Abstract
Petroleum-based polymers are not susceptible to microorganisms because of its high molecular weight. Acid treatments convert the polymers into a more oxidized form having low molecular weight. The present in-vitro degradation study focuses on the potential of Cephalosporium species to degrade acid-treated polystyrene (PS) and low-density polyethylene (LDPE) films. A weight loss of around 12% and 13% was achieved for PS and LDPE films respectively in eight weeks of treatment with Cephalosporium species. Fourier transform infrared spectroscopy analysis showed the formation of hydroxyl and carbonyl groups in nitric acid treated PS and LDPE films, respectively. Scanning electron microscopy indicated modifications in the surface morphology of PS and LDPE films after chemical and microbial treatment. An increase in crystallinity of pre-treated polymer samples was observed after fungal treatment. The observations of present study confirmed the enzymatic deterioration and assimilation of pre-treated PS and LDPE samples by the microbial species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plastic materials have become an integral part of daily life due to its low cost, toughness, durability, and lightweight. Petroleum-based plastics like polystyrene and polyethylene are widely used in the food industry, agriculture, biomedical field, packaging industries, construction, and automotive industries (Andrady and Neal 2009; Sánchez 2020). The application of plastic materials in different fields also leads to the accumulation of massive amounts of waste plastic. India generates around 9.46 million tons of polymer wastes yearly, which corresponds to around 25,940 tons each day, which comprises 71.68% of polyethylene and polystyrene (Mehta 2017). Most of the plastics (90%) are of single-use and discarded without any proper waste management, which pollutes the natural environment.
Three basic methods such as soil burial, incineration, and recycling are adopted to overcome the problems of plastic waste accumulation in the environment. Burial of plastics in the soil leads to reduction in the land fertility, discharge of various pollutants, and release of greenhouse gases (Muenmee et al. 2016). Petroleum-based polymers such as polyethylene and polystyrene have shown partial decomposition after 32 years of soil burial (Otake et al. 1995). This may be due to lack of oxygen in the dump yard which limits the degradation (Massardier-Nageotte et al. 2006). Waste plastics can be eliminated by the process of incineration, but the incineration process generates unwanted hazardous by-products such as carbon mono oxide, dioxins, NOx, SO2, and toxic products, which imparts harm effects on the environment (Röper and Koch 1990). In the other way to minimize plastic waste, the used products are recycled to manufacture other products. However, the quality of the product decreases after each recycling due to the removal of additives from the product and makes the process of recycling inefficient. Also, the higher cost of recycling further limits the practice of recycling (Zheng et al. 2005).
Other methods such as thermal degradation, thermo-oxidative degradation, chemical degradation and photodegradation were also adopted to degrade the plastics. These processes are environmentally unfriendly and it reduces plastic waste at a high cost. Therefore, it is a need to search for an alternative technique, which is cost-effective and environmentally friendly. Biodegradation is an environmental friendly technique adopted in the reduction of plastic waste. Further, microorganisms comprise more than 60% of earth’s biomass (Fraser et al. 2000) and its capability to utilize plastics as a source of carbon suggesting one prominent technique to tackle the plastic waste disposal.
Biodegradation of polymers occurs through three steps such as biodeterioration, biofragmentation, and assimilation. In the biodeterioration step, microorganisms come in contact with polymers and during the biofragmentation step, the secretion of enzymes from microorganisms depolymerizes the polymers into fragments. In the assimilation step, these fragments can pass through the outer semi-permeable membrane of microorganisms, and microorganisms utilize these smaller molecules as energy sources in the form of carbon (Chaudhary et al. 2021). The pathway for polymer biodegradation depends on the availability of oxygen. CO2, H2O, and microbial biomass are end products of an aerobic degradation process. In contrast, biomass, CO2, CH4, and H2O are the major end products of the anaerobic biodegradation process (Barlaz et al. 1989).
Petroleum-based polymers are not susceptible to microorganisms because of its high molecular weight, hydrophobicity and macromolecular nature of polymers (Schlemmer et al. 2009; Krueger et al. 2017). These polymers have shown minimal degradation against microorganisms. Modifications in the structure of polymers either through oxidization of polymers or to make a biodegradable polymer composite is necessary to facilitate fast biodegradation. Using treatments with ultraviolet radiation (UV; Zahra et al. 2010), heat (Awasthi et al. 2017) and chemicals (Rajandas et al. 2012) result in the generation of free radicals in the polymers which cleave the polymer chains. These pre-treatments convert the polymers into a more oxidized form having low molecular weight.
Syranidou et al. (2017) and Yang et al. (2018) have reported the biodegradation of pure polystyrene samples. Pre-treatments with ultraviolet (UV) radiation, gamma radiation, ozonation and nitric acid could be a viable approach to improve biodegradation in polystyrene samples. Several studies have demonstrated improved biodegradation in polystyrene samples after ultraviolet (UV) radiation (Ojeda et al. 2009), gamma radiation (Ali and Abdel Ghaffar 2017) and ozonation (Tian et al. 2017) treatments. However, the influence of nitric acid treatment on the decomposition of polystyrene films in presence of Cephalosporium sp. has not been reported elsewhere.
Brown et al. (1974) reported the growth of Cephalosporium sp. in mineral salt medium (MSM) on oxidized polystyrene and polyethylene samples. Further, the potential of Cephalosporium sp. to decompose pure polystyrene and acid treated high-density polyethylene (HDPE) films were reported by Chaudhary and Vijayakumar (2020a) and Chaudhary and Vijayakumar (2020b). In the present study, acid treated polystyrene (PS) and acid treated low-density polyethylene (LDPE) films are utilized for the degradation study in the presence of pure fungal culture. Further, pre-treatments using acid treatment are performed before the biodegradation study in order to insert functional group to ease the process of biodegradation.
Experimental methods
Nitric acid treatment of polystyrene and LDPE samples
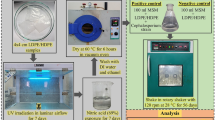
Polystyrene (PS) films were prepared by the similar method as reported by Chaudhary and Vijayakumar (2020a). Then the films were dipped in nitric acid (69%) solution in a glass beaker for 1 week. After 1 week, the samples were retrieved from the beaker and washed several times with distilled water and ethanol solution. Then the acid treated films were kept in a hot air oven at 60 °C for 6 h for the removal the moistures.
Source of fungal culture
Fungal strain of Cephalosporium sp. (NCIM 1251) was obtained from the National Collection of Industrial Microorganism (NCIM), NCL, Pune, India. Cephalosporium sp. was preserved in potato dextrose agar (PDA) at 28 °C and was kept at 4 °C.
Biodegradation study
100 mL of MSM was kept in a conical flask along with pre-treated polymer films. Positive (mineral salt media + pre-treated polymer + fungus) and negative (mineral salt media + pre-treated polymer) controls were prepared in laminar air flow chamber for the in-vitro degradation study. Then, the flasks were kept in an incubator for 56 days at 120 rpm and 28 °C.
Analysis of biodegradation
Weight loss measurement
Variations in the weight of pre-treated polymers were measured using Japan, BL-220H apparatus after 56 days of incubation with Cephalosporium species. The loss in weight was determined by the following formula:
where P1 = Initial weight of pre-treated polymer, P2 = Weight of pre-treated polymer after treatment with Cephalosporium sp.
Measurement of pH, TDS, conductivity
WENSER LMMP-30 apparatus was utilized to determine the pH, total dissolved solids (TDS) and conductivity of MSM.
Characterization techniques
Fourier transform infrared spectroscopy (FTIR) analyzer was utilized to identify the presence of functional group in polymer samples (Shimadzu, FTIR-8400). Thermogravimetric analyzer (TGA) was utilized to observe the thermal stability of the PS samples after each treatment (Shimadzu, TGA-50). Variations in surface texture after each treatment was analyzed through scanning electron microscopy (SEM; ZEISS, EVO 18). X-ray diffraction (XRD; Rigaku, Miniflex) studies were carried out to determine the changes in peaks related to crystalline and amorphous phases.
Results and discussion
Weight loss measurement
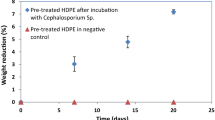
The weight loss measurements of the pre-treated PS films showed that the culture Cephalosporium sp. could deteriorate pre-treated PS films and pre-treated low-density polyethylene (LDPE) films by 12.22 ± 0.82% and 13.15 ± 0.44% respectively in 56 days. The exposure of polymers to acid treatment converts the hydrophobic nature of polymers into hydrophilic by the addition of functional groups which enables the microorganisms to instigate the biodeterioration process (Sheik et al. 2015). During pre-treatment process, polymer films get oxidized by the nitric acid and subsequently these oxidized portions were utilized by the microorganisms and lead to weight loss in the polymers. Thus, the weight loss suggests the physical breakdown of the plastics and affirms the potential ability of Cephalosporium sp. to deteriorate pre-treated polymer films. Similar observations in the weight loss for LDPE and high impact polystyrene (HIPS) treated with the microorganisms has been reported by Sheik et al. (2015) and Mohan et al. (2016).
Measurement of pH, TDS, and conductivity of mineral salt media
The values of pH, TDS and conductivity of the mineral salt media (MSM) measured at different intervals of the biological treatment are recorded in Table 1. The initial pH value of MSM was 7.01 ± 0.01, which reduced to 5.35 ± 0.09 when the pre-treated PS samples were exposed to Cephalosporium sp. for 4 weeks of incubation and the pH value further reduced to 4.30 ± 0.06 after 8 weeks of fungal treatment. However, a relative increase in TDS and conductivity values was observed in pre-treated PS films. The proteins, enzymes, and other metabolites secreted by the microbes in the mineral salt media have caused an improvement in the value of TDS and conductivity of MSM (Cassidy et al. 2001). The higher values of TDS and conductivity after 8 weeks of exposure to the microorganisms indicate that the Cephalosporium sp. could able to assimilate the pre-treated PS films. Similar observations were obtained for pre-treated LDPE films. The pH value of MSM media in pre-treated LDPE films which was initially at 7.01 ± 0.01 get reduced to around 5.65 ± 0.06 and 4.54 ± 0.19 after 4 weeks and 8 weeks of microbial treatment, respectively. This gradual shift in the pH toward the acidic region is due to the release of acids such as pentadecanoic acid, 2,6,10,14-tetramethyl-, methyl ester, 9-Octadecanoic acid, ethyl ester and enzymes by the Cephalosporium species (Gu 2003; Chaudhary and Vijayakumar 2020a). Unlike pH, the increase in TDS content and the conductivity of the MSM was observed. Further, the nitric acid treatment made LDPE samples more susceptible to Cephalosporium sp. and therefore it releases large amounts of enzymes, acids, antioxidants and this result in large value of TDS as compared to PS films.
Fourier transform infrared spectroscopy (FTIR)
FTIR spectrum for untreated polystyrene sample shows peaks at 3026, 2922, 2850, 1601, 1492, 1450, and 754 wavenumbers (Fig. 1a). The peak at 3026 cm−1 corresponds to aromatic C-H stretching whereas the peak at 1601 cm−1 corresponds to C=C vinyl group respectively (Jeyakumar et al. 2013; Ojeda et al. 2009). Characteristics transmittance peak around 2920 cm−1 represents CH2 asymmetric stretching. Further, peaks at 2850 (CH2 symmetric stretching), 1492, 1450 (benzene ring) and 754 cm−1 (substituted benzene derivative) have also appeared in FTIR spectrum in the polystyrene sample (Gu 2003; Sarmiento et al. 2016). Figure 1b shows sharp peaks at 1743 cm−1 and 1155 cm−1 for polystyrene sample treated with nitric acid. The peaks at 1743 cm−1 and 1155 cm−1 are related to carbonyl group and vibrations in the CH group, respectively. Similar observations were reported by Hace et al. (1996) for the nitric acid treated polystyrene sample. The peak at 3392 cm−1 corresponding to the hydroxyl group was detected after Cephalosporium sp. exposure in acid-treated polystyrene surface (Fig. 1c). Chaudhary and Vijayakumar (2020c) have shown a similar formation of peaks from 3200 to 3600 cm−1 in polystyrene samples kept under soil burial for 3 months. The formation of a new peak in pre-treated polystyrene suggests the chain scissions, cleavage of chains, and variations in the polymer chain length. Further, the formation of the peak is related to variations in macromolecular segments of the polymer that causes the hydrolysis process in the polymers (Sheik et al. 2015). Thus, the FTIR analysis indicates the deterioration of the pre-treated polystyrene surface by Cephalosporium sp. fungal culture.
The characteristics transmittance bands at 2914, 2848, 1465, 1373, and 719 cm−1 are visible in pure LDPE samples (Fig. 2a). The peaks around 2914 and 2848 cm−1 correspond to CH2 asymmetric stretching and CH2 symmetric stretching, respectively. Sharp bands appeared around 1465, 1373, and 719 cm−1 are related to bending, wagging and rocking deformation, respectively (Gulmine et al. 2002). The presence of a new peak at 1650 cm−1 in LDPE films after nitric acid treatment is seen which is related to the nitro group (–ON=O) (Fig. 2b). Similar observations have been reported by Trilla et al. (1983) for the polyethylene samples etched with nitric acid. The presence of a sharp peak at 1714 cm−1, corresponds to the carbonyl group is seen in the spectrum of pre-treated LDPE films after exposure to microbial treatment (Fig. 2c). Volke-Seplveda et al. (2002) observed similar peak at 1715 cm−1 for the pre-treated LDPE polymers after incubation with Aspergillus niger. Thus, the formation of peak after biological treatment suggests that the LDPE samples were deteriorated by Cephalosporium species.
Thermogravimetric analysis (TGA)
TGA thermogram shows the onset of degradation temperature at 370 °C for pure polystyrene film whereas the nitric acid treatment enhances the onset degradation temperature to 385 °C (Fig. 3a, b). This enhancement in onset degradation temperature is related to structural modifications and crosslinking reactions in polymer films induced by nitric acid treatment (Fig. 3b). Hace et al. (1996) reported similar observations in the enhancement of thermal property in acid-treated PS. The TGA analysis also showed slight change in the degradation temperature of acid-treated PS after the fungal attack (Fig. 3c). This change in thermal stability is due to the generation of low-molecular-weight compounds by the enzymatic scission of the polymeric chain (Jeyakumar et al. 2013).
Pure LDPE samples showed an onset degradation temperature at 437 °C whereas etching with nitric acid increased the onset degradation temperature to 468 °C for LDPE films (Fig. 4a, b). The percentage of amorphous and crystalline phases determines the degree of crystallinity in the semi-crystalline polymers like polyethylene (Li et al. 2019). The treatment of polyethylene with nitric acid leads to deterioration of amorphous phases and results in the higher percentage of crystalline phase. This increase in crystalline content increases the onset degradation temperature as highly crystalline polymer exhibits a higher melting point (Avalos-Belmontes et al. 2009). Further, the microbial exposure reduced the onset degradation temperature to 441 °C for pre-treated LDPE films (Fig. 4c). The reduction in degradation temperature is due to fungal actions in the void portion of LDPE films which caused chain scission in the polymers (Satlewal et al. 2008). Thus, the changes observed after microbial treatment reveals the utilization of pre-treated LDPE films by Cephalosporium species.
Scanning electron microscopy (SEM)
SEM micrograph for pure polystyrene sample shows a smooth, homogeneous, continuous, and compact pattern whereas nitric acid treatment induced cracks, holes, and rough surface patterns in polystyrene samples (Fig. 5a, b). Variations in the surface patterns depend on the diffusion of nitric acid into the polystyrene. Therefore, the diffusion rate through polystyrene pores is the deciding factor that determines the extent of chemical degradation in the polymer. Hace et al. (1996) have reported similar morphological variations in nitric acid treated polystyrene samples. Cephalosporium sp. hyphae along with spores are visible in the pre-treated PS samples after fungal treatment (Fig. 5c). Similar observations were reported by Syranidou et al. (2017) and Sekhar et al. (2016) when polystyrene samples were exposed to microbial treatment (Sekhar et al. 2016; Syranidou et al. 2017). This observation suggests the adherence of fungal culture to the pre-treated PS films that leads to deterioration of pre-treated PS films.
Figure 6a shows a neat and smooth texture for pure LDPE samples. Few protuberances with tiny holes in the LDPE samples are seen for the nitric acid treated films which turned the surface texture into rougher as compared to the pure LDPE samples (Fig. 6b). This indicates that the exposure to nitric acid induces structural changes in the LDPE films. Wang et al. (2009) observed similar observations in the LDPE films after acid etching. Further, the biological treatment of films created cracks, holes, surface deformation, grooves, hyphae penetration, and rough texture in the acid-treated LDPE films (Fig. 6c). Hyphae penetration is interpreted as the attachment of fungal culture to the polymer surface and utilized this surface as a carbon material for their metabolic activities (Ojha et al. 2017). Thus, the SEM study confirms the usage of pre-treated LDPE films by the microorganisms.
X-ray diffraction (XRD)
A broad peak around 2θ = 20 is seen for pure polystyrene sample which indicates the amorphous nature of polymer (Fig. 7a; Al-Shabanat 2012). The intensity of the peak increased after nitric acid treatment, which signifies an increase in the crystallinity of sample due to the rearrangements in the polymeric chains (Fig. 7b). Similar increase in the crystallinity of LDPE films after nitric acid treatment was reported by Wang et al. (2009). The intensity of peak further increased after microbial treatment and implies the utilization of amorphous component of polymer by the microorganism (Gleadall et al. 2012). This leads to increase in the proportions of crystalline phase in the pre-treated polystyrene sample (Fig. 7c).
In case of LDPE, XRD spectra shows a sharp peak at 2θ = 22.14 which corresponds to 110 reflections (Fig. 8a; Musuc et al. 2013). This reflection represents the orthorhombic crystal structure of LDPE. XRD spectra shows increase in the intensity of peak at 2θ = 22.14 for nitric acid treated LDPE samples as compared to pure sample (Fig. 8a, b). Nitric acid treatment causes deterioration of amorphous segments of pre-treated LDPE films which developed a highly crystalline structure (Avalos-Belmontes et al. 2009). Further, a significant increase in the intensity of peak is seen for pre-treated LDPE films after fungal treatment (Fig. 8c). It is due to utilization of amorphous phases of pre-treated LDPE films by Cephalosporium sp. and resulted in higher proportions of crystalline phases in the sample (Volke-Seplveda et al. 2002).
Conclusion
Polystyrene (PS) and low-density polyethylene (LDPE) were reported to be non-bio degradable due to its recalcitrant nature toward microorganisms. The present work showed a weight reduction of 12.22 ± 0.82% and 13.15 ± 0.44% in the pre-treated PS and pre-treated LDPE films after incubation of eight weeks with Cephalosporium species. Decrease in pH value and increase in TDS and conductivity value of the mineral salt medium after microbial treatment indicated that the Cephalosporium sp. utilizes the polymer for its metabolic activities. Acid treatment resulted in formation of carbonyl group in PS films, whereas nitro group was formed in LDPE films. Decrease in the thermal stability after microbial exposure confirms the formation of low molecular weight compounds as analyzed by TGA. Changes in the surface textures and increase in the crystallinity has been observed through SEM and XRD, respectively. These findings confirmed the capability of Cephalosporium sp. to assimilate pre-treated PS and pre-treated LDPE films.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Ali HE, Abdel Ghaffar AM (2017) Preparation and effect of gamma radiation on the properties and biodegradability of poly(styrene/starch) blends. Radiat Phys Chem 130:411–420. https://doi.org/10.1016/J.RADPHYSCHEM.2016.09.006
Al-Shabanat M (2012) Electrical studies of nanocomposites consisting of MWNTs and polystyrene. J Polym Res. https://doi.org/10.1007/S10965-011-9795-Z
Andrady AL, Neal MA (2009) Applications and societal benefits of plastics. Philos Trans R Soc B Biol Sci 364:1977–1984. https://doi.org/10.1098/rstb.2008.0304
Avalos-Belmontes F, Zapata-Gonzalez I, Ramos-De Valle LF et al (2009) Thermo-oxidative degradation of HDPE as a function of its crystalline content. J Polym Sci Part B Polym Phys 47:1906–1915. https://doi.org/10.1002/POLB.21785
Awasthi S, Srivastava P, Singh P, et al (2017) Biodegradation of thermally treated high-density polyethylene (HDPE) by Klebsiella pneumoniae CH001. 3 Biotech 7:1–10. https://doi.org/10.1007/s13205-017-0959-3
Barlaz MA, Schaefer DM, Ham RK (1989) Bacterial population development and chemical characteristics of refuse decomposition in a simulated sanitary landfill. Appl Environ Microbiol 55:55–65. https://doi.org/10.1128/aem.55.1.55-65.1989
Brown BS, Mills J, Hulse JM (1974) Chemical and biological degradation of waste plastics. Nature 250:161–163. https://doi.org/10.1038/250161A0
Cassidy DP, Werkema DD, Sauck W et al (2001) The effects of LNAPL biodegradation products on electrical conductivity measurements. J Environ Eng Geophys 6:47–52. https://doi.org/10.4133/JEEG6.1.47
Chaudhary AK, Vijayakumar RP (2020a) Studies on biological degradation of polystyrene by pure fungal cultures. Environ Dev Sustain 22:4495–4508. https://doi.org/10.1007/S10668-019-00394-5
Chaudhary AK, Vijayakumar RP (2020b) Effect of chemical treatment on biological degradation of high-density polyethylene (HDPE). Environ Dev Sustain 22:1093–1104. https://doi.org/10.1007/S10668-018-0236-6
Chaudhary AK, Vijayakumar RP (2020c) Synthesis of polystyrene/starch/CNT composite and study on its biodegradability. J Polym Res 27:187
Chaudhary AK, Chaitanya K, Vijayakumar RP (2021) Synergistic effect of UV and chemical treatment on biological degradation of polystyrene by cephalosporium strain NCIM 1251. Arch Microbiol 203:2183–2191. https://doi.org/10.1007/s00203-021-02228-3
Fraser CM, Eisen JA, Salzberg SL (2000) Microbial genome sequencing. Nature 406:799–803. https://doi.org/10.1038/35021244
Gleadall A, Pan J, Atkinson H (2012) A simplified theory of crystallisation induced by polymer chain scissions for biodegradable polyesters. Polym Degrad Stab 97:1616–1620. https://doi.org/10.1016/J.POLYMDEGRADSTAB.2012.06.023
Gu JD (2003) Microbiological deterioration and degradation of synthetic polymeric materials: recent research advances. Int Biodeterior Biodegrad 52:69–91. https://doi.org/10.1016/S0964-8305(02)00177-4
Gulmine JV, Janissek PR, Heise HM, Akcelrud L (2002) Polyethylene characterization by FTIR. Polym Test 21:557–563. https://doi.org/10.1016/S0142-9418(01)00124-6
Hace D, Kovacevic V, Pajc-Liplin D (1996) Thermally stimulated oxidative degradation of high impact polystyrene with nitric acid. Polym Eng Sci 36:1140–1151. https://doi.org/10.1002/PEN.10508
Jeyakumar D, Chirsteen J, Doble M (2013) Synergistic effects of pretreatment and blending on fungi mediated biodegradation of polypropylenes. Bioresour Technol 148:78–85. https://doi.org/10.1016/J.BIORTECH.2013.08.074
Krueger MC, Seiwert B, Prager A et al (2017) Degradation of polystyrene and selected analogues by biological Fenton chemistry approaches: opportunities and limitations. Chemosphere 173:520–528. https://doi.org/10.1016/J.CHEMOSPHERE.2017.01.089
Li D, Zhou L, Wang X et al (2019) Effect of crystallinity of polyethylene with different densities on breakdown strength and conductance property. Materials. https://doi.org/10.3390/MA12111746
Massardier-Nageotte V, Pestre C, Cruard-Pradet T, Bayard R (2006) Aerobic and anaerobic biodegradability of polymer films and physico-chemical characterization. Polym Degrad Stab 91:620–627. https://doi.org/10.1016/j.polymdegradstab.2005.02.029
Mehta AK (2017) Assessment & quantification of plastics waste generation in major cities. Cent Pollut Control BOARD (CPCB), India, pp 1–94
Mohan AJ, Sekhar VC, Bhaskar T, Nampoothiri KM (2016) Microbial assisted high impact polystyrene (HIPS) degradation. Bioresour Technol 213:204–207. https://doi.org/10.1016/j.biortech.2016.03.021
Muenmee S, Chiemchaisri W, Chiemchaisri C (2016) Enhancement of biodegradation of plastic wastes via methane oxidation in semi-aerobic landfill. Int Biodeterior Biodegrad 113:244–255. https://doi.org/10.1016/j.ibiod.2016.03.016
Musuc AM, Badea-Doni M, Jecu L et al (2013) FTIR, XRD, and DSC analysis of the rosemary extract effect on polyethylene structure and biodegradability. J Therm Anal Calorim 114:169–177. https://doi.org/10.1007/S10973-012-2909-Y
Ojeda T, Freitas A, Dalmolin E et al (2009) Abiotic and biotic degradation of oxo-biodegradable foamed polystyrene. Polym Degrad Stab 94:2128–2133. https://doi.org/10.1016/J.POLYMDEGRADSTAB.2009.09.012
Ojha N, Pradhan N, Singh S et al (2017) Evaluation of HDPE and LDPE degradation by fungus, implemented by statistical optimization. Sci Rep. https://doi.org/10.1038/SREP39515
Otake Y, Kobayashi T, Asabe H et al (1995) Biodegradation of low-density polyethylene, polystyrene, polyvinyl chloride, and urea formaldehyde resin buried under soil for over 32 years. J Appl Polym Sci 56:1789–1796. https://doi.org/10.1002/app.1995.070561309
Rajandas H, Parimannan S, Sathasivam K et al (2012) Analysis method A novel FTIR-ATR spectroscopy based technique for the estimation of low-density polyethylene biodegradation. Polym Test 31:1094–1099. https://doi.org/10.1016/j.polymertesting.2012.07.015
Röper H, Koch H (1990) The role of starch in biodegradable thermoplastic materials. Starch Stärke 42:123–130. https://doi.org/10.1002/star.19900420402
Sánchez C (2020) Fungal potential for the degradation of petroleum-based polymers: an overview of macro- and microplastics biodegradation. Biotechnol Adv 40:107501. https://doi.org/10.1016/j.biotechadv.2019.107501
Sarmiento AM, Guzmán HL, Morales G et al (2016) Expanded polystyrene (EPS) and waste cooking oil (WCO): from urban wastes to potential material of construction. Waste Biomass Valoriz 7:1245–1254. https://doi.org/10.1007/S12649-016-9511-7
Satlewal A, Soni R, Zaidi M et al (2008) Comparative biodegradation of HDPE and LDPE using an indigenously developed microbial consortium. J Microbiol Biotechnol 18:477–482
Schlemmer D, Sales MJA, Resck IS (2009) Degradation of different polystyrene/thermoplastic starch blends buried in soil. Carbohydr Polym 75:58–62. https://doi.org/10.1016/J.CARBPOL.2008.06.010
Sekhar VC, Nampoothiri KM, Mohan AJ et al (2016) Microbial degradation of high impact polystyrene (HIPS), an e-plastic with decabromodiphenyl oxide and antimony trioxide. J Hazard Mater 318:347–354. https://doi.org/10.1016/J.JHAZMAT.2016.07.008
Sheik S, Chandrashekar KR, Swaroop K, Somashekarappa HM (2015) Biodegradation of gamma irradiated low density polyethylene and polypropylene by endophytic fungi. Int Biodeterior Biodegrad 105:21–29. https://doi.org/10.1016/J.IBIOD.2015.08.006
Syranidou E, Karkanorachaki K, Amorotti F et al (2017) Biodegradation of weathered polystyrene films in seawater microcosms. Sci Rep. https://doi.org/10.1038/S41598-017-18366-Y
Tian L, Kolvenbach B, Corvini N et al (2017) Mineralisation of 14C-labelled polystyrene plastics by Penicillium variabile after ozonation pre-treatment. N Biotechnol 38:101–105. https://doi.org/10.1016/J.NBT.2016.07.008
Trilla R, Perena JM, Fatou JG (1983) Thermal and mechanical properties of oriented polyethylene treated with nitric acid. Polym J 15:803–809. https://doi.org/10.1295/polymj.15.803
Volke-Seplveda T, Saucedo-Castaeda G, Gutirrez-Rojas M et al (2002) Thermally treated low density polyethylene biodegradation by Penicillium pinophilum and Aspergillus niger. J Appl Polym Sci 83:305–314. https://doi.org/10.1002/app.2245
Wang H, Chen SJ, Zhang J (2009) Surface treatment of LLDPE and LDPE blends by nitric acid, sulfuric acid, and chromic acid etching. Colloid Polym Sci 287:541–548. https://doi.org/10.1007/s00396-009-2000-9
Yang SS, Wu WM, Brandon AM et al (2018) Ubiquity of polystyrene digestion and biodegradation within yellow mealworms, larvae of Tenebrio molitor Linnaeus (Coleoptera: Tenebrionidae). Chemosphere 212:262–271. https://doi.org/10.1016/J.CHEMOSPHERE.2018.08.078
Zahra S, Abbas SS, Mahsa MT, Mohsen N (2010) Biodegradation of low-density polyethylene (LDPE) by isolated fungi in solid waste medium. Waste Manag 30:396–401. https://doi.org/10.1016/j.wasman.2009.09.027
Zheng Y, Yanful EK, Bassi AS (2005) A review of plastic waste biodegradation. Crit Rev Biotechnol 25:243–250. https://doi.org/10.1080/07388550500346359
Funding
This study has no funding from any sources.
Author information
Authors and Affiliations
Contributions
All of the three authors have equal contribution in the experimental work, analysis and preparation of manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chaudhary, A.K., Chitriv, S.P. & Vijayakumar, R.P. Influence of nitric acid on biodegradation of polystyrene and low-density polyethylene by Cephalosporium species. Arch Microbiol 204, 489 (2022). https://doi.org/10.1007/s00203-022-03089-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-03089-0