Abstract
The global initiatives of monitoring and conserving pollinators require worldwide assessments with comparable data sets collected through standardized methods. The use of pan traps is a passive method widely applied to sample flower visitors, standing out for its simplicity. Despite its wide use to sample pollinator diversity, the influence of color on trap efficiency is not well understood. The available studies are particularly scarce in the tropics and have generated divergent results. The main goal of the present study was to assess whether blue, yellow and white pan traps are complementary to sample Hymenoptera community. For this, we placed 49 sample units of blue, white and yellow pan traps in agricultural and natural (savanna-like) areas in Chapada Diamantina, Bahia, Brazil. We found that the species richness from blue and yellow pan traps were not significantly different, but both were significantly greater than the species richness from white pan traps. However, bees were significantly more attracted to the blue pan traps and wasps to the yellow ones; thus, color attractiveness was group-specific. Pan traps of different color showed low species composition overlap with 61 % of species collected exclusively in one of the three pan trap colors, and the species composition in the blue traps differed consistently from that in the traps of the other colors. In the article we discuss the implication of the results and defend the combined use of pan traps with different colors as a solution for the differential variable sample bias.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, the decline of pollination services in both natural and agricultural environments has stimulated an interest in pollinator conservation, generating a demand for good-quality baseline information regarding the implementation and the monitoring of pollinator conservation initiatives (Harding et al. 2001; Nielsen et al. 2011; Garibaldi et al. 2013; Ekroos et al. 2014). The effectiveness of local environmental policies depends on a long-term evaluation of their impacts (Harding et al. 2001). Furthermore, global initiatives require worldwide assessments, with comparable standardized data sets (Lebuhn et al. 2013; Garibaldi et al. 2013). Combined, these demands can be not only very expensive and time consuming but also affected by the taxonomic group of interest and field conditions, such as vegetation type and characteristics, as well as by management conditions (Harding et al. 2001; Ekroos et al. 2014). These issues pose a real challenge for standardization that can only be overcome by good sampling methods that must be simple, replicable, and easily adopted in a broad variety of studies and, most importantly, their biases must be well known (Gotelli and Colwell 2001; Roulston et al. 2007).
In this context, passive methods can be extremely valuable tools because they are economical, could allow a high sampling effort and avoid the biases associated with collectors’ skills (Wilson et al. 2008; Westphal et al. 2008; Tuell and Isaacs 2009). One of the most common passive methods for collecting flower-visiting insects is the pan trap (Toler et al. 2005; Roulston et al. 2007; Westphal et al. 2008; Vrdoljak and Samways 2012). Pan traps are colored bowls containing water and detergent, the latter is used to break the water surface tension forcing the insects drop down to the bottom of the trap (Disney et al. 1982). The color of pan traps attracts diverse groups of flying insects not all of them are pollinators, but some of which could confuse the traps with natural flowers, causing their capture (Disney et al. 1982; LeBuhn et al. 2003; Popic et al. 2013). Pan traps are used in a wide variety of colors, most commonly yellow, blue and white, with and without ultraviolet reflection (Toler et al. 2005; Westphal et al. 2008; Vrdoljak and Samways 2012). This method is efficient in sampling species richness; however, some studies indicate that this method is not bias free (Disney et al. 1982; Droege et al. 2010; Baum and Wallen 2011; Heneberg and Bogusch 2014). In addition, the technique may be limited in capturing larger or more robust insects (Toler et al. 2005; Roulston et al. 2007; Wilson et al. 2008), and its efficiency in attracting specific species or groups differs with trap color (Disney et al. 1982; Chittka and Thomson 2001; Campbell and Hanula 2007; Vrdoljak and Samways 2012). There is evidence for cases of nearly complete avoidance of these traps by some insect species regardless being extremely abundant in their environment (Heneberg et al. 2014).
Despite the wide use of pan traps to sample Hymenoptera, the influence of color on trap efficiency is not well understood. Pan trap color combinations can influence the capture results, although the existing studies have reached divergent conclusions, with controversy in the literature regarding their implications (Toler et al. 2005; Campbell and Hanula 2007; Missa et al. 2008; Abrahamczyk et al. 2010; Vrdoljak and Samways 2012). There are few studies comparing color efficiency, most of which were conducted in temperate and subtropical zones, especially in North America, leaving an important gap in the tropics (Abrahamczyk et al. 2010; Gollan et al. 2011; Bashir et al. 2013; Gonçalves and Oliveira 2013; Saunders and Luck 2013; Heneberg and Bogusch 2014; Joshi et al. 2015). Therefore, it is necessary to investigate more diverse environments and regions around the world to adequately test and understand pan trap color efficiency (Gonçalves and Oliveira 2013).
Bees and wasps (Hymenoptera: Anthophila and several other Aculeata) are considered important pollinator groups for several wild and crop angiosperm plants (Michener 2007; Le Féon et al. 2010). The attraction of bees and wasps to certain colors is affected by visual stimulation and their inherent instincts (Leong et al. 1999; Chittka and Thomson 2001). Even though the preference for colors may vary between genera of the same family, species of the same genus, or even the sexes of the same species (Chittka and Thomson 2001; Weiss and Reice 2005; Heneberg and Bogusch 2014), such preferences can not only address physiological requirements but also be modified by prior experience and/or learning (Chittka and Thomson 2001; Gumbert and Kunze 2001).
To optimize the effort required to sample pollinators and save resources, studies using pan traps to assess a pollinator community should employ traps with complementary color combinations. This complementarity allows the sampling of a broader spectrum of flower-visiting species and, therefore, reduces the trap attraction bias. Knowledge regarding the color combinations more attractive to specific taxonomic groups is also a useful tool in the assessment of biodiversity. The potential applications for the traps are diverse, such as the study of pollinator behavior, the design of management and conservation strategies and the accumulation of evidence to better evaluate the decline of pollinators.
Thus, the main goal of this study was to investigate whether there is community complementarity among white, blue ultraviolet and yellow ultraviolet pan traps to sample Hymenoptera. We addressed four questions: (1) Which pan trap color attracts more bee and wasp species and individuals? (2) Is the use of different pan trap colors complementary to sample a Hymenoptera community? (3) Are there significant differences in the composition of bee and wasp communities sampled by different colors? (4) How do the sampling effort and the spatial distribution of sampling units affect pan trap efficiency?
Materials and methods
Study area
The study was performed between January 2011 and June 2012 in the agricultural area of Mucugê-Ibicoara (41°28′40″W and 13°09′10″S), which is adjacent to the Chapada Diamantina National Park and located in the central region of the State of Bahia, Brazil. This region primarily comprises a mosaic of intensive annual crops and a gradient of savanna formations ranging from grassy to woody savanna to semideciduous forest (Veloso et al. 1991). The climate is type Aw (Kottek et al. 2006), with a dry winter and rainy summer. The average annual temperature is 29 °C, with an average monthly rainfall of 1281 mm (INMET 2013).
Sampling units and experimental design
In an area of approximately 197,930 ha, we selected 49 sampling units to capture the environmental variation in this study region (Fig. 1). The goal of the selection was to randomize the effect of confounding variables, such as geographical distribution, vegetation type and landscape characteristics that could affect the performance of traps and the sampling design. To assess the effect of sampling design on trap performance, we adopted two alternative designs. The first, named the Proximal Approach (PA), contained ten sampling units located at least 200 m apart in the cover types semideciduous forest, grassy-woody savanna and agricultural crops. The second, called the Broad Approach (BA), consisted of forty sampling units that were separated by at least 3 km from one another along a structural gradient of native vegetation, which varied from grassy to woody savanna to forested savannah.
Location of the study area. (a) Map of South America highlighting the country of Brazil and Bahia State. (b) Map of Bahia State highlighting the Mucugê-Ibicoara “agricultural pole” and Chapada Diamantina National Park. (c) Delimitation of the agriculture pole and sampling units selected for the broad approach. (d) Sampling units for the proximal approach
The community of Hymenoptera was sampled with a passive collection method based on colored pan traps in shades attractive to the group, including blue—UVA (near ultraviolet), yellow—UVA and white—UVA (LeBuhn et al. 2003; Westphal et al. 2008; Vrdoljak and Samways 2012). The spectral properties of the pan traps such as reflectance and fluorescence were detailed in the support information (Online Resources 1 and 2). The traps were exposed in the field for a period of 24 h and installed 1 m above the ground using a cane support (Fig. 2a). At each sampling unit, the orientation of the pan traps was rotated to eliminate a systematic effect of color position. The arrangement of traps in the field and the sampling effort also varied between the two sampling designs: in the BA, the pan traps were placed in the field forming a triangle with sides of 15 m, and the traps of the three different colors were positioned equidistant from each other 5 m apart at the triangle vertices, for a total of nine pan traps (Fig. 2b). Each sampling unit was evaluated bimonthly in two blocks of twenty units, which were alternated throughout the year between the months of January to September 2011. In the PA, each sampling unity was composed by an 85 m-long transect with 15 pan traps positioned in groups of three (of different colors) to form five equilateral triangles with 5 m-long sides. The triangles were separated by 15 m (LeBuhn et al. 2003) (Fig. 2c). The collected insects were trapped monthly between April 2011 and June 2012. The total sampling hours were 43,200 h for the Proximal Approach and 33,696 h for the Broad Approach.
Pan trap installation in the field. a For the broad approach, each of three sets of three differently colored pan traps was positioned 15 m from the other sets at the vertices of a triangle with 15 m sides. b For the proximal approach, five sets of three differently colored traps arranged in triangles with 5 m sides were installed on 85 m transects oriented in a north–south direction. c Photograph showing a trap installed in the field. (Color figure online)
The collected hymenopteran specimens were identified by the expert zoologists Dr. Favízia Freitas de Oliveira and Me. Thiago Mahlmann Vitoriano Lopes Muniz to at least the genus level for the bees, according to Michener (2007), and to the family level for most of the wasps. Because of taxonomic difficulties in identifying all the insects, only the bees and wasps were considered for the analyses, and morphospecies designations were used. The specimens were deposited at the Natural History Museum/Zoology of the Federal University of Bahia State, Brazil (MHNBA/MZUFBA).
Statistical analysis
To avoid the necessity of compliance with the assumptions of normality and homoscedasticity, we used nonparametric analyses. All the statistical analyses described in this section were performed with the R statistical program, version 2.15.3 (R Development Core Team 2009). In order to deal with the Type I error inflation by serial non-independent statistical tests the Bonferroni’s correction was applied on the base significance level α = 0.05 (Underwood 1997).
Complementarity of pan trap colors
To investigate whether the three pan trap colors used in this study show complementarity, we compared the numbers of bee and wasp morphospecies and their total abundances among the insects found in the blue—UVA, yellow—UVA and white—UVA traps. For these comparisons, we used Friedman’s test for randomized blocks, followed by a post hoc, Wilcoxon, test for paired samples to identify the source of treatment differences. In these analyses, the colors were treated as sample units, and the blocks were the original 49 collection areas. Therefore, these analyses were performed with n = 147. Furthermore, to assess the differences in the composition of the capture among the blue—UVA, yellow—UVA and white—UVA traps, we performed an analysis of similarities (ANOSIM) using 1,000,000 permutations and the Bray-Curtis coefficient. To graphically evaluate the existence of groupings in relation to the colors of the traps, we used the same distance matrix to perform nonmetric multidimensional scaling (NMDS) for three dimensions with 1000 interactions. These analyses were performed separately using the data from the proximal (n = 30) and the broad (n = 117) approaches as well as those pooled from both approaches (n = 147). Two sampling units whose captures were composed solely of singletons were omitted from these analyses. Both analyses were performed with the package ‘vegan’.
To determine whether specific morphospecies, genera, tribes, and families are preferentially captured in traps of a certain color, we performed the Friedman test for a randomized block design on the abundance of the insect taxa captured by the traps of the three colors, followed by a post hoc, Wilcoxon, test for paired samples to identify the source of treatment differences. We used data pooled from the two design approaches, with the number of samples ranging between 15 and 120 per group. Each analysis included only those groups represented in at least five sample units and containing at least nine individuals.
The significance level with the Bonferroni correction for the Friedman’s and the corresponding post hoc, Wilcoxons’ tests was α’ = 0.017. Similarly, for the global and Pair to pair ANOSIM tests the significance level with the Bonferroni correction was also α’ = 0.017 (the results of the pair-to-pair tests are presented in the Online Resources 4).
Spatial distribution of the sampling units
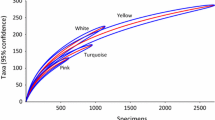
To evaluate the effect of sampling effort on sampling efficiency, we built species accumulation curves for the bee and wasp communities based on the individuals gathered using the three sampling designs (PA, BA and combined). To determine the sampling sufficiency of the three approaches for estimating the bee, wasp and combined communities, the richness estimator Jackknife 2 was calculated. The reason for choosing this richness estimator is that Jackknife 2 is one of the less susceptible to sample coverage and has high precision even at low sampling intensities in comparison to other estimators (Hortal et al. 2006). The ‘vegan’ packages was used for these analyses.
Results
Bee and wasp communities
We collected 2662 individuals of 301 flying hymenopteran morphospecies, with 2239 bee specimens. The bees were represented by 160 morphospecies. The most representative bee family was Apidae, with almost 56 % of the bee capture, followed by the families Halictidae with 38.8 % and Andrenidae with almost 5 %; Colletidae and Megachilidae together represented less than 0.5 %. The wasps were represented by 141 morphospecies belonging to 14 families; most of the individuals belonged to Vespidae (almost 32.9 %), followed by Crabronidae (23.6 %), Tiphiidae (15.6 %), Pompilidae (9.9 %), Scoliidae (4.4 %), Bethylidae and Ichneumonidae (3.5 % each) and Chalcididae (2.4 %). Other less representative families included Braconidae, Evaniidae, Sphecidae, Ampulicidae, Mutillidae and Trigonalidae (Table 1).
Complementarity of pan trap colors
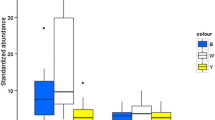
We found variation in the sampling efficiency of the differently colored pan traps. In general, the blue traps collected a greater abundance and richness of morphospecies, when compared with traps of the other colors (Table 2; Fig. 3a, b). At the genus and tribe levels, the collections in the blue traps also showed greater richness (Table 1). Bee abundance and richness were highest in blue traps, driving the pattern seen for total insect catches (Table 2; Fig. 3c, d). In contrast, wasp abundance and richness were highest in yellow traps (Table 1; Fig. 3e, f).
Box plot comparison of the number of morphospecies (a–c) and the abundance (d–f) of Hymenoptera found in collections from blue with ultraviolet, white and yellow with ultraviolet pan traps for bees and wasps combined (a, d), only bees (b, e) and only wasps (c, f). The colors are represented along the x axis by the respective initials of blue, white and yellow. (Color figure online)
Despite the differences in efficiency, the colors were complementary for sampling Hymenoptera. Approximately 62 % of the collected morphospecies were unique to one of the three colors, whereas only 19 % of the morphospecies were collected by all three colors (Fig. 4a). Considering the total community, the yellow and blue traps differed only slightly regarding the number of unique morphospecies captured, whereas the white traps collected the fewest unique morphospecies. Considering only bees, 48 % of the morphospecies were exclusive to one trap color, and the blue traps showed the highest number of exclusive morphospecies, approximately 28 % (Fig. 4b). For wasps, 77 % of the morphospecies were exclusive to one trap color, and 44 % of these morphospecies were collected exclusively in yellow traps (Fig. 4c).
Apparently, the wasp morphospecies showed greater color specificity, when compared with the bee morphospecies. However, the bees and wasps also differed regarding the number of individuals collected per morphospecies. On average, the bees showed approximately 14 (median = 3) individuals per morphospecies, whereas the wasps showed only 3 (median = 1). In addition, 66 % of the bee morphospecies were represented by more than one individual, whereas only 31 % of the wasp morphospecies had two or more representatives.
The non-color-exclusive morphospecies also showed frequency differences among the three collections from the differently colored pan traps. Considering the bees, 77 (48 %) morphospecies had a higher abundance in the blue traps (Fig. 4). However, of the 40 morphospecies tested, only 11 (58 %) showed a statistically significant preference for blue. At the bee genus and tribe levels, these results remained consistent, with only 6 of the 23 genera (26 %) and 5 of the 13 tribes (39 %) showing a statistically significant preference for blue. Wasps showed a general preference for yellow, 74 wasp morphospecies (52 %) had a higher abundance in the yellow traps (additional data is available in Online Resource 3).
The complementarity of the pan trap colors was also evident for the community composition, which varied significantly more between colors than within colors for all three sampling designs (Fig. 5). As indicated by the lack of overlap between the notches of the boxplots, the community composition in the blue pan traps were significantly differentiated from the other colors in all tested conditions (Fig. 5a–c). The yellow pan traps were significantly different from those of the other colors only in the broad approach (Fig. 5b). In addition, the white pan traps were found to be different only when using the proximal sampling approach (Fig. 5c). This pattern can also be visualized on the ordinations, where the blue dots are more grouped and separated from the yellow ones for the three sampling designs (Fig. 5d–f). Likewise, the pair-to-pair post hoc analysis of similarities confirmed the observed results of the main tests (see Online Resources 4 for more details).
Differences in community composition assessed using blue, white and yellow pan traps and two plotting methods: ANOSIM (a–c) and NMDS (d–f). Plots a and d, b and e, and c–f are from combined, broad and proximal sampling designs, respectively. The ANOSIM test is statistically significant when the distance between colors is greater than the distance within at least one color. The lack of overlap of the box plot notches indicate that the dissimilarity medians are significantly different at a 95 % confidence interval. (Color figure online)
Spatial distribution of the sampling units
The species accumulation curves did not differ among the three experimental designs. In addition, the results for the estimated morphospecies richness showed little variation among the sampling designs (Fig. 6). Furthermore, the broad approach performed slightly better than the proximal approach, collecting 52 % of the estimated morphospecies (Fig. 6). However, qualitative and quantitative differences occurred in the representativeness of the sampling designs for bees and wasps. The curves for bees exhibited an inflection point, whereas those for wasps remained almost linear (Fig. 6). In addition, the relationship between the estimated and observed morphospecies richness was better for bees than for wasps in the three sampling designs (Fig. 6). This evidence indicates that the bees were proportionally better represented than the wasps.
Species accumulation curves for bees and wasps gathered from Hymenoptera communities using the three sampling designs (broad, proximal and combined). The vertical lines represent standard deviations for the rarefaction procedure. The observed (OR) and estimated (Jackknife 2) species richness are shown inside each graphic
Discussion
Our results clearly show that the yellow—UVA, blue—UVA and white—UVA pan traps are complementary to each other for sampling bee and wasp communities. Based on these results in comparison with previous studies, we suggest that a combination of pan traps with different colors can reduce the interference by environmental conditions in the pan trap sampling results. Below we explain the logic behind this claim and how it can be especially important for studies with a wide geographical distribution, as well as for long term studies. We also found a strong group-specific bias, with the bees preferentially attracted by the blue traps and the wasps mostly preferring the yellow ones. This differentiation was true for richness, total abundance, the number of species attracted exclusively to each color and the absolute frequency of each species, with few exceptions. However, differences in taxonomic group preferences found between studies may reveal an even more important bias.
There is no consensus in the literature regarding the colors preferred by each group. Many studies reported no significant differences in color attractiveness (Toler et al. 2005; Wilson et al. 2008), whereas those that reported differences disagreed on the color preference (yellow (Abrahamczyk et al. 2010; Gollan et al. 2011), yellow and white (Vrdoljak and Samways 2012; Heneberg and Bogusch 2014), blue (Stephen and Rao 2007), blue and white (Campbell and Hanula 2007) and white (Romey et al. 2007)). Some authors argue that the preferences for traps of certain colors are innate characteristics (Heneberg and Bogusch 2014). However, behavioral studies indicate that the preferences of pollinators for certain colors are subject to learning mechanisms and the experience of organisms (Chittka and Thomson 2001; Gumbert and Kunze 2001). An evaluation of innate preferences is possible only with inexperienced, newly hatched individuals in a controlled environment (Lunau and Maier 1995). Under these conditions, the foraging theory states that the memory of Hymenoptera ensures that individuals prefer resources that offer a higher reward-to-cost ratio (Chittka and Thomson 2001; Reser et al. 2012). Therefore, innate preference is unlikely to be observed in uncontrolled field studies.
Accordingly, preferences for pan trap colors probably are highly influenced by the local floral phenology and/or the periodicity of agricultural crops. Another consequence that follows from this proposition is that the differences found between groups of potential flower visitors in their color preferences may reflect a niche differentiation present in the region. Such niche differentiation between pollinator groups is plausible given the common modularity in plant-pollinator networks (Olesen et al. 2007; Bascompte and Jordano 2007; Bastolla et al. 2009). Thus, bees that visit mostly blue flowers should be preferentially collected in blue pan traps, and bees whose favorite flowers are yellow should be collected in greater numbers by yellow traps. For example, Saunders and Luck (2013) found that white traps placed in almond orchards (with white flowers) captured more Hymenoptera than the white traps in native vegetation habitats.
Another source of bias is the visual perceptibility of the pan traps in different environments. Behavioral studies indicate that the contrast between color signals and the background, along with the purity of the visual signal, determine the color detection capacity of Hymenoptera (Chittka and Thomson 2001). Accordingly, specific vegetation types can favor the detection of colored pan traps by Hymenoptera (Saunders and Luck 2013). Abrahamczyk et al. (2010) found that the effectiveness of yellow and blue traps is affected by forest cover; yellow pan traps performed better inside forests, perhaps because yellow traps are more perceptible than blue ones in these places (with lower light intensity). Stephen and Rao ( 2007) used yellow and blue traps to sample in sunflower orchards and concluded that blue traps capture more bees, a fact that could be explained by the contrast between blue traps and the yellow background of sunflowers.
Together, the complex interaction between the contrast with background, innate and learned color preferences can help to explain why the preference for color varies so much between studies. Saunders and Luck (2013) proposed that the preferences for pan trap colors is habitat-specific. However, the evidence discussed here shows that the preferences for pan traps colors could vary along a year, between years, even in the same area as well as among areas (Joshi et al. 2015). This make the trap color preference context-dependent rather than habitat-dependent. In fact, it is that myriad of confounding factors resulting from complex interactions with the characteristics of the local vegetation that makes the combined use of different colored traps so relevant. The three colors, yellow—UVA, blue—UVA and white—UVA, cover the extremes of the wavelengths visible to Hymenoptera (Chittka and Menzel 1992; Chittka et al. 1994; Vorobyev 1999). Therefore, the three colors in conjunction would ensure that at least one of them would be compatible with the preference of flower visitors, buffering the effect of local conditions on monitoring Hymenoptera. Otherwise, sampling from even very similar communities in different conditions can generate completely different results. However, it is important to consider the tradeoffs between the use of three or more different colors that presented different efficiency (i.e. number of individuals and species) in a given condition in comparison to just triple the number of the most attractive color.
For example, suppose that 10 yellow traps attracted 100 individuals of 20 species, and 10 blue traps attracted 10 individuals of 8 species, of which 5 are present in both traps. In this scenario, if we use 20 traps of the most efficient colors, we may end up with 200 individuals of 20 species. Therefore, the use of two colors instead of one apparently would not be justified. Now suppose that in other study we aim to evaluate the effect of reforestation in the potential pollinator richness. For this, we sampled ten areas, five reforested and five non-reforested, with five blue pan traps in each area, and end up with two times more bees in the non-reforested areas in comparison to the reforested areas. The point here is that, given what was discussed above, we have no confidence that the difference between these two treatments is given by the difference in species richness between environments, or if it is given by a difference in bias of the blue pan traps between the two conditions analyzed. If two or three pan trap colors were used instead of one, we would lower the chances of compromising the sampling by a systematic effect of the sampling bias from the pan trap colors. Bear in mind that maximizing sampling is rarely the aim in ecological studies and even if this is the case one cannot be confident that the chosen color is the right one.
We also found evidence for an effect of sampling design on the complementarity of pan trap colors. The collections in the blue traps differed significantly from those in the other traps independently of the sampling design employed, but the differences encountered for the white and yellow traps depended on the sampling design. The specific effect of sampling design on the hymenopteran response to yellow and white traps may be associated with the behavior characteristics of the groups predominantly collected in traps of those colors. Generally, wasps present a gregarious distribution, which generates considerable heterogeneity in their sampling (Richter 2000; O’Neill 2001; Polidori et al. 2010). Therefore, the use of spatially grouped traps combined with widely distributed samples would generate results with high variability. This proposition is supported by the high variability observed in the wasp community composition in both the broad and proximal sampling approach. The white traps collected mainly morphospecies of solitary bees in the Apidae and Halictidae families, which are relatively small, possibly with low home range and were widely distributed in the studied region. In this case, a more spatially clustered sampling compared with a more dispersed can promote a better representation of these Hymenoptera.
However, our data indicated no differences among the sampling designs regarding the efficiency of the traps (observed richness/estimated richness). This result suggests that the efficiency of pan traps is less sensitive to the spatial distribution of samples (clustered or dispersed) than to the sampling effort (number of traps). One can make this conclusion because the major difference between the sampling designs was the spatial distribution of the pan traps, whereas the number of pan traps exposed per sample unit was almost the same. Moreover, the absence of an asymptote in the pan trap sampling indicates that this method was insufficient to exhaust the diversity of Hymenoptera in the tropical savanna (region studied), despite the considerable sampling effort that we expended. Such results are common in tropical environments independently of the sampling techniques and can be attributed to the relatively high species diversity in comparison to other regions. However, similar performances of pan traps are reported in the literature for other regions too (Heneberg and Bogusch 2014). Therefore, it is advisable for the survey of diversity to combine the use of pan traps with other collection devices, such as malaise, trap nests and hand-net (Bashir et al. 2013; Spafford and Lortie 2013). In addition, the results obtained from pan traps must be interpreted with caution when the interest is specifically pollinators. Not all insects collected with pan traps are potential pollinators (Popic et al. 2013). Therefore, to drive conclusions about pollination based on pan trap results it is essential to filter the results selecting only the groups that are known as floral visitors and potential pollinators.
Conclusion
The presented evidence clearly shows that given the large numbers of factors involved, it is very difficult to determine, a priori, the possible effectiveness of a single color of pan trap and their bias. Therefore, we recommend the combined use of three differently colored pan traps (blue—UVA with, yellow—UVA with white—UVA) in studies that aim to monitor and compare flower visitors on a global scale. However, for studies aiming to extensively survey potential pollinators and flower visitor diversity, a combination of pan traps with other collection methods, such as trap nests and entomological net, is recommended.
References
Abrahamczyk S, Steudel B, Kessler M (2010) Sampling Hymenoptera along a precipitation gradient in tropical forests: the effectiveness of different coloured pan traps. Entomol Exp Appl 137:262–268. doi:10.1111/j.1570-7458.2010.01063.x
Bascompte J, Jordano P (2007) Plant-animal mutualistic networks: the architecture of biodiversity. Annu Rev Ecol Evol Syst 38:567–593. doi:10.1146/annurev.ecolsys.38.091206.095818
Bashir MA, Saeed S, Sajjad A (2013) Monitoring hymenoptera and diptera pollinators in a sub-tropical forest of southern Punjab, Pakistan. Pakistan J Agric Sci 50:359–366
Bastolla U, Fortuna MA, Pascual-García A et al (2009) The architecture of mutualistic networks minimizes competition and increases biodiversity. Nature 458:1018–1020. doi:10.1038/nature07950
Baum KA, Wallen KE (2011) Potential bias in pan trapping as a function of floral abundance. J Kansas Entomol Soc 84:155–159. doi:10.2317/JKES100629.1
Campbell JW, Hanula JL (2007) Efficiency of Malaise traps and colored pan traps for collecting flower visiting insects from three forested ecosystems. J Insect Conserv 11:399–408. doi:10.1007/s10841-006-9055-4
Chittka L, Menzel R (1992) The evolutionary adaptation of flower colours and the insect pollinators’ colour vision. J Comp Physiol A 171:171–181. doi:10.1007/BF00188925
Chittka L, Thomson JD (2001) Cognitive ecology of pollination animal behavior and floral evolution. Cambridge University Press, Cambridge
Chittka L, Shmida A, N Troje, Menzel R (1994) Ultraviolet as a component of flower reflections, and the colour perception of Hymenoptera. Vision Res 34:1489–1508
Disney RHL, Erzinclioglu YZ, Henshaw DJC et al (1982) Collecting methods and the adequacy of attempted fauna surveys, with reference to the Diptera. F Stud 5:607–621
Droege S, Tepedino VJ, Lebuhn G et al (2010) Spatial patterns of bee captures in North American bowl trapping surveys. Insect Conserv Divers 3:15–23. doi:10.1111/j.1752-4598.2009.00074.x
Ekroos J, Olsson O, Rundlöf M et al (2014) Optimizing agri-environment schemes for biodiversity, ecosystem services or both? Biol Conserv 172:65–71. doi:10.1016/j.biocon.2014.02.013
Garibaldi LA, Steffan-Dewenter I, Winfree R et al (2013) Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science. doi:10.1126/science.1230200
Gollan JR, Ashcroft MB, Batley M (2011) Comparison of yellow and white pan traps in surveys of bee fauna in New South Wales, Australia (Hymenoptera: Apoidea: Anthophila). Aust J Entomol 50:174–178. doi:10.1111/j.1440-6055.2010.00797.x
Gonçalves RB, Oliveira PS (2013) Preliminary results of bowl trapping bees (Hymenoptera, Apoidea) in a southern Brazil forest fragment. J Insect Biodivers 1:1–9. doi:10.12976/jib/2013.1.2
Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4:379–391. doi:10.1046/j.1461-0248.2001.00230.x
Gumbert A, Kunze J (2001) Colour similarity to rewarding model plants affects pollination in a food deceptive orchid, Orchis boryi. Biol J Linn Soc 72:419–433. doi:10.1006/bijl.2000.0510
Harding EK, Crone EE, Elderd BD et al (2001) The scientific foundations of habitat conservation plans: a quantitative assessment. Conserv Biol 15:488–500. doi:10.1046/j.1523-1739.2001.015002488.x
Heneberg P, Bogusch P (2014) To enrich or not to enrich? Are there any benefits of using multiple colors of pan traps when sampling aculeate Hymenoptera? J Insect Conserv 18:1123–1136. doi:10.1007/s10841-014-9723-8
Heneberg P, Bogusch P, Astapenková A (2014) Reed galls serve as an underestimated but critically important resource for an assemblage of aculeate hymenopterans. Biol Conserv 172:146–154. doi:10.1016/j.biocon.2014.02.037
Hortal J, Borges PAV, Gaspar C (2006) Evaluating the performance of species richness estimators: sensitivity to sample grain size. J Anim Ecol 75:274–287. doi:10.1111/j.1365-2656.2006.01048.x
INMET (2013) Banco de Dados Meteorológicos para Ensino e Pesquisa. In: 29/04/2013. http://www.inmet.gov.br/portal/index.php?r=bdmep/bdmep. Accessed 2 Nov 2014
Joshi NK, Leslie T, Rajotte EG et al (2015) Comparative trapping efficiency to characterize bee abundance, diversity, and community composition in apple orchards. Ann Entomol Soc Am 108:785–799. doi:10.1093/aesa/sav057
Kottek M, Grieser J, Beck C et al (2006) World Map of the Köppen-Geiger climate classification updated. Meteorol Z 15:259–263. doi:10.1127/0941-2948/2006/0130
Le Féon V, Schermann-Legionnet A, Delettre Y et al (2010) Intensification of agriculture, landscape composition and wild bee communities: a large scale study in four European countries. Agric Ecosyst Environ 137:143–150. doi:10.1016/j.agee.2010.01.015
LeBuhn G, Griswold T, Minckley R, et al (2003) A standardized method for monitoring bee populations—the bee inventory (BI) Plot. http://online.sfsu.edu/beeplot/pdfs/BeePlot2003.pdf. Accessed 1 Jan 2015
Lebuhn G, Droege S, Connor EF et al (2013) Detecting insect pollinator declines on regional and global scales. Conserv Biol 27:113–120. doi:10.1111/j.1523-1739.2012.01962.x
Leong JM, Thorp RW, Entomology E (1999) Colour-coded sampling: the pan trap colour preferences of oligolectic and nonoligolectic bees associated with a vernal pool plant. Ecol Entomol 24:329–335. doi:10.1046/j.1365-2311.1999.00196.x
Lunau K, Maier EJ (1995) Innate colour preferences of flower visit. J Comp Physiol A 177:1–19
Michener CD (2007) The bees the world. The Johns Hopkins University Press, Baltimore
Missa O, Basset Y, Alonso A et al (2008) Monitoring arthropods in a tropical landscape: relative effects of sampling methods and habitat types on trap catches. J Insect Conserv 13:103–118. doi:10.1007/s10841-007-9130-5
Nielsen A, Steffan-Dewenter I, Westphal C et al (2011) Assessing bee species richness in two Mediterranean communities: importance of habitat type and sampling techniques. Ecol Res 26:969–983. doi:10.1007/s11284-011-0852-1
O’Neill KM (2001) Solitary wasps: behavior and natural history. Cornell University Press, Ithaca
Olesen JM, Bascompte J, Dupont YL, Jordano P (2007) The modularity of pollination networks. Proc Natl Acad Sci U S A 104:19891–19896. doi:10.1073/pnas.0706375104
Polidori C, Giordani I, Mendiola P et al (2010) Emergence and dispersal relative to natal nest in the digger wasp Stizus continuus (Hymenoptera: Crabronidae). C R Biol 333:255–264. doi:10.1016/j.crvi.2009.11.014
Popic TJ, Davila YC, Wardle GM (2013) Evaluation of common methods for sampling invertebrate pollinator assemblages: net sampling out-perform pan traps. PLoS one 8:e66665. doi:10.1371/journal.pone.0066665
Reser DH, Wijesekara Witharanage R, Rosa MGP, Dyer AG (2012) Honeybees (Apis mellifera) learn color discriminations via differential conditioning independent of long wavelength (green) photoreceptor modulation. PLoS one 7:e48577. doi:10.1371/journal.pone.0048577
Richter MR (2000) Social wasp (Hymenoptera: Vespidae) foraging behavior. Annu Rev Entomol 45:121–150. doi:10.1146/annurev.ento.45.1.121
Romey AWL, Ascher JS, Powell DA, Yanek M (2007) Impacts of logging on midsummer diversity of native bees (Apoidea) in a northern hardwood forest impacts of logging on midsummer diversity of native bees (Apoidea) in a northern hardwood forest. J Kansas Entomol Soc 80:327–338. doi:10.2317/0022-8567(2007)80[327:IOLOMD]2.0.CO;2
Roulston TH, Smith SA, Brewster AL (2007) A comparison of pan trap and intensive net sampling techniques for documenting a bee (Hymenoptera: Apiformes) fauna. J Kansas Entomol Soc 80:179–181. doi:10.2317/0022-8567(2007)80[179:ACOPTA]2.0.CO;2
Saunders ME, Luck GW (2013) Pan trap catches of pollinator insects vary with habitat. Aust J Entomol 52:106–113. doi:10.1111/aen.12008
Spafford RD, Lortie CJ (2013) Sweeping beauty: is grassland arthropod community composition effectively estimated by sweep netting? Ecol Evol 3:3347–3358. doi:10.1002/ece3.688
Stephen WP, Rao S (2007) Sampling native bees in proximity to a highly competitive food resource (Hymenoptera: Apiformes). J Kansas Entomol Soc 80:369–376. doi:10.2317/0022-8567(2007)80[369:SNBIPT]2.0.CO;2
Toler T, Evans EW, Tepedino VJ (2005) Pan-trapping for bees (Hymenoptera: Apiformes) in Utah’s west desert: the importance of color diversity. Pan-Pac Entomol 81:103–113
Tuell JK, Isaacs R (2009) Elevated pan traps to monitor bees in flowering crop canopies. Entomol Exp Appl 131:93–98. doi:10.1111/j.1570-7458.2009.00826.x
Underwood AJ (1997) Experiments in ecology: their logical design and interpretation using analysis of variance. Cambridge University Press, Cambridge
Veloso HP, Lima ALRR, Lima JCA (1991) Classificação da Vegetação Brasileira, Adaptada a um Sistema Universal. Fundação Instituto Brasileiro de Geografia e Estatística, Rio de Janeiro
Vorobyev M (1999) Evolution of flower colors–a model against experiments reply to comments by Chittka. Naturwissenschaften 86:598–600
Vrdoljak SM, Samways MJ (2012) Optimising coloured pan traps to survey flower visiting insects. J Insect Conserv 16:345–354. doi:10.1007/s10841-011-9420-9
Weiss JM, Reice SR (2005) The aggregation of impacts: using species-specific effects to infer community-level disturbances. Ecol Appl 15:599–617. doi:10.1890/02-5100
Westphal C, Bommarco R, Carré G et al (2008) Measuring bee diversity in different european habitats and biogeographical regions. Ecol Monogr 78:653–671. doi:10.1890/07-1292.1
Wilson JS, Griswold T, Messinger OJ (2008) Sampling bee communities (Hymenoptera: Apiformes) in a desert landscape: are pan traps sufficient? J Kansas Entomol Soc 81:288–300. doi:10.2317/JKES-802.06.1
Acknowledgments
We thank the Higher Education Personnel Improvement Coordination (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, CAPES), National Council for Scientific and Technological Development, CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq), the Global Environmental Facility (GEF), the Food and Agriculture Organization of the United Nations (FAO), the United Nations Environmental Programme (UNEP) and the Brazilian Biodiversity Fund (Fundo Brasileiro para a Biodiversidade, FUNBIO) for the financial support. In addition we thank the team of the Laboratory of Biology and Ecology of Bees (Laboratório de Biologia e Ecologia de Abelhas LABEA), for their help during the fieldwork. We also thank the POLINFRUT network for their support and rich discussions. We gratefully acknowledge the essential taxonomic support given by Thiago Mahlmann, who assisted in bee identification.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Moreira, E.F., Santos, R.L.d., Penna, U.L. et al. Are pan traps colors complementary to sample community of potential pollinator insects?. J Insect Conserv 20, 583–596 (2016). https://doi.org/10.1007/s10841-016-9890-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-016-9890-x