using the Fourier transform infrared spectroscopy (FTIR) (Shimadzu FTIR-8400), scanning electron microscopy (SEM) (Zeiss, EVO 18), and thermogravimetric analysis (TGA) (Shimadzu TGA-50) respectively.
Results and discussion
Weight loss measurement
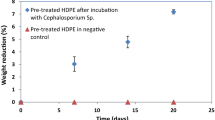
In the present study, pre-treated LDPE and HDPE samples incubated with Cephalosporium strain showed a weight loss of 24.53 ± 0.73% and 18.22 ± 0.31% respectively. It was also observed that there was no weight reduction in the sample inoculated without the Cephalosporium strain. The earlier studies reported weight loss of 13% and 7.18% when nitric acid–treated LDPE and HDPE were incubated with Cephalosporium strain for 56 and 20 days respectively (Chaudhary & Vijayakumar, 2020a; Chaudhary et al., 2022). From these observations, it is evident that the UV treatment and nitric acid exposure enhanced the biodegradability of LDPE and HDPE. In another study, Awasthi et al. (2017b) reported a similar weight reduction of 18.4% in a thermally treated HDPE sample by Klebsiella pneumoniae after an incubation period of 60 days. Thus, the weight reduction in the polyethylene samples incubated with the Cephalosporium strain could be solely attributed to the enzymatic attack by the fungal species.
Changes in pH, total dissolved solids (TDS), conductivity of MSM
The properties such as pH, TDS, and conductivity of the mineral salt medium were determined at regular intervals during the biodegradation period (Table 1). The pH value of MSM media gradually shifted from neutral to acidic side during the biodegradation studies. Also, during this period there was an increase in the TDS content of the MSM. Similar to the TDS content, there was an increase in the conductivity of the medium to a higher value by the end of 8 weeks. The changes in pH, TDS, and conductivity values are due to the secretion of enzymes and chemical substances by the Cephalosporium strain into the MSM during the degradation period (Gu, 2003 and Chaudhary and Vijayakumar (2020b)). These property changes confirm that the microorganisms are active and responsible for the degradation of pre-treated polyethylene samples (Cassidy et al., 2001).
Fourier transform infrared spectroscopy (FTIR)
The FTIR spectrum of the pure LDPE sample showed characteristic peaks at 719, 1373, 1465, 2848, and 2914 cm−1 (Fig. 2a), and the assignments to these characteristic peaks are listed in Table 2. Continuous bombardment of UV rays on LDPE films led to the formation of new peak at 1016 cm−1 and peak with low intensity at 1745 cm−1 as observed in the FTIR spectrum of the UV-treated LDPE sample (Fig. 2b). These peaks are related to the transvinylene -CH = CH- vibrations and the C = O stretching respectively (Vasilets et al., 2004). Furthermore, the treatment of the UV-treated LDPE film with nitric acid showed the formation of major peaks at 1714, 1627, and 1048 cm−1 (Fig. 2c). These peaks are related to the carbonyl, -C = C-, and -CH = CH- groups respectively. The formation of these peaks confirms the oxidation of the LDPE samples (Hasan et al., 2007). Later, the biological treatment of the pre-treated LDPE films with the Cephalosporium strain resulted in the formation of new peaks at 3390 and 1645 cm−1 (Fig. 2d). The peaks at 3390 and 1645 cm−1 correspond to the hydroxyl and C = C groups respectively (Jamil et al., 2017). Thus, the formation of new bands after fungal treatment indicates the changes in the LDPE chemical structure, and thus provides the necessary evidence to confirm the capability of fungal culture to deteriorate pre-treated LDPE films.
Alternately, in the FTIR spectrum of the pure HDPE films, characteristic peaks were observed at wave numbers of 2912, 2846, 1463, 1371, and 721 cm−1 (Fig. 3a). The UV pre-treatment modified the HDPE surface as indicated by the new peak formed at 1259 cm−1 which represents the -C-O stretching (Fig. 3b) (Jeon & Kim, 2013). On further exposure to the nitric acid pre-treatment, new peaks appeared at 1724 and 1597 cm−1 (Fig. 3c). These peaks at 1724 and 1597 cm−1 correspond to the carbonyl group (Hasan et al., 2007; Jamil et al., 2017; Jeon & Kim, 2013; Rajandas et al., 2012). Also, the shifting of characteristic peaks was observed after the acid treatment. The peak at 2912 cm−1 shifted to 2970 cm−1 whereas the peak at 2846 cm−1 shifted to 2864 cm−1. Hasan et al. (2007) reported a similar formation of peaks in UV- and acid-treated LDPE films. The formation of carbonyl groups after the combined effect of UV and nitric acid is the prerequisite for the initiation of the degradation process which weakens the segments of polyethylene chains (Albertsson, 1978). Peaks at 1724 and 1597 cm−1 disappeared after treatment with Cephalosporium strain and suggest the disruption of polymer chains (Fig. 3d). The disappearance of peaks implies that the fungal culture underwent a hydrolysis process that has distressed the carbonyl chain of pre-treated HDPE samples (Satlewal et al., 2008). Furthermore, new peaks at 2382 and 2645 cm−1 were visible after fungal treatment which corresponds to CH2 stretching. Similar observations were reported by Soni et al. (2009) where vibrations in CH2 stretching were seen between 919 to 2364 cm−1 in LDPE polymer after indigenous microbial treatment (Soni et al., 2009). Disappearance, formation, and shifting of peaks in pre-treated HDPE samples after microbial treatment suggest the effective usage of microorganisms to disintegrate pre-treated HDPE.
Thermogravimetric analysis (TGA)
The TGA thermogram of the pure LDPE sample showed an onset degradation temperature of 417 °C (Fig. 4a). A similar single steep degradation profile around 400–500 °C for LDPE samples was earlier reported by Bhatia et al. (2014). After subjecting to UV irradiation, the LDPE samples showed around 11 °C reduction in the onset degradation temperature (Fig. 4b) which indicates that the thermal stability of the sample decreased after UV treatment. This decrease in thermal stability can be related to the chain scissions and cross-linking reactions occurred in the polymer matrix (Miltz & Narkis, 1976; Novotný et al., 2018). On further treatment with nitric acid, the LDPE films had shown an increase in the onset degradation temperature to 441 °C (Fig. 4c). Hace et al. (1996) reported a similar increase in the thermal stability of polystyrene films post the nitric acid treatment. The changes in degradation temperature after UV and acid treatments can be attributed to the structural changes incorporated in the polymer matrix as observed in the FTIR studies. Figure 4d shows the onset degradation temperature at around 466 °C for the pre-treated LDPE film after 8 weeks of incubation with the Cephalosporium strain. This increase in degradation temperature is due to the addition of functional groups in the inoculated film sample as evidenced by the FTIR studies. Novotný et al. (2018) reported similar observations when linear low-density polyethylene (LLDPE) samples were exposed to Bacillus amyloliquefaciens. Thus, this variation in thermal stability after microbial treatment clearly suggests the consumption of oligomers by the fungal culture.
The TGA thermogram of pure HDPE samples showed an onset degradation temperature of 464 °C (Fig. 5a). The UV pre-treatment reduced this temperature to 450 °C indicating a decline in the thermal stability of the HDPE samples (Fig. 5b). This decrease in thermal stability can be assigned to the -C-O stretching as observed in the FTIR studies (Novotný et al., 2018). Furthermore, exposure to the nitric acid treatment resulted in an increase in the onset degradation temperature to 463 °C (Fig. 5c). This increase in thermal stability can be correlated to the deterioration of amorphous phases present in the UV-treated HDPE samples that caused homogenization in the crystalline part of the HDPE. Avalos-Belmontes et al. (2009) reported a similar improvement in the thermal stability of HDPE after the digestion in nitric acid. After 8 weeks of incubation with the fungal culture, the onset degradation temperature further improved to 470 °C for the pre-treated HDPE (Fig. 5d). After treatment with nitric acid, the attacked parts of the HDPE dissolved in the mineral salt media which increased the thermal stability of samples (Novotný et al., 2018). Thus, the TGA analysis confirms the ability of fungal culture to utilize pre-treated HDPE films.
Scanning electron microscopy (SEM)
The SEM images of virgin LDPE samples showed a smooth and homogeneous texture without any irregularities or deformations; however, tiny holes and cracks were observed after the bombardment with ultraviolet radiation (Fig. 6a, b). Changes in the surface texture are due to the destruction of amorphous phases by the UV pre-treatment without compromising the crystalline regions of LDPE films (Vasilets et al., 2004). The texture of the nitric acid–treated film appeared to be even more rugged when compared with the UV-treated film texture. (Fig. 6c). The surface deterioration resulted from subjecting the LDPE to UV and chemical treatments is attributed to the oxidation of LDPE polymeric chains. The oxidation of the long polymeric polyethylene chains caused several chain scissions and bond-breaking which eventually appeared as surface irregularities. Wang et al. (2009) reported similar observations for nitric acid–treated LDPE films. Furthermore, pre-treated LDPE films after biological treatment showed the formation of tiny holes, pits, and grooves (Fig. 6d). Also, there were several trenches on the surface of these samples indicating the erosion of the polymer material. The enzymes and proteins secreted by the Cephalosporium strain broke down the polymer surface into smaller sized carbon molecules. The microbial assimilation and enzymatic attack might collectively lead to this degradation of the pre-treated LDPE surface. Thus, the variations in the surface texture are interpreted as the consumption of pre-treated LDPE films by the fungal culture to regulate their metabolism.
Similar to the LDPE films, the pure HDPE films also are characterized by an extremely smooth, continuous, and homogeneous surface texture as observed via SEM (Fig. 7a). Treatment with UV irradiation has partially deteriorated the polymer surface (Fig. 7b). There were many micro-cracks developed in several regions of UV-treated HDPE samples. Continuous exposure to UV helps to increase the hydrophilicity and oxygen content of polymers by incorporating the -C-O groups in the polymer matrix (Wu et al., 2003). These incorporated structural changes in the polymer matrix led to the formation of different types of surface irregularities on the HDPE surface (Teare et al., 2001). Treatment with nitric acid transformed the surface texture and showed the formation of craters and trenches at multiple locations throughout the polymer surface (Fig. 7c). In addition, the surface looked highly perforated with many holes and pits. This is also a consequence of the oxidation of polymeric carbon through the insertion of carbonyl groups into the polymer matrix (Garaeva et al., 2010). There was clear evidence of surface erosion after the fungal attack as shown in Fig. 7d, e. Adherent biomass at some locations as observed via SEM confirmed the colonization and microbial adhesion on the pre-treated HDPE surfaces. The coarser surface accommodated with several pits, holes, craters, protrusions, and trenches can be interpreted as an outcome of colonization and localized enzymatic attack by Cephalosporium strain at several locations on the HDPE polymer surface. Thus, the SEM analysis confirmed the consumption of pre-treated HDPE samples by Cephalosporium strain.
Comparison of biodegradation of LDPE and HDPE
Studies reported on biodegradation of LDPE and HDPE by various microorganisms as reported in the literature are compared in Table 3. The results obtained from the present study on synergic effect of UV- and nitric acid–treated LDPE and HDPE showed higher weight loss efficiency as compared to the study on only nitric acid–treated samples. The literature also revealed that the extent of degradation varied based on the type of microorganisms and the degradation time. Thus, the observations of the present study confirm that the pre-treatment with UV irradiation and nitric acid is considerably effective pre-treatment for enhancing the biodegradation of LDPE and HDPE using Cephalosporium strain.
Conclusions
Polyethylene samples were made bio-susceptible by oxidation through UV irradiation and nitric acid treatment. These pre-treated films were incubated with Cephalosporium strain NCIM 1251 in a nutrient medium for 8 weeks and obtained a significant polymer weight reduction. Furthermore, the enhanced biodegradability of LDPE and HDPE films when pre-treated with UV irradiation and nitric acid exposure showed the effectiveness of synergic treatment. The formation of new functional groups after microbial degradation was observed via FTIR analysis, while the TGA analysis showed an increase in the thermal stability of pre-treated polymer samples. These observations confirmed the enzymatic deterioration and assimilation of pre-treated polymer samples. The SEM images after biological treatment showed deteriorated surfaces with several cavities, grooves, and eroded regions. Thus, the present study confirms that the synergetic effect of UV and acid treatment could be helpful to enhance the biological degradation of polyethylene samples.
Code availability
Not applicable.
References
Albertsson, A. C. (1978). Biodegradation of synthetic polymers. II. A limited microbial conversion of 14C in polyethylene to 14CO2 by some soil fungi. Journal of Applied Polymer Science, 22(12), 3419–3433. https://doi.org/10.1002/app.1978.070221207
Avalos Belmontes, F., Zapata Gonzalez, I., Ramos De Valle, L. F., Zitzumbo Guzman, R., & Alonso Romero, S. (2009). Thermo-oxidative degradation of HDPE as a function of its crystalline content. Journal of Polymer Science Part B: Polymer Physics, 47(19), 1906–1915. https://doi.org/10.1002/POLB.21785
Awasthi, S., Srivastava, N., Singh, T., Tiwary, D., & Mishra, P. K. (2017a). Biodegradation of thermally treated low density polyethylene by fungus Rhizopus oryzae NS 5. 3 Biotech, 7(1). https://doi.org/10.1007/S13205-017-0699-4
Awasthi, S., Srivastava, P., Singh, P., Tiwary, D., & Mishra, P. K. K. (2017b). Biodegradation of thermally treated high-density polyethylene (HDPE) by Klebsiella pneumoniae CH001. 3 Biotech, 7(5), 1–10. https://doi.org/10.1007/s13205-017-0959-3
Bhatia, M., Girdhar, A., Tiwari, A., & Nayarisseri, A. (2014). Implications of a novel Pseudomonas species on low density polyethylene biodegradation: an in vitro to in silico approach. SpringerPlus, 3(1). https://doi.org/10.1186/2193-1801-3-497
Brown, B. S., Mills, J., & Hulse, J. M. (1974). Chemical and biological degradation of waste plastics. Nature, 250(5462), 161–163. https://doi.org/10.1038/250161a0
Cassidy, D. P., Werkema, D. D., Sauck, W., Atekwana, E., Rossbach, S., & Duris, J. (2001). The effects of LNAPL biodegradation products on electrical conductivity measurements. Journal of Environmental and Engineering Geophysics, 6(1), 47–52. https://doi.org/10.4133/JEEG6.1.47
Chaudhary, A. K., Chaitanya, K., & Vijayakumar, R. P. (2021). Synergistic effect of UV and chemical treatment on biological degradation of Polystyrene by Cephalosporium strain NCIM 1251. Archives of Microbiology, 203(5), 2183–2191. https://doi.org/10.1007/s00203-021-02228-3
Chaudhary, A. K., Chitriv, S. P., & Vijayakumar, R. P. (2022). Influence of nitric acid on biodegradation of polystyrene and low - density polyethylene by Cephalosporium species. Archives of Microbiology, 204(8), 1–10. https://doi.org/10.1007/s00203-022-03089-0
Chaudhary, A. K., & Vijayakumar, R. P. (2020a). Effect of chemical treatment on biological degradation of high-density polyethylene (HDPE). Environment, Development and Sustainability, 22(2), 1093–1104. https://doi.org/10.1007/s10668-018-0236-6
Chaudhary, A. K., & Vijayakumar, R. P. (2020b). Studies on biological degradation of polystyrene by pure fungal cultures. Environment, Development and Sustainability, 22(5), 4495–4508. https://doi.org/10.1007/S10668-019-00394-5
Chemical and Petrochemical Statistics at a Glance - 2021. (2021). https://chemicals.nic.in/sites/default/files/ChemicalandPetrochemicalStatisticsataGlance-20170.pdf.
Das, M. P., Kumar, S., & Das, J. (2018). Fungal-mediated deterioration and biodegradation study of low-density polyethylene (LDPE) isolated from municipal dump yard in Chennai, India. Energy, Ecology and Environment, 3(4), 229–236. https://doi.org/10.1007/s40974-018-0085-z
Farzi, A., Dehnad, A., & Fotouhi, A. F. (2019). Biodegradation of polyethylene terephthalate waste using Streptomyces species and kinetic modeling of the process. Biocatalysis and Agricultural Biotechnology, 17, 25–31. https://doi.org/10.1016/J.BCAB.2018.11.002
Garaeva, S. R., Aydin, A. A., Aydin, A., Yalçin, B., Fatullaeva, P. A., & Medzhidov, A. A. (2010). Composition, properties, and application of products formed in oxidation of polyethylene by nitric acid. Russian Journal of Applied Chemistry, 83(1), 97–101. https://doi.org/10.1134/S1070427210010192
Ghatge, S., Yang, Y., Ahn, J. H., & Hur, H. G. (2020). Biodegradation of polyethylene: A brief review. Applied Biological Chemistry, 63(1). https://doi.org/10.1186/s13765-020-00511-3
Gu, J. D. (2003). Microbiological deterioration and degradation of synthetic polymeric materials: Recent research advances. International Biodeterioration and Biodegradation, 52(2), 69–91. https://doi.org/10.1016/S0964-8305(02)00177-4
Hace, D., Kovacevic, V., & Pajc-liplin, D. (1996). Thermally stimulated oxidative degradation of high impact polystyrene with nitric acid. Polymer Engineering and Science, 36(8), 1140–1151. https://doi.org/10.1002/pen.10508
Hasan, F., Shah, A. A., Hameed, A., & Ahmed, S. (2007). Synergistic effect of photo- and chemical treatment on the rate of biodegradation of low density polyethylene by Fusarium sp. AF4. Journal of Applied Polymer Science, 105(3), 1466–1470. https://doi.org/10.1002/APP.26328
Jamil, S. U. U., Zada, S., Khan, M., Sajjad, W., Rafiq, M., Alishah, A., & Hasan, F. (2017). Biodegradation of polyethylene by bacterial strains isolated from Kashmir cave, Buner, Pakistan. Journal of Cave and Karst Studies, 79(1), 73–80. https://doi.org/10.4311/2015MB0133
Jeon, H. J., & Kim, M. N. (2013). Isolation of a thermophilic bacterium capable of low-molecular-weight polyethylene degradation. Biodegradation, 24(1), 89–98. https://doi.org/10.1007/s10532-012-9560-y
Koutny, M., Lemaire, J., & Delort, A. M. (2006). Biodegradation of polyethylene films with prooxidant additives. Chemosphere, 64(8), 1243–1252. https://doi.org/10.1016/J.CHEMOSPHERE.2005.12.060
Kowalczyk, A., Chyc, M., Ryszka, P., & Latowski, D. (2016). Achromobacter xylosoxidans as a new microorganism strain colonizing high-density polyethylene as a key step to its biodegradation. Environmental Science and Pollution Research, 23(11), 11349–11356. https://doi.org/10.1007/s11356-016-6563-y
Li, D., Zhou, L., Wang, X., He, L., & Yang, X. (2019). Effect of crystallinity of polyethylene with different densities on breakdown strength and conductance property. Materials, 12(11). https://doi.org/10.3390/MA12111746
Maroof, L., Khan, I., Yoo, H. S., Kim, S., Park, H. T., Ahmad, B., & Azam, S. (2021). Identification and characterization of low density polyethylenedegrading bacteria isolated from soils of waste disposal sites. Environmental Engineering Research, 26(3), 0–2. https://doi.org/10.4491/eer.2020.167
Miltz, J., & Narkis, M. (1976). The effect of ultraviolet radiation on chemically crosslinked low-density polyethylene. Journal of Applied Polymer Science, 20(6), 1627–1633. https://doi.org/10.1002/APP.1976.070200620
Muhonja, C. N., Makonde, H., Magoma, G., & Imbuga, M. (2018). Biodegradability of polyethylene by bacteria and fungi from Dandora dumpsite Nairobi-Kenya. PLoS One, 13(7), 1–17. https://doi.org/10.1371/journal.pone.0198446
Mukherjee, S., RoyChaudhuri, U., & Kundu, P. P. (2017). Anionic surfactant induced oxidation of low density polyethylene followed by its microbial bio-degradation. International Biodeterioration and Biodegradation, 117, 255–268. https://doi.org/10.1016/J.IBIOD.2017.01.013
Munir, E., Harefa, R. S. M., Priyani, N., & Suryanto, D. (2018). Plastic degrading fungi Trichoderma viride and Aspergillus nomius isolated from local landfill soil in Medan. IOP Conference Series: Earth and Environmental Science, 126(1). https://doi.org/10.1088/1755-1315/126/1/012145
Novotný, Č, Malachová, K., Adamus, G., Kwiecień, M., Lotti, N., Soccio, M., et al. (2018). Deterioration of irradiation/high-temperature pretreated, linear low-density polyethylene (LLDPE) by Bacillus amyloliquefaciens. International Biodeterioration and Biodegradation, 132, 259–267. https://doi.org/10.1016/J.IBIOD.2018.04.014
Ojha, N., Pradhan, N., Singh, S., Barla, A., Shrivastava, A., Khatua, P., et al. (2017a). Evaluation of HDPE and LDPE degradation by fungus, implemented by statistical optimization. Scientific Reports, 7, 39515. https://doi.org/10.1038/srep39515
Ojha, N., Pradhan, N., Singh, S., Barla, A., Shrivastava, A., Khatua, P., et al. (2017b). Evaluation of HDPE and LDPE degradation by fungus, implemented by statistical optimization. Scientific Reports, 7. https://doi.org/10.1038/SREP39515
Rajandas, H., Parimannan, S., Sathasivam, K., Ravichandran, M., & Yin, L. S. (2012). A novel FTIR-ATR spectroscopy based technique for the estimation of low-density polyethylene biodegradation. Polymer Testing, 31(8), 1094–1099. https://doi.org/10.1016/j.polymertesting.2012.07.015
Satlewal, A., Soni, R., Zaidi, M., Shouche, Y., & Goel, R. (2008). Comparative biodegradation of HDPE and LDPE using an indigenously developed microbial consortium. Journal of Microbiology and Biotechnology, 18(3), 477–482.
Shabani, F., Kumar, L., & Esmaeili, A. (2015). A modelling implementation of climate change on biodegradation of Low-Density Polyethylene (LDPE) by Aspergillus niger in soil. Global Ecology and Conservation, 4, 388–398. https://doi.org/10.1016/J.GECCO.2015.08.003
Sheik, S., Chandrashekar, K. R., Swaroop, K., & Somashekarappa, H. M. (2015). Biodegradation of gamma irradiated low density polyethylene and polypropylene by endophytic fungi. International Biodeterioration and Biodegradation, 105, 21–29. https://doi.org/10.1016/J.IBIOD.2015.08.006
Singh, R., & Pant, D. (2016). Polyvinyl chloride degradation by hybrid (chemical and biological) modification. Polymer Degradation and Stability, 123, 80–87. https://doi.org/10.1016/J.POLYMDEGRADSTAB.2015.11.012
Skariyachan, S., Setlur, A. S., Naik, S. Y., Naik, A. A., Usharani, M., & Vasist, K. S. (2017). Enhanced biodegradation of low and high-density polyethylene by novel bacterial consortia formulated from plastic-contaminated cow dung under thermophilic conditions. Environmental Science and Pollution Research, 24(9), 8443–8457. https://doi.org/10.1007/s11356-017-8537-0
Sojobi, A. O., Nwobodo, S. E., & Aladegboye, O. J. (2016). Recycling of polyethylene terephthalate (PET) plastic bottle wastes in bituminous asphaltic concrete. Cogent Engineering, 3(1). https://doi.org/10.1080/23311916.2015.1133480
Sojobi, A., & Owamah, H. (2014). Evaluation of the suitability of low-density polyethylene (LDPE) waste as fine aggregate in concrete. Nigerian Journal of Technology, 33(4), 409. https://doi.org/10.4314/njt.v33i4.1
Sojobi, A. O., & Zayed, T. (2022). Impact of sewer overflow on public health: A comprehensive scientometric analysis and systematic review. Environmental Research, 203. https://doi.org/10.1016/J.ENVRES.2021.111609
Soni, R., Kapri, A., Zaidi, M. G. H., & Goel, R. (2009). Comparative biodegradation studies of non-poronized and poronized LDPE using indigenous microbial consortium. Journal of Polymers and the Environment, 17(4), 233–239. https://doi.org/10.1007/s10924-009-0143-x
Teare, D. O. H., Emmison, N., Ton-That, C., & Bradley, R. H. (2001). Effects of serum on the kinetics of CHO attachment to ultraviolet-ozone modified polystyrene surfaces. Journal of Colloid and Interface Science, 234(1), 84–89. https://doi.org/10.1006/jcis.2000.7282
Tribedi, P., & Dey, S. (2017). Pre-oxidation of low-density polyethylene (LDPE) by ultraviolet light (UV) promotes enhanced degradation of LDPE in soil. Environmental Monitoring and Assessment, 189(12). https://doi.org/10.1007/s10661-017-6351-2
Tribedi, P., & Sil, A. K. (2013). Low-density polyethylene degradation by Pseudomonas sp. AKS2 biofilm. Environmental Science and Pollution Research, 20(6), 4146–4153. https://doi.org/10.1007/S11356-012-1378-Y
Vasilets, V. N., Kuznetsov, A. V., & Sevastianov, V. I. (2004). Vacuum ultraviolet treatment of polyethylene to change surface properties and characteristics of protein adsorption. Journal of Biomedical Materials Research - Part A, 69(3), 428–435. https://doi.org/10.1002/JBM.A.30005
Wang, H., Chen, S. J., & Zhang, J. (2009). Surface treatment of LLDPE and LDPE blends by nitric acid, sulfuric acid, and chromic acid etching. Colloid and Polymer Science, 287(5), 541–548. https://doi.org/10.1007/s00396-009-2000-9
Wu, S., Zhang, J., & Xu, X. (2003). Studies on high density polyethylene (HDPE) functionalized by ultraviolet irradiation and its application. Polymer International, 52(9), 1527–1530. https://doi.org/10.1002/pi.1251
Author information
Authors and Affiliations
Contributions
Ashutosh Kr Chaudhary: conceptualization, methodology, data curation, investigation, writing—original draft. Shubham P. Chitriv: data curation, investigation, draft revising. Kundrapu Chaitanya: data curation, investigation, draft revising. R. P. Vijayakumar: conceptualization, validation, resources, supervision, draft revising.
Corresponding author
Ethics declarations
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chaudhary, A.K., Chitriv, S.P., Chaitanya, K. et al. Influence of ultraviolet and chemical treatment on the biodegradation of low-density polyethylene and high-density polyethylene by Cephalosporium strain. Environ Monit Assess 195, 395 (2023). https://doi.org/10.1007/s10661-023-10982-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-10982-8