Abstract
The Virginia opossum (Didelphis virginiana) is widespread in the USA, ranging south through Latin America. The ecology of opossums is such that they are in frequent contact with soils, suggesting that they may function as a valuable bioindicator for chemical contamination in terrestrial environments. Surprisingly, there have been virtually no toxicology studies on opossums. Here, we provide the first analysis of metal contaminants in opossum liver tissues. Liver samples were obtained from 471 opossums, collected from 2003 to 2006, at four sites in North Florida and South Georgia, USA, and concentrations of copper, lead, nickel, selenium, and zinc were measured. We found little evidence of age differences in the concentration of any of the metals. However, there were at least some significant differences between years, males and females, and between sites for each metal, although the pattern of these differences was not always consistent across metals. Concentrations of metals in liver tissue were positively correlated with one another, primarily of each metal (except Pb) with zinc. Reference levels of metal contaminants are not available for opossums, but concentrations of Cu, Ni, Pb, and Zn in our samples were for the most part significantly higher than those reported from liver tissues of nine-banded armadillos (Dasypus novemcinctus) collected at the same sites and in the same years. Data from other small mammals studied elsewhere further indicate that metal concentrations in opossums were high, but at this time, it is not possible to determine if these elevated levels generated toxicity. The substantial temporal and spatial variation we found in metal concentrations suggests that determination of baseline levels for opossums may not be straightforward. Nonetheless, this is the first study quantifying metal accumulation in the livers of Didelphis virginiana and, as such, provides an important starting point for future research.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metals are naturally occurring components of sediment. As sediment composition varies in different geographical areas, so does the concentration of metals. However, anthropogenic contributions of metals to the environment are generally much greater than natural contributions (Eisler 1988, 1993, 1997, 1998). Metals enter natural systems via industrial effluent, agricultural and storm water runoff, sewage treatment discharge, fossil fuel combustion, ore smelting and refining, mining processes, and various metal-working enterprises (Baird 1995). The influence of anthropogenic disturbance on natural habitats is a growing area of research (Pyati et al. 2012), particularly as it pertains to effects on wildlife. Some metals are essential for animals in trace quantities for normal physiological processes; however, at elevated concentrations, both essential and nonessential metals may become toxic and can cause adverse effects (Guidotti et al. 1997; Mullally et al. 2004). Assessment of adverse effects requires knowledge of baseline metal tissue concentrations. Unfortunately, with a few exceptions (Burger et al. 2000; Shore and Rattner 2001; Gaines et al. 2002; Wijnhoven et al. 2007; Mariniakova et al. 2011; Jarvis et al. 2013), this information is largely unknown for most wildlife species.
When metals enter an animal, they may be used in various physiological processes, they can accumulate in tissues, such as the liver, feathers, or hair, or they can be eliminated through excretion (Phelps et al. 1980; Goede and De Bruin 1984, 1986). The liver is a vital organ for the detoxification and excretion of various chemicals, and through this process, contaminants may accumulate in the liver, particularly after chronic exposure (Casarett et al. 2008; Nwokocha et al. 2012; Roggeman et al. 2014). Liver analysis is often performed in ecotoxicological studies of mammals in order to characterize metal exposure (Damek-Poprawa and Sawicka-Kapusta 2004; Sánchez-Chardi et al. 2007; Levengood and Heske 2008; Jarvis et al. 2013; Salińska et al. 2013). Furthermore, previous studies have correlated metal tissue concentrations in various terrestrial animals with liver diseases (Cook et al. 1991; Suzuki et al. 1996; Cesur et al. 2005; Avsaroglu et al. 2005).
Didelphis virginiana, the Virginia opossum, is a small (adults typically weigh 0.8–2.5 kg; Gardner 1973) terrestrial marsupial whose ecological lifestyle makes exposure to soil-borne metal contaminants likely (Shore and Rattner 2001). Specifically, while foraging and resting, D. virginiana is often in intimate contact with soils. Likewise, its dietary preferences lead to consumption of prey that also have considerable contact with soils (Hunsaker and Shupe 1977). A further reason for conducting toxicology studies of D. virginiana is that it is widely distributed throughout the western hemisphere (Hunsaker 1977) and is often found in urban areas (Harmon et al. 2005). Thus, because of its distribution and ecology, D. virginiana represents an ideal species in which to study the impacts of urbanization and anthropogenic pollution. However, as mentioned earlier, studying such impacts requires as a first step knowledge of the baseline levels of metal concentrations in opossum tissues. The goal of this study was to provide such data and evaluate the potential of opossums as a bioindicator for metal contaminants in terrestrial habitats.
Methods
Opossums were collected from four different locations in south Georgia and north Florida between 2003 and 2006 as part of a larger study examining the effects of removal of nest predators on northern Bobwhite (Colinus virginianus; see McDonough et al. 2007; Jarvis et al. 2013). In 2003, opossums were collected from the eastern side of Pinebloom Plantation near Albany, Georgia (31° 59′/84° 34′), and Pebble Hill Plantation near Thomasville, Georgia (30° 78′/84° 06′). In 2004–2006, this experiment was repeated within the western portion of Pinebloom Plantation and at Tall Timbers Research Station, located near Tallahassee, Florida (30° 66′/84° 20′). Opossums at all sites were euthanized by technicians from the US Department of Agriculture-Wildlife Services between March 1 and September 30 of each year.
Liver samples were obtained from each collected animal and stored in vials at −20 °C until they were analyzed. In addition, a tooth was taken and used for age determination by Matson’s Laboratories (Milltown, MT). However, this was only done for males in 2003 (n = 77), whereas it was done in all 4 years of the study for most females (n = 280).
Metal analysis
Liver samples were thawed and then weighed to obtain wet weights (mean ± SE = 1.00 ± 0.45 g). Subsequently, samples were dried in an oven for 2–3 h at 80 °C and weighed again to determine dry weight (dw). The mean dw ± SE for the liver samples was 0.125 ± 0.023 g. Dried liver samples were digested with trace metal grade nitric acid (Fischer Scientific, Pittsburgh, PA, USA) and then heated in a 60 °C water bath for at least 24 h. After full digestion, samples were diluted with deionized Milli-Q® water and then analyzed for copper (Cu), lead (Pb), nickel (Ni), selenium (Se), and zinc (Zn) content using atomic absorption spectrophotometry (AAS; PerkinElmer AAnalysts 800, Norwalk, CT, USA) with flame or graphite furnace detection (detection limit ∼1 μg/L for all metals). These particular metals have been detected in the liver tissues of other mammals in previous studies in our laboratory (Jarvis et al. 2013). Certified standards from Ricca Chemical Company (Arlington, TX with 3 % HCl) were used for each calibration, and recalibration was performed after every 40 samples. Two types of lobster hepatopancreas from National Research Council, Canada [LUTS-1 (Non defatted) and TORT-3] were used as reference materials (three replicates each) and treated the same way as the samples to determine metal extraction efficiencies. Efficiencies for LUTS-1 and TORT-3 were 99.5 and 95.8 % for Cu, 95.7 and 92.6 % for Pb, 84.4 and 91.7 % for Ni, 98.8 and 90.8 % for Se, and 99.4 and 96.2 % for Zn, respectively. Data are reported as milligram metal per kilogram dw tissue.
Statistical analysis
All the data were analyzed for normality and equality of variance using a Shapiro-Wilk’s test and a Bartlett’s test, respectively. We initially ran Spearman rank order correlations of metal concentrations versus age in years in order to determine whether we could pool data across age groups. Because most age data came from females (see above), we analyzed the data first using just data from females (age range = 0–7 years old; 0 refers to young of the year) and found no significant relationships (all P > 0.13). Inclusion of data from males (age range = 0–6 years old) changed the results slightly, with concentrations of Pb showing a marginally significant positive correlation with age (Rho corrected for ties = 0.11, Z = 2.06, P = 0.04), but all other correlations were not significant (all P > 0.09). Consequently, in all of the remaining analyses, we pooled data across age classes.
We next ran a two-way ANOVA to examine differences between sites and males versus females across all years. Finally, for the two sites sampled in multiple years (Pinebloom West and Tall Timbers), we performed ANOVAs to examine differences between years (data pooled across sexes; note that we could not include Pinebloom East and Pebble Hill in these analyses because year and site differences were confounded). For all ANOVAs, Bonferroni-Dunn tests were used for post hoc pair-wise comparisons.
We used Pearson product-moment analyses to determine whether metal concentrations were correlated with one another. Because we did find some sex differences in the concentration of certain metals, we performed these analyses separately for males and females as well as with pooled data from both sexes. For all these analyses, data were pooled across years and sites.
Reference values of metal contaminants are currently not available for opossums. As an attempt to gain some insight into whether the metal concentrations we found in opossums were extreme, we compared our values with those from a previous study of nine-banded armadillos (Dasypus novemcinctus; see Jarvis et al. 2013). Armadillos are ecologically very similar to opossums (Loughry and McDonough 2013), so our prediction was that metal concentrations in the liver tissues of each species would mirror one another. Not only that, but the armadillo tissues were collected from the same sites in the same years (Jarvis et al. 2013), which led us to expect that the two species were similarly exposed to whatever contaminants were present. Because of potential sex and site differences in metal concentrations, we used a series of unpaired t tests to compare the concentrations of Cu, Ni, Pb, and Zn (Se was not measured in armadillos) separately for males and females at each site and in each year (but pooled across age groups). See Jarvis et al. (2013) for sample sizes for armadillos. All statistical tests were conducted using Statview 4.01.
Results
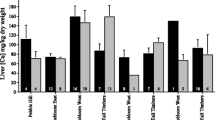
In total, we analyzed metal concentrations in liver tissues from 471 opossums. Figures 1, 2, 3, 4, and 5 depict the concentrations of each metal for male and female opossums at each site and in each year of the study. Statistically, we found substantial differences between sites for every metal (Table 1); however, significant differences between male and female concentrations were only found for Cu (Table 1). Post hoc pair-wise comparisons of site differences revealed that, for Cu, concentrations were higher at Tall Timbers than Pebble Hill (P = 0.005; Fig. 1), for Pb, Pinebloom East had higher concentrations than both Pinebloom West (P = 0.004) and Tall Timbers (P = 0.002; Fig. 2), for Ni, Pebble Hill and Pinebloom East exhibited higher concentrations than either Pinebloom West or Tall Timbers (all P < 0.0001; Pinebloom West and Tall Timbers did not differ from one another nor did Pebble Hill versus Pinebloom East; Fig. 3), and for Se, Pinebloom West had higher concentrations than Tall Timbers (P = 0.004) but lower than Pinebloom East and Pebble Hill (both P < 0.0001; Fig. 4). Tall Timbers also had lower concentrations of Se than Pinebloom East and Pebble Hill (both P < 0.0001; Fig. 4). Finally, for Zn, Pebble Hill had higher concentrations than any other site (all P < 0.0001; Fig. 5). Likewise, Pinebloom East had higher concentrations than either Tall Timbers or Pinebloom West (both P < 0.0001; Fig. 5).
For Tall Timbers and Pinebloom West, which were sampled in three successive years, we found significant year-to-year variation in the concentrations of every metal (Table 1). Although in most cases, we did not find significant differences in metal concentrations between these two sites (see above), and to be conservative, we analyzed yearly variation separately for each site. However, post hoc pair-wise comparisons between years at each site revealed very similar patterns. For example, concentrations of Cu were higher at both sites in 2006 than in either 2004 or 2005 (all P < 0.0001; Fig. 1). Likewise, at both sites, Se was higher in 2004 than either of the other 2 years (all P < 0.0001; Fig. 4) and Zn concentrations were lower in 2005 than either 2004 or 2006 (all P < 0.0001; Fig. 5). The only site-specific differences involved Ni, which was higher at Tall Timbers in 2005 than in 2004 (P = 0.016) but exhibited no significant yearly variation at Pinebloom West (Table 1; Fig. 3) and Pb, which did not vary significantly at Tall Timbers (Table 1) but was higher in 2006 than in 2004 at Pinebloom West (P = 0.007; Fig. 2).
Regardless of whether data from both sexes were pooled or examined separately, the concentrations of at least some metals were positively correlated with one another (Table 2). The strongest associations were of each metal (except Pb) with Zn, but there was also evidence of significant relationships of Cu, Ni, and Se with Pb and of Ni with Se (Table 2).
Comparison of the data obtained in this study with that of nine-banded armadillos collected at the same sites and in the same years showed that, for the four metals studied in common (Cu, Ni, Pb and Zn), opossums exhibited significantly higher metal concentrations in most cases (Table 3). Indeed, of the 64 total possible comparisons, 46 revealed statistically significant differences between opossums and armadillos, and 42 of these 46 involved instances where metal concentrations were higher in opossums (Table 3).
Discussion
This study was by necessity retrospective, exploiting the availability of opossums collected for other purposes. Thus, while we did uncover strong differences in metal concentrations between sites, years and in comparison with concentrations in nine-banded armadillos, it is difficult to devise compelling explanations for these results. For example, we are unaware of any land management practices that might account for differences in metal concentrations between sites or in different years. Similarly, all sites were relatively close to one another so it is difficult to envision how climatic or other environmental conditions could have contributed to variation between sites. Climatic conditions also did not vary dramatically across years, which would seem to then rule them out in explaining year-to-year differences. Finally, no data are available from any of our study sites on environmental concentrations of metals. Thus, we cannot evaluate whether the concentrations found in opossum liver tissues reflect processes of bioaccumulation or biomagnification, or simply current environmental conditions.
As stated at the outset, our primary goal in this study was to provide the first documentation of metal concentrations in the livers of wild opossums and, despite the drawbacks just mentioned, we were successful in this regard. Ideally, our hope is that these data can be used as a baseline for future studies. However, the substantial temporal and spatial variation in metal concentrations that we uncovered suggests that determination of baseline levels for opossums may not be straightforward. Indeed, the most extreme example of this comes from differences at Pinebloom, where not only were there differences between the western and eastern portions of the same property, but also between years at Pinebloom West (it is worth noting that, given the temporal variation at Pinebloom West, it is possible that differences between Pinebloom East and Pinebloom West may also represent temporal, rather than spatial, variation). Overall, our results indicate that considerable fine-scale variation in metal concentrations can occur in opossums, which may mean that identifying general reference levels is not realistic and all reference levels will have to be site-specific.
Previous literature describing metal concentrations in the Virginia opossum is scarce. Burger et al. (1994) examined Pb concentration in hair samples from D. virginiana in Costa Rica and reported levels more than an order of magnitude lower than what we observed in the present study. This may represent yet another instance of spatial (and/or temporal) variation or, more likely, that the liver is a better bioindicator of metal exposure than hair in these animals.
Opossums had higher concentrations of metals than did armadillos across all sites (Table 3). This suggests that opossums generally suffer higher levels of exposure to metal contaminants. A partial explanation for this may come from the more omnivorous diet of opossums (armadillos largely prey on soil-dwelling invertebrates such as ants and beetles; Loughry and McDonough 2013). A more diverse food base may lead to an increased probability of contact with a contaminated food item. Alternatively, armadillos may be more effective at clearing contaminants from the liver; however, this is purely speculation at the moment as no data are available on this issue.
Aside from armadillos, data from other small mammals, albeit studied elsewhere, further indicate that the metal concentrations we obtained for opossums are somewhat high. For example, Burger et al. (2000) reported metal concentrations of 12.9 μg/g Cu, 0.48 μg/g Pb, and 2.34 μg/g Se in the livers of racoons collected from the Department of Energy’s Savannah River Site in South Carolina (see also Gaines et al. 2002). Elevated metal concentrations, such as we have reported here, were found by Wijnhoven et al. (2007) for Cd, Cu, Pb, and Zn in the liver, kidney, and muscle of voles, mice, and shrews in floodplain areas of the Netherlands, and by Mariniakova et al. (2011) for Cd, Fe, Pb, and Zn in the bones of the yellow-necked mouse, wood mouse, bank vole, and common vole from polluted areas in Slovakia.
The question of whether the seemingly high metal concentrations we observed in opossums were extreme enough to exert toxicity is difficult to answer given the lack of reference data for this species. Shore and Rattner (2001) reported metal concentrations in the livers of other mammals, comparable to those reported here and suggested that they may have reached levels capable of causing deleterious effects. Toxic effects to mammals are extensive and can include carcinogenicity, teratogenicity, impaired reproduction, cardiovascular and pulmonary diseases, immunosuppression, nephrotoxicity, and neurotoxicity (Wren 1986; Goyer 1996). While we did not specifically autopsy the animals in our study for any of these effects, we will mention that we observed no obvious pathologies during dissections to obtain tissue samples. Thus, although we cannot be definitive, the available anecdotal evidence indicates that the metal concentrations in opossums did not generate noticeably adverse effects.
To summarize, metal accumulation was observed in the livers of Virginia opossums at concentrations equivalent to or higher than those reported in other mammals. Differences were observed in metal accumulation due to gender, site, and temporal variation; however, age did not influence liver metal concentrations. This study highlights the potential use of D. virginiana as a bioindicator species for metal accumulation in terrestrial environments. Furthermore, because of its distribution and diet, D. virginiana is ideal for studying the impacts of urbanization. More research is needed to determine the toxicity of the accumulated metals in these animals and the relationship between environmental metal concentrations and accumulated tissue metal concentrations.
References
Avsaroglu, D. T., Inal, T. C., Demir, M., Attila, G., Acarturk, E., Evlice, Y. E., & Kayrin, L. (2005). Biochemical indicators and cardiac functions tests in chronic alcohol abusers. Croatian Medical Journal, 46, 233–237.
Baird, C. (1995). Environmental chemistry (2nd ed.). New York: W.H. Freeman and Company.
Burger, J., Marquez, M., & Gochfeld, M. (1994). Heavy metals in the hair of opossum from Palo Verde, Costa Rica. Archives of Environmental Contamination and Toxicology, 27, 472–476.
Burger, J., Lord, C. G., Yurkow, E. J., McGrath, L., Gaines, K. F., Brisbin, I. L., Jr., & Gochfeld, M. (2000). Metals and metallothionein in the liver of raccoons: utility for environmental assessment and monitoring. Journal of Toxicology and Environmental Health Part A, 60, 243–261.
Casarett, L. J., Doull, J., & Klaasen, C. D. (2008). Casarett and Doull’s toxicology: the basic science of poisons (7th ed.). New York: McGraw-Hill.
Cesur, S., Cebeci, S. A., Kavas, G. O., Aksaray, S., & Tezeren, D. (2005). Serum copper and zinc concentrations in patients with chronic hepatitis. British Journal of Infection Control, 51, 38–40.
Cook, C. C. H., Walden, R. J., Graham, B., Gillham, C., Davies, S., & Prichard, B. N. C. (1991). Trace element and vitamin deficiency in alcoholic and control subjects. Alcohol and Alcoholism, 26, 541–548.
Damek-Poprawa, M., & Sawicka-Kapusta, K. (2004). Histopatological changes in liver, kidneys, and testes of bank voles environmentally exposed to heavy metals emissions from the steelworks and zinc smelter in Poland. Environmental Research, 96, 72–78.
Eisler, R. (1988). Lead hazards to fish, wildlife, and invertebrates: a synoptic review. Biology of Reproduction U.S Fish Wildlife Service, 85, 1–94.
Eisler, R. (1993). Zinc hazards to fish, wildlife, and invertebrates: a synoptic review. Biology of Reproduction U.S Fish Wildlife Service, 10, 33–47.
Eisler, R. (1997). Copper hazards to fish, wildlife, and invertebrates: a synoptic review. Biology Science Report USGS/BRD/BSR, 33, 1–98.
Eisler, R. (1998). Nickel hazards to fish, wildlife, and invertebrates: a synoptic review. Biology of Reproduction U.S Fish Wildlife Service, 34, 1–95.
Gaines, K. F., Romanek, C. S., Boring, C. S., Lord, C. G., Gochfeld, M., & Burger, J. (2002). Using raccoons as an indicator species for metal accumulation across trophic levels: a stable isotope approach. Journal of Wildlife Management, 66, 811–821.
Gardner, A. L. (1973). The systematics of the genus Didelphis (Marsupialia:Dedelphidae) in North and Middle America. Special Publication the Museum Texas Tech University Texas, 4, 1–81.
Goede, A. A., & De Bruin, M. (1984). The use of bird feathers as a monitor for metal pollution. Environmental Pollution (Amsterdam, Netherlands), 37, 287–308.
Goede, A. A., & De Bruin, M. (1986). The use of bird feathers for indicating heavy metal pollution. Environmental Monitoring and Assessment, 7, 249–256.
Goyer, R. (1996). Toxic effects of metals. In C. D. Klaassen (Ed.), Casarett & Doull’s toxicology (pp. 691–736). New York: McGraw-Hill.
Guidotti, T. L., Audette, R. J., & Martin, C. J. (1997). Interpretation of the trace metal analysis profile for patients occupationally exposed to metals. Occupational Medicine, 47, 497–503.
Harmon, L. J., Bauman, K., McCloud, M., Parks, J., Howell, S., & Losos, J. B. (2005). What free-ranging animals do at the zoo: a study of the behaviour and habitat use of opossums (Didelphis virginiana) on the grounds of the St. Louis Zoo. Zoo Biology, 24, 197–213.
Hunsaker, D., II. (1977). Ecology of New World marsupials. In D. Hunsaker II (Ed.), The biology of marsupials (pp. 95–158). New York: Academic Press.
Hunsaker, D., II, & Shupe, D. (1977). Behavior of new world marsupials. In D. Hunsaker II (Ed.), The biology of marsupials (pp. 279–348). New York: Academic Press.
Jarvis, T. A., Lockhart, J. M., Loughry, W. J., & Bielmyer, G. K. (2013). Metal accumulation in wild nine-banded armadillos. Ecotoxicology, 22, 1053–1062.
Levengood, J. M., & Heske, E. J. (2008). Heavy metal exposure, reproductive activity, and demographic patterns in white-footed mice (Peromyscus leucopus) inhabiting a contaminated floodplain wetland. The Science of the Total Environment, 389, 320–328.
Loughry, W. J., & McDonough, C. M. (2013). The nine-banded armadillo: a natural history. Norman: University of Oklahoma Press.
Mariniakova, M., Omelka, R., Stawarz, R., & Formicki, G. (2011). Accumulation of lead, cadmium, nickel, iron, copper, and zinc in bones of small mammals from polluted areas in Slovakia. Polish Journal of Environmental Studies, 21, 153–158.
McDonough, C. M., Lockhart, J. M., & Loughry, W. J. (2007). Population dynamics of nine-banded armadillos: insights from a removal experiment. Southeastern Naturalist, 6, 381–392.
Mullally, A. M., Vogelsang, G. B., & Moliterno, A. R. (2004). Wasted sheep and premature infants: the role of trace metals in hematopoiesis. Blood Reviews, 18, 227–234.
Nwokocha, C. R., Owu, D. U., Nwokocha, M. I., Ufearo, C. S., & Iwuala, M. O. E. (2012). Comparative study on the efficacy of Allium sativum (garlic) in reducing some heavy metal accumulation in liver of Wistar rats. Food and Chemical Toxicology, 50, 222–256.
Phelps, R. W., Clarkson, T. W., Kershaw, T. G., & Wheatley, B. (1980). Interrelationships of blood and hair mercury concentrations in a North American population exposed to methylmercury. Archives of Environmental Health, 35, 161–168.
Pyati, R., Bielmyer, G.K., Chalk, S., McCarthy, D., McCarthy, H., Pinto, G., Sonnenberg, L., & Welsh, P. (2012). Case Study: St. Johns River Basin, USA. In: Fourth United Nations World Water Development Report, World Water Assessment Programme 2012, UNESCO Publishing, France.
Roggeman, S., De Boeck, G., De Cock, H., Blust, R., & Bervoets, L. (2014). Accumulation and detoxification of metals and arsenic in tissues of cattle (Bos taurus), and the risks for human consumption. The Science of the Total Environment, 466–467, 175–184.
Salińska, A., Włostowski, T., & Oleńska, E. (2013). Differential susceptibility to cadmium-induced liver and kidney injury in wild and laboratory-bred bank voles Myodes glareolus. Archives of Environmental Contamination and Toxicology, 65, 324–331.
Sánchez-Chardi, A., Marques, C. C., Nadal, J., & Da Luz Mathias, M. (2007). Metal bioaccumulation in the greater white-toothed shrew, Crocidura russula, inhabiting an abandoned pyrite mine site. Chemosphere, 67, 121–130.
Shore, R. F., & Rattner, B. A. (Eds.). (2001). Ecotoxicology of wild mammals. Ecotoxicology and environmental toxicology series. New York: John Wiley and Sons Ltd.
Suzuki, K., Oyama, R., Hayashi, E., & Arakawa, Y. (1996). Liver disease and essential trace elements. Nihon Rinsho, 54, 85–92.
Wijnhoven, S., Leuven, R. S. E. W., Van der Velde, G., Jungheim, G., Koelemij, E. I., De Vries, F. T., Eijsackers, H. J. P., & Smits, A. J. M. (2007). Heavy-metal concentrations in small mammals from a diffusely polluted floodplain: importance of species-and location-specific characteristics. Archives of Environmental Contamination and Toxicology, 52, 603–613.
Wren, C. D. (1986). A review of metal contamination and toxicity in wild mammals. I. Mercury. Environmental Research, 40, 210–244.
Acknowledgments
Funding for lab analyses was provided by a Valdosta State University faculty research seed Grant to JML and an equipment Grant to GKB-F for the atomic absorption spectrophotometer. We thank USDS-Georgia Wildlife Services personnel for allowing access to the specimens they collected. Finally, we thank the handling editor and two anonymous reviewers for their useful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lockhart, J.M., Siddiqui, S., Loughry, W.J. et al. Metal accumulation in wild-caught opossum. Environ Monit Assess 188, 317 (2016). https://doi.org/10.1007/s10661-016-5327-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-016-5327-y