Abstract
Vane trapping is one of the most effective methods for sampling flower-visiting arthropods. Despite its importance in pollinator studies, the effects of trap color on the abundance and richness of pollinators are less understood. To test this, we conducted a 3-season field experiment over 2 years with two types of vane traps: yellow and colorless. We set up twelve traps each in three field sites within the Lower Rio Grande Valley in south Texas, planted with Vigna unguiculata, Crotalaria juncea, Raphanus raphanistrum, and Sorghum drummondii. At each site, six colorless vane and six yellow vane traps were placed equidistant from each other. The experiment was replicated three times across three seasons, first during the pre-flowering season, when the crops were in full bloom, and when there was no crop on the field. In total, we collected 1912 insects, out of which 76.7% were pollinators. Generalized Linear Regression analyses showed that yellow traps consistently attracted significantly more arthropods and pollinators, but these differences were also season dependent. Furthermore, we noticed that Hymenoptera, followed by Coleoptera, were the most prevalent orders in both the yellow vane and colorless vane traps. Interestingly, although there was no significant difference in species richness of the arthropods in the yellow and colorless vane traps, our results suggest that trap color plays a significant role in capturing pollinators, including non-target arthropods. Our data add another line of evidence suggesting that trap color should be accounted for designing experiments that estimate pollinator and arthropod community diversity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arthropods contribute significantly to biodiversity in agroecosystems by driving critical ecosystem processes such as pollination, herbivory, and multi-trophic interactions (Meyer et al., 2011; Yang & Gratton, 2014). Both management and conservation practices in agroecosystems depend on reliable and consistent estimation of arthropods, both at species richness and diversity, and abundance (Kaur et al., 2020; Martinez et al., 2020; McLeod, 2000; Sane et al., 1999). In addition, estimating arthropods in the agroecosystems is essential in devising policy, conservation efforts, and monitoring arthropod decline over time (Karamaouna et al., 2021). However, the estimation of arthropods in the agroecosystems tends to be inconsistent and can vary due to many factors- including trap methodologies, an area that is poorly understood.
Various active or passive sampling techniques have been used to estimate and evaluate the population density and diversity of arthropods. Active sampling of arthropods involves using sweep nets and transect walks to estimate the population of arthropods in each area (Steffan-Dewenter & Schiele, 2008; Taki et al., 2018). However, this sampling technique is quite rigorous and time-consuming as accurate results entirely depend on the investigator's skill and experience (Prendergast et al., 2020). Passive sampling (e.g., trap) is easy to install and does not require the input of the investigator in collecting arthropod samples once the traps have been installed correctly (McCravy, 2018). They also allow for a more diverse range of arthropod collection through multiple time periods to eliminate investigator bias (McCravy, 2018). Examples include pan traps (Gonzalez et al., 2020; Shrestha et al., 2019; Sircom et al., 2018; Kariyat et al., 2018, sticky traps (Dimitrova et al., 2020; Kariyat et al., 2012), malaise traps (Srisuka et al., 2021; Volpato et al., 2020), pitfall traps (Umair Sial et al., 2022; Jiménez-Carmona et al., 2019; Kariyat et al., 2018, and colored vane traps (Hall & Reboud, 2019; Hall, 2018; Stephen & Rao, 2005). These traps are installed passively but actively use their colors to attract different arthropods belonging to various taxonomic groups (Acharya et al., 2021; Hall & Reboud, 2019; Moreira et al., 2016).
Trap color is an essential factor to consider while estimating arthropods and can influence arthropod diversity (Acharya et al., 2021; Cai et al., 2021; Hoback et al., 1999). The response of arthropods to trap color has also been found to vary significantly based on taxonomic group and ecology (Hall & Reboud, 2019; Skvarla & Dowling, 2017; Vrdoljak & Samways, 2012). For example, the commonly used colors for sampling pollinators are blue, white, and yellow (Abrahamczyk et al., 2010; McCravy, 2018). However, arthropods are more attracted to blue and yellow traps, although there is no consensus on which color is more appealing to a particular taxa or different habitat types (Abrahamczyk et al., 2010; Saunders & Luck, 2013). This has been linked to variations in reflectivity and vibrancy caused by a lack of uniform color and design quality (Shrestha et al., 2019). Dimitrova et al. (2020) compared the effectiveness of yellow and transparent sticky traps to evaluate arthropod diversity in an olive agroecosystem. They realized that the total number of arthropods captured in yellow traps exceeded the number trapped in transparent sticky traps. A similar experiment conducted in southern Australia tested the effectiveness of yellow and blue vane traps for sampling wild bees in both open and wooden habitats. They found that blue vane traps more effectively attracted wild bees than yellow vane traps (Hall, 2018). In an experiment to test the relative performance of different sampling methods for native bees, Prendergast et al. (2020) showed that the blue vane traps were more efficient than the yellow vane traps in sampling native bees. Taken together, these studies clearly demonstrate the importance of trap color for estimating arthropod diversity but also highlight the inconsistency in results.
There is no argument for the fact that pest and pollinator management needs a sound estimation of the prevailing arthropod community (Cai et al., 2021; Martinez et al., 2020). In addition to the odor of plant volatiles, arthropods employ visual cues, especially color, to locate their hosts for feeding and laying eggs (Schoonhoven et al., 2005). Some rely on vision and olfaction cues in locating their hosts, while others rely only on visual stimuli (Kerr et al., 2017; Kariyat et al., 2021). For example, an experiment that evaluated the population of Lygus lineolaris showed that both olfactory cues and visual stimuli played a vital role in their host selection (Prokopy et al., 1979). Arthropods such as Empoasca fabae and Altica engstroemi rely only on visual stimuli when they search for their host (Bullas-Appleton et al., 2004). In laboratory color-choice tests and field tests, Cai et al. (2021) showed that Protaetia brevitarsis adults (a major pest of many fruits and vegetable crops, including grapes and tomatoes) were more attracted to red traps. Clearly, the results from such experiments will improve the effectiveness of traps for capturing herbivores in integrated pest control programs.
Color is one of the most important visual characteristics utilized by pollinating insects (Kariyat et al., 2021; van der Kooi et al., 2019). The display of colorful flowers elicits various responses among pollinators (Renoult et al., 2014). For example, an experiment that assessed the most preferred cue used by pollinators showed that pollinators in two-choice bioassays showed a strong bias for visual cues over olfactory cues (Barragán-Fonseca et al., 2020). Also, the intensity of light and the reflectance of trap colors directly affect the population of pollinators that are attracted to the traps (Joshi et al., 2015; Vrdoljak & Samways, 2012). Pollinators such as bees can identify different colors and even discriminate against color textures, although this varies by species. Bees direct their attention to a source based on the color intensity and chromaticity of illumination (Koethe et al., 2018; Lotto & Chittka, 2005). Also, they use their olfactory signal if the source is at ~ 30 cm and their visual cues if they are close to flowers (Streinzer et al., 2009).
Taken together, agroecosystems can benefit from a better estimation of pollinators, and trapping methodologies, and should also explore whether non-target insects are disproportionately targeted by trap design, including their color. To test this, we used yellow and colorless vane traps to estimate the population of pollinators and non-target arthropods in three agroecological fields in the Lower Rio Grande Valley, Texas. The following questions were asked: (1) Can trap color affect arthropod diversity assessment? (2) If color affects trapping, will the traps differentially attract pollinators and non-pollinators?
Methods
Study sites
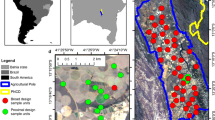
This experiment was conducted on three different agricultural fields in the Lower Rio Grande Valley. Our first site (26°18′12.2″N 97°50′13.1″W) had a mix of cowpea (Vigna unguiculata Fabaceae) and sunn hemp (Crotalaria juncea L.) (Fabaceae). The second site (26°24′35.1″N 98°27′52.8″W) also had a mix of cowpea (Vigna unguiculata Fabaceae) and radish (Raphanus raphanistrum Brassicaceae). Our third site (26°20′32.6″N 98°31′51.6″W) was planted with cowpea (Vigna unguiculata Fabaceae) and sorghum-sudangrass (Sorghum drummondii Poaceae).
Sampling
Sampling in each site was conducted three times over 2 years. The first was carried out during September 2021 (fall), when the crops were at the pre-flowering stage, the second sampling was conducted in during December 2021 (winter), when the crops were in full bloom, and finally during July 2022 (summer), post-harvesting when there no crops/plants on the fields. At each site, six yellow vane and six colorless vane traps (BanfieldBio INC Woodinville WA, USA; both traps had blue funnel and different in the color of the jar- yellow or colorless) were installed at 1.2–1.5 m above the ground on wooden sticks (Fig. 1A, B) and distanced 36 m from each other to prevent spatial autocorrelation (Greenleaf et al., 2007; Prendergast et al., 2020). The diameter and height of the traps used were 12 cm and 15 cm, respectively. The yellow and colorless vane traps were filled with a soap water solution to almost one-fourth of the traps to reduce surface tension (Kariyat et al., 2012, 2018) and were collected after four days of their setup in the field. Captured arthropods were transferred to containers containing 70% ethanol for identification. The sampled arthropods were classified into their orders and functional group (pollinators).
Shannon-Weiner diversity index
The Shannon-Weiner diversity index was used to test the diversity of arthropods in the yellow and colorless vane traps during different seasons (fall, winter, and summer). The diversity index was computed using the formula below:
Here,
\(H\mathrm{^{\prime}}\) = Shannon diversity index
Pi = Relative number of each species
\(ni\) = Species number i
N = total number of species
Statistical analysis
Since most of the data were insect count, we used Generalized Regression with Poisson distribution, and means were separated using the Tukey HSD test. All analyses were carried out using SAS JMP (JMP®, Version < 15 >. SAS Institute Inc., Cary, NC, 1989–2021), and plots were built using GraphPad Prism (GraphPad Software, version 9.0.0 for Windows, La Jolla, CA, USA, www.graphpad.com). The total number of arthropods was assessed and separated into different taxonomic orders and functional roles across different seasons. The mean numbers of total arthropods, pollinators, parasitoids, and taxonomic orders were included as response variables, while trap color, season, and their interactions were included as factors.
Results
General results on orders and families collected
We collected a total of 1912 arthropods, out of which 1466 were pollinators. The major orders found were Hymenoptera (76.7%) followed by Coleoptera (11.3%), Lepidoptera (5.6%), Diptera (2.2%), and Hemiptera (0.8%). A few insects of orders Collembola (0.49%), Blattodea (0.18%), Embioptera (0.06%), and Neuroptera (0.06%), were also found but in much fewer numbers. Most pollinators found were from Megachilidae, Xylocopidae, Vespidae, and Halictidae. Also, most of the Coleopterans found were from families Meloidae and Melyridae.
In general, yellow vane traps captured a higher number of total arthropods, which was ~ 68% of the total arthropods collected, compared to the colorless vane trap, which collected ~ 32% of the total arthropods. Furthermore, the yellow vane trap captured more pollinators (~ 70%) than the colorless vane traps (~ 30%).
Regression analyses
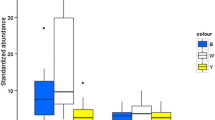
The total number of arthropods and pollinators collected differed between the yellow vane and colorless vane traps. The yellow vane trap was by far the most effective in sampling pollinators and lepidopterans. There was a significant difference in the total number of arthropods (Table 1; Fig. 2A; P < 0.0001) and pollinators (Table 1; Fig. 3A; P < 0.0001) caught in the yellow vane traps. Among the orders, Lepidopterans were significantly attracted to the yellow vane traps (Table 1; Fig. 4C; P < 0.0001). However, trap color did not significantly affect the attraction of insects belonging to the orders Coleoptera (Table 1; Fig. 4A; P = 0.0921), Diptera (Table 1; Fig. 4B; P = 0.3038), and Hemiptera (Table 1; Fig. 4D; P = 0.5129).
A Results of the abundance of total arthropods pooled from yellow vane and colorless traps. Data was analyzed using generalized regression models and means were separated Tukey’s post hoc tests at a P value of 0.05. Different letters denote statistical significance at P < 0.05. B Results of the interaction of trap color and season on the total arthropods pooled from the yellow and colorless vane traps. Data was analyzed with generalized regression models and means were separated Tukey’s post hoc tests at a P value of 0.05. Different letters denote statistical significance at P < 0.05. C Results of the effect of season on total arthropods pooled from the yellow and colorless vane traps. Data was analyzed with generalized regression models and means were separated Tukey’s post hoc tests at a P value of 0.05. Different letters denote statistical significance at P < 0.05
A Results of the abundance of pollinators pooled from the yellow and colorless vane traps. Data was analyzed with generalized regression models means were separated Tukey’s post hoc tests at a P value of 0.05. Different letters denote statistical significance at P < 0.05. B Results of the interaction of trap color and season on the pollinators pooled from the yellow and colorless vane traps. Data was analyzed with generalized regression models and means were separated Tukey’s post hoc tests at a P value of 0.05. Different letters denote statistical significance at P < 0.05. C Results of the effect of season on pollinators pooled from the yellow and colorless vane traps. Data was analyzed with generalized regression models and means were separated Tukey’s post hoc tests at a P value of 0.05. Different letters denote statistical significance at P < 0.05
A Results of the abundance of the total Coleoptera pooled from the yellow and colorless vane traps. Data was analyzed with generalized regression model means and were separated Tukey’s post hoc tests at a P value of 0.05. Different letters denote statistical significance at P < 0.05. B Results of the abundance of the total Diptera pooled from the yellow and colorless vane traps. Data was analyzed with generalized regression models and means were separated Tukey’s post hoc tests at a P value of 0.05. Different letters denote statistical significance at P < 0.05. C Results of the effect of season on Dipterans pooled from the yellow and colorless vane traps. Data was analyzed with generalized regression models and means were separated Tukey’s post hoc tests at a P value of 0.05. Different letters denote statistical significance at P < 0.05. D Results of the abundance of the total Lepidoptera pooled from the yellow and colorless vane traps. Data was analyzed with generalized regression models and means were separated Tukey’s post hoc tests at a P value of 0.05. Different letters denote statistical significance at P < 0.05. E Results of the abundance of the total Hemipterans pooled from the yellow and colorless vane traps. Data was analyzed with generalized regression models and means were separated Tukey’s post hoc tests at a P value of 0.05. Different letters denote statistical significance at P < 0.05. F Results of the interaction of trap color and season on the Lepidopterans pooled from the yellow and colorless vane traps. Data was analyzed with generalized regression models and means were separated Tukey’s post hoc tests at a P value of 0.05. Different letters denote statistical significance at P < 0.05
The season in which the sampling was carried out had a significant effect on the total number of arthropods (Table 2; Fig. 2C; P < 0.0001) and pollinators (Table 2; Fig. 3C; P < 0.0001) captured in the traps. There were significantly more arthropods and pollinators captured in the fall of 2021 in the yellow and colorless vane traps. However, the season had no significant effect on the population of the insect orders, even though their numbers were higher in the fall of 2021, except for the Dipterans. (Fig. 4C). There was a significant trap color x season interaction on the total number of arthropods (Table 3; Fig. 2B; P = 0.0004) and pollinators (Table 3; Fig. 3B; P = 0.0001). The yellow vane traps significantly captured more arthropods and pollinators than the colorless vane trap across the seasons. There was no significant difference in the numbers of the insect orders captured in the yellow vane, and colorless vane traps across the seasons except for the Lepidopterans (Table 3; Fig. 4E; P < 0.0001).
Shannon diversity index
The Shannon-Weiner diversity index estimate showed no significant difference in the diversity of arthropods in yellow or colorless vane traps across the seasons. However, the maximum diversity index equitability was 0.52 for the yellow traps during the fall of 2021. Therefore, we concluded that the trap color and season did not affect the species richness or the diversity of the arthropods in the yellow vane or colorless vane traps (Tables 4 and 5).
Discussion
This study demonstrates that trap color is essential in assessing arthropod community dynamics in agroecosystems, adding another line of evidence on how trap color must be accounted in field estimation of arthropods. Through 3 field seasons spread over 2 years, we demonstrate that the yellow vane traps captured significantly more arthropods than the colorless vane traps through repeated sampling. There was also a significant trap color x season interaction on the total number of arthropods and even pollinators captured in the yellow vane and colorless vane traps. Arthropods, especially the pollinators and Lepidopterans, showed a marked preference for the yellow vane traps over the colorless vane traps across the seasons. A large number of arthropods were collected across the survey periods, particularly from certain families, mainly considered pollinators (Megachilidae, Xylocopidae, Vespidae, and Halictidae). Interestingly, lepidopterans displayed a marked color preference for yellow vane traps among the sampled orders. However, there was no significant difference in the species diversity of the arthropods in the yellow vane and the colorless vane trap. Our results indicate that trap color is an essential factor in understanding the distribution and demography of arthropods in the agroecosystem.
Our results that show a significantly higher number of pollinators in the yellow vane traps disagree with some other vane trap studies targeting arthropods in the agroecosystem (Stephen & Rao, 2005; Hall, 2018; Hall & Reboud, 2019) but is consistent with the results of Prokopy & Owens (1979), Dimitrova et al., 2020, which reported that arthropods are more attracted to yellow traps. The U.V. reflectance of traps has been reported as a factor responsible for arthropod attractiveness, especially bees and wasps, to the yellow vane traps (Acharya et al., 2022; Koski & Ashman, 2014). However, there was no significant difference in the number of insects from the orders: Diptera, Hemiptera, and Coleoptera in the yellow or colorless vane traps.
Habitat type, together with trap color, can influence arthropod trapping in agroecosystems (Saunders & Luck, 2013). Although most pollinators are considered generalist foragers, the composition of pollinators assemblages is believed to have evolved in response to environmental characteristics like habitat shape, plant diversity, or the dominant floral resource color (Bates et al., 2011; Wenninger & Inouye, 2008). Therefore, pollinator assemblages may respond to colored traps differently depending on their habitat. The fields sampled in this study differ in some ways. The fields comprised different cover crops like cowpea, radish, sorghum-sudangrass, and sunflower. Although each cover crops have different growth patterns and floral resources (Martinez et al., 2020). We found that pollinators and Lepidopterans were caught in yellow vane traps more often than in the colorless vane traps across the three fields. Furthermore, the yellow vane traps caught more pollinators and total arthropods than the colorless vane traps. This could be due to the yellow vane traps' high reflectivity and the contrast between trap color and the predominant colors of the plants in the field (green) (Abrahamczyk et al., 2010; Joshi et al., 2015).
Pollinating insects require two essential habitats to perform ecosystem services: nesting habitat and foraging habitat (Olsson et al., 2015). However, the proximity of these habitats to the pollinators is essential for pollination (Kline & Joshi, 2020). Therefore, landscape structure characteristics such as the shape of patches, availability of flowers, plant arrangement, and type of plants significantly affect the population and visitation of pollinators (Turley et al., 2022; Syrbe & Walz, 2012). Additionally, the availability of floral resources and pollinators’ foraging behavior could also be impacted by variations in seasonal weather patterns and extreme weather patterns (Straka et al., 2014). We surveyed the fields three times, the first few days after germination of the plants (fall 2021; no flowers), when the plants were in full bloom (winter 2021) and when there were no plants on the fields (summer 2022). We noticed that there were significantly greater numbers of pollinators in the fall and winter of 2021 across the three fields. This could, however, be because pollinators such as bees are more abundant in mild to moderately warm weather conditions than in harsh or extremely hot conditions, which is associated with fewer flowering plants (Abrahamczyk et al., 2011; Koch, 2002). Furthermore, the abundance and diversity of pollinators such as bees and wasps are influenced by a variety in the number of flower and food plant species which were more abundant during the fall and winter seasons (Abrahamczyk et al., 2011).
In 2018, we developed (Kariyat et al., 2018) a comprehensive and inexpensive trapping technique to quantify the field arthropod community that uses both volatile and contact cues for trapping. Therefore, a combination of this trapping technique together with the vane traps can be used for the complete quantification of arthropod diversity in agroecosystems. Previous studies have also confirmed that trap color is a significant factor in the survey of pollinators and non-bee pollinators in agroecosystems. However, here we show and confirm that trap color is a significant factor in estimating arthropods in the agroecosystem. The vane traps (yellow and colorless) are best suited for estimating pollinators alone because no single trap can be efficiently used to capture all arthropods in every habitat in the agroecosystem.
Conclusions
The study revealed four vital findings: (1) Trap color is a significant factor in estimating the population of arthropods in the agroecosystem. (2) Season is an essential factor to consider when estimating the population of arthropods in the agroecosystem. (3) The yellow vane trap significantly captured more arthropods, especially pollinators and Lepidopterans, than the colorless vane traps. (4) The yellow vane trap is best suited for the estimation of pollinators in the agroecosystem.
Data availability
All raw data will be made available on request.
References s
Abrahamczyk, S., Kluge, J., Gareca, Y., et al. (2011). The influence of climatic seasonality on the diversity of different tropical pollinator groups. PLoS ONE, 6, e27115. https://doi.org/10.1371/journal.pone.0027115
Abrahamczyk, S., Steudel, B., & Kessler, M. (2010). Sampling Hymenoptera along a precipitation gradient in tropical forests: The effectiveness of different coloured pan traps. Entomologia Experimentalis Et Applicata, 137, 262–268. https://doi.org/10.1111/j.1570-7458.2010.01063.x
Acharya, R. S., Burke, J. M., Leslie, T., et al. (2022). Wild bees respond differently to sampling traps with vanes of different colors and light reflectivity in a livestock pasture ecosystem. Scientific Reports, 12, 9783. https://doi.org/10.1038/s41598-022-10286-w
Acharya, R. S., Leslie, T., Fitting, E., et al. (2021). Color of pan trap influences sampling of bees in livestock pasture ecosystem. Biology. https://doi.org/10.3390/biology10050445
Barragán-Fonseca, K. Y., van Loon, J. J. A., Dicke, M., & Lucas-Barbosa, D. (2020). Use of visual and olfactory cues of flowers of two brassicaceous species by insect pollinators. Ecological Entomology, 45, 45–55. https://doi.org/10.1111/een.12775
Bates, A. J., Sadler, J. P., Fairbrass, A. J., et al. (2011). Changing bee and hoverfly pollinator assemblages along an urban-rural gradient. PLoS ONE, 6, e23459. https://doi.org/10.1371/journal.pone.0023459
Bullas-Appleton, E. S., Otis, G., Gillard, C., & Schaafsma, A. W. (2004). Potato leafhopper (Homoptera: Cicadellidae) varietal preferences in edible beans in relation to visual and olfactory cues. Environmental Entomology, 33, 1381–1388. https://doi.org/10.1603/0046-225X-33.5.1381
Cai, H., Zhang, T., Su, Y., et al. (2021). Influence of trap color, type, and placement on capture efficacy for protaetia brevitarsis (Coleoptera: Scarabaeidae). Journal of Economic Entomology, 114, 225–230. https://doi.org/10.1093/jee/toaa259
Dimitrova, A., Milošević, M., Spanos, T., et al. (2020). Yellow or transparent? Comparison of sticky traps for monitoring functional arthropod diversity in an olive agroecosystem. Animal Biodiversity and Conservation. https://doi.org/10.32800/abc.2020.43.0159
Gonzalez, V. H., Osborn, A. L., Brown, E. R., et al. (2020). Effect of pan trap size on the diversity of sampled bees and abundance of bycatch. Journal of Insect Conservation, 24, 409–420.
Greenleaf, S. S., Williams, N. M., Winfree, R., & Kremen, C. (2007). Bee foraging ranges and their relationship to body size. Oecologia, 153, 589–596. https://doi.org/10.1007/s00442-007-0752-9
Hall, M. (2018). Blue and yellow vane traps differ in their sampling effectiveness for wild bees in both open and wooded habitats. Agricultural and Forest Entomology, 20, 487–495. https://doi.org/10.1111/afe.12281
Hall, M. A., & Reboud, E. L. (2019). High sampling effectiveness for non-bee flower visitors using vane traps in both open and wooded habitats. Austral Entomology, 58, 836–847. https://doi.org/10.1111/aen.12416
Hoback, W. W., Svatos, T. M., Spomer, S. M., & Higley, L. G. (1999). Trap color and placement affects estimates of insect family-level abundance and diversity in a Nebraska salt marsh. Entomologia Experimentalis Et Applicata, 91, 393–402. https://doi.org/10.1046/j.1570-7458.1999.00507.x
Jiménez-Carmona, F., Carpintero, S., & Reyes-López, J.-L. (2019). The digging-in effect on ant studies with pitfall traps: Influence of type of habitat and sampling time. Entomologia Experimentalis Et Applicata, 167, 906–914. https://doi.org/10.1111/eea.12834
Joshi, N. K., Leslie, T., Rajotte, E. G., et al. (2015). Comparative trapping efficiency to characterize bee abundance, diversity, and community composition in apple orchards. Annals of the Entomological Society of America, 108, 785–799. https://doi.org/10.1093/aesa/sav057
Karamaouna, F., Jaques, J. A., & Kati, V. (2021). Practices to conserve pollinators and natural enemies in agro-ecosystems. Insects, 12, 31. https://doi.org/10.3390/insects12010031
Kariyat, R., Chavana, J., & Kaur, J. (2018). An Inexpensive and comprehensive method to examine and quantify field insect community influenced by host plant olfactory cues. Bio-protocol. https://doi.org/10.21769/BioProtoc.2967
Kariyat, R. R., Bentley, T. G., Nihranz, C. T., et al. (2021). Inbreeding in Solanum carolinense alters floral attractants and rewards and adversely affects pollinator visitation. American Journal of Botany, 108, 74–82. https://doi.org/10.1002/ajb2.1594
Kariyat, R. R., Mauck, K. E., De Moraes, C. M., et al. (2012). Inbreeding alters volatile signalling phenotypes and influences tri-trophic interactions in horsenettle (Solanum carolinense L.). Ecology Letters, 15, 301–309. https://doi.org/10.1111/j.1461-0248.2011.01738.x
Kaur, J., Chavana, J., Soti, P., et al. (2020). Arbuscular mycorrhizal fungi (AMF) influences growth and insect community dynamics in Sorghum-sudangrass (Sorghum x drummondii). Arthropod-Plant Interactions, 14, 301–315. https://doi.org/10.1007/s11829-020-09747-8
Kerr, J., Kelly, D., Bader, M., Brockerhoff, E. (2017). Olfactory cues, visual cues, and semiochemical diversity interact during host location by invasive forest beetles. Journal of Chemical Ecology, 43(1), 17–25. ISSN: 1573-1561. https://doi.org/10.1007/s10886-016-0792-x
Kline, O., & Joshi, N. K. (2020). Mitigating the effects of habitat loss on solitary bees in agricultural ecosystems. Agriculture. https://doi.org/10.3390/agriculture10040115
Koch, F. (2002). Charles D. Michener (2000). The bees of the world. – Baltimore and London (The John Hopkins University Press). – 913 S., 48 Farbfotos und zahlr. s/w Illustrationen. ISBN 0-8018-6133-0 (hardcover). US$ 165. Zoosystematics and Evolution, 78, 353–353. https://doi.org/10.1002/mmnz.20020780209
Koethe, S., Banysch, S., Alves-dos-Santos, I., & Lunau, K. (2018). Spectral purity, intensity and dominant wavelength: disparate colour preferences of two Brazilian stingless bee species. PLoS ONE, 13, e0204663. https://doi.org/10.1371/journal.pone.0204663
Koski, M. H., & Ashman, T.-L. (2014). Dissecting pollinator responses to a ubiquitous ultraviolet floral pattern in the wild. Functional Ecology, 28, 868–877. https://doi.org/10.1111/1365-2435.12242
Lotto, R. B., & Chittka, L. (2005). Seeing the light: Illumination as a contextual cue to color choice behavior in bumblebees. Proceedings of the National Academy of Sciences, 102, 3852–3856. https://doi.org/10.1073/pnas.0500681102
Martinez, L., Soti, P., Kaur, J., et al. (2020). Impact of cover crops on insect community dynamics in organic farming. Agriculture. https://doi.org/10.3390/agriculture10060209
McCravy, K. W. (2018). A review of sampling and monitoring methods for beneficial arthropods in agroecosystems. Insects. https://doi.org/10.3390/insects9040170
McLeod, P. J. (2000). Comparison of insect sampling techniques in snap bean. Journal of Vegetable Crop Production, 6, 37–43. https://doi.org/10.1300/J068v06n01_05
Meyer, S. T., Leal, I. R., Tabarelli, M., Wirth, R. (2011). Ecosystem engineering by leaf-cutting ants: nests of Atta cephalotes drastically alter forest structure and microclimate. Ecological Entomology, 36(1), 14–24. https://doi.org/10.1111/j.1365-2311.2010.01241.x
Moreira, E. F., da Santos, R. L., S, Penna UL, et al. (2016). Are pan traps colors complementary to sample community of potential pollinator insects? Journal of Insect Conservation, 20, 583–596. https://doi.org/10.1007/s10841-016-9890-x
Olsson, O., Bolin, A., Smith, H. G., & Lonsdorf, E. V. (2015). Modeling pollinating bee visitation rates in heterogeneous landscapes from foraging theory. Ecological Modelling, 316, 133–143. https://doi.org/10.1016/j.ecolmodel.2015.08.009
Prendergast, K. S., Menz, M. H. M., Dixon, K. W., & Bateman, P. W. (2020). The relative performance of sampling methods for native bees: An empirical test and review of the literature. Ecosphere. https://doi.org/10.1002/ecs2.3076
Prokopy, R. J., Adams, R. G., Hauschild, K. I. (1979). Visual responses of tarnished plant bug 1 adults on apple. Environmental Entomology, 8(2), 202–205. https://doi.org/10.1093/ee/8.2.202
Renoult, J. P., Valido, A., Jordano, P., & Schaefer, H. M. (2014). Adaptation of flower and fruit colours to multiple, distinct mutualists. New Phytologist, 201, 678–686. https://doi.org/10.1111/nph.12539
Sane, I., Alverson, D. R., & Chapin, J. W. (1999). Efficiency of conventional sampling methods for determining arthropod densities in close-row soybeans. Journal of Agricultural and Urban Entomology, 16, 65–84.
Saunders, M. E., & Luck, G. W. (2013). Pan trap catches of pollinator insects vary with habitat. Australian Journal of Entomology, 52, 106–113. https://doi.org/10.1111/aen.12008
Schoonhoven, L. M., van Loon, J. J. A., & Dicke, M. (2005). Insect-plant biology (2nd ed.). Oxford University Press, Oxford.
Shrestha, M., Garcia, J. E., Chua, J. H. J., et al. (2019). Fluorescent pan traps affect the capture rate of insect orders in different ways. Insects. https://doi.org/10.3390/insects10020040
Sircom, J., Arul Jothi, G., & Pinksen, J. (2018). Monitoring bee populations: Are eusocial bees attracted to different colours of pan trap than other bees? Journal of Insect Conservation, 22, 433–441. https://doi.org/10.1007/s10841-018-0071-y
Skvarla, M. J., & Dowling, A. P. G. (2017). A comparison of trapping techniques (Coleoptera: Carabidae, Buprestidae, Cerambycidae, and Curculionoidea excluding Scolytinae). Journal of Insect Science, 17, 7. https://doi.org/10.1093/jisesa/iew098
Srisuka, W., Sulin, C., Aupalee, K., et al. (2021). Community structure, biodiversity and spatiotemporal distribution of the black flies (Diptera: Simuliidae) using malaise traps on the highest mountain in Thailand. Insects. https://doi.org/10.3390/insects12060504
Steffan-Dewenter, I., & Schiele, S. (2008). Do resources or natural enemies drive bee population dynamics in fragmented habitats. Ecology, 89, 1375–1387. https://doi.org/10.1890/06-1323.1
Stephen, W. P., & Rao, S. (2005). Unscented color traps for non-Apis bees (Hymenoptera: Apiformes). Journal of the Kansas Entomological Society, 373–380.
Straka, J., Černá, K., Macháčková, L., et al. (2014). Life span in the wild: The role of activity and climate in natural populations of bees. Functional Ecology, 28, 1235–1244. https://doi.org/10.1111/1365-2435.12261
Streinzer, M., Paulus, H. F., & Spaethe, J. (2009). Floral colour signal increases short-range detectability of a sexually deceptive orchid to its bee pollinator. Journal of Experimental Biology, 212, 1365–1370. https://doi.org/10.1242/jeb.027482
Syrbe, R.-U., & Walz, U. (2012). Spatial indicators for the assessment of ecosystem services: Providing, benefiting, and connecting areas and landscape metrics. Ecological Indicators, 21, 80–88. https://doi.org/10.1016/j.ecolind.2012.02.013
Taki, H., Murao, R., Mitai, K., & Yamaura, Y. (2018). The species richness/abundance–area relationship of bees in an early successional tree plantation. Basic and Applied Ecology, 26, 64–70. https://doi.org/10.1016/j.baae.2017.09.002
Turley, N. E., Biddinger, D. J., Joshi, N. K., López‐Uribe, M. M. (2022). Six years of wild bee monitoring shows changes in biodiversity within and across years and declines in abundance. Ecology and Evolution, 12(8). https://doi.org/10.1002/ece3.9190
Umair Sial, M., Zeeshan Majeed, M., Atiq, A., et al. (2022). Differential efficacy of edaphic traps for monitoring arthropods diversity in subtropical regions. Journal of King Saud University - Science, 34, 101686. https://doi.org/10.1016/j.jksus.2021.101686
van der Kooi, C. J., Dyer, A. G., Kevan, P. G., & Lunau, K. (2019). Functional significance of the optical properties of flowers for visual signalling. Annals of Botany, 123, 263–276. https://doi.org/10.1093/aob/mcy119
Volpato, A., Ahmed, K. S. D., Williams, C. D., et al. (2020). Using Malaise traps to assess aculeate Hymenoptera associated with farmland linear habitats across a range of farming intensities. Insect Conservation and Diversity, 13, 229–238. https://doi.org/10.1111/icad.12383
Vrdoljak, S., & Samways, M. (2012). Optimising colored pan traps to survey flower visiting insects. Journal of Insect Conservation, 16, 1–10. https://doi.org/10.1007/s10841-011-9420-9
Wenninger, E., & Inouye, R. (2008). Insect community response to plant diversity and productivity in a sagebrush-steppe ecosystem. Journal of Arid Environments, 72, 24–33. https://doi.org/10.1016/j.jaridenv.2007.04.005
Yang, L. H., & Gratton, C. (2014). Insects as drivers of ecosystem processes. Current Opinion in Insect Science, 2, 26–32. https://doi.org/10.1016/j.cois.2014.06.004
Acknowledgements
The authors thank the United States Department of Agriculture for financial support through the USDA-NRCS Conservation Innovation Trials On-Farm Trials Agreement NR213A750013G034. We also thank Daphne Zapsas, Manish Gautam, Omar Montalvo Juan Raygoza, and Cruz Salinas for fieldwork and sample collection. And, the authors also thank the reviewers and editor for helpful comments on the manuscript.
Funding
United States Department of Agriculture for financial support through the USDA-NRCS Conservation Innovation Trials On-Farm Trials Agreement NR213A750013G034 to AR and RK.
Author information
Authors and Affiliations
Contributions
AF, RK, and AR designed the study. AF, SK, and AV collected and analysed the data. AF wrote the first draft and RK edited it. All authors contributed to revisions.
Corresponding author
Ethics declarations
Ethics approval
All authors have read, understood, and have complied as applicable with the statement on "Ethical responsibilities of Authors" as found in the Instructions for Authors and are aware that with minor exceptions, no changes can be made to authorship once the paper is submitted.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fajemisin, A., Kaur, S., Vasquez, A. et al. Can trap color affect arthropod community attraction in agroecosystems? A test using yellow vane and colorless traps. Environ Monit Assess 195, 366 (2023). https://doi.org/10.1007/s10661-023-10972-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-10972-w