Abstract
Bobcats (Lynx rufus) are wide-ranging mammals found throughout the continental USA. As carnivores near the top of their food chain, bobcats would seem to be a useful bioindicator of metal pollution in terrestrial environments. However, there is very limited research on bobcats in toxicology studies. Here, we offer the first analysis of metal (copper, selenium, silver, and zinc) contaminants in the livers of wild bobcats. Liver tissues from 120 adult bobcats (i.e., estimated to be ≥1 year old) were collected from 2003 to 2006 at four sites in Georgia and Florida, USA that experienced relatively similar levels of human disturbance. We found no differences in metal concentrations between males and females. At two of the sites sampled over three consecutive years, there was substantial year-to-year variation in the concentrations of Cu, Se, and Zn. We also documented some variation between sites, but only between sites sampled in different years, which may reflect additional temporal, rather than spatial, variation. Concentrations of Cu and Ag were significantly positively correlated with one another, as were concentrations of Se and Zn. Contrary to expectation, there were no significant relationships between body weight and metal concentrations. Finally, comparison with results from previous metal toxicology studies of nine-banded armadillos (Dasypus novemcinctus) and Virginia opossums (Didelphis virgianus), collected from the same sites during the same years, showed differential patterns of accumulation across species, suggesting that ecological lifestyle is an important influence on metal accumulation. This study provides reference levels of metal contaminants in the liver of bobcats as well as insight into metal accumulation in a top level carnivore.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthropogenic activity has led to an increase in environmental contamination, which can disrupt natural ecosystems (Bilandzic et al. 2012; Pyati et al. 2012). In particular, metal pollution is common in many developed areas due to intensive forestry, mining, urban development, and industrial and agricultural practices, with elevated levels of heavy metals reported in both aquatic and terrestrial environments (Wang et al. 2011; Lombardi et al. 2012; Feleafel and Mirdad 2013). Metal accumulation in wildlife may occur as a result of excess metals in the environment, which in turn can lead to toxicity when concentrations exceed certain threshold levels (Eisler 1993, 1997; Goyer 1996; Guidotti et al. 1997; Riley et al. 2003; Mullally et al. 2004). Additionally, metal accumulation may also increase the susceptibility of animals to infectious diseases (Nriagu and Skaar 2015). Monitoring contaminants in wildlife is important for many reasons, including: (1) to provide early warning of potential human health risks, (2) determine possible cleanup techniques, (3) evaluate the success of past remediation strategies, and (4) assess the general health of an ecosystem (Burger et al. 2000).
However, evaluating every species for exposure to every type of toxicant is unrealistic and extremely tedious. Therefore, a more reasonable approach is to assess certain sentinel species as the primary bioindicators for multiple members of particular ecosystems (Jarvis et al. 2013).
Among mammals, bobcats (Lynx rufus) have certain qualities that make them an ideal bioindicator species for terrestrial toxicology studies. First, they are the most common wildcat and are widely distributed throughout the entire continental USA, as well as northern Mexico and southern parts of Canada (Roberts and Crimmins 2010). Second, they are medium-sized, non-migratory animals that are adaptable to various terrains but tend to avoid human-altered habitats. Indeed, bobcats were the mammals least associated with urban environments when compared with coyotes, raccoons, foxes, skunks, armadillos, and opossums (Gaines et al. 2002). Because bobcats are less likely to come into direct contact with human products and wastes, they would seem to provide a more accurate assessment of the contaminant levels in natural environments than would more opportunistic animals (Gaines et al. 2002). Finally, while many toxicology studies have been conducted on omnivores (Wijnhoven et al. 2007; Mariniakova et al. 2011; Lockhart et al. 2016), far fewer have examined mammalian carnivores (Cumbie and Jenkins 1974; Cumbie 1975; Millan et al. 2008). The principal finding of these earlier studies was that carnivores tended to have higher heavy metal concentrations than omnivores and herbivores within an area, perhaps due to higher exposure to soil contamination while foraging, or through consumption of contaminated prey (Cumbie and Jenkins 1974; Wijnhoven et al. 2007). Bobcats feed on many soil-dwelling species of rodents and rabbits, as well as deer (Jones and Smith 1979; Larivière and Walton 1997); therefore, studying the concentration of metals in bobcats may provide insight into patterns of bioaccumulation and biomagnification in food webs (Gaines et al. 2002).
The present study is part of an ongoing series of analyses with the intent of documenting metal accumulation in a mammal assemblage from south Georgia and north Florida, USA. Previously, two species with extensive direct contact with soils (via burrowing and/or consumption of soil-dwelling prey) were examined: the nine-banded armadillo (Dasypus novemcinctus; Jarvis et al. 2013) and Virginia opossum (Didelphis virginianus; Lockhart et al. 2016). These species provide useful information on metal accumulation in an insectivore (armadillo) and omnivore (opossum), but information is still lacking on patterns of accumulation in species near the top of the food chain, such as the bobcat. As stated previously, one might expect patterns of metal accumulation in carnivorous bobcats to be very different, in part because of differences in ecological lifestyle (which may result in different levels of exposure to contaminants), as well as differences in how metals are processed within each species, which may reflect the relative importance of processes such as biomagnification.
Essential metals are necessary for animals in trace quantities for normal physiological processes; however, at elevated concentrations, both essential and nonessential metals may become toxic and can cause adverse effects (Guidotti et al. 1997; Mullally et al. 2004). Upon entering an animal, metals may be used in various physiological processes, accumulate in tissues, or they can be eliminated through excretion (Casarett et al. 2008). For many studies, the liver is chosen to assess metal accumulation because it is an important organ for processing, detoxifying, and excreting toxicants, and metal accumulation has been reported in this organ following chronic exposure (Nwokocha et al. 2012; Jarvis et al. 2013; Roggeman et al. 2014). Additionally, metal accumulation in the liver of mammals has been associated with liver toxicity and oxidative stress (Cook et al. 1991; Suzuki et al. 1996; Avsaroglu et al. 2005; Cesur et al. 2005; Flora et al., 2008).
Metal tissue concentrations in several small mammals are well known (Eisler 1985, 1993, 1996, 1997; Burger et al. 2000; Shore and Rattner 2001; Gaines et al. 2002; Wijnhoven et al. 2007; Mariniakova et al. 2011; Jarvis et al. 2013); however, reports about top level carnivores are much scarcer. To date, mercury is the only metal which has been extensively studied in top level carnivores, including bobcats (Cumbie and Jenkins 1974; Cumbie 1975; Millan et al. 2008). Here, we provide the first analysis of four other common metals: copper (Cu), selenium (Se), silver (Ag), and zinc (Zn). This information should be valuable in providing baseline, or reference, values for these metals in bobcats that can be used in future studies. In addition, because the bobcats were collected from four different sites over 4 years (2003–2006), we were able to analyze both spatial and temporal variation in metal concentrations. Finally, we compare our values against those obtained previously for armadillos and opossums, collected in the same areas and during the same years, in order to evaluate how trophic position influences patterns of metal accumulation.
Methods
Sample collection
Bobcats were collected from four locations in Georgia and Florida, USA between 2003 and 2006. The project was conducted as a byproduct of a Wildlife Management and Gamebird Restoration Project conducted by the University of Georgia, Auburn University, Tall Timbers Research Station, Joseph Jones Ecological Research Station, and the USDA-Wildlife Services from 2001 to 2006. The objective of that larger research effort was to determine whether spring and summer trapping to remove mammalian nest predators of quail (northern bobwhite, Colinus virginianus) would provide landowners with a means of enhancing wildlife production and populations on their land (McDonough et al. 2007). The project was conducted on private properties in the area of Albany and Thomasville, Georgia. Predators were removed by technicians of the USDA – Georgia Wildlife Services from 1 March to 30 September of each year by either trapping or shooting, using standard protocols (Lockhart, unpublished data). As a consequence of this activity, each year, a large number of mammalian carcasses were thus available for scientific study. We were fortunate to be given access to these animals for necropsy starting in 2003. However, it is important to stress that we had no role in the larger study design nor were we involved in the capture and euthanasia of animals.
For the present study, we used samples that were collected from the eastern portion of Pinebloom Plantation, located near Albany, GA, and Pebble Hill Plantation near Thomasville, GA in 2003. We then used samples that were collected on the western portion of Pinebloom Plantation and at Tall Timbers Research Station, located near Tallahassee, FL from 2004 to 2006 (for more details, including a map of the sampling sites, see Jarvis et al. 2013; Lockhart et al. 2016). All sites were managed for wildlife (primarily game species for hunting) with prescribed burns and other routine management activities; Pinebloom and Pebble Hill were also managed as pine plantations. Overall, the anecdotal impression was that the level of human disturbance occurring at each site was reasonably similar.
Metal analyses
After euthanasia, a portion of liver tissue was obtained from each animal collected. Liver samples were kept frozen at −20 °C in vials until analyzed. Upon analysis, liver samples were weighed for their wet weight (ww), dried at 80 °C for at least 24 h, and then weighed again for their dry weights (dw). The mean ± SD of the dw and ww samples were 0.29 ± 0.12 and 1.05 ± 0.34 g, respectively, yielding a ww/dw ratio of 3.62. Once dried, liver samples were placed in 15-ml polypropylene centrifuge tubes, trace metal grade nitric acid (Fisher Scientific, Pittsburgh, PA, USA) was added to the tubes, which were then placed in a water bath at 75 °C for 6–8 h until fully digested. Samples were then passed through a 47-mm filter to ensure that no undigested particles remained within the tube. The digested samples were diluted with 18 mΩ Milli-Q water and analyzed for metals using atomic absorption spectrophotometry (GFAAS; Perkin Elmer AAnalysts 800, Norwalk, CT, USA) with flame or graphite furnace detection (detection limit =1 ppb). Certified 1 g/mL metal standards was used, and recalibration was performed every 40 samples. Standards and samples were analyzed in triplicate. To determine average extraction efficiencies, we treated three replicates of a certified reference material (LUTS-1, lobster hepatopancreas) as samples. Efficiencies for LUTS-1 were 99.5 % for Cu, 98.8 % for Se, 99.4 % for Zn, and 84.1 % for Ag. This method of digestion and metal analysis has proven reliable and effective in several other studies in our laboratory (Main et al. 2010; Jarvis et al. 2013; Lockhart et al. 2016).
Statistical analyses
All data were analyzed for normality and equality of variance using a Shapiro-Wilk’s test and a Bartlett’s test, respectively. We then performed five sets of analyses. First, we used a two-way ANOVA to examine variation in metal concentrations across sites and between males and females. Bonferroni-Dunn tests were used to conduct post-hoc, pair-wise comparisons. It is important to point out that we were unable to examine age differences in our bobcat data. Unlike our previous studies of armadillos and opossums, there were very few accurate age estimates available for the bobcats. Although body weight has been used as a predictor of general age categories (juvenile, yearling, adult) in armadillos (Loughry and McDonough 1996), this was not useful for bobcats because all animals weighed at least 3.32 kg (mean ± SD = 6.93 ± 1.67 kg, range = 3.32–11.25 kg, n = 107). These weights suggest that all of our bobcat specimens were probably ≥1 year old (Crowe 1975; Larivière and Walton 1997). Thus, for the purposes of this study, we assume that all samples came from adult or near-adult individuals. Bobcats can exhibit sexual dimorphism in body weight (Crowe 1975; Litvaitis et al. 1984), which is supported by our data (mean ± SD weight for males =7.66 ± 1.62 kg, females = 6.10 ± 1.32 kg, t = 5.39, P < 0.0001). Even so, sex differences in body weight did not appear to influence our results because we found no differences in metal concentrations between males and females (see “Results”) and obtained the same pattern of results when each sex was analyzed separately. Consequently, we only report results using pooled data from both sexes in all subsequent analyses.
For the next analysis, we used a one-way ANOVA to examine temporal (i.e., year-to-year) variation at the two sites that were sampled in three consecutive years (Pinebloom West and Tall Timbers). Because we found no evidence of differences in metal concentrations between these two sites (see “Results”), data were pooled not only across males and females but also across the two sites for this analysis.
The third analysis entailed a series of Pearson product-moment tests in order to determine if metal concentrations were correlated with one another. We also used Pearson correlations to examine relationships between metal concentrations and body weight. Other studies have reported negative correlations between levels of metal accumulation and body condition in aquatic animals (Bielmyer et al. 2005, 2006). Here, we use body weight as a proxy for body condition. For all correlation analyses, data were pooled across sites and sexes.
Finally, we used an ANOVA to compare metal concentrations in the livers of bobcats against those obtained in the previous studies of armadillos and opossums, collected at the same sites and in the same years. It was not our intent to provide a detailed analysis of species differences, rather just a general picture of how they might differ. Thus, data were pooled across all sites and individuals for this analysis. Note that, because a different set of metals was examined in each species, the analysis was limited to comparisons of Cu and Zn across all three species, and Se for bobcats versus opossums. All statistical analyses were performed using Statview 4.01.
In all that follows, bobcat liver metal concentrations are presented as mg metal/kg tissue dw, means are presented ± SE, and statistical significance is recognized at P ≤ 0.05.
Results
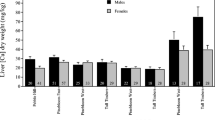
There was no evidence of sex differences in liver concentrations of any metal (Table 1; Figs. 1, 2, 3, and 4). However, with the marginal exception of Cu, concentrations of all metals differed between sites (Table 1; Figs. 1, 2, 3, and 4). There was also one significant sex × site interaction (Zn, see Table 1 and Fig. 4). While these results indicate substantial spatial variation in metal concentrations, it is important to note that all post-hoc comparisons involved differences between sites sampled in 2003 versus sites sampled subsequently (Table 1). There were no cases where sites sampled in the same year(s) differed from one another. Given the extensive temporal variation we found in metal concentrations (see below), we cannot discount the hypothesis that the site differences we have found represent a further instance of temporal, rather than spatial, variation.
Temporal variation was found in the concentrations of all metals except Ag, with Cu concentrations much higher in 2004 than in 2005 or 2006 at Tall Timbers and Pinebloom West (Table 1; Fig. 1). However, Se concentrations were lower at the same sites in 2004 than either of the succeeding years (Table 1, Fig. 2), while Zn concentrations differed significantly in all pair-wise comparisons between years (Table 1, Fig. 4).
Pair-wise correlations among metal concentrations uncovered only two significant relationships: levels of Cu and Ag were positively correlated with one another, as were Se and Zn (Table 2). In contrast to our expectation, there was no significant relationship between body weight and any metal concentration (r values ranged from 0.01 for Cu to 0.07 for Se and Ag, all P > 0.53; because of missing data for weight and/or metal concentration, sample sizes ranged from 76 for Ag to 105 for Cu).
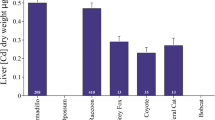
There were dramatic differences in metal concentrations between species (Fig. 5), with strongly significant differences in each case (ANOVA, each P < 0.0001). Bobcats exhibited much higher levels of Cu compared to both opossums and armadillos (Bonferroni-Dunn tests, both P < 0.0001; the latter two species did not differ from one another). Concentrations of Zn were also higher in bobcats than armadillos (Bonferroni-Dunn test, P < 0.0001) but did not differ from those found in opossums (Fig. 5). Interestingly, opossums had higher concentrations of Se than did bobcats (F = 103.42, P < 0.0001), and also higher levels of Zn than did armadillos (Bonferroni-Dunn test, P < 0.0001).
Comparison of liver metal accumulation in three species sampled at the same sites and in the same years. Note that armadillos were not tested for selenium. Sample sizes are provided within each bar. Different letters indicate significant differences between species for each metal. Armadillo and opossum metal concentrations were reported in past studies in our laboratory (Jarvis et al. 2013; Lockhart et al. 2016)
Discussion
As with our previous studies, our analyses of metal accumulation in the livers of bobcats are inherently retrospective, exploiting the availability of animals collected for other purposes. Consequently, we do not have much in the way of ancillary data that might help to explain the patterns we have observed. Nonetheless, our results have value in presenting the first baseline, or reference, values for Cu, Se, Ag, and Zn in bobcats and, as such, set the stage for future investigations. However, it is important to bear in mind that the temporal variation we have documented means that there may be no consistent, universal standard set of values for this species (Lockhart et al. 2016). Also, the fact that concentrations of some metals were strongly correlated with one another (Table 2) indicates values may not be completely independent and may reflect environmental exposure.
Although we cannot fully explain the temporal differences in metal concentrations that we found at Tall Timbers and Pinebloom West (see Table 1), one plausible contributing factor is immigration. It seems likely that the extensive predator removal program at each site rapidly depleted the resident population of bobcats (see also McDonough et al. 2007; Jarvis et al. 2013). Consequently, metal concentrations of animals collected in subsequent years (2005 and 2006) may reflect the prior exposures of individuals off-site. If so, this argument provides a somewhat ironic twist to our proposal earlier (see “Results”) that differences in metal concentrations between sites might actually reflect additional temporal, as opposed to spatial, variation because the temporal variation we found at Tall Timbers and Pinebloom West might in fact result from spatial variation in the exposure of immigrating bobcats to metals at previous locations.
Aside from extensive temporal variation, our other major finding was the dramatic difference in patterns of metal accumulation between bobcats, armadillos and opossums (Fig. 5). This suggests that the unique ecological lifestyle of each species leads to differential levels of exposure to particular metals, and/or that the species differ substantially in how metals are processed in the body (Cumbie and Jenkins 1974; Wijnhoven et al. 2007). Regardless, the fact that there were species differences in the concentrations of some metals would seem to refute the hypothesis that liver metal concentrations merely reflect ambient levels in the environment. If such were the case, one would expect all species to have similar values, which they clearly did not.
Of the metals analyzed in this study, Cu, Zn, and Se are essential, and Ag is a nonessential element to most organisms. Because of their abundance, Cu and Zn both accumulate to some degree in biota (Eisler 1993, 1997). Both metals act as vital constituents of many enzymes and are necessary for normal growth and other somatic cell functions (Prasad 1979; Aaseth and Norseth 1986; Eisler 1993, 1997). However, in excess, both Cu and Zn may exert toxicity (Eisler 1993, 1997). Animals containing elevated tissue Cu concentrations are generally found in areas with more anthropogenic impact, such as those treated with Cu-containing herbicides, near smelters, and from heavily urbanized and industrialized areas (Eisler 1997). Increased Zn levels in the environment are primarily due to corrosion of Zn alloys and galvanized surfaces, and erosion of agricultural soils (Eisler 1993).
Like Cu and Zn, Se is also nutritionally important; however, there is relatively less known about Se deficiency and the function of Se in organisms (Eisler 1985). Excess Se enters the environment primarily through the combustion of fossil fuels, metal smelting, and other industrial processes; however, natural sources can be very high in some areas also (Eisler 1985). Increased Se exposure may cause adverse growth, survival, and reproduction in mammals; however, Se sensitivity can be highly variable in different classes of organisms (Eisler 1985).
Increased Ag concentrations in biota have been reported near sewage outfalls, electroplating plants, and mine waste sites, and prior to the last decade, photo processing and development was the major anthropogenic source of Ag contamination in the environment (Eisler 1996; Luoma et al. 1999). Silver uptake in mammals can generally occur through inhalation, ingestion, and movement across mucous membranes (Eisler 1996). Ag has been shown to accumulate in mammalian tissues and increased accumulation has been correlated with increased age of the individual (Eisler 1996). Ag has been shown to inhibit enzymes by mimicking essential elements, resulting in toxicity, even after exposure to low levels (Luoma et al. 1999).
The high concentrations of some metals in bobcat livers lead to concerns about whether these levels were high enough to be toxic (Riley et al. 2003). Obviously, without previous reference values, this is a difficult question to answer. There are three indirect lines of evidence which suggest that the bobcats in our study were not suffering from toxicity. First, studies of metal accumulation in other species of mammals have reported concentrations as high as or higher than those we obtained for bobcats without detecting toxic effects (Eisler 1993; Shore and Rattner 2001; Millan et al. 2008). For example, the liver copper concentration in terrestrial mammals such as the dog (336 mg/kg dw), horse (10–51.3 mg/kg dw), muskox (67 mg/kg dw), and caribou (68 mg/kg dw) that were not suffering from toxicity (Eisler 1997) were within the range reported here. In contrast, the liver copper concentrations in mammals (impalas, cattle) found dead near contaminated sites ranged from 444 to 1078 mg/kg dw (Eisler 1997), which were substantially higher than the Cu concentrations we found. Likewise, liver zinc concentrations in normal (symptom-free) cattle, horses, bank voles, and goats ranged from 23 to 150 mg/kg dw, comparable to our study, whereas zinc-poisoned cattle and horses had liver zinc concentrations of 2000 and 402 mg/kg dw, respectively (Eisler 1993). Fleming et al. (1979) reported Se liver concentrations in the woodchuck, Marmota monax, ranging from 0.4 to 10.7 mg/kg dw, similar to the range reported in our study; however, the higher concentrations were closer to a fly ash landfill. In our study, the higher Se concentrations observed in 2005 and 2006 at Pinebloom West and Tall Timbers were similar to those found in woodchucks collected near the landfill (Fleming et al. 1979), suggesting that perhaps the bobcat were exposed to increased levels of Se. However, it is also possible that there could be species differences that account for the differences in Se accumulation. Ag concentration in the livers of various terrestrial mammals was reportedly less than 50 mg/kg dw (Eisler 1996), which is within the range of that observed in this study (i.e., <16 mg/kg Ag).
A second line of evidence in support of the hypothesis that the metal concentrations we found in bobcats were not toxic concerns the fact that, although we did not systematically autopsy the animals in our study, in collecting tissue samples we did not notice many glaring pathologies, which would suggest direct toxic effects were not occurring. Finally, the fact that metal concentrations were not correlated with body weight further supports the idea that metals were not negatively impacting individual condition. Admittedly, none of these lines of evidence is particularly compelling on its own, but collectively they seem to indicate an absence of direct toxic effects from the four metals examined. Even so, we cannot eliminate the possibility of indirect effects, such as increased susceptibility to infectious diseases (Nriagu and Skaar 2015).
The only other study of metal accumulation in bobcats was by Cumbie and Jenkins (1974), who presented a detailed analysis of mercury concentrations (from hair and tissue samples) in various mammals from Georgia, Florida, and South Carolina. Our research provides an important complement to this work by providing data on concentrations of other metals in a mammal assemblage located in an ecologically similar part of the United States. With regard to bobcats, their high trophic position, coupled with other features of their ecology, suggest that they may be useful bioindicators of metal bioaccumulation in terrestrial ecosystems. Further research is needed to determine if the metals accumulated are toxic to bobcats, and if anthropogenic activities are related to tissue concentrations.
References
Aaseth, J., & Norseth, T. (1986). Copper. In L. Friberg, G. F. Nordberg, & V. B. Vouk (Eds.), Handbook on the toxicology of metals. Second edition. Volume II: specific metals (pp. 233–254). New York: Elsevier.
Avsaroglu, D. T., Inal, T. C., Demir, M., Attila, G., Acarturk, E., Evlice, Y. E., & Kayrin, L. (2005). Biochemical indicators and cardiac functions tests in chronic alcohol abusers. Croatian Medical Journal, 46, 233–237.
Bielmyer, G. K., Tomasso, J., Gatlin, D., Isely, J., & Klaine, S. J. (2005). Responses of hybrid striped bass to waterborne and dietary copper in freshwater and saltwater. Comparative Biochemistry and Physiology Part C., 140, 131–137.
Bielmyer, G. K., Tomasso, J., & Klaine, S. J. (2006). Physiological responses of hybrid striped bass to aqueous copper in freshwater and saltwater. Archives of Environmental Contamination and Toxicology, 50, 531–538.
Bilandzic, N., Sedak, M., Dokic, M., & Simic, B. (2012). Metal concentrations in tissues of wild boar of continental Croatia. International Journal of Environment and Pollution, 2, 6–9.
Burger, J., Lord, C. G., Yurkow, E. J., & McGrath, L. (2000). Metals and metallothionein in the liver of raccoons: utility for environmental assessment and monitoring. Journal of Toxicology and Environmental Health, 60, 243–261.
Casarett, L. J., Doull, J., & Klaasen, C. D. (2008). Casarett and Doull’s toxicology: the basic science of poisons (7th ed.). New York: McGraw-Hill.
Cesur, S., Cebeci, S. A., Kavas, G. O., Aksaray, S., & Tezeren, D. (2005). Serum copper and zinc concentrations in patients with chronic hepatitis. British Journal of Infection Control, 51, 38–40.
Cook, C. C. H., Walden, R. J., Graham, B., Gillham, C., Davies, S., & Prichard, B. N. C. (1991). Trace element and vitamin deficiency in alcoholic and control subjects. Alcohol, 26, 541–548.
Crowe, D. M. (1975). Aspects of ageing, growth, and reproduction of bobcats from Wyoming. Journal of Mammalogy, 56, 177–198.
Cumbie, P. M. (1975). Mercury in hair of bobcats and raccoons. Journal of Wildlife Management, 39, 419–425.
Cumbie, P. M., Jenkins, J. H. (1974). Mercury accumulation in native mammals of the southeast. In Proceedings of the 28th Annual Conference of the Southeastern Association of Game and Fish Commissioners. United States.
Eisler, R. (1985). Selenium hazards to fish, wildlife, and invertebrates: a synoptic review. U.S. Geological Survey, Biological Resources Division, Biological Science Report, 5, 1–40.
Eisler, R. (1993). Zinc hazards to fish, wildlife, and invertebrates: a synoptic review. U.S. Fish and Wildlife Service, Biological Science Report, 10, 106.
Eisler, R. (1996). Silver hazards to fish, wildlife, and invertebrates: a synoptic review. U.S. Geological Survey, Biological Resources Division, Biological Science Report, 32, 1–63.
Eisler, R. (1997). Copper hazards to fish, wildlife, and invertebrates: a synoptic review. U.S. Geological Survey, Biological Resources Division, Biological Science Report, 33, 1–98.
Feleafel, M. N., & Mirdad, Z. M. (2013). Hazard and effects of pollution by lead on vegetable crops. Journal of Agricultural and Environmental Ethics, 26, 547–567.
Fleming, W. J., Gutenmann, W. H., & Lisk, D. J. (1979). Selenium in tissues of woodchucks inhabiting fly ash landfills. Bulletin of Environmental Contamination and Toxicology, 21, 1–3.
Flora, S. J., Mittal, M., & Mehta, A. (2008). Heavy metal induced oxidative stress and its possible reversal by chelation therapy. Indian Journal of Medical Research, 128, 501–523.
Gaines, K. F., Romanek, C. S., Boring, C. S., Lord, C. G., Gochfeld, M., & Burger, J. (2002). Using raccoons as an indicator species for metal accumulation across trophic levels: a stable isotope approach. Journal of Wildlife Management, 66, 811–821.
Goyer, R. (1996). Toxic effects of metals. In C. D. Klaassen (Ed.), Casarett & Doull’s toxicology (pp. 691–736). New York: McGraw-Hill.
Guidotti, T. L., Audette, R. J., & Martin, C. J. (1997). Interpretation of the trace metal analysis profile for patients occupationally exposed to metals. Occupational Medicine, 47, 497–503.
Jarvis, T. A., Lockhart, J. M., Loughry, W. J., & Bielmyer, G. K. (2013). Metal accumulation in wild nine-banded armadillos. Ecotoxicology, 22, 1053–1062.
Jones, J. H., & Smith, N. S. (1979). Bobcat density and prey selection in Central Arizona. Journal of Wildlife Management, 43, 666–672.
Larivière, S., & Walton, L. R. (1997). Lynx rufus. Mammalian Species, 563, 1–8.
Litvaitis, J. A., Stevens, C. L., & Mautz, W. W. (1984). Age, sex, and weight of bobcats in relation to winter diet. Journal of Wildlife Management, 48, 632–635.
Lockhart, J. M., Siddiqui, S., Loughry, W. J., & Bielmyer-Fraser, G. K. (2016). Metal accumulation in wild-caught opossum. Environmental Monitoring and Assessment, 188, 317.
Lombardi, G., Lanzirotti, A., Qualls, C., Socola, F., Ali, A. M., & Appenzeller, O. (2012). Five hundred years of mercury exposure and adaptation. Journal of Biomedicine and Biotechnology, 2012, 1–10.
Loughry, W. J., & McDonough, C. M. (1996). Are road kills valid indicators of armadillo population structure? American Midland Naturalist, 135, 53–59.
Luoma, S. N., Hogstrand, C., Bell, R. A., Bielmyer, G. K., Galvez, F., LeBlanc, G. A., Lee, B. G., Purcell, T. W., Santore, R. C., Santschi, P. H., & Shaw, J. R. (1999). In A. W. Andren & T. W. Bober (Eds.), Silver in the environment: transport, fate, and effects (pp. 65–97). Madison, Wisconsin, USA: University of Wisconsin Sea Grant.
Main, W. P. L., Ross, C., & Bielmyer, G. K. (2010). Copper accumulation and oxidative stress in the sea anemone, Aiptasia pallida, after waterborne copper exposure. Comparative Biochemistry and Physiology Part C, 151, 216–221.
Mariniakova, M., Omelka, R., Stawarz, R., & Formicki, G. (2011). Accumulation of lead, cadmium, nickel, iron, copper, and zinc in bones of small mammals from polluted areas in Slovakia. Polish Journal of Environmental Studies, 21, 153–158.
McDonough, C. M., Lockhart, J. M., & Loughry, W. J. (2007). Population dynamics of nine-banded armadillos: insights from a removal experiment. Southeastern Naturalist, 6, 381–392.
Millan, J., Mateo, R., Taggart, M. A., Lopez-Bao, J. V., Viota, M., Monsalve, L., Camarero, P. R., Blazquez, E., & Jimenez, B. (2008). Levels of heavy metals and metalloids in critically endangered Iberian lynx and other wild carnivores from southern Spain. Science of the Total Environment, 399, 193–201.
Mullally, A. M., Vogelsang, G. B., & Moliterno, A. R. (2004). Wasted sheep and premature infants: the role of trace metals in hematopoiesis. Blood Reviews, 18, 227–234.
Nriagu, J. O., & Skaar, E. P. (2015). Trace metals and infectious diseases. Cambridge, MA: MIT Press.
Nwokocha, C. R., Owu, D. U., Nwokocha, M. I., Ufearo, C. S., & Iwuala, M. O. E. (2012). Comparative study on the efficacy of Allium sativum (garlic) in reducing some heavy metal accumulation in liver of wistar rats. Food and Chemical Toxicology, 50, 222–256.
Prasad, A. S. (1979). Clinical, biochemical, and pharmacological role of zinc. Annual reviews in pharmacology and. Toxicology, 20, 393–426.
Pyati, R., Bielmyer, G. K., Chalk, S., McCarthy, D., McCarthy, H., Pinto, G., Sonnenberg, L., Welsh, P. (2012). Case study: St. Johns river basin, USA. In: Fourth United Nations World Water Development Report, World Water Assessment Programme 2012. UNESCO Publishing, France.
Riley, M. R., Boesewetter, D. E., Kim, A. M., & Francisco, P. S. (2003). Effects of metals Cu, Fe, Ni, V, and Zn on rat lung epithelial cells. Toxicology, 190, 171–182.
Roberts, N. M., & Crimmins, S. H. (2010). Bobcat population status and management in North America: evidence of large-scale population increase. Journal of Fish and Wildlife Management, 2, 169–174.
Roggeman, S., de Boeck, G., De Cock, H., Blust, R., & Bervoets, L. (2014). Accumulation and detoxification of metals and arsenic in tissues of cattle (Bos taurus), and the risks for human consumption. Science of the Total Environment, 466-467, 175–184.
Shore, R. F., & Rattner, B. A. (Eds.) (2001). Ecotoxicology of wild mammals. Ecotoxicology and environmental toxicology series. New York: Wiley.
Suzuki, K., Oyama, R., Hayashi, E., & Arakawa, Y. (1996). Liver disease and essential trace elements. Nihon Rinsho, 54, 85–92.
Wang, B. S., Goodkin, N. F., Angeline, N., Switzer, A. D., You, C. F., & Hughen, K. (2011). Temporal distributions of anthropogenic Al, Zn, and Pb in Hong Kong Porites Coral during the last two centuries. Marine Pollution Bulletin, 63, 508–515.
Wijnhoven, S., Leuven, R. S. E. W., van der Velde, G., Jungheim, G., Koelemij, E. I., de Vries, F. T., Eijsackers, H. J. P., & Smits, A. J. M. (2007). Heavy-metal concentrations in small mammals from a diffusely polluted floodplain: importance of species-and location-specific characteristics. Archives of Environmental Contamination and Toxicology, 52, 603–613.
Acknowledgments
The authors would like to thank the USDA-WS for collection of the samples, and Samreen Siddiqui and Eric Barber for assistance in analyzing some of the samples. Thanks also to Corey Anderson for advice on statistical analyses. We are grateful for the comments of two anonymous reviewers in improving this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thomason, R.K., Lockhart, J.M., Loughry, W.J. et al. Metal accumulation in bobcats in the Southeastern USA. Environ Monit Assess 188, 565 (2016). https://doi.org/10.1007/s10661-016-5587-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-016-5587-6