Abstract
Beneficial plant–microbe interactions in the rhizosphere have been found to enhance plant growth and development. Arbuscular mycorrhizal fungi (AMF), a major group among these microbes, have been found to improve plant fitness through mycorrhizal symbiosis. Despite being well documented in various natural and domesticated study systems, few studies have examined whether AMF also has cascading effects on other traits, such as influencing insect community dynamics through attraction/repulsion of beneficial and harmful insects. To test this, we planted Sorghum-sudangrass (Sorghum x drummondii), a fast-growing annual grain/forage crop, either inoculated with commercial AMF mix or left as control in lab and field experiments. We hypothesized that AMF would enhance plant growth and influence the recruitment of insect herbivores and their natural enemies due to possible alterations in plant defense pathways. Our results suggest that while AMF-inoculated plants had significantly better germination, growth, and establishment; they also experienced a lower initial incidence of Spodoptera frugiperda, a major herbivore on Sorghum in the Lower Rio Grande Valley. In addition, our insect community trapping experiment revealed that AMF-inoculated plants attracted significantly more beneficial insects (predators and parasitoids) and a lower number of damaging herbivores. Taken together, our field and lab data show that AMF can not only positively influence plant growth traits but can also provide defenses against herbivores by selectively attracting beneficial insects and repelling herbivores, with implications for sustainable pest management strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soil microorganisms are a critical component of the rhizosphere. Associations of beneficial microbes such as arbuscular mycorrhizal fungi (AMF) and plants date back millions of years (Reynolds et al. 2003). AMF are highly cosmopolitan and associate with nearly 80% of terrestrial autotrophs (Smith and Read 2008; Fontana et al. 2009; Vannette and Hunter 2009). Mutualistic associations of AMF with their host plants have been found to influence plant growth and fitness by the exchange of resources (Goverde et al. 2000; Smith and Read 2008; Fontana et al. 2009; Vannette and Hunter 2009; Kempel et al. 2010; Smith et al. 2011), which has been associated with increased yield (Anderson 1988) in various crops like maize (Zea mays), potato (Solanum tuberosum), yam (Dioscorea alata; Begum et al. 2019; Posta and Duc 2019) cowpea (Vigna unguiculata), flax (Linum usitatissimum; Posta and Duc 2019), and pepper (Capsicum annuum; Kaya et al. 2009). Moreover, they improve host plants’ tolerance to abiotic stresses like drought, salinity, and heavy metals, and can modify plant defenses (Bennett et al. 2006; Fiorilli et al. 2009; Kempel et al. 2010; Jung et al. 2012). Few studies have also documented that AMF can modulate plant interactions with herbivores (Kempel et al. 2010), their natural enemies and pollinators (Pineda et al. 2010; Willis et al. 2013). Therefore, it is possible that AMF can have cascading effects on plant–insect interactions (Gehring and Bennett 2009; Khaitov et al. 2015), an area of research that warrants more attention.
As the major biotic stress, herbivorous insects either damage plant tissues and/or act as vectors for pathogens. While plants inoculated with mycorrhizae can defend better against root herbivores (Gange 2007; Gehring and Bennett 2009), few studies also suggest that they usually harbor higher proportions of sucking insects (e.g., aphids) and lower proportions of chewing insects (Gange et al. 2002). Previous findings also report that generalist herbivores may perform poorly on the mycorrhizae-inoculated plants (Rabin and Pacovsky 1985; Gange and West 1994; Kempel et al. 2010), while the specialists can overcome such changes, and even gain from the association (Gehring and Whitham 2002; Gehring and Bennett 2009; Kempel et al. 2010). Fall armyworm (Spodoptera frugiperda; FAW) (J.E. Smith) (Lepidoptera: Noctuidae) is one such leaf-chewing generalist herbivore that can potentially be impacted by mutualism between AMF and the host plants (Mukherjee 2017). FAW is a polyphagous insect pest (Chapman 1999; Lange et al. 2018) that mainly feeds on grasses (Gramineae) and is an active forager (Buntin 1986), distributed worldwide, and has been considered as one of the most destructive crop pests (Degen et al. 2012; Padhee and Prasanna 2019).
AMF possibly alters the host plant defense chemistry by changing its nutritional status (Gange and West 1994; West 1995; Gosling et al. 2006), thereby mediating a wide range of species interactions. Consequently, AMF has been speculated to play a key role in shaping the organization and composition of ecological communities (van der Putten 2007, 2009; Hartley and Gange 2009). As one of the key mediators of insect–plant interactions, plants emit a range of constitutive volatile compounds that either repel or attract herbivores, pollinators, and predators/parasitoids (Moraes et al. 1998; Chen et al. 2019). However, under herbivory they emit herbivore-induced plant volatiles (HIPVs) (Pare and Tumlinson 1999; Kariyat et al. 2012; Ye et al. 2018) that can vary in quality and quantity from constitutive volatiles (Rowan 2011). Consequently, HIPVs have been found to act as signals to herbivores indicating that the host plant is already infested and is less suitable for feeding (Kariyat et al. 2014). More importantly, these volatiles can also increase the recruitment of natural enemies that either parasitize or predate the herbivores (Pare and Tumlinson 1999; Dicke and Baldwin 2010), altering the tri-trophic interactions—a sustainable pest management approach that has gained momentum recently (Hill et al. 2018). Due to the ability of AMF to modify plant chemistry (Laird and Addicott 2007; Pozo and Azcón-Aguilar 2007; Hill et al. 2018), it is possible to expect that AMF can also alter multi-trophic interactions through plant–herbivore–natural enemy community dynamics.
Sorghum-sudangrass (Sorghum x drummondii) is a forage species from Sudan and southern Egypt that is well adapted to dry and hot climates. It is a common cover crop grown in the summer season in various agricultural ecosystems worldwide (Hariprasanna and Patil 2015; Venkateswaran et al. 2019), including the United States. The species can act as a natural weed suppressant due to its dense canopy (Soti and Racelis 2020). However, the plant along with its congener Sorghum bicolor is also a host to a wide range of insect herbivores (Kariyat et al. 2019). Interestingly, there is limited understanding on the dynamics of insect community associated with this species, and more importantly, whether mycorrhizal association can potentially alter these interactions. Since the few studies on AMF-insect–plant interactions have reported varied results with different study systems (Johnson et al. 1997; Reynolds et al. 2005; Fontana et al. 2009), using Sorghum-sudangrass as our host plant species, we examined whether the commercial AMF has cascading effects on plant growth and development, herbivory, and insect community dynamics, in an organic cropping system in Lower Rio Grande Valley in south Texas. We employed a combination of field and lab experiments to answer these questions. We hypothesized that AMF-inoculated plants will have better growth traits than the non-inoculated control plants, herbivore will be lower on the AMF-inoculated plants as compared to the control and that AMF-inoculated plants will attract more beneficial insects and lower number of herbivores.
Materials and methods
The field experiment was conducted at a 21-acre organic farm owned and operated by PPC farms in Mission, Texas, United States, 78,572 (26.168425, − 98.313547). The field was sown with seeds of two treatments—Sorghum-sudangrass seeds (Super sugar sudex variety, Green Cover Seed company, USA) inoculated with AMF (Wildroot® Organic Mighty Mycorrhizal Concentrate USA), and seeds without AMF inoculation (control), separated in the middle by a fallow land area, such that area under cover for each treatment is 4.5, 4.5, and 2 acres, respectively. Details of mycorrhizal species included in the commercial mix are included in the supplementary file (Data S1). Each seed lot of 22.67 kg was inoculated with 200 g of AMF (as recommended by the manufacturer) along with 60 ml water to make the powdered AMF formulation adhere to the seeds. The seeds were sown on ridges maintaining the seed rate of 22.67 kg/acre, during early summer of 2018. AMF-inoculated seeds, as well as control seeds, were each sown on 106 ridges separately (9.5 acres per treatment), separated in the middle by 22 ridges (2 acres) of fallow; however, the experiments and observations were based on 4.5, 4.5, and 2 acres for seeds with AMF inoculation, without inoculation, and fallow land area, respectively. Seeds were covered with 0.5 cm of soil after broadcasting and the field was flood irrigated immediately after sowing.
Plant growth traits (field)
Various growth traits were recorded at early season (30–40 Days After Planting-DAP), mid-season (45–55 DAP), and late season (60 DAP and after), as described below:
Plant height
We recorded the height of 60 plants per treatment using the measuring tape from the base of the plant to the tip of the youngest leaf, during the mid-season. For this, we selected six rows randomly from each treatment, out of which 10 plants per row were further randomly selected. Additional height measurements were taken in the late season and recorded from another 100 plants per treatment using the same method.
Number of fully opened leaves
Total number of fully opened leaves per plant were recorded from 60 randomly selected plants per treatment (irrespective of any damage) during the mid-season. The same was recorded from 100 random plants per treatment twice during late season, using a 1 m2 quadrat made from PVC (Polyvinyl Chloride, Lowes Inc, Edinburg, Texas) pipes. The quadrat was randomly thrown 10 times into different directions per treatment. For each throw, data were recorded from 10 plants randomly selected from within the quadrat.
Plant density
During the mid-season, the quadrat was thrown five times and eight times in the different directions within the AMF-inoculated and control plots, respectively. The total number of plants contained in the quadrat was recorded. For the second density measurement, we doubled the sample size to 10 throws per treatment, and the number of plants in the quadrat was recorded during the late season. A third set of data was recorded again in late season.
Plant girth
To continue measuring the growth traits of the plant, girth of the plants from each treatment was recorded at the base of the plant. Data were recorded twice during the late season. The quadrat was thrown 10 times in the different directions within each treatment. Girth of 10 randomly selected plants contained in the quadrat was recorded using a digital Vernier caliper (Gyros® DIGI-SCIENCE™). It was calibrated before recording measurement from each plant. Similar data were also recorded for any throw even with less than 10 plants inside the quadrat.
Plant defense traits
Number of leaves damaged
The field was surveyed for the number of insect-damaged leaves, more specifically, for the damage caused by the FAW. For this, 60 plants were randomly selected per treatment and carefully observed for any foliar damage done by FAW larvae such as ragged feeding on the foliage and the presence of small holes (Fig. S1) during the early season. Similarly, observations were recorded from 100 randomly selected plants per treatment during the late season.
Presence of fall armyworm
In addition to damage assessment, the treatments were also observed for the presence of FAW using two characteristic features—the actual presence of the FAW and/or the presence of caterpillar frass on the leaf whorls. During the early season, we examined 100 plants randomly selected per treatment and recorded the number of plants having the presence of fall armyworms or its frass, or both. During the late season, the quadrat was randomly thrown 10 and 8 times within the AMF-inoculated and control plots, respectively. The total number of plants contained in the quadrat was also recorded for signs of caterpillar incidence. The parameter was recorded again during the late season, again observing 100 randomly selected plants per treatment.
Insect community
To examine the insect community diversity associated with AMF-inoculated and non-inoculated Sorghum-sudangrass, a trapping method comprising three types of traps was employed (Figs. S2, S3). During the early season, six cages were set up diagonally (3–4 rows apart) in both treatments and fallow (n = 18), covering an area of ~ 75 m × 20 m in each plot. To build the cage, hardwire material (0.635 cm mesh size, 0.61 × 3.05 m—Lowe’s, Blue Hawk, catalog number: 492388, model: 840147) was folded into a cylindrical shape (90 cm tall × 76 cm diameter) and fastened with the zip ties. The top of the cage was fitted with the aluminum pie pan (22.2 cm dia. × 2.9 cm) fastened with two zip ties (28 cm) at the diametrically opposite ends. For sticky traps, white colored bridal veil nets (25 cm × 30 cm) (Hobby Lobby, catalog number: 852640) covered with odorless tangle foot sticky glue (Tangle-Trap® Sticky Coating, catalog number: 300000676, Part No. LB8249) were placed uniformly opposite to each other on the cage, in the field. The sheets were secured with rubber bands placed at the top and bottom of the sheets, around the cage. For pitfall traps, two 266 ml clear plastic cups (Solo, Walmart, 554949033) were placed diametrically opposite to each other at the base of each cage in two holes dug (same size as the cup) around it, such that holes are situated on the ridges. The pitfall traps and aluminium pie pan traps were filled with water and Micro-90 odorless detergent (Cole-Parmer, catalog number:SK-18100-05) to trap insects. For details of the cage design, please see Kariyat et al. (2018), and the supplementary files (Figs. S2, S3). The following day, pie pan traps and pitfall traps were re-filled with soap water to replenish the water lost to evaporation. On the third day, the traps were removed and collected from the field (Kariyat et al. 2012, 2018). Each bridal veil was carefully removed and placed between two labeled sheets—an A4 size white sheet at the base and a clear acetate sheet at the top. Based on phylogeny and feeding guild, the insects trapped were identified in orders (and families when possible) (Kariyat et al. 2012) including predatory wasps and parasitoids (Hymenoptera), generalist and specialist herbivorous beetles (Coleoptera), caterpillars and adult moths and butterflies (Lepidoptera), true herbivorous bugs (Hemiptera) and flies (Diptera) (Kariyat et al. 2012). The experiment was repeated later in the season following the same procedure.
Seedling germination and establishment (lab)
In addition to the field experiments, we performed a series of controlled lab experiments to examine the germination and seedling establishment rates at different AMF concentrations: 0.1032 g/500 seeds (recommended rate), two times the recommended rate (0.206 g/500 seeds), half the recommended rate (0.052 g/500 seeds), and a control (no AMF). Mean weight for 500 seeds (weighing balance-Accuris instruments, Bloodbankdepot®) was estimated (11.714 g) to calculate the AMF required for each treatment. For each treatment, seeds were placed in separate vials containing a slurry made of required amount of AMF and 500 μL of di-water. Control seeds were inoculated with di-water without AMF. Each vial containing inoculated seeds was vortexed for 30 s for uniform inoculation. Seeds were sown in trays (51.435 cm × 25.4 cm) containing sterilized potting mixture (Berger- custom blend, Graco Fertilizer Company, Georgia, USA) and placed in an incubator (Sheldon Manufacturing, INC.) at a 25 °C temperature and 16 h day/8 h night cycle. To ensure that each tray received an equal amount of light, they were rotated daily within the incubator. Number of emerged seedlings was recorded for three consecutive days, since the first day of emergence and final readings were recorded at 11th day after sowing. In addition, the length of seedlings (cm) was recorded at 10 days after emergence.
Dry biomass measurements (field and lab)
To compare the shoots and roots biomass between both treatments, 30 plants per treatment from the field were uprooted along their roots near the time of crop termination. After separating the aerial parts and roots of each plant sample, the roots were washed to remove any attached soil from the field. Following this, the samples were allowed to dry in an oven (Quincy lab.INC, Fisher Scientific, USA) at 70 °C for 2 days and weighed for dry biomass. Similar procedure was followed for the laboratory raised seedlings to analyze the difference between treatments for dry biomass, number of seedlings germinated and successfully established.
Root staining and microscopy
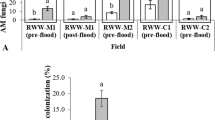
A modified light microscopy-based staining method (Mcgonigle et al. 1990) was followed to detect the colonization of plant roots by AMF. Five fine root fragments (1.5 cm) were collected from five random plants per treatment, uprooted from the field. The root cuttings were immersed in 10% KOH solution for 3–4 days to remove tannins in the roots and then gently rinsed with di-water twice. This was followed with immersing the roots in alkaline H2O2 bleach for 30 min and then rinsing with di-water twice. Next, the cuttings were drenched in 1% HCl for 30 min and gently rinsed with di-water. The processed roots were then stained overnight with a mixture of Trypan blue ink and acidified glycerol and later rinsed with di-water to clear the excess ink off the roots. To perform light microscopy, the stained root cuttings were mounted over slides and examined carefully for any vesicles, arbuscules or hyphal threads, under × 100 to × 400 magnification (Olympus BX53 upright microscope; Olympus camera adaptor U-TV1XC, C- mount; Software: LC micro 2.2, Olympus Soft Imaging Solutions, USA) (Fig. 1).
Statistical analysis
All analyses were performed using the statistical software JMP (Statistical Analysis Software Institute, NC, USA). The data for height parameter were analyzed with Mann–Whitney U test (non-parametric test) as it did not satisfy normality assumptions despite transformations. Two-tailed t tests were used to compare the mean number of fully opened leaves in AMF-inoculated and non-inoculated treatments. Plant density was also analyzed using Mann–Whitney U test because the data did not meet normality assumptions. Two-tailed t tests were used to test compare the plant girth measurements between both treatments. Data for FAW-damaged leaves in the mid-season were analyzed with Mann–Whitney U test (non-normal data), while two-tailed t tests were used for late season observations. For insect trapping experiments, pooled data from both collections were analyzed using univariate analyses. The counts for the orders Coleoptera and Diptera satisfied normality assumptions, so were analyzed with One-Way ANOVA, while Hemiptera and Hymenoptera counts were analyzed with non-parametric Kruskal Wallis tests to compare insect diversity among both treatments and the fallow (Table 1). To reconfirm our univariate analyses (count data), we also ran a multivariate linear discriminant analysis to identify the separation of the main insect orders and the treatments from the insect diversity data. In our first analysis, we compared AMF and non-AMF treatments over the four insect orders of interest (Hymenoptera, Coleoptera, Diptera, and Hemiptera). A canonical plot was built with biplot axes using variables from the linear combination of covariates from the treatment groups and insect orders. We followed this by adding the fallow treatment and built additional canonical plot and used Wilks’ lambda to test the significance between the treatment groups. The logistical constraints of working in a farmer’s field affected our ability to do replicated field trials, so detailed confirmation assays were carried out in lab. Data on seedling germination and establishment in the lab studies were analyzed using χ2 tests for each pairwise comparisons among control seedlings without AMF and the three groups of seedlings with different AMF inoculations. χ2 test was also used to examine if the data for FAW incidence/ presence at various stages are independent in both the treatments and whether there is significant difference in the presence of FAW between both the treatments. Lab studies data including shoot and root length and dry biomass were all normally distributed and were analyzed with One-Way ANOVA and, field dry biomass data analyses were performed using two-tailed t test. All the ANOVA analyses that had three treatment groups (AMF, non-AMF or control and fallow) were also subjected to appropriate post hoc tests to examine the significance of all pairwise combinations using Tukey or Dunn’s tests for parametric and non-parametric tests, respectively. More details of the statistics are provided in Table 2.
Results
Growth traits
The results from the field experiment revealed that AMF-inoculated plants were significantly taller (29.6%) than non-inoculated plants (Mann–Whitney U test; U = 1343, P = 0.0160) (Fig. 2a, Table 1). No significant difference was found for the number of fully opened leaves between the treatments (Two-tailed t test; P = 0.3505) during the mid-season of plant growth (Fig. 2b, Table 1). However, plants inoculated with AMF also produced significantly more leaves (Two-tailed t tests; P < 0.0001; P = 0.0002) than the control plants in the mid-season (Fig. 2c), and towards the late season (Fig. 2d). Control plants were significantly denser Mann–Whitney U test; U = 55, P < 0.0001) than plants inoculated with AMF (Fig. 2e, Table 1); while AMF-inoculated plants had significantly higher girth by 73.65% and 54% (Fig. 2f, g, Table 1) than the control during the final two development stages (Two-tailed t tests; P < 0.0001).
Results of growth traits comparisons between control and arbuscular mycorrhizal fungi (AMF)-inoculated Sorghum-sudangrass (Sorghum x drummondii) in field. a Mean height (cm); mean number of fully opened leaves at (b) mid-season (45 DAP), c at 50 DAP, d late season (60 DAP); e mean plant density/m2; mean girth (cm) at f late season (60 DAP) and g late season (70 DAP) are reported. Mean and standard error of the results of Mann–Whitney U test of the mean height between plants (y-axis) (in cm), two-tailed t tests to examine the number of opened leaves between plants (y-axes) (in cm), Mann–Whitney U tests of mean density of plants (in plants per m2) (y-axis), two-tailed t tests data analysis of measurement for girth of plants (in cm) (y-axis) in control and AMF treatment (x-axis) are represented. Statistically significant differences are represented by different lowercase alphabetical letters at P < 0.05, while ns denotes non-significant results
Defense traits
Damage assessment in field showed that AMF-inoculated plants suffered lower damage by FAW larvae than control plants, during the mid-season of the crop (Mann–Whitney U test, U = 1616, P = 0.0045; Fig. 3a, Table1). However, there was no significant difference for the number of FAW-damaged leaves between two treatments later in the season (Two-tailed t test; P = 0.8749; Fig. 3a, Table 1). Consistent with this, our early-season and late-season herbivore observations in field also showed that plants inoculated with AMF had lower incidence of FAW (χ2 = 4.261, P = 0.0390; χ2 = 11.30, P = 0.0008). However, the third set of data recorded during the late season, shows no significant difference (χ2 = 2.079, P = 0.1493) for the presence of FAW between both treatments (Fig. 3b, Table 1). Our insect community trapping data also show interesting trends. The data collected at 40 DAP and 60 DAP were examined and grouped by the major insect orders into damaging herbivores or beneficial insects. In total, we collected ~ 6400 insects. Our results showed a notable impact of AMF on the insect community composition. No significant results were found for affinity of Coleoptera insects (One-Way ANOVA; P = 0.7520; Fig. 3c, Table 1) to either AMF inoculated or control; however, we found specialist herbivorous beetles such as the Diabrotica spp. and Epitrix spp. (family: Chrysomelidae), and some generalist detrivorous beetles (families: Carabidae and Staphylinidae) that are particularly not harmful for the crops, in the traps. Interestingly, Hemipteran insects displayed a lower affinity to the AMF-inoculated plants relative to the control (Kruskal–Wallis test; P = 0.0034; Fig. 3d, Table 1) that included herbivores such as leaf hoppers and shield bugs (families: Cicadellidae and Pentatomidae). However, we found both beneficial (families: Tachinidae and Syrphidae) and herbivorous Dipterans (families: Cecidomyiidae and Bradysia spp.) in significantly higher numbers on control plants, and fallow when compared to AMF-inoculated plants (One-Way ANOVA; P = 0.0335; Fig. 3e, Table 1). More interestingly, Hymenoptera were found in significantly higher numbers (Kruskal–Wallis test; P = 0.0056; Fig. 3f, Table 1) on the plants incorporated with AMF. Winged beneficial Hymenopterans, largely comprising of the parasitoids in Braconidae, Ichneumonidae, predatory wasps, and ants, were largely driven towards the AMF inoculated Sorghum-sudangrass. Overall, AMF was positively associated with beneficial insects and negatively associated with damaging herbivores.
Results of defense traits comparisons among different treatments of Sorghum-sudangrass (Sorghum x drummondii). a Fall armyworm damage; b number of fall armyworm/frass observed; c–f mean number of Coleopteran, Hemipteran, Dipteran, and Hymenopteran insects, respectively. Mean and standard error of the results of Mann–Whitney U test and two-tailed t test for fall armyworm damage during mid and late season, respectively (y-axis), in control and AMF treatment (x-axis) are represented; One-Way ANOVA tests to examine mean number of coleopterans and dipterans (y-axis) and Kruskal–Wallis test to examine mean number of hemipteran and hymenopteran insect diversity (y-axis) in control, AMF-inoculated and fallow plot (x-axis) are represented. Statistically significant differences are represented by different lowercase alphabetical letters at P < 0.05, while ns denotes non-significant results
Our multivariate statistics with discriminant analyses also reinforced these results. The outer ellipse on the canonical plot (95% confidence level for each mean) clearly showed that the groups separated out without overlapping, showing significant differences and with distinct separation between the beneficial Hymenoptera clustered at the AMF when compared to other groups. Wilks’ lambda had a value of 0.408 and a P < 0.0001, showing significant treatment differences and robustness of the group separation in terms of a direct measure of the proportion of variance in the combination of dependent variables that is unaccounted for by the independent variable (Eigen value = 1.4487, F value = 7.23; Fig. 4a). For second replication, we built a similar plot and conducted analyses but with additional treatment of fallow (mostly infested by weedy grasses). Like the previous model, we found that the AMF treatment separated from the non-AMF and from the fallow treatments, while non-AMF and fallow overlapped their ellipses (Eigen value = 1.3655, F value = 11.22; Fig. 4b). In addition, Wilks’ lambda had a value of 0.389 and a P < 0.0001, clearly showing significant differences in treatments. Taken together, the analyses clearly show that AMF plants varied from both other treatments by attracting higher number of Hymenoptera insects and lower number of other insect groups.
Canonical plot depiction of insect community attraction. Canonical plots were constructed for a linear discriminant analysis for the attraction of four insect orders of interest (Hymenoptera, Coleoptera, Diptera, and Hemiptera) with two biplot axes that has the two canonical variables from the linear combination of covariates from the treatment groups and insect orders. The outer ellipse represents 95% confidence interval for each mean and the colored dots represent the treatments. The length and direction of each ray that represents the covariates in the biplot indicate the degree of association of the corresponding covariate with the first two canonical variables. a Represents the plot for AMF and non-AMF comparisons, and b represents the plot for AMF, non-AMF, and fallow treatments. Non-overlapping ellipses and canonical details calculated from the overall pooled within-group covariance matrix shows that the treatments differ from each other in their attractiveness towards the insect orders (Table 2)
Seedling germination and establishment in lab
To confirm the effect of AMF on Sorghum-sudangrass under sterilized soil conditions (without the presence of any native AMF in field), we conducted laboratory experiments at different concentrations of AMF inoculum (Fig. 5, Table 2). We found that seeds inoculated with twice the recommended rate of AMF germinated significantly more seedlings than control seeds (χ2 = 15.97, P < 0.0001). However, we did not find any significant difference between seeds inoculated at recommended rate and control seeds (χ2 = 0.0083, P = 0.9272). Moreover, we found significant difference comparing half the recommended rate against control seeds, where the control seeds germinated better (χ2 = 167.069, P < 0.0001). While comparing the germination effects among the different rates of AMF, we found that seeds inoculated at twice the recommended rate of AMF germinated significantly better than seeds inoculated at both the recommended rate and half the recommended rate of AMF (χ2 = 55.85, P < 0.0001 and χ2 = 26.29, P < 0.0001). However, we also found that seeds inoculated with half the recommended rate germinated better than seeds inoculated at the recommended rate of AMF (χ2 = 7.883, P = 0.0050) (Fig. 5a). Taken together, our results suggest that AMF in general significantly improved the germination rate of Sorghum sudangrass.
Results of separate pairwise comparisons following the χ2 tests of growth assays conducted in lab (χ2). a Seedling germination and b seedling establishment; at three AMF concentration levels and control. X-axis represents the number of seedlings. Different treatments have been represented by different colors in the graph. Significant differences are represented by different lowercase alphabetical letters at P < 0.05 while asterisks (*) denote the significance at P ≤ 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001
However, some of this effect was lost at establishment stage (Fig. 5b). Results from seedling establishment (3 weeks after seeding) suggest that seeds inoculated at recommended rate and half the recommended rate of AMF established significantly better than control (χ2 = 4.161, P = 0.0414; χ2 = 4.536, P = 0.0332). Moreover, the establishment rates were significantly higher for seeds inoculated with double the recommended rate against control (χ2 = 8.228, P = 0.0041) (Fig. 5, Table 2).
Field and lab biomass
For the samples collected from field, the plants inoculated with AMF had significantly higher shoot dry biomass (Two-tailed t tests; P = 0.0128; Fig. 6a, Table 1), while no significant difference was found for root dry biomass between the two treatments (Two-tailed t tests; P = 0.7684; Fig. 6b, Table 1). Nevertheless, the overall dry biomass was significantly higher for AMF-inoculated plants (Two-tailed t tests; P = 0.0245; Fig. 6c, Table 1). Lab experiments conducted under sterilized soil conditions, free of native mycorrhizae, suggest that inoculation with different concentrations of commercial AMF produce seedlings with significantly higher dry biomass (One-Way ANOVA, P = 0.0006; Fig. 6d–f). Tukey’s multiple comparisons test suggests that the seedlings with twice the recommended rate of AMF (double concentration) significantly gained more biomass than rest of the treatments (Tukey’s multiple comparisons, P = 0.0012; P = 0.0029). However, the difference between dry biomass of seedlings with AMF at recommended rate and control showed no significant difference (Tukey’s multiple comparisons, P = 0.9396; Fig. 6d–f). Also, seedlings with twice the recommended rate of AMF had higher shoot length than seedlings with recommended rate and control treatments (Tukey’s multiple comparisons, P = 0.0013; P = 0.0299). Shoot length among seedlings at the recommended rate of AMF, half the recommended rate of AMF and control treatments (Tukey’s multiple comparisons, P = 0.1159; P = 0.7358; P = 0.6520), and between half the recommended rate and double the recommended rate of AMF were not significantly different (Tukey’s multiple comparisons, P = 0.2578) (Fig. 6d). Similarly, we did not find any significant differences for root length between half the recommended rate and twice the recommended rate of AMF (Tukey’s multiple comparisons, P = 0.1454). Additionally, no significant differences were found in root length between the seedings with recommended rate and twice the recommended rate of AMF (Tukey’s multiple comparisons, P = 0.5508). However, seedlings with half the recommended rate had very significantly higher root length than the seedlings with recommended rate of AMF (Tukey’s multiple comparisons, P = 0.0023). Not surprisingly, roots of seedlings with half the recommended rate and twice the recommended rate of AMF were significantly longer than the roots of control seedlings (Tukey’s multiple comparisons, P < 0.0001; P = 0.0103) (Fig. 6e). Taken together, our data show the positive effect of AMF on total biomass of the plants from the field and seedlings from the lab, along with positive concentration-dependent effects on plant growth.
Results of plant biomass analysis from field collected samples and lab experiments. a Shoot dry biomass (g), b root dry biomass (g), and c total dry biomass of the plants (g) from field; d shoot length (cm), e root length (cm), and f total dry biomass of the plants (g) from the lab experiments. Mean and Standard error of the results of the two tailed t tests of plant dry biomass data collected from the field experiments (y-axis) and one-way ANOVA of plant dry biomass data collected from the lab experiments (y-axis) at three AMF concentration levels. X-axes and different colors represent different treatments comprising of different AMF concentration levels at which the seeds were inoculated. Significant differences are represented by lowercase alphabetical letters at P < 0.05, while ns denotes non-significant results
Light microscopy for arbuscules detection
The results for the light microscopy clearly showed arbuscules in the roots fragments of AMF colonized plants from the field, thereby, suggesting a successful colonization of Sorghum-sudangrass roots by AMF fungi. There were little to no arbuscules found in the roots of plants without AMF inoculation (Fig. 1).
Discussion
Our findings demonstrate that AMF can provide both overall growth as well as defense benefits to plants against herbivores. Improved growth traits in our study system can be attributed to the increased availability of nutrients by AMF to the host plants (Lynch 1990; Roesti et al. 2006; Smith and Read 2008). In our study, AMF-inoculated plants were significantly taller than control plants in resonance with various recent and past studies. For example, Murrell et al. (2019), showed that AMF colonization increases the growth in cover crops (Murrell et al. 2019). Similarly, Bi et al. (2018) recorded increased height in AMF-inoculated Amygdalus pedunculata, a native tree species used for ecological restoration, than the control plants (Bi et al. 2018). We also recorded significantly higher girth in AMF inoculated plants than the control plants (Fig. 2f, g), suggesting a positive impact of AMF on plant vigor (Siddiqui et al. 2008). Not surprisingly, AMF-inoculated plants grow vigorous over the course of the season (Zangerl and Rutledge 1996). Young seedlings have been found to invest their resources more towards height increment before diverting it to leaf production (Weiner 1994; Nagashima 1995; Nagashima and Hikosaka 2011), and in our experiments we see that the plants inoculated with AMF had a higher number of leaves than control only post the initial growth stage (Fig. 2c, d).
In a recent study (Murrell et al. 2019), AMF-inoculated plants were found to invest more in growth and defenses simultaneously. Similarly, in our study, the number of insect-damaged leaves during the mid-season and FAW incidence until the mid-season were significantly lower in the AMF-inoculated plants than control (Fig. 3a, b). AMF possibly alters the chemical composition of plants by changing their nutrient pool (Weiner 1994). Therefore, healthy and nutrient-rich (Zangerl et al. 2007; Mithöfer et al. 2018; Formenti and Rasmann 2019) AMF-inoculated plants allocated more resources to defend against herbivores, in this case against FAW. We speculate that AMF-inoculated plants either produced defense chemicals to hinder the FAW feeding/development or activated signaling molecules that mediate defense pathways in the species. Additionally, the negative impact of mycorrhizal-plant association on herbivores through altered carbon:nitrogen ratio has been reported previously (Bryant et al. 1983). They speculated AMF-inoculated plants invest higher in carbon-based secondary metabolites, thereby discouraging herbivores (Bryant et al. 1983; Kempel et al. 2010). A recent study confirmed the activation of jasmonic acid signaling pathway in various AMF-inoculated cover crops under the attack of European corn borer (Ostrinia nubilalis), a polyphagous lepidopteran pest (Murrell et al. 2019), with similar feeding habit as FAW. However, we also found that FAW was able to establish itself and colonized the entire field towards the later crop growth stages, including AMF plants (Fig. 3a, b). It is possible that AMF helps the crop protect itself against herbivores during the initial establishment stages, thereby allowing the host to allocate more resources for fitness (Formenti and Rasmann 2019) and once established on the non-inoculated plants, FAW eventually damage the AMF plants as well as in the late season.
More interestingly, our insect community trapping experiment recorded twice at 40 and ~ 60 DAP showed that AMF inoculated plants attracted lower number of harmful Hemipteran and Dipteran herbivores (Fig. 3d, e) but significantly more natural enemies of Hymenoptera (parasitoids and predators) (Kariyat et al. 2012) than control plants (Fig. 3f). Therefore, AMF indirectly defends the host plants against herbivores by recruiting more natural enemies, through tri-trophic interactions (Hempel et al. 2009). In fact, a study has documented increased number of hymenopteran insects visiting the mycorrhizae-inoculated plants than the control plants (Gange and Smith 2005). However, we found no difference among the number of coleopterans trapped in the treatments including both generalist (predatory and detrivorous) and specialist (herbivorous) beetles. We speculate that constitutive and/or induced volatile compounds produced by the plants from each treatment regulated the movement of both herbivores and beneficial arthropods (parasitoids and predators) (Kariyat et al. 2012). Therefore, AMF possibly helps inoculated plants to alter their volatiles to attract the beneficial insects. However, it is unclear if the increased defenses in the inoculated plants are a resultant of increased nutrients acquisition and consequently more available resources towards the plant defenses or its direct association with the plant roots. In contrast, less vigorous and resource-limited control plants are possibly more susceptible to herbivory, for example, through reduced induction of defensive plant volatile compounds such as terpenes (Heil 2008; Kariyat et al. 2012). Taken together, our data validate the efficacy of AMF inoculated crops to attract beneficial arthropods and repel damaging insects.
To confirm that the effects observed in our experiments were primarily due to inoculated commercial AMF on Sorghum-sudangrass and not overpowered by natural AMF present in the soil systems (Torrecillas et al. 2011; Berruti et al. 2016), we conducted various laboratory experiments at different concentrations of AMF inoculum under sterilized soil conditions (without the presence of native AMF). Not surprisingly, we found a concentration-dependent effect on the germination of seeds inoculated at different rates of AMF: Seeds inoculated with twice the recommended rate of AMF germinated and established better than control seeds. However, seeds inoculated with double the recommended rate performed better than other two AMF rates, which further strengthens the premise of concentration-dependent effect of AMF on seedling germination and establishment. These findings confirm the results for growth traits obtained from the field study and the often-documented positive results of AMF on plant growth in various other studies, except Maighal et al. (2016) which showed that AMF negatively affects seed viability in the soil (Maighal et al. 2016). However, very recently, concentration-dependent effects of AMF have also been found in Medicago truncatula against pea aphids (Acyrthosiphon pisum) (Garzo et al. 2018). Notably, more research in this field under both lab and field conditions is warranted—to understand the optimum concentration of commercial AMF to reap both growth and defense benefits. It is quite clear that AMF can successfully alter plant chemistry that can modulate defense responses, possibly through plant volatiles and secondary defense metabolites; an area we are currently exploring.
Recently, AMF-inoculated crops were reported to have increased shoot and root dry biomass of 80.8% and 73.6%, respectively, in a total of 146 and 91 experiments used in their analyses (Berruti et al. 2016). In our study, field grown AMF-inoculated plants had greater aerial dry biomass than control (Fig. 6a) possibly due to acquisition of more nutrients (Roesti et al. 2006). Additionally, for seedlings grown under controlled lab conditions (without the presence of native AMF), seedling growth was found to be positively associated with AMF concentration (Fig. 6d), even during the initial establishment stages. Consistent with our results, Bi et al. (2018) also recorded increased root and shoot growth in AMF-inoculated Amygdalus pedunculata trees (Bi et al. 2018). Also, since during initial stage plant tends to invest more energy in elongation rather than secondary growth, (Weiner 1994; Nagashima 1995; Nagashima and Hikosaka 2011) we found similar results to the total dry biomass results for the seedlings grown in lab (Fig. 6f). Similarly, higher biomass for mycorrhizal wheat has also been previously documented in some studies (Al-Karaki et al. 2003; Zhu et al. 2015).
Conclusions
Overall, our results suggest that AMF boosts the crop health and vigor. But more importantly, AMF repels damaging herbivores while selectively attracting natural enemies in the initial crucial stages of crop growth and development (Weiner 1994). Our results also show that AMF effects are clearly visible in early stages through germination, establishment, growth, and herbivore defenses. The mechanisms underlying these effects warrant immediate and detailed examination.
References
Al-Karaki G, Mcmichael B, Zak J (2003) Field response of wheat to arbuscular mycorrhizal fungi and drought stress. Mycorrhiza 14:263–269. https://doi.org/10.1007/s00572-003-0265-2
Anderson AJ (1988) Mycorrhizae-host specificity and recognition. Phytopathology 78:375–378
Begum N, Qin C, Ahanger MA et al (2019) Role of arbuscular mycorrhizal fungi in plant growth regulation: implications in abiotic stress tolerance. Front Plant Sci. https://doi.org/10.3389/Fpls.2019.01068
Bennett AE, Alers-Garcia J, Bever JD (2006) Three-way interactions among mutualistic mycorrhizal fungi, plants, and plant enemies: hypotheses and synthesis. Am Nat 167:141. https://doi.org/10.2307/3491257
Berruti A, Lumini E, Balestrini R, Bianciotto V (2016) Arbuscular mycorrhizal fungi as natural biofertilizers: lets benefit from past successes. Front Microbiol. https://doi.org/10.3389/fmicb.2015.01559
Bi Y, Zhang Y, Zou H (2018) Plant growth and their root development after inoculation of arbuscular mycorrhizal fungi in coal mine subsided areas. Int J Coal Sci Technol 5:47–53. https://doi.org/10.1007/s40789-018-0201-x
Bryant JP, Chapin FS, Klein DR (1983) Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 40:357. https://doi.org/10.2307/3544308
Buntin GD (1986) A review of plant response to fall armyworm, Spodoptera frugiperda (J. E. Smith), injury in selected field and forage crops. Fla Entomol 69:549. https://doi.org/10.2307/3495389
Chapman JW (1999) Fitness consequences of cannibalism in the fall armyworm, Spodoptera frugiperda. Behav Ecol 10:298–303. https://doi.org/10.1093/beheco/10.3.298
Chen Y, Martin C, Mabola JCF et al (2019) Effects of Host plants reared under elevated CO2 concentrations on the foraging behavior of different stages of corn leaf aphids Rhopalosiphum maidis. Insects 10:182. https://doi.org/10.3390/insects10060182
Degen T, Bakalovic N, Bergvinson D, Turlings TCJ (2012) Differential performance and parasitism of caterpillars on maize inbred lines with distinctly different herbivore-induced volatile emissions. PLoS ONE. https://doi.org/10.1371/journal.pone.0047589
Dicke M, Baldwin IT (2010) The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help’. Trends Plant Sci 15:167–175. https://doi.org/10.1016/j.tplants.2009.12.002
Fiorilli V, Catoni M, Miozzi L et al (2009) Global and cell-type gene expression profiles in tomato plants colonized by an arbuscular mycorrhizal fungus. New Phytol 184:975–987. https://doi.org/10.1111/j.1469-8137.2009.03031.x
Fontana A, Reichelt M, Hempel S et al (2009) The Effects of arbuscular mycorrhizal fungi on direct and indirect defense metabolites of Plantago lanceolata L. J Chem Ecol 35:833–843. https://doi.org/10.1007/s10886-009-9654-0
Formenti L, Rasmann S (2019) Mycorrhizal fungi enhance resistance to herbivores in tomato plants with reduced jasmonic acid production. Agronomy 9:131. https://doi.org/10.3390/agronomy9030131
Gange A (2007) Insect-mycorrhizal interactions: patterns, processes, and consequences. In: Oghushi T, Craig TP, Price PW (eds) Ecological communities: plant mediation in indirect interaction webs. Cambridge University Press, London, pp 124–144
Gange AC, West HM (1994) Interactions between arbuscular mycorrhizal fungi and foliar-feeding insects in Plantago lanceolata L. New Phytol 128:79–87. https://doi.org/10.1111/j.1469-8137.1994.tb03989.x
Gange AC, Smith AK (2005) Arbuscular mycorrhizal fungi influence visitation rates of pollinating insects. Ecol Entomol 30:600–606. https://doi.org/10.1111/j.0307-6946.2005.00732.x
Gange AC, Stagg PG, Ward LK (2002) Arbuscular mycorrhizal fungi affect phytophagous insect specialism. Ecol Lett 5:11–15. https://doi.org/10.1046/j.1461-0248.2002.00299.x
Garzo E, Rizzo E, Fereres A, Gomez SK (2018) High levels of arbuscular mycorrhizal fungus colonization on Medicago truncatula reduces plant suitability as a host for pea aphids (Acyrthosiphon pisum). Insect Sci 27:99–112. https://doi.org/10.1111/1744-7917.12631
Gehring C, Bennett A (2009) Mycorrhizal fungal–plant–insect interactions: the importance of a community approach. Environ Entomol 38:93–102. https://doi.org/10.1603/022.038.0111
Gehring CA, Whitham TG (2002) Mycorrhizae-herbivore interactions: population and community consequences. In: Heijden MGA, Sanders IR (eds) Mycorrhizal ecology. Springer Verlag, Heidelberg, pp 295–320
Gosling P, Hodge A, Goodlass G, Bending G (2006) Arbuscular mycorrhizal fungi and organic farming. Agric Ecosyst Environ 113:17–35. https://doi.org/10.1016/j.agee.2005.09.009
Goverde M, Heijden MVD, Wiemken A et al (2000) Arbuscular mycorrhizal fungi influence life history traits of a lepidopteran herbivore. Oecologia 125:362–369. https://doi.org/10.1007/s004420000465
Hariprasanna K, Patil JV (2015) Sorghum: origin, classification, biology and improvement. Sorghum Mol Breed. https://doi.org/10.1007/978-81-322-2422-8_1
Hartley SE, Gange AC (2009) Impacts of plant symbiotic fungi on insect herbivores: mutualism in a multitrophic context. Annu Rev Entomol 54:323–342. https://doi.org/10.1146/annurev.ento.54.110807.090614
Heil M (2008) Indirect defence via tritrophic interactions. New Phytol 178:41–61. https://doi.org/10.1111/j.1469-8137.2007.02330.x
Hempel S, Stein C, Unsicker SB et al (2009) Specific bottom–up effects of arbuscular mycorrhizal fungi across a plant–herbivore–parasitoid system. Oecologia 160:267–277. https://doi.org/10.1007/s00442-009-1294-0
Hill EM, Robinson LA, Abdul-Sada A et al (2018) Arbuscular mycorrhizal fungi and plant chemical defence: effects of colonisation on aboveground and belowground metabolomes. J Chem Ecol 44:198–208. https://doi.org/10.1007/s10886-017-0921-1
Johnson NC, Graham JH, Smith FA (1997) Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol 135:575–585. https://doi.org/10.1046/j.1469-8137.1997.00729.x
Jung SC, Martinez-Medina A, Lopez-Raez JA, Pozo MJ (2012) Mycorrhiza-induced resistance and priming of plant defenses. J Chem Ecol 38:651–664. https://doi.org/10.1007/s10886-012-0134-6
Kariyat RR, Mauck KE, Moraes CMD et al (2012) Inbreeding alters volatile signalling phenotypes and influences tri-trophic interactions in horsenettle (Solanum carolinense L.). Ecol Lett 15:301–309. https://doi.org/10.1111/j.1461-0248.2011.01738.x
Kariyat RR, Scanlon SR, Moraski RP et al (2014) Plant inbreeding and prior herbivory influence the attraction of caterpillars (Manduca sexta) to odors of the host plant Solanum carolinense (Solanaceae). Am J Bot 101:376–380. https://doi.org/10.3732/ajb.1300295
Kariyat R, Chavana J, Kaur J (2018) an inexpensive and comprehensive method to examine and quantify field insect community influenced by host plant olfactory cues. Bio-Protocol. https://doi.org/10.21769/bioprotoc.2967
Kariyat RR, Gaffoor I, Sattar S et al (2019) Sorghum 3-deoxyanthocyanidin flavonoids confer resistance against corn leaf aphid. J Chem Ecol 45:502–514. https://doi.org/10.1007/s10886-019-01062-8
Kaya C, Ashraf M, Sonmez O et al (2009) The influence of arbuscular mycorrhizal colonisation on key growth parameters and fruit yield of pepper plants grown at high salinity. Scientia Hortic 121:1–6. https://doi.org/10.1016/j.scienta.2009.01.001
Kempel A, Schmidt AK, Brandl R, Schädler M (2010) Support from the underground: Induced plant resistance depends on arbuscular mycorrhizal fungi. Funct Ecol 24:293–300. https://doi.org/10.1111/j.1365-2435.2009.01647.x
Khaitov B, Patiño-Ruiz JD, Pina T, Schausberger P (2015) Interrelated effects of mycorrhiza and free-living nitrogen fixers cascade up to aboveground herbivores. Ecol Evolut 5:3756–3768. https://doi.org/10.1002/ece3.1654
Laird RA, Addicott JF (2007) Arbuscular mycorrhizal fungi reduce the construction of extrafloral nectaries in Vicia faba. Oecologia 152:541–551. https://doi.org/10.1007/s00442-007-0676-4
Lange ESD, Farnier K, Degen T et al (2018) Parasitic wasps can reduce mortality of teosinte plants infested with fall armyworm: support for a defensive function of herbivore-induced plant volatiles. Front Ecol Evolut. https://doi.org/10.3389/fevo.2018.00055
Lynch JM (1990) The rhizosphere. Wiley, Chichester
Maighal M, Salem M, Kohler J, Rillig MC (2016) Arbuscular mycorrhizal fungi negatively affect soil seed bank viability. Ecol Evolut 6:7683–7689. https://doi.org/10.1002/ece3.2491
Mcgonigle TP, Miller MH, Evans DG et al (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol 115:495–501. https://doi.org/10.1111/j.1469-8137.1990.tb00476.x
Mithöfer A, Boland W, Maffei ME (2018) Chemical ecology of plant-insect interactions. Annu Plant Rev Online. https://doi.org/10.1002/9781119312994.apr0369
Moraes CMD, Lewis WJ, Paré PW et al (1998) Herbivore-infested plants selectively attract parasitoids. Nature 393:570–573. https://doi.org/10.1038/31219
Mukherjee D (2017) Microorganisms: role for crop production and its interface with soil agroecosystem. Plant-Microbe Interact Agro-Ecol Perspect. https://doi.org/10.1007/978-981-10-5813-4_17
Murrell EG, Ray S, Lemmon ME et al (2019) Cover crop species affect mycorrhizae-mediated nutrient uptake and pest resistance in maize. Renew Agric Food Syst. https://doi.org/10.1017/s1742170519000061
Nagashima H (1995) Relationships between height, diameter and weight distributions of Chenopodium album plants in stands: effects of dimension and allometry. Ann Bot 75:181–188. https://doi.org/10.1006/anbo.1995.1010
Nagashima H, Hikosaka K (2011) Plants in a crowded stand regulate their height growth so as to maintain similar heights to neighbours even when they have potential advantages in height growth. Ann Bot 108:207–214. https://doi.org/10.1093/aob/mcr109
Padhee A, Prasanna B (2019) The emerging threat of fall armyworm in India. Indian Farm 59:51–54
Paré PW, Tumlinson JH (1999) Plant volatiles as a defense against insect herbivores. Plant Physiol 121:325–332. https://doi.org/10.1104/pp.121.2.325
Pineda A, Zheng S-J, Loon JJV et al (2010) Helping plants to deal with insects: the role of beneficial soil-borne microbes. Trends Plant Sci 15:507–514. https://doi.org/10.1016/j.tplants.2010.05.007
Posta K, Duc NH (2019) Benefits of arbuscular mycorrhizal fungi application to crop production under water scarcity. IntechOpen. https://doi.org/10.5772/intechopen.86595
Pozo MJ, Azcón-Aguilar C (2007) Unraveling mycorrhiza-induced resistance. Curr Opin Plant Biol 10:393–398. https://doi.org/10.1016/j.pbi.2007.05.004
Rabin LB, Pacovsky RS (1985) Reduced larva growth of two lepidoptera (Noctuidae) on excised leaves of soybean infected with a mycorrhizal fungus. J Econ Entomol 78:1358–1363. https://doi.org/10.1093/jee/78.6.1358
Reynolds HL, Packer A, Bever JD, Clay K (2003) Grassroots ecology: plant–microbe–soil interactions as drivers of plant community structure and dynamics. Ecology 84:2281–2291. https://doi.org/10.1890/02-0298
Reynolds HL, Hartley AE, Vogelsang KM et al (2005) Arbuscular mycorrhizal fungi do not enhance nitrogen acquisition and growth of old-field perennials under low nitrogen supply in glasshouse culture. New Phytol 167:869–880. https://doi.org/10.1111/j.1469-8137.2005.01455.x
Roesti D, Gaur R, Johri B et al (2006) Plant growth stage, fertilizer management and bio-inoculation of arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria affect the rhizobacterial community structure in rain-fed wheat fields. Soil Biol Biochem 38:1111–1120. https://doi.org/10.1016/j.soilbio.2005.09.010
Rowan DD (2011) Volatile Metabolites. Metabolites 1:41–63. https://doi.org/10.3390/metabo1010041
Siddiqui ZA, Akhtar MS, Futai K (2008) Mycorrhizae: sustainable agriculture and forestry. Springer, Dordrecht
Smith SE, Read DJ (2008) Mycorrhizal symbiosis. Academic Press, San Diego
Smith SE, Jakobsen I, Grønlund M, Smith FA (2011) Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol 156:1050–1057. https://doi.org/10.1104/pp.111.174581
Soti P, Racelis A (2020) Cover crops for weed suppression in organic vegetable systems in semiarid subtropical Texas. Org Agr. https://doi.org/10.1007/s13165-020-00285-4
Torrecillas E, Alguacil MDM, Roldán A (2011) Differences in the AMF diversity in soil and roots between two annual and perennial gramineous plants co-occurring in a Mediterranean, semiarid degraded area. Plant Soil 354:97–106. https://doi.org/10.1007/s11104-011-1047-9
van der Putten WH, Klironomos JN, Wardle DA (2007) Microbial ecology of biological invasions. ISME J 1:28–37
van der Putten WH, Bardgett RD, de Ruiter PC et al (2009) Empirical and theoretical challenges in aboveground–belowground ecology. Oecologia 161:1–14
Vannette RL, Hunter MD (2009) Mycorrhizal fungi as mediators of defence against insect pests in agricultural systems. Agric Forest Entomol 11:351–358. https://doi.org/10.1111/j.1461-9563.2009.00445.x
Venkateswaran K, Elangovan M, Sivaraj N (2019) Origin, domestication and diffusion of Sorghum bicolor. Breed Sorghum Divers End Uses. https://doi.org/10.1016/b978-0-08-101879-8.00002-4
Weiner J (1994) Competition and allometry in Kochia scoparia. Ann Bot 73:263–271. https://doi.org/10.1006/anbo.1994.1031
West HM (1995) Soil phosphate status modifies response of mycorrhizal and non-mycorrhizal Senecio vulgaris L. to infection by the rust Puccinia lagenophorae Cooke. New Phytol 129:107–116. https://doi.org/10.1111/j.1469-8137.1995.tb03014.x
Willis A, Rodrigues BF, Harris PJC (2013) The ecology of arbuscular mycorrhizal fungi. Crit Rev Plant Sci 32:1–20. https://doi.org/10.1080/07352689.2012.683375
Ye M, Veyrat N, Xu H et al (2018) An herbivore-induced plant volatile reduces parasitoid attraction by changing the smell of caterpillars. Sci Adv. https://doi.org/10.1126/sciadv.aar4767
Zangerl AR, Rutledge CE (1996) The probability of attack and patterns of constitutive and induced defense: a test of optimal defense theory. Am Nat 147:599–608. https://doi.org/10.1086/285868
Zangerl AR, Khan GS, Chaudhry AK (2007) Effect of spacing and plant density on the growth of poplar (Populus deltoides) trees under agro-forestry system. Pak J Agric Sci 44:321–327
Zhu X, Song F, Liu S, Liu F (2015) Arbuscular mycorrhiza improve growth, nitrogen uptake, and nitrogen use efficiency in wheat grown under elevated CO2. Mycorrhiza 26:133–140. https://doi.org/10.1007/s00572-015-0654-3
Acknowledgements
Lindsey Richards, Habraham Lopez, and Stephanie Kasper for their immense help with getting the field experiments done; Anwar Garza for providing the land, labor, and equipment for the field experiments; Paloma Flores for assisting in lab experiments; Dr. Lekshmi Sasidharan for statistics; and Lili Martinez for assisting in field trap setup and collection.
Funding
This research was funded by following grants awarded to Dr. Rupesh Kariyat: UTRGV Transforming the world–Strategic plan award, UT system Rising Star Award, and Startup funds.
Author information
Authors and Affiliations
Contributions
Conceptualization: RRK, PGS, and JK.; methodology: RRK and JK; software: RRK.; validation: RRK, JK, and JC; formal analysis: RRK; investigation: JK and JC.; resources: RRK.; data curation: JK and JC.; writing—original draft preparation, JK, PGS, and RRK; writing—review and editing, JK and RRK; supervision: RRK; project administration: RRK.; funding acquisition: RRK.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Handling Editor: Joe Louis.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kaur, J., Chavana, J., Soti, P. et al. Arbuscular mycorrhizal fungi (AMF) influences growth and insect community dynamics in Sorghum-sudangrass (Sorghum x drummondii). Arthropod-Plant Interactions 14, 301–315 (2020). https://doi.org/10.1007/s11829-020-09747-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11829-020-09747-8