Abstract
Monitoring pollinator health and pollination is among the top priorities to safeguard pollinators and secure pollinator services. Assessments of sampling methods are therefore essential for developing a standardized protocol for long-term pollinator monitoring. Pan trapping is a popular technique to survey pollinators, but limited information is available on the effect of pan trap diameter on the abundance, richness, and body size of sampled bees, as well as on the abundance of bycatch (non-targeted arthropods). We conducted experiments using four diameters of yellow pan traps in three habitats (a semi-natural phrygana habitat, a roadside, and a salt flat) on the Greek island of Lesvos during a 10-day period in late June/early July of 2017 and 2018. We found that pan traps of 4, 7, 10, and 12 cm captured a similar richness of bees and have little or no effect on the abundance estimates of bees and flies. Pan trap diameter did not affect body size of collected bees. Bycatch accounted for 62.8% of the arthropods collected and increased with the diameter of the pan traps in the phrygana and roadside habitats. According to literature, many researchers, especially outside Europe, use pan traps of various diameters (7–34 cm), volumes (96.1–2000 ml), and shapes (round, square, rectangular, or hexagonal). To reduce potential negative effects on populations of other beneficial arthropods, as well as to minimize processing effort and costs, we recommend using small pan traps (7 cm), unless standardized pan trapping protocols have already been adopted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Declines of bee populations and species across the world have raised environmental and economic concerns, as bees play a critical role in the maintenance of ecosystems, plant reproduction, food security, and social and cultural values (Berenbaum et al. 2006; Biesmeijer et al. 2006; Klein et al. 2007; Potts et al. 2010, 2016). Pollinator loss will negatively affect global human diet and health, crop market economies, and farmer and beekeeper livelihoods. For example, micronutrient deficiencies caused by a diet low in fruits, vegetables, nuts, and seeds, resulting from the lack of pollinators, are likely to affect the global rate of preventable diseases, such as ischemic heart disease (Smith et al. 2015; Potts et al. 2016). Recently, the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES) assessed the global status of pollinators and pollination. While reinforcing the multifaceted relevance of pollinators to human well-being and the alarming large-scale declines, it also highlighted the urgent need for developing long-term monitoring of pollinators and pollination services (IPBES 2016). Indeed, monitoring pollinator health and pollination is among the ten priorities suggested to governments worldwide to safeguard our pollinators and secure pollinator services (Dick et al. 2016).
One of the most common methods to survey and monitor pollinators is pan trapping. This passive sampling method, consisting of plastic bowls filled with soapy water, has allowed a rapid assessment of the richness, diversity, abundance, and phenology of bee communities across a number of habitats and landscapes of both tropical and temperate environments. Pan trapping generally performs well in comparison to other sampling methods (e.g., Westphal et al. 2008), and pan traps are readily available, inexpensive, easily replicated, and not collector biased (e.g., Westphal et al. 2008; Wilson et al. 2008; Tuell and Isaacs 2009; Droege et al. 2010; Ulyshen et al. 2010; Nielsen et al. 2011; Geroff et al. 2014). The shapes and sizes of pan traps vary greatly among published studies. For example, researchers have used containers ranging from rectangular trays (e.g., Easton and Goulson 2013; Andersson et al. 2017), ice-cream bowls (Larsen et al. 2014) and buckets (e.g., Rubene et al. 2015), to plastic party bowls (e.g., Wilson et al. 2008).
Several studies have assessed the abundance and composition of the captured bees using multiple colors and sizes of pan traps, height above ground, and even the use of contrasting patterns drawn at the bottom of the bowls to resemble nectar guides (e.g., Droege 2005; Wilson et al. 2008, 2016; Heneberg and Bogusch 2014; Gonzalez et al. 2016; Hall 2016). Such studies have provided sound evaluations of this popular sampling method, which is essential for developing a standardized protocol for long-term pollinator monitoring. However, in most cases information is still limited and geographically restricted. For example, only two studies have assessed the effect of pan trap size on the abundance of captured bees. Both studies took place in North America and their results are difficult to compare because of differences in their experimental design. The study in eastern USA (Droege 2005) used seven sizes of pan traps ranging from 0.7 oz. to 12 oz. and did not detect any effect on the abundance of the collected bees. In contrast, the other study in western USA (Wilson et al. 2016) only used three sizes of pan traps (3.5, 8.0, and 20 oz.) and found that larger pan traps (20 oz.) collected significantly more bees than smaller traps.
In addition to abundance, assessing the effect of pan trap size on bee body size is relevant, as ecological studies increasingly use changes in this functional trait to understand how bee communities and species respond to changes in the landscape (e.g., Wray et al. 2014; Renauld et al. 2016; Normandin et al. 2017). While height above ground appears to have an effect on the size of bees collected in pan traps (Gonzalez et al. 2016), the study of Wilson et al. (2016) suggests that pan trap size does not. However, the latter study used total body length as proxy of body size, which is a weak size estimator in bees when compared to the intertegular distance (hereafter ITD) (Cane 1987). Body length is a more variable measurement than ITD because the bee’s metasoma can retract or extend after bees drown in the pan traps. Thus, studies at different locations and using a more appropriate estimator of body size are necessary to gain a better understanding of the effect of pan trap size on captured bees.
Although increasing sample size might be desirable in short-term surveys, the effect of pan trap size on the abundance and richness of collected bees remains unknown. If large pan traps capture more bees, then they are more likely to catch a higher richness than smaller traps. Similarly, the effect of pan trap size on non-target arthropods or bycatch remains unknown. If large pan traps increase the chances of capturing more bees, they might also increase the chances of capturing more bycatch. Scientists have used pan traps to survey other insects besides bees and these traps capture a wide range of arthropods that include several insect orders and spiders (e.g., Easton and Goulson 2013; Gervais et al. 2018). Such a bycatch, which is often discarded or left unanalyzed, might negatively affect populations of other arthropods, including beneficial predators (e.g., spiders) and pollinators such as flies and beetles. It might also influence survey effectiveness by reducing space for target species and by increasing costs and processing time of sample contents (Spears and Ramirez 2015; Spears et al. 2016).
In this paper, we test if (1) large pan traps increase abundance and richness of collected bees; (2) large pan traps increase abundance of bycatch arthropods; and (3) large pan traps capture larger bees. Because large pan traps have a larger diameter and thus a greater surface area than small pan traps, we expect the former to increase the chances of capturing a higher abundance and richness of bees and abundance of bycatch. Considering that the majority of bees are small (e.g., Bullock 1999), we also expect large pan traps to increase the chances of capturing larger species. We conducted our experiments on the Greek island of Lesvos, one of the world’s regions of highest bee diversity (e.g., Nielsen et al. 2011), and assessed body size differences using ITD. In addition, we examined the effect of pan trap diameter on the most abundant insect orders in our samples (Hymenoptera excluding bees, Coleoptera, Diptera, and Hemiptera) and reviewed the pan trap dimensions and volumes employed in bee surveys globally during a 5-year period (2014–2018). We conducted the latter literature survey to contextualize our results because the shapes and sizes vary greatly in published studies. Finally, we offer conservation recommendations based on our findings.
Materials and methods
Study sites and sampling design
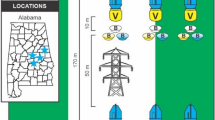
We conducted this work in three habitats around Kalloni Bay on the Greek island of Lesvos that we predicted to differ in their composition of bees, as judged by their location and type of vegetation (Fig. 1a–c). We chose these contrasting habitats because we wanted to determine if results were independent of the bee community composition. Our first site (“phrygana”) consisted of a semi-natural phrygana habitat in Achladeri, near Ancient Pyrra (39º 09′ 17.40″ N, 26º 16′ 32.74″ E, 1.3 m), at a time of the year characterized by abundant, flowering shrubs and bushes of Vitex agnus-castus L. (Lamiaceae) and sparse patches of Centaurea solstitialis L. (Asteracea). Our second site (“roadside”) was an unmanaged roadside 6 km southeast of Kalloni (39º 12′ 43.95″ N, 26º 16′ 15.21″ E, 2.0 m), with abundant plants of Daucus carota L. (Apiaceae) and Echium italicum L. (Boraginaceae), about 30 m wide, and bordered by an olive grove. Our last site (“salt flat”), located 1 km west of Skala Kallonis (39º 12′ 25.99″ N, 26º 11′ 57.58″ E, − 1.0 m), was a salt flat with abundant plants of Salicornia sp. (Amaranthaceae). At each site, we set up three transects, each consisting of 24 pan traps of the following four sizes (Fig. 1d): 4.0 cm × 3.2 cm in upper diameter and height (29.6 ml, 1 oz.), 7.0 cm × 3.5 cm (96.1 ml, 3.25 oz.), 10.3 cm × 3.8 cm (147.9 ml, 5 oz.), and 12 cm × 4.5 cm (354.9 ml, 12 oz.). Hereafter, pan traps of 4, 7, 10, and 12 cm in diameter. Thus, at each site we placed 72 pan traps, 18 of each diameter (6 of each diameter per transect).
Habitats surveyed (a–c) and pan trap sizes (d) used to sample bees in Kalloni Bay, Lesvos Island, Greece. a semi-natural phrygana habitat (east Mediterranean low scrub), b unmanaged roadside, c salt flat, d pan traps used in the surveys of 4 cm, 7 cm, 10 cm, and 12 cm in upper diameter, respectively
We arranged pan traps in a sequential pattern (smallest to largest) and spaced those 5 m apart, the minimum distance at which pan traps do not interfere with each other (Droege 2005). Transects were at least 10 m apart from each other (see Supporting information, Fig. 1S). We used plastic bowls (Solo® plastics Soufflé Cup) and spray-painted them fluorescent yellow (Rust-Oleum®). We chose yellow because preliminary observations suggest that it is the most effective color to capture bees at the study area during this time of the year (V.H. Gonzalez., pers. obs.). We chose to compare plastic party bowls of those four diameters because they are readily available, are easy to carry and deploy in the field, as well as commonly used in pollinator surveys. We filled each pan trap with soapy water to break the water tension.
We collected all arthropods and refilled pan traps with soapy water every two days. We aggregated data within and across transects, and kept trap diameter per sampling event per habitat. We sampled the phygana habitat from June 28 to July 8, 2017, and the other two habitats from June 12 to June 22, 2018. We counted and sorted to the order level all arthropods captured in the traps, except for thrips and collembolans. These arthropods were often abundant in the samples but difficult to quantify because of their small size. To assess the composition of the captured bees among traps, we determined them to morphospecies (67–72% of total number at each site) or species level.
Bee body size
We estimated bee body size by measuring the minimum ITD (Cane 1987) with an ocular micrometer on a Leica S6E stereomicroscope. For each habitat type, we measured at least one specimen of all species or morphospecies collected per pan trap diameter. Because Lasioglossum malachurum (Kirby) (Halictidae: Halictini) was the most common species captured in all traps (see results below), we randomly chose at least 25 specimens of this species from each pan trap diameter per habitat. We measured 626 bee specimens from all habitats (phrygana = 317, roadside = 134, salt flat = 175).
Pan trap size in the literature
To establish the pan trap size more commonly used in bee surveys, we conducted a literature search on the Web of Science database for articles published between 2014 and 2018 with the search terms “pan trap bees”. We retrieved 101 publications and screened them for: (1) pan trap dimensions; (2) pan trap volume; and (3) country where surveys were conducted. We excluded conference abstracts, reviews, and publications that did not include bees in their samples. For comparisons, we converted reported dimensions and volumes to centimeters and milliliters. When authors did not provide pan trap dimensions but referred to previous works for details, we made an effort to find those references to extract the information.
Data analyses
We conducted statistical analyses in R (R Core Team 2018) and created boxplots and histograms using GraphPad Prism version 7.04 (GraphPad Software, San Diego, CA, USA). To examine the effect of pan trap diameter and habitat type on the abundance of bees and bycatch, including the most abundant insect orders in our samples, we used a Generalized Linear Mixed Effect Model (GLMM) with Negative Binomial Distribution. We chose this test because our count data did not follow a Normal nor a Poisson distribution and were overdispersed. To test for differences in species richness of bees collected at different pan trap diameter, we used a GLMM with a Poisson distribution. To examine the effect of habitat and pan trap diameter on the bees’ ITD, we used a Generalized Linear Model (GLM) with normal distribution. In these models, habitat type and pan trap diameter served as fixed factors. To avoid potential effects of temporal autocorrelation, we considered sampling event as a random factor in all GLMMs. We implemented these models using the lme4 and glmmTMB packages (Bates et al. 2015; Brooks et al. 2017) and assessed the significance of fixed effects using a Type II Wald χ2 test with the car package (Fox and Weisberg 2019). When factors and factor interactions were significant, we used the lsmeans package (Lenht 2016) to conduct multiple pairwise comparisons with Bonferroni adjustment to assess for differences among groups.
To assess species diversity per pan trap diameter, we estimated the Shannon–Wiener and Simpson (1-D) indices using the vegan package (Oksanen et al. 2019). To assess the similarity among pan trap diameter, we estimated the Morisita-Horn index using both abundance and occurrence (presence/absence) data with SpadeR package (Chao et al. 2016). We used occurrence data because one bee species, L. malachurum, dominated the samples of the habitats surveyed and could potentially affect abundance-based estimations. To assess for differences in these indices among pan trap sizes, we calculated a 95% confidence intervals with a non-parametric bootstrap with replacement (1000 times). Average values are given with standard error and sample size.
Results
Bee abundance and diversity
We sampled 13,155 arthropods of which the majority of them were hymenopterans (52.1%), coleopterans (20.4%), dipterans (11.6%), and hemipterans (10.3%). The remaining percentage (5.6%) corresponded to arachnids, blattodeans, orthopterans, lepidopterans, and mantises. Bees accounted for 37.2% of all arthropods collected. Across habitats, the percentage of bees collected by each trap diameter ranged from 7.88 to 77.78% (\(\overline{x}\) = 39.63 ± 1.98, N = 60, 4 pan trap diameters × 3 habitats × 5 collection events per site) per collection event. Bee abundance was different among habitat types (Wald χ2 = 60.432, DF = 2, P < 0.001) and among diameters of pan traps (χ2 = 27.286, DF = 3, P < 0.001). The interaction between habitat type and pan trap diameter was also significant (χ2 = 14.431, DF = 6, P = 0.025). Pairwise comparisons with Bonferroni adjustment indicated that the lowest abundance of bees were captured at the salt flat and the highest at the phrygana (DF = 1, P < 0.001). Pan trap diameter had an effect on bee abundance only at the roadside. At this habitat, all diameters of pan traps captured similar numbers of bees, except the smallest pan trap that captured less bees than pan traps of 10 cm and 12 cm (Table 1, Fig. 2).
Boxplots showing abundance of bees and bycatch arthropods by pan trap diameter within each habitat sampled per collection event (N = 5 events per habitat) on Lesvos Island, Greece. Boxplots display medians and quartiles. For each habitat, groups with different letters above boxplots are significantly different. See text and Table 1 for significance values of pairwise comparisons
Pan traps collected between 1 and 13 species of bees (\(\overline{x}\) = 7.145 ± 0.29, N = 60) per collection event and there were no differences among habitats (χ2 = 2.403, DF = 2, P = 0.301) nor pan trap diameters (χ2 = 1.704, DF = 3, P = 0.636). The interaction between the type of habitat and the diameter of pan trap was also not significant (χ2 = 6.89, DF = 6, P = 0.331) (Fig. 3a). During our surveys, L. malachurum was the most common species in all habitats, accounting for 85.0%, 91.7%, 56.7% of all bees collected in the phrygana, roadside, and salt flat habitats, respectively. Both Shannon and Simpson’s indices were similar among trap sizes in all habitats, as their confidence intervals overlapped (Table 2, see Supporting information, Fig. 2S). The Morisita-Horn index, either using abundance or occurrence data, was close to 1 between pairs of pan trap diameters in all habitats and their confidence intervals also overlapped (Fig. 4, see Supporting information, Fig. 3S). The number of shared species between pairs of pan trap diameters differed among habitats. In the phrygana, pan traps of 4 cm and 7 cm shared the most species, whereas in the roadside and salt flat habitats pan traps of 4 cm and 12 cm and 7 cm and 12 cm shared the most species (Fig. 4).
Boxplots showing the number of species and morphospecies of bees captured per collection event (N = 5 events per habitat) for each pan trap diameter (a) and intertegular distance (ITD) of bees collected at each pan trap diameter (b). Values above error bars indicate the total number of specimens measured per pan trap diameter. Boxplots display medians and quartiles
Venn diagrams showing the number of shared species and mean value of Morisita-Horn similarity index using abundance data from each bee community collected with four diameters of pan traps at three habitats on Lesvos Island, Greece (phrygana, roadside, and salt flat). Circles represent pan traps of different diameters. The two values at the intersection between circles represent the number of shared species (top value) and mean value of Morisita-Horn similarity index (bottom value)
Bee ITD
ITD ranged from 0.59 to 5.53 mm (\(\overline{x}\) = 1.61 ± 0.64, N = 626 specimens) and was similar among habitats (χ2 = 1.489, DF = 2, P = 0.475) and among pan trap diameters (χ2 = 3.338, DF = 3, P = 0.342) (Fig. 3b). We did not find an interaction between the type of habitat and the diameter of pan trap on the bees’ ITD (χ2 = 6.843, DF = 6, P = 0.336).
Bycatch abundance
Across habitats, the percentage of bycatch ranged from 22.22 to 92.12% (\(\overline{x}\) = 60.37 ± 1.99, N = 60) per collection event. Bycatch abundance varied among habitats (χ2 = 85.086, DF = 2, P < 0.001) and pan trap diameter (χ2 = 88.360, DF = 3, P < 0.001). There was not interaction between habitat type and pan trap diameter (χ2 = 6.221, DF = 6, P = 0.398). Pairwise comparisons with Bonferroni adjustment detected differences among all habitat types (DF = 1, P < 0.001 for all comparisons), with the lowest abundance of bycatch captured at the salt flat and the highest at the roadside. At the phrygana and roadside habitats, bycatch abundance was similar between pan traps of 4 cm and 7 cm and between pan traps of 10 cm and 12 cm, the last trap diameter capturing more bycatch than the first two. Pan trap diameter did not have an effect on bycatch abundance at the salt flat habitat (Table 1, Fig. 2).
The abundance of the most common insect orders collected in our surveys varied depending on the habitat type and diameter of the pan trap. The interaction between habitat type and pan trap diameter was only significant for non-Anthophila Hymenoptera and Hemiptera (Table 3). Non-Anthophila Hymenoptera were more abundant at the phrygana habitat, Coleoptera and Hemiptera were more abundant at the roadside habitat, and Diptera at the salt flat habitat (Fig. 5, Table 1S). Pan traps of all diameters captured similar abundance of Diptera in all habitats, except at the roadside where pan traps of 12 cm collected more than pan traps of 4 cm (Fig. 5c). For the remaining insect groups, abundance tend to increase with the diameter of the pan traps, particularly in the habitat(s) where each group was most commonly collected. In those habitats, pan traps of 12 cm only or both 10 cm and 12 cm captured more than pan traps of other diameters (Fig. 5a, b, d).
Boxplots showing abundance of the most common arthropod groups collected by each pan trap diameter within each habitat per collection event (N = 5 events per habitat) on Lesvos Island, Greece. Boxplots display median and quartiles. For each habitat, groups with different letters above boxplots are significantly different. See Table 1S for significance values of pairwise comparisons
Pan trap size usage
We found 93 publications (\(\overline{x}\) = 18.60 ± 1.87, 14–25 publications per year) that matched our criteria and used pan traps to survey or monitor bees (see Supporting information, Table 2S). About 45% (N = 42) of these publications are from studies conducted in North America, mostly from the US. Neither pan trap dimensions nor volume was available in 25% of these publications. Some (34%) provided pan trap diameter (upper diameter and sometimes lower) and often included depth or height of the trap. In a few cases (14% of publications reviewed), authors provided both the dimensions and volume of the traps. Pan trap dimensions, volume, and shape varied among studies (Fig. 6), especially those conducted outside of Europe (see Supporting information, Table 2S). Pan trap volume ranged from 96.1 ml to 2000 ml (\(\overline{x}\) = 397.3 ± 47.5, N = 52 publications) or 3.25 oz. to 67.6 oz. (\(\overline{x}\) = 13.43 ± 1.61, N = 52), upper diameter from 7.25 cm to 34.00 cm (\(\overline{x}\) = 15.05 ± 1.18, N = 32), and depth (height) from 3.00 cm to 13.50 cm (\(\overline{x}\) = 8.09 ± 0.76, N = 22). Most studies used rounded plastic bowls, but some employed 2 L square ice-cream containers (Larsen et al. 2014), rectangular aluminum trays (Andersson et al. 2017), buckets (Rubene et al. 2015), and hexagonal weighing trays (Gezon et al. 2015).
Discussion
In the habitats surveyed, pan trap diameter had little or no effect on the abundance of captured bees (Fig. 2a, Table 1). Pan traps of all diameters caught a similar number of species (Fig. 3a) and had comparable diversity indices (Table 2), as well as high similarity indices (Fig. 4). Thus, these results are not consistent with our expectations that pan traps with large diameters increase the chances of capturing a higher abundance and number of species when compared with pan traps of small diameters. These results provide support to previous observations by Droege (2005) in eastern North America that pan trap size has little to no effect on the abundance of collected bees. However, we carried out short-term experiments that took place towards the end of the main activity period of bees. As a result, the overall availability of flowers, as well as the composition of the bee fauna active at the time, may not have been representative of the sampled habitats. Bees are more likely to encounter pan traps when floral resources are scarce (Cane et al. 2000; Wilson et al. 2008; Baum and Wallen 2011) and, under such conditions, all colored pan traps would appear attractive to them regardless of the pan trap diameter. These observations are strengthened by the high number of bee specimens relative to the small number of species detected (26–43 spp.). Although Nielsen et al. (2011) surveyed bees from March through July on Lesvos’ phrygana habitats, they collected a comparable number of specimens, but they recorded a three times higher level of species richness. Thus, our results may only apply under these conditions and future studies should assess the impact of floral phenology.
The sweat bee L. malachurum was the most common species in all habitats, accounting for 56.7% to 91.7% of all collected bees. This is an obligately eusocial bee species that is common in transformed environments and widely distributed in Europe and northern Africa. It builds subterranean nests in hard, exposed soils, sometimes forming dense aggregations of more than 1000 nests. Each nest consists of as few as four workers in the spring, but it might contain up to 80 workers in the summer (Knerer 1992). Thus, the high abundance of this species in our pan traps can be explained by the type of habitats we surveyed, which were within or near rural environments, as well as by the time of year (late June/early July), particularly if traps were located near nest aggregations. The high abundance of L. malachurum at our study sites could have influenced the chances of capturing other species by reducing the available space within the pan traps, especially in traps of small diameter. However, traps of all diameters captured a similar number of species and did not differ in their diversity nor similarity indices, the latter calculated using both abundance and occurrence data (Table 2, see Supporting information, Figs. 2S, 3S). In addition, excluding L. malachurum from the analyses revealed differences only in the abundance of the remaining bee species among habitats (χ2 = 53.007, DF = 2, P < 0.001), not among pan trap diameters (χ2 = 1.861, DF = 3, P = 0.602) or the interaction between these two factors (χ2 = 9.779, DF = 6, P = 0.134). Thus, the little to no effect of pan trap diameter on the abundance estimates of bees did not result from the high dominance of L. malachurum in our samples.
We found no effect of pan trap diameter on the body size of captured bees (Fig. 3b), as estimated from their ITD. Thus, our results are not consistent with our expectations that large pan traps increase the chances of capturing larger bees and they support previous observations by Wilson et al. (2016) despite using body length as proxy of body size. Although large pan traps may increase the chances of capturing larger bees, all our pan traps were at ground level. Foraging ability depends on body size (Greenleaf et al. 2007) and bees tend to forage in the horizontal stratum (Gumbert and Kunze 1999; Cane et al. 2000). Thus, pan traps placed at the same level, regardless of their diameter, may capture bees of a particular range of body size. Indeed, at least one study (Gonzalez et al. 2016) demonstrates that pan trap height, even as small as 70 cm above ground, influences the body size of the collected bees. Large-bodied bees were rare on our traps and, in one occasion, the largest trap (12 cm) at the phrygana habitat captured a single individual of the carpenter bee Xylocopa violacea (L.) (Apidae, Xylocopini). This single record does not reflect the abundance of that carpenter bee in the area, as we frequently found it throughout the day foraging at inflorescences of Vitex agnus-castus L. (Lamiaceae). The effect of pan trap diameter at different heights above ground is so far unknown.
The effect of pan trap diameter on the abundance of bycatch is one of the most significant results of our work, as it has practical implications for the development of logistically feasible and environmentally sustainable pan trapping. While pan trap diameter had little or no effect on the abundance of bees captured, it significantly influenced bycatch abundance in the phrygana and roadside. In these habitats, the abundance of bycatch increased with pan trap diameter (Fig. 2b), which means greater chances of affecting local populations of arthropods, as well as higher costs and longer processing time of sample contents. In contrast, pan trap diameter did not have an effect on the abundance of bycatch at the salt flat (Fig. 2b), which might be the result of its depauperate arthropod fauna due to it being a less structurally complex habitat when compared with the other two (Fig. 1). Except for the abundance of Diptera (Fig. 5c), which exhibited a similar pattern to that of bees, the abundance of the remaining insect groups increased with pan trap diameter, particularly in those habitats where they were most commonly collected (Fig. 5a,b,d). Thus, these results are consistent with our expectations that pan traps with large diameters increase the chances of capturing a higher abundance of bycatch when compared with small pan traps. They also suggest that pan trap performance by diameter might also be context-dependent, similar to the performance of pan traps by color depending on the type of habitat and bee community (e.g., Heneberg and Bogusch 2014).
Although pan traps of all diameters captured similar abundances of Diptera at the phrygana and salt flat habitats, pan traps of 12 cm collected more specimens than pan traps of 4 cm at the roadside (Fig. 5c). Thus, pan trap diameter at this habitat had at least some effect on the abundance of this insect group. Diptera is a highly diverse insect order with taxa ranging from pollinators to agricultural pests, and thus responses to pan traps might be different depending on the taxonomic group. For example, although Diptera appears to be attracted to non-florescent pan traps (Shrestha et al. 2019), color preference varies among families and even genera (e.g., Campbell and Hanula 2007). Some taxa respond to white and blue while others respond to yellow, such as the olive fruit fly Bactrocera oleae (Rossi), an agricultural pest efficiently collected using yellow sticky traps (e.g., Burrack et al. 2008). Hover flies (Syrphidae) and bombyliid flies (Bombyliidae) are among the most commonly collected taxa with yellow and blue pan traps (Campbell and Hanula 2007; Saunders and Luck 2013) and, considering their relationships with flowers, they may respond to similar visual cues and exhibit similar responses to pan traps as bees. However, we do not know the taxonomic composition of Diptera in our samples, which precluded us from conducting further analyses. Nevertheless, our results contribute significantly to the dearth of information regarding passive sampling protocols for monitoring non-bee pollinators, which have been under-represented in pollination studies (Hall and Reboud 2019).
Our literature review from papers published between 2014 and 2018 revealed that pan trap dimensions, volume, and shape vary among studies. However, it appears that researchers prefer two diameters of pan traps, 7 cm and 12–16 cm (Fig. 6). It seems researchers do not use smaller traps (4 cm), except in an experimental context as in Droege (2005) and the present study. Although we do not know the cause of this variation in the size of pan traps, it might simply reflect differences in the material available locally to researchers for pan traps. For instance, while bee surveys in the U.S. often use readily available 7 cm Solo® plastics cups, bowls of this volume are not often easy to find in other countries. Much larger plastic bowls (15–18 cm) are usually the only option available to researchers (V.H. Gonzalez, pers. obs.).
Conservation implications and future directions
Given the increasing popularity of pan trapping for surveying and monitoring bees, establishing an optimal size of pan trap that maximizes catches while reducing impact on the local arthropod fauna, as well as monetary costs, is highly relevant. In our study, bycatch accounted for 62.8% of the arthropods collected, and we showed that it increases with pan trap diameter in two of the habitats sampled. We also demonstrated that pan trap diameter does not affect the abundance and richness of the captured bees and abundance of flies, although the short duration and timing of our experiments could have influenced our results. In addition, we only used one pan trap color and thus the effect of pan trap diameter for other colors remains unknown. Future studies should address these limitations as well as differential responses at lower taxonomic levels (family or genera) of the insect orders analyzed in the present study. The latter aspect is particularly important for Diptera, as several taxa are key pollinators that sometimes entirely replace bees and their role in pollination in some environments and ecosystems (e.g., Lefebvre et al. 2018).
If future studies support our results, then to minimize bycatch in both bee and non-bee pollinator surveys, namely flies, researchers might want to use bowls of 7 cm in upper diameter and about 4 cm in depth (3.25 oz.). This size of bowl uses less plastic, water, and paint than those of 10 cm and 12 cm, making it more sustainable and easier to carry and deploy in the field. For example, Shapiro et al. (2014) suggest that 30 bowls per transect is the minimum number of bowls that maximizes sampling efficiency. Thus, a single 30-bowl transect of 7 cm cups requires about 2.9 L (or 2.2 L if cups are ¾ filled) per collection event. Using larger bowls (10 cm and 12 cm) would increase by 1.5 and 3.7 times the amount of water required per transect. We do not recommend using bowls of 4 cm mainly because these were less stable than other traps and the water often evaporated between collection periods. However, using a propylene glycol solution can prevent evaporation (Thomas 2008), but this is not always available and also increases costs. Finally, an important aspect to consider is comparability with previous studies. Especially in Europe, where the vast majority of researchers use standardized pan trapping protocols (Westphal et al. 2008), we advocate their use to ensure continuity and comparability of results.
References
Andersson P, Koffman A, Sjödin E, Johansson V (2017) Roads may act as barriers to flying insects: species composition of bees and wasps differs on two sides of a large highway. Nat Conserv 18:41–59
Bates D, Mächler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Baum KA, Wallen KE (2011) Potential bias in pan trapping as a function of floral abundance. J Kans Entomol Soc 84:155–159
Berenbaum MR, Bernhardt P, Buchmann S, Calderone NW, Goldstein P, Inouye DW, Kevan P, Kremen C, Medellin R, Ricketts TH, Robinson GE, Snow AA, Swinton S, Thien LB, Thompson FC (2006) Status of pollinators in North America. National Academies Press, Washington, DC
Biesmeijer JC, Roberts SPM, Reemer M, Ohlemüller R, Edwards M, Peeters T, Schaffers AP, Potts SG, Kleukers R, Thomas CD, Settele J, Kunin WE (2006) Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313:351–354
Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Mächler M, Bolker BM (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9:378–400
Bullock SH (1999) Relationship among body size, wing size and mass in bees from a tropical dry forest in México. J Kans Entomol Soc 72:426–439
Burrack HJ, Connell JH, Zalom FG (2008) Comparison of olive fruit fly (Bactrocera oleae (Gmelin)) (Diptera: Tephritidae) captures in several commercial traps in California. Int J Pest Manage 54:227–234
Campbell JW, Hanula JL (2007) Efficiency of malaise traps and colored pan traps for collecting visiting insects from three forested ecosystems. J Insect Conserv 11:399–408
Cane JH (1987) Estimation of bee size using intertegular span (Apoidea). J Kans Entomol Soc 60:145–147
Cane JH, Minckley RL, Kervin LJ (2000) Sampling bees (Hymenoptera: Apiformes) for pollinator community studies: pitfalls of pan-trapping. J Kans Entomol Soc 73:225–231
Chao A, Ma KH, Hsieh TC, and Chiu C-H (2016) SpadeR: species-richness prediction and diversity estimation with R. R package version 0.1.1. https://CRAN.R-project.org/package=SpadeR
Dick LV, Viana B, Bommarco R, Brosi B, Arizmendi M, Cunningham SA, Galetto L, Hill R, Lopes AV, Pires C, Taki H, Potts SG (2016) Ten policies for pollinators. What governments can do to safeguard pollination services. Science 354(6315):975–976
Droege S (2005) The bee inventory plot. https://online.sfsu.edu/~beeplot. Accessed 21 Mar 2018
Droege S, Tepedino VJ, Lebuhn G, Link W, Minckley RL, Chen Q, Conrad C (2010) Spatial patterns of bee captures in North American bowl trapping surveys. Insect Conserv Divers 3:15–23
Easton AH, Goulson D (2013) The neonicotinoid insecticide Imidacloprid repels pollinating flies and beetles at field-realistic concentrations. Plos ONE 8(1):e54819. https://doi.org/10.1371/journal.pone.0054819
Fox J, Weisberg S (2019) An R companion to applied regression. Sage, Thousand Oaks
Geroff RK, Gibbs J, McCravy KW (2014) Assessing bee (Hymenoptera: Apoidea) diversity of an Illinois restored tallgrass prairie: methodology and conservation considerations. J Insect Conserv 18:951–964
Gervais A, Chagnon M, Fournier V (2018) Diversity and pollen loads of flower flies (Diptera: Syrphidae) in cranberry crops. Ann Entomol Soc Am 111:326–334
Gezon ZJ, Wyman ES, Ascher JS, Inouye DW, Irwin RE (2015) The effect of repeated, lethal sampling on wild bee abundance and diversity. Methods Ecol Evol 6:1044–1054
Gonzalez VH, Park KE, Çakmak I, Hranitz JM, Barthell JF (2016) Pan traps and bee size in unmanaged urban habitats. J Hymenopt Res 51:241–247
Greenleaf SS, Williams NM, Winfree R, Kremen C (2007) Bee foraging ranges and their relationships to body size. Oecologia 153:589–596
Gumbert A, Kunze J (1999) Inflorescence height affects visitation behavior of bees—a case study of an aquatic plant community in Bolivia. Biotropica 31:466–477
Hall HG (2016) Color preferences of bees captured in pan traps. J Kans Entomol Soc 89:273–276
Hall MA, Reboud EL (2019) High sampling effectiveness for non-bee flower visitors using vane traps in both open and wooded habitats. Austral Entomol 58:836–847
Heneberg P, Bogusch P (2014) To enrich or not to enrich? Are there any benefits of using multiple colors of pan traps when sampling aculeate Hymenoptera? J Insect Conserv 18:1123–1136
IPBES (2016) Summary for policymakers of the assessment report of the intergovernmental science-policy platform on biodiversity and ecosystem services on pollinators, pollination and food production. In: Potts SG, Imperatriz-Fonseca VL, Ngo HT, Biesmeijer JC, Breeze TD, Dicks LV, Garibaldi LA, Hill R, Settele J, Vanbergen AJ, Aizen MA, Cunningham SA, Eardley C, Freitas BM, Gallai N, Kevan PG, Kovacs-Hostyanszki A, Kwapong PK, Li J, Li X, Martins DJ, Nates-Parra G, Pettis JS, Rader R, Viana BF (eds) Secretariat of the intergovernmental science-policy platform on biodiversity and ecosystem services. Bonn, Germany
Klein MA, Vaissière BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T (2007) Importance of pollinators in changing landscapes for world crops. Proc R Soc [Biol] 274:303–313
Knerer G (1992) The biology and social behaviour of Evylaeus malachurus (K.) (Hymenoptera; Halictidae) in different climatic conditions of Europe. Zool Jahrb Syst 119:261–290
Larsen N, Minor MA, Cruickshank RH, Robertson AW (2014) Optimising methods for collecting Hymenoptera, including parasitoids and Halictidae bees, in New Zealand apple orchards. J Asia Pac Entomol 17:375–381
Lefebvre V, Villemant C, Fontaine C, Daugeron C (2018) Altitudinal, temporal and trophic partitioning of flower-visitors in Alpine communities. Sci Rep 8:4706. https://doi.org/10.1038/s41598-018-23210-y
Lenth RV (2016) Least-squares means: the R package lsmeans. J Stat Softw 69:1–33
Nielsen A, Steffan-Dewenter I, Westphal C, Messinger O, Potts SG, Roberts SPM, Settele J, Szentgyörgyi H, Vaissière BE, Vaitis M, Woyciechowski M, Bazos I, Biesmeijer JC, Bommarco R, Kunin WE, Tscheulin T, Lamborn E, Petanidou T (2011) Assessing bee species in two Mediterranean communities: importance of habitat type and sampling techniques. Ecol Res 26:969–983
Normandin E, Vereecken NJ, Buddle CM, Fournier V (2017) Taxonomic and functional trait diversity of wild bees in different urban settings. PeerJ 5:e3051. https://doi.org/10.7717/peerj.3051
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, StevensMH, Szoecs E, Wagner H (2019) vegan: community ecology package. R package version 2.5-6. https://CRAN.R-project.org/package=vegan
Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE (2010) Global pollinator declines: trends, impacts and drivers. Trends Ecol Evol 25:345–353
Potts SG, Imperatriz-Fonseca V, Ngo HT, Aizen MA, Biesmeijer JC, Breeze TD, Dicks LV, Garibaldi LA, Hill R, Settele J, Vanbergen AJ (2016) Safeguarding pollinators and their values to human well-being. Nature. https://doi.org/10.1038/nature20588
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org
Renauld M, Hutchinson A, Loeb G, Poveda K, Connelly H (2016) Landscape simplification constrains adult size in a native ground-nesting bee. PLoS ONE 11(3):e0150946. https://doi.org/10.1371/journal.pone.0150946
Rubene D, Schroeder M, Ranius T (2015) Estimating bee and wasp (Hymenoptera: Aculeata) diversity on clear-cuts in forest landscapes—an evaluation of sampling methods. Insect Conserv Diver 8:261–271
Saunders ME, Luck GW (2013) Pan trap catches of pollinator insects vary with habitat. Aust J Entomol 52:106–113
Shapiro LH, Tepedino VJ, Minckley RL (2014) Bowling for bees: optimal sample number for “bee bowl” sampling transects. J Insect Conserv 18:1105–1113
Shrestha M, Garcia JE, Chua JH, Howard SR, Tscheulin T, Dorin A, Nielsen A, Dyer AG (2019) Fluorescent pan traps affect the capture rate of insect orders in different ways. Insects 10:40. https://doi.org/10.3390/insects10020040
Smith MR, Singh GM, Mozaffarian D, Myers SS (2015) Effects of decreases of animal pollinators on human nutrition and global health: a modelling analysis. Lancet 386:1964–1972
Spears LR, Ramirez RA (2015) Learning to love leftovers. Using by-catch to expand our knowledge in entomology. Am Entomol 61:168–173
Spears LR, Looney C, Ikerd H, Koch JB, Griswold T, Strange JP, Ramirez RA (2016) Pheromone lure and trap color affects bycatch in agricultural landscapes of Utah. Environ Entomol 45(4):1009–1016
Thomas DB (2008) Nontoxic antifreeze for insect traps. Entomol News 119:361–365
Tuell JK, Isaacs R (2009) Elevated pan traps to monitor bees in flowering crop canopies. Entomol Exp Appl 131:93–98
Ulyshen MD, Soon V, Hanula JL (2010) On the vertical distribution of bees in a temperate deciduous forest. Insect Conserv Diver 3:222–228
Westphal C, Bommarco R, Carré G, Lamborn E, Morison N, Petanidou T, Potts SG, Roberts SP, Szentgyörgyi H, Tscheulin T, Vaissière BE, Woyciechowski M, Biesmeijer JC, Kunin WE, Settele J, Steffan-Dewenter I (2008) Measuring bee diversity in different European habitats and biogeographical regions. Ecol Monogr 78(4):653–671
Wilson JS, Griswold T, Messinger OJ (2008) Sampling bee communities (Hymenoptera: Apiformes) in a desert landscape: are pan traps sufficient? J Kans Entomol Soc 81:288–300
Wilson JS, Jahner JP, Starley L, Calvin CL, Ikerd H, Griswold T (2016) Sampling bee communities using pan traps: alternative methods increase sample size. J Insect Conserv 20:919–922
Wray JC, Neame LA, Elle E (2014) Floral resources, body size, and surrounding landscape influence bee community assemblages in oak-savannah fragments. Ecol Entomol 39:83–93. https://doi.org/10.1111/een.12070
Acknowledgements
We are indebted to fellow REU program participants for their help in the field: Amanda Fernandez, Taylor Salaguinto, Erika Metzler, Vilnery Rivera, Connor Chambers, Claudia Cordero, Kristie Shirley, Sebastian Silva, Sarah Markland, Alexandria Ambrose, and Jordan Ellis; Stephen T. Tapsak for the picture of the pan traps; Amy R. Comfort, Daphne Mayes, Mariano Lucia, Piotr Nowicki, Oliver Schweiger, and two anonymous reviewers for comments and suggestions that significantly improved this manuscript. National Science Foundation REU program (DBI 1560389) provided financial support for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gonzalez, V.H., Osborn, A.L., Brown, E.R. et al. Effect of pan trap size on the diversity of sampled bees and abundance of bycatch. J Insect Conserv 24, 409–420 (2020). https://doi.org/10.1007/s10841-020-00224-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-020-00224-4