Abstract
Metal pollution commonly occurs in many terrestrial environments and may pose a threat for the animals inhabiting such areas. Here, we present concentrations of six metals (cadmium [Cd], copper [Cu], nickel [Ni], lead [Pb], selenium [Se], and zinc [Zn]) in the liver tissues of seven species of mammals obtained from a study that examined the impact of mesopredator removal on northern bobwhite (Colinus virginianus) populations. A total of 1326 samples were collected from 2003 to 2006 at four sites in southwest Georgia and north Florida from nine-banded armadillos (Dasypus novemcinctus), bobcats (Lynx rufus), feral cats (Felis catus), coyotes (Canis latrans), grey foxes (Urocyon cinereoargenteus), opossums (Didelphis virginiana), and raccoons (Procyon lotor). Data from armadillos, bobcats, opossums, and raccoons were published previously to examine age, sex, spatial (between sites), and temporal (between years) variation. In this paper, we present similar comparisons for the remaining three species as well as comparisons of metal concentrations among all seven species. Concentrations of Cu and Pb exhibited strong negative relationships with body weight in coyotes, while Ni was positively correlated with weight in feral cats. Concentrations of these metals, as well as the other two tested (Cd and Zn), were not significantly correlated with one another in any of the three species. The only sex difference in liver metal concentrations was observed in female feral cats, which had higher levels of Pb than did males. Coyotes exhibited significant differences in Cu concentrations between sites and between years (2005 versus 2006). We also found significant differences between sites in Pb concentrations for both feral cats and grey foxes. There were significant differences in metal concentrations among all seven species for all metals except Cd. With the exception of Cd and Se (tested only in bobcats and opossums), a three-way ANOVA with species, year, and site as the three factors revealed significant differences among species for every metal but only a single main effect of year for Cu, and no main effects of site. In sum, our results provide an extensive survey of metal concentrations in a diverse assemblage of mammals and suggest that metal accumulation may be heavily influenced by species identity, which in turn may reflect ecological lifestyle.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increasing anthropogenic activity has contributed excess concentrations of metals in the environment (Pyati et al. 2012; Bielmyer-Fraser et al. 2017). Sources of metals include localized applications of pesticides, fertilizers, and biosolids for agriculture, forestry and timber management practices, industrial pollution, and urban development (Burger et al. 2002; Kendall et al. 2010; Wuana and Okieimen 2011). Because of these activities, animals may accumulate metals in tissues and organs, which can exceed threshold levels and result in toxicity (Shore and Rattner 2001).
Numerous studies have focused on the accumulation of various metals in individual species of terrestrial mammals (e.g., Burger et al. 2002; Jarvis et al. 2013; Lockhart et al. 2016; Thomason et al. 2016; Hough et al. 2020) or the accumulation of one or a few metals in several species (Hunter and Johnson 1982; Wijnhoven et al. 2007). Fewer studies have examined the concentrations of multiple metals in a species assemblage (Phelps and McBee 2009; Mann et al. 2011; Gall et al. 2015). Such an analysis can be useful in assessing how trophic position or ecological lifestyle (Sibly and Brown 2007) affects the accumulation of metals, as well as provide a clearer and more detailed picture of the health of an ecosystem (Burger and Gochfeld 2001; Gaines et al. 2002). For such comparisons, the ideal situation is to test samples from a set of species occupying the same habitat(s) at the same time.
In a series of papers, we have previously reported metal concentrations in the liver tissues of nine-banded armadillos (Dasypus novemcinctus; Jarvis et al. 2013), opossums (Didelphis virginiana; Lockhart et al. 2016), bobcats (Lynx rufus; Thomason et al. 2016), and raccoons (Procyon lotor; Hough et al. 2020). All animals were collected from four sites in southwest Georgia and north Florida during the same time period (2003–2006). Here, we conclude these analyses by reporting data from three additional species that were not tested previously: feral cats (Felis catus), coyotes (Canis latrans), and grey foxes (Urocyon cinereoargenteus). In addition to analyzing data from each of these three species separately, we compared patterns of metal accumulation across all seven species that were tested. Our study is thus unique in providing a broad overview of the accumulation of multiple metals in a relatively large assemblage of terrestrial mammals that range in trophic position from species that are primarily insectivores (armadillos) to species that are almost exclusively carnivores (feral cats and bobcats).
Materials and methods
Sample collection
All seven species of mammals were collected from Pebble Hill Plantation, located near Thomasville, Georgia, and the eastern portion of Pinebloom Plantation, near Albany Georgia, in 2003. Additional specimens were collected from Tall Timbers Research Station, near Tallahassee, Florida, and the western portion of Pinebloom Plantation between 2004 and 2006. All animals were harvested as part of a wildlife management and gamebird restoration project—of which we were not part—focused on increasing northern bobwhite (Colinus virginianus) populations by removing mesopredators (McDonough et al. 2007). The sampling sites have bottomland hardwood forests, upland pine, and open fields as the main habitats, and are primarily managed for wildlife and timber, although Pinebloom Plantation also has areas that are managed for agricultural crops. All sites are relatively rural with minimal development; however, it should be noted that there is a US Environmental Protection Agency (EPA)-designated Superfund site (since 1989) in Albany Georgia (US EPA 2016).
Between 1 March and 30 September of each year, US Department of Agriculture Wildlife Services technicians obtained specimens of each species by trapping or shooting. Animals were weighed and sexed by the technicians, and various tissues were collected and frozen at − 20 °C in polypropylene vials (with no preservative) until analyzed. We chose to examine metal concentrations in liver samples because this organ is the main site of detoxification (Klaassen 2013).
Metal analysis for feral cats, coyotes, and grey foxes
The same procedures were used as in our previous studies (Jarvis et al. 2013; Lockhart et al. 2016; Thomason et al. 2016; Hough et al. 2020). Using an analytical balance, wet weights (ww) were measured from thawed liver samples, followed by a 24-h drying period in an 80 °C oven to measure dry weights (dw). Dried samples were then digested with trace metal grade nitric acid (Fisher Scientific, Pittsburgh, PA) in a 60 °C water bath for at least 24 h, or until fully digested. Digested samples were diluted with 18 mΩ Milli-Q® water, and metal analysis was conducted using atomic absorption spectrophotometry (Perkin Elmer AAnalysts 800, Norwalk, CT) using flame and graphite furnace detection (detection limit = 1–2 ppb). Liver samples were analyzed against certified 1 g/mL metal standards dissolved in 2% HCl (Fisher Chemical, Fairlawn, NJ). Both liver samples and metal standards were analyzed in duplicates, and recalibration occurred every 40 samples. As a standard reference material, lobster hepatopancreas (LUTS-1 (non-defatted) and TORT-3) was analyzed (three replicates each) to measure metal extraction efficiencies. Data are reported as μg metal per g dw tissue.
Statistical analyses
Table 1 provides a summary of the sampling effort for all seven species. Originally, samples were available for all species at all sites in all years. However, because of a freezer failure, almost all samples from 2003 and 2004 were lost for feral cats, coyotes, grey foxes, and raccoons. Note also that, for a variety of reasons, species were not all tested for the same set of metals. All seven species were tested for two metals: Cu and Zn (Table 1). Most species were tested for three other metals: Cd, Pb, and Ni; Se was only tested in bobcats and opossums. We do not report data here on metals that were only sampled in one species: aluminum (Al) in armadillos (Jarvis et al. 2013) and silver (Ag) in bobcats (Thomason et al. 2016).
Our first set of statistical analyses examined variation in metal concentrations separately for feral cats, coyotes, and grey foxes. For feral cats and grey foxes, body weight data suggested that all the animals were adults (weight range for feral cats = 2.62–4.88 kg; grey foxes = 3.35–4.74 kg; see Fritzell and Haroldson 1982, Davison and Stromsten 1947), thereby eliminating the possibility of examining age differences in metal concentrations. In contrast, some coyotes were small enough that they were likely juveniles (weights < 8.1 kg; Bekoff and Gese 2003) but there were so few of them (N = 4) that age comparisons were not possible. Instead, we performed linear regressions of metal concentrations with body weight for each species on the assumption that weight is a reasonable surrogate for age, with larger animals being older.
Next, we determined whether metal concentrations were correlated with one another in each species by performing Pearson product-moment correlations between each possible pair of metals. Finally, we compared differences in metal concentrations between males and females, sites (Pinebloom West versus Tall Timbers), and years (2005 versus 2006) with a series of t tests. Sample sizes were not sufficient to run a more preferable three-way ANOVA.
Comparisons of metal concentrations among all seven species were done in three ways. First, we used data from all animals in all years in a one-way ANOVA in order to examine overall differences between species. Bonferroni-Dunn tests were used to identify significant post hoc pairwise comparisons. Note that results comparing Se concentrations between the two species tested for this metal (bobcat and opossum, see Table 1) have already been published (Thomason et al. 2016) and so they are not repeated here. Second, as described above, data for feral cats, coyotes, grey foxes, and raccoons were restricted to 2005 and 2006 due to loss of samples from earlier years. We thus used data from just those 2 years in a three-way ANOVA in which species, site, and year were the three factors. This analysis allowed us to determine if there were consistent differences across species due to spatial and temporal effects, as well as whether there were any significant interactions among these factors. Unlike the first analysis, we did include Se here because site and year differences were not examined previously. Even though more years and sites were available for the analysis of Se, we still restricted the data to just 2005 and 2006 in order to make the results comparable with those of the other metals. Finally, we reanalyzed data from armadillos in which we had examined variation in metal concentrations due to age by creating discrete age categories (juvenile, yearling, and adult) from weight data. In order to compare patterns across species as much as possible, we performed regression analyses of metal concentrations with body weight for armadillos, as was done for five of the other species. Opossums were the one species where we could not perform such an analysis because we had no weight data for those specimens. Instead, relationships between metal concentrations and age were based on age estimates (in years) from measurements of tooth annuli (Lockhart et al. 2016).
Results
Feral cats, coyotes, and grey foxes
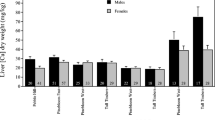
We found three significant relationships between body weight and metal concentrations (Table 2). Specifically, Cu and Ni exhibited strong negative relationships with weight in coyotes but Pb was positively correlated with weight in feral cats (Table 2). There were no significant correlations between metal concentrations for any of the three species (all P > 0.10; Table 3). The only significant sex difference in metal concentrations was that female feral cats had higher levels of Pb than did males (Table 4). Coyotes exhibited strong differences in liver Cu concentrations between sites and years, with the highest Cu concentrations observed at Pinebloom West in 2006 (Table 4). Grey foxes had a significant difference between sites for Pb liver concentrations, as well as a marginally significant difference between sites for Cu liver concentrations, with the highest concentrations of both metals observed at Pinebloom West (Table 4). There also was a marginally significant difference in Pb liver concentrations between sites for feral cats, with the highest concentrations observed at Tall Timbers (Table 4).
Comparative analyses
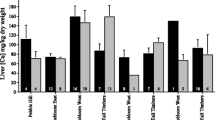
Using data from all species and all years, there were highly significant differences in metal concentrations between species for every metal except Cd (Figs. 1, 2, 3, 4, and 5). Taking the pairwise post hoc comparisons for each metal in turn, armadillos had significantly lower concentrations of Cu than bobcats, feral cats, coyotes, and raccoons (all P < 0.0004) as did opossums (all P < 0.002; Fig. 2). In addition, bobcats had significantly higher Cu levels than did coyotes, grey foxes, and raccoons (all P < 0.0001; Fig. 2). For Pb and Ni, opossums had significantly higher concentrations than any other species (all P < 0.0001; Figs. 3 and 4; note that bobcats were not tested for either metal). Armadillos had higher concentrations of Pb than did raccoons (P = 0.0002; Fig. 3) but lower levels of Zn than any other species (all P < 0.0001; Fig. 5). Raccoons had higher concentrations of Zn than both bobcats and opossums (both P < 0.0001; Fig. 5).
Liver Cu concentration (mean ± SE) for seven species of mammals, using data from all sites and all years. Sample size given within each bar. Results of a one-way ANOVA comparing species means: F6, 1305 = 57.77, P < 0.0001. Different letters indicate species that statistically differed from one another in post hoc pairwise comparisons
Liver Pb concentration (mean ± SE) for six species of mammals, using data from all sites and all years (bobcats were not tested). Sample size given above each bar. Results of a one-way ANOVA comparing species means: F5, 1164 = 69.10, P < 0.0001. Different letters indicate species that statistically differed from one another in post hoc pairwise comparisons
Liver Ni concentration (mean ± SE) for six species of mammals, using data from all sites and all years (bobcats were not tested). Sample size given within each bar. Results of a one-way ANOVA comparing species means: F5, 1136 = 48.35, P < 0.0001. Different letters indicate species that statistically differed from one another in post hoc pairwise comparisons
Liver Zn concentration (mean ± SE) for seven species of mammals, using data from all sites and all years. Sample size given within each bar. Results of a one-way ANOVA comparing species means: F6, 1292 = 50.81, P < 0.0001. Different letters indicate species that statistically differed from one another in post hoc pairwise comparisons
When we restricted the data to 2005 and 2006, we once again found a strong main effect of species for every metal except Cd and Se (Table 5). Cd did exhibit a marginally significant three-way interaction between species, site, and year, but no other comparisons were significant (Table 5). In contrast, Cu showed a significant main effect of year (means ± SE, 2005 = 44.65 ± 2.02 μg/g dw, N = 402; 2006 = 53.56 ± 2.59 μg/g dw, N = 440), as well as a significant species × year interaction and a marginally significant species × site interaction (Table 5). These interaction terms appeared to be largely due to differences in metal concentrations between sites and years for feral cats, coyotes, and grey foxes (see Table 4), and, in the case of the species × year interaction, opossums (see Lockhart et al. 2016). Zn also showed a significant species × year interaction as well as a significant year × site interaction (Table 5). The year × site interaction is somewhat puzzling because the mean values for each site in each year were not markedly different from one another (mean ± SE, Pinebloom West, 2005 = 124.86 ± 4.31 μg/g dw, N = 219; 2006 = 132.79 ± 4.78 μg/g dw, N = 238; Tall Timbers, 2005 = 131.38 ± 6.05 μg/g dw, N = 180; 2006 = 145.40 ± 4.46 μg/g dw, N = 201). The species × year interaction likely resulted from the fact that Zn concentrations increased from 2005 to 2006 for three species (armadillos, bobcats, and opossums—more than quadrupling in the case of armadillos, Jarvis et al. 2013) but decreased for the remaining four (Hough et al. 2020 and Table 4). Interestingly, there was no difference in Se concentrations between bobcats and opossums using data from just 2005 and 2006, in contrast to the strong difference reported by Thomason et al. (2016) based on data from all sites and years. Presumably, this difference reflects the influence of the earlier years of sampling, which leads us to question if the two species really differ from one another or whether the difference is an artifact of sampling year (or site).
In armadillos, a significant positive relationship between weight and levels of Pb was observed (r = 0.17, F = 6.24, P = 0.013, N = 207) but significant negative relationships were obtained for Cu and Zn (r = − 0.14, F = 3.92, P = 0.05 and r = − 0.20, F = 8.44, P = 0.004 respectively; Cd and Ni were not significant, both P > 0.10; N = 207 for all comparisons). These results confirm the earlier analysis of Jarvis et al. (2013) who found higher levels of Pb in adult compared with juvenile armadillos but the negative relationships are novel. This is likely because Jarvis et al. (2013) included animals from other sites in Florida that were excluded from the present analyses because the latter were restricted to just sites where all species were sampled.
Discussion
Table 6 provides a general summary of the results of this study, combined with the previous findings of Jarvis et al. (2013), Lockhart et al. (2016), Thomason et al. (2016), and Hough et al. (2020). For the most part, differences in metal concentrations between males and females were rare (Table 6). In contrast, we documented substantial spatial and temporal variation in metal concentrations, as well as some strong relationships between metal concentrations and body weight (Table 6). In a number of cases, the differences between sites and years were confounded because significant differences occurred between 2003 and subsequent years and the two sites sampled in 2003 were different than the two sites sampled in the remaining 3 years (see Table 1). Nonetheless, as Table 5 documents, we found some differences between sites and years even when the data were restricted to just the two sites sampled in 2005 and 2006. Unfortunately, as we have acknowledged previously, the biggest weakness of our studies is that they are retrospective because we exploited the availability of a large number of animals that were collected for a different purpose. Consequently, we do not have much information, including details of land management practices, that might help to explain the different patterns observed at the different sites from 2003 to 2006. Thus, the main value of our data is in providing reference values for the various metals we tested in these species and in documenting various sources of potential variation in metal concentrations.
Another notable feature of Table 6 is that differences in metal concentrations due to body weight, sex, site, and year were not consistent across species. For example, males sometimes had larger values than did females (e.g., Cu in opossums; Pb and Zn in raccoons) but the reverse also occurred (e.g., Pb in armadillos and feral cats). Likewise, adults had higher concentrations of Pb in armadillos, but we found strong negative relationships between some metal concentrations and body weight in armadillos, coyotes, and raccoons, which would suggest that smaller (and presumably younger) animals experienced higher levels of accumulation. Additionally, differences were not consistent for a particular metal. Aside from the lack of any site differences in Cd in the five species tested for that metal, for every other metal, some species showed differences among sites while others did not. Given the strong effects of species identity that we found when comparing metal concentrations across species, coupled with the lack of many other significant influences (Figs. 1, 2, 3, 4, and 5 and Table 5), we interpret these results to suggest that the unique ecological lifestyle of each species plays a critical role in determining its pattern of metal accumulation.
Presumably, one fundamental feature of a species’ ecological lifestyle is its trophic position. We sampled animals with very different diets. Armadillos are primarily insectivorous (McDonough and Loughry 2008). Opossums also eat insects, but their diet typically includes a broader range of other items (Gardner 1982; Kasparian et al. 2002). While the remaining species are all classified as carnivores, there is considerable variation among them in how much they rely on meat in their diet, with species such as coyotes, grey foxes, and raccoons being far more opportunistic and omnivorous (Bekoff 1977; Saunders 1988; Davis and Schmidly 1994; Kasparian et al. 2002; Bekoff and Gese 2003). It seems reasonable to suppose that the differences in liver metal concentrations in these species are at least in part influenced by these dietary differences.
There are at least two other possible explanations for differences in metal concentrations among species. The first is that accumulation is due to how metals are processed internally. Once inside an organism, metals can be utilized for internal physiological processes, such as metabolism, altered to a non-toxic form, or excreted from the body. Any or all of these could influence how much of the contaminant is retained in tissues (Laskowski 1991; Gaines et al. 2002; Grey 2002; Mann et al. 2011). Second, species may differ in their level of exposure to metals in the physical environment. For example, armadillos and opossums experience relatively high and intimate contact with soils while foraging and burrowing, and species also differ in the extent to which they utilize aquatic habitats (e.g., raccoons versus bobcats). Of course, it is probably most realistic to assume that all of these factors work together to determine patterns of metal accumulation within a particular species. An important task for the future will be to determine the relative importance of each in explaining the patterns we have documented here.
Unlike our previous studies, we found no significant pairwise correlations between metal concentrations for feral cats, coyotes, or grey foxes, perhaps because of small sample sizes (Table 3). Hough et al. (2020) discussed the pattern of correlations between metals found in armadillos, opossums, bobcats, and raccoons. Given that our current results add nothing new to those patterns, we will not repeat that discussion here. Instead, we focus on patterns of accumulation for the five metals that were tested in most of our seven species of mammals.
Cd has been shown to accumulate in freshwater, marine, and small terrestrial animal species that occupy relatively low trophic positions (Ferard et al. 1983; Eisler 1985; Janssen et al. 1993; Gall et al. 2015; Bielmyer-Fraser et al. 2018). However, Laskowski (1991) proposed that metals, such as Cd, do not accumulate in higher concentrations in mammals at successive trophic levels because of the interplay between various factors such as homeostatic regulation processes and level of environmental exposure. If so, this leads to the expectation that differences among species in Cd concentrations should be minimal. Our results are consistent with this hypothesis because we did not find any differences among species in Cd concentrations (Fig. 1). However, it is worth noting that there were several species-specific differences in Cd levels, specifically in raccoons and armadillos (Table 6).
We found that bobcats had significantly higher levels of Cu compared with any of the other species and that the species at presumed lower trophic levels, armadillos and opossums, had the lowest Cu concentrations (Fig. 2). Our results also indicated that younger animals were more likely to have higher Cu concentrations than older ones, because armadillos, coyotes, and raccoons had negative relationships between Cu concentration and body weight (Table 6; Hillis and Parker 1993; Eisler 1997). Younger animals may have higher liver Cu concentrations because they typically have higher metabolic rates (which require more Cu) than older, adult animals (Blagojević et al. 2012). Additionally, as an essential element, Cu is needed for physiological processes such as tissue growth and development (Eisler 1997). As such, it is likely that younger animals would have greater concentrations of Cu present in tissues than older animals (Hillis and Parker 1993). While site and year differences in Cu concentrations were observed for four of the seven species (Table 6), we are unaware of any specific land management practices at these sites that could have generated these differences. Elevated tissue concentrations at certain sites in some years might suggest that these locations experienced increased anthropogenic impacts from Cu-containing pesticides or industrial byproducts (Eisler 1997).
Pb, like Cd, is a non-essential metal that bioaccumulates in organisms with increasing age (Eisler 1988; Gall et al. 2015). Our results partially confirm this pattern because Pb concentrations were positively related to body weight in two species (armadillo and feral cat; Table 6) and are consistent with other studies which found increased Pb accumulation in older mammals (Sánchez-Chardi and Nadal 2007; Sánchez-Chardi et al. 2007; Gall et al. 2015). In addition to this, our results indicated that females had higher concentrations of Pb than did males in armadillos and feral cats, but males had higher concentrations than females in raccoons (Table 6). The sex difference in feral cats may be an artifact of small sample size (see Table 4) but we have no compelling reason that would explain the opposing patterns in armadillos and raccoons. We also found that opossums had significantly higher concentrations of Pb than the other species examined (recall that bobcats were not analyzed; Table 1), and armadillos had higher levels than did raccoons (Fig. 3). These differences might reflect the more insect-based diet of these two species, as well as their high levels of contact with potentially contaminated soils (e.g., by burrowing). Like Cu, Pb concentrations varied among our study sites (Table 6). As several of the sites are managed for wildlife hunting, it seems plausible that differences in Pb concentrations between sites might reflect differential exposure to Pb-based shot.
Opossums had significantly higher liver Ni concentrations when compared with the other species (bobcats not tested; Fig. 4). This difference is difficult to explain because all of the other species had similar levels of Ni, which suggests that Ni does not biomagnify at higher trophic levels, and/or that each species had similar exposure to Ni. At this time, we cannot explain why opossums were so different from the other species examined. Likewise, we cannot explain why opossums exhibited variation in Ni concentrations among sites and years, or the among year difference found in armadillos (Table 6).
As an essential element, Zn is needed for physiological processes and normal growth and development. However, excess Zn in the environment from various anthropogenic sources, such as runoff from agricultural lands, wood combustion, and galvanized surfaces, can result in increased Zn accumulation in the tissues of some birds and mammals (Eisler 1993; Gall et al. 2015). In our study, armadillos had the lowest Zn concentrations, while raccoons had higher levels than bobcats and opossums (Fig. 5). Just as with Ni, explaining these differences in Zn liver concentrations is challenging because there seems to be no clear relationship between metal concentrations and the trophic position of each species. Differences in accumulation of these metals may instead reflect differences in their physiological requirements among the animals.
Conclusions
We have now analyzed metal concentrations in the liver tissues of seven species of mammals collected from the same locations and in the same years. Although reference values are lacking for some of these species, particularly the carnivores, our data generally fall within the range of previously published values. Unfortunately, we were unable to identify any consistent patterns that would help to explain how and why the metals accumulated as they did in the species we studied. There seemed to be few clear links with trophic position, possibly because these species exist in a complex food web that makes it difficult to discern distinct trophic levels (Gaines et al. 2002). However, the results do suggest that the unique ecological lifestyle of each species may play a critical role in determining its pattern of metal accumulation. An important contribution of our studies is the generation of reference values for the concentrations of eight metals (six described here) in the liver tissues of these species. We have also documented multiple sources of variation in liver metal concentrations that may help in formulating plans for studies of metal toxicology in species assemblages in other ecosystems.
References
Bekoff, M. (1977). Canis latrans. Mammalian Species, 79, 1–9.
Bekoff, M., & Gese, E. M. (2003). Coyote (Canis latrans). In G. A. Feldhamer, B. C. Thompson, & J. A. Chapman (Eds.), Wild mammals of North America: biology, management and conservation (pp. 467–481). Baltimore, Maryland: The Johns Hopkins University Press.
Bielmyer-Fraser, G. K., Waters, M. N., Duckworth, C. G., Patel, P., Webster, B., Blocker, A., Crummey, C. H., Duncan, A., Nwokike, S., Picariello, C., Ragan, J. T., Schumacher, E. L., Tucker, R., Tuttle, E., & Wiggins, C. (2017). Assessment of metal contamination in the biota of four rivers experiencing varying degrees of human impact. Environmental Monitoring and Assessment, 189, 23.
Bielmyer-Fraser, G. K., Harper, B., Picariello, C., & Albritton-Ford, A. (2018). The influence of salinity and water chemistry on acute toxicity of cadmium to two euryhaline fish species. Comparative Biochemistry and Physiology C, 214, 23–27.
Blagojević, J., Jovanović, V., Stamenković, G., Jojić, V., Bugarski-Stanojević, V., Adnađević, T., & Vujošević, M. (2012). Age differences in bioaccumulation of heavy metals in populations of the black-striped field mouse, Apodemus agrarius (Rodentia, Mammalia). International Journal of Environmental Research, 6, 1045–1052.
Burger, J., & Gochfeld, M. (2001). On developing bioindicators for human and ecological health. Environmental Monitoring and Assessment, 66, 23–46.
Burger, J., Gaines, K. F., Lord, C. G., Brisbin Jr., I. L., Shukla, S., & Gochfeld, M. (2002). Metal levels in raccoon tissues: differences on and off the Department of Energy’s Savannah River site in South Carolina. Environmental Monitoring and Assessment, 74, 67–84.
Davis, W., & Schmidly, D. (1994). The mammals of Texas. Texas Parks and Wildlife Department. Austin: Texas.
Davison, A., & Stromsten, F. A. (1947). Mammalian anatomy with special reference to the cat. New York: McGraw-Hill.
Eisler, R. (1985). Cadmium hazards to fish, wildlife, and invertebrates: a synoptic review. Biological Report 85(1.2), Contaminant Hazards Review No. 2, U.S. Fish and Wildlife Service.
Eisler, R. (1988). Lead hazards to fish, wildlife, and invertebrates: a synoptic review. Biological Report 85, Contaminant Hazards Review No. 14, U.S. Fish and Wildlife Service.
Eisler, R. (1993). Zinc hazards to fish, wildlife, and invertebrates: a synoptic review. Biological Report 85, Contaminant Hazards Review No. 26, U.S. Fish and Wildlife Service.
Eisler, R. (1997). Copper hazards to fish, wildlife, and invertebrates: a synoptic review. Biological Science Report USGS/BRD/BSR, Contaminant Hazards Review No. 33, U.S. Geological Survey, Biological Resources Division.
Ferard, J. F., Jouany, J. M., Truhaut, R., & Vasseur, P. (1983). Accumulation of cadmium in a freshwater food chain experimental model. Ecotoxicology and Environmental Safety, 7, 43–52.
Fritzell, E. K., & Haroldson, K. J. (1982). Urocyon cinereoargenteus. Mammalian Species, 189, 1–8.
Gaines, K. F., Rommanek, C. S., Boring, C. S., Lord, C. G., Gochfeld, M., & Burger, J. (2002). Using raccoons as an indicator species for metal accumulation across trophic levels: a stable isotope approach. Journal of Wildlife Management, 66, 811–821.
Gall, J. E., Boyd, R. S., & Rajakaruna, N. (2015). Transfer of heavy metals through terrestrial food webs: a review. Environmental Monitoring and Assessment, 187, 201.
Gardner, A. (1982). Virginia opossum (Didelphis virginiana). In G. A. Feldhamer, B. C. Thompson, & J. A. Chapman (Eds.), Wild mammals of North America: biology, management and conservation (pp. 3–36). Baltimore, Maryland: Johns Hopkins University Press.
Grey, J. S. (2002). Biomagnification in marine systems: The perspective of an ecologist. Marine Pollution Bulletin, 45, 46–52.
Hillis, T. L., & Parker, G. H. (1993). Age and proximity to local ore-smelters as determinants of tissue metal levels in beaver (Castor canadensis) of the Sudbury (Ontario) area. Environmental Pollution, 80, 67–72.
Hough, S., Lockhart, J. M., Loughry, W. J., Bielmyer-Fraser, G. K. (2020). Metal accumulation in wild-caught raccoons in Southeastern USA (In press).
Hunter, B. A., & Johnson, M. S. (1982). Food chain relationships of copper and cadmium in contaminated grassland ecosystems. Oikos, 38, 108–117.
Janssen, M. P. M., Ma, W. C., & Van Straalen, N. M. (1993). Biomagnification of metals in terrestrial ecosystems. Science of the Total Environment, 134, 511–524.
Jarvis, T. A., Lockhart, J. M., Loughry, W. J., & Bielmyer, G. K. (2013). Metal accumulation in wild nine-banded armadillos. Ecotoxicology, 22, 1053–1062.
Kasparian, M. A., Hellgren, E. C., & Ginger, S. M. (2002). Food habits of the Virginia opossum during raccoon removal in the cross timbers ecoregion, Oklahoma. Proceedings of the Oklahoma Academy of Science, 82, 73–78.
Kendall, R. J., Lacher, T. E., Cobb, G. P., & Cox, S. B. (Eds.). (2010). Wildlife toxicology: emerging contaminant and biodiversity issues. Boca Raton, Florida: CRC Press.
Klaassen, C. D. (Ed.). (2013). Casarett and Doull’s toxicology: the basic science of poisons. New York: McGraw-Hill.
Laskowski, R. (1991). Are the top carnivores endangered by heavy-metal biomagnification? Oikos, 60, 387–390.
Lockhart, J. M., Siddiqui, S., Loughry, W. J., & Bielmyer-Fraser, G. K. (2016). Metal accumulation in wild-caught opossum. Environmental Monitoring and Assessment, 188, 317.
Mann, R. E., Vijver, M. G., & Peijnenburg, W. J. G. M. (2011). Metals and metalloids in terrestrial systems: bioaccumulation, biomagnification and subsequent adverse effects. In F. Sánchez-Bayo, P. J. van den Brink, & R. M. Mann (Eds.), Ecological impacts of toxic chemicals (pp. 43–62). Sharjah: Bentham.
McDonough, C. M., & Loughry, W. J. (2008). Behavioral ecology of armadillos. In S. F. Vizcaíno & W. J. Loughry (Eds.), The biology of the Xenarthra (pp. 281–293). Gainesville: University Press of Florida.
McDonough, C. M., Lockhart, J. M., & Loughry, W. J. (2007). Population dynamics of nine-banded armadillos: insights from a removal experiment. Southeastern Naturalist, 6, 381–392.
Phelps, K. L., & McBee, K. (2009). Ecological characteristics of small mammal communities at a superfund site. American Midland Naturalist, 16, 57–68.
Pyati, R., Bielmyer, G. K., Chalk, S., McCarthy, D., McCarthy, H., Pinto, G., Sonnenberg, L., & Welsh, P. (2012). Case study: St. Johns River Basin, USA. Fourth United Nations World Water Development Report, World Water Assessment Programme 2012. Paris: UNESCO Publishing.
Sánchez-Chardi, A., & Nadal, J. (2007). Bioaccumulation of metals and effects of landfill pollution in small mammals. Part I. The greater white-toothed shrew, Crocidura russula. Chemosphere, 68, 703–711.
Sánchez-Chardi, A., López-Fuster, M. J., & Nadal, J. (2007). Bioaccumulation of lead, mercury, and cadmium in the greater white-toothed shrew, Crocidura russula, from the Ebro Delta (NE Spain): sex- and age-dependent variation. Environmental Pollution, 145, 7–14.
Saunders, D. A. (1988). Adirondack mammals. Albany, New York: State University of New York, College of Environmental Science and Forestry.
Shore, R. F., & Rattner, B. A. (Eds.). (2001). Ecotoxicology of wild mammals. Chichester: John Wiley and Sons.
Sibly, R. M., & Brown, J. H. (2007). Effects of body size and lifestyle on evolution of mammal life histories. Proceedings of the National Academy of Sciences, 104:17707-17712.
Thomason, R. K., Lockhart, J. M., Loughry, W. J., & Bielmyer-Fraser, G. K. (2016). Metal accumulation in bobcats in the Southeastern USA. Environmental Monitoring and Assessment, 188, 565.
U.S. Environmental Protection Agency. (2016). Six-year review report, fourth five-year review report for Firestone Tire & Rubber Co. (Albany Plant) GAD990855074. U.S. Environmental Protection Agency, Region 4. Atlanta, GA. September 2016.
Wijnhoven, S., Leuven, R. S. E. W., van der Velde, G., Jungheim, G., Koelemij, E. I., de Vries, F. T., Eijsackers, H. J. P., & Smits, A. J. M. (2007). Heavy-metal concentrations in small mammals from a diffusely polluted floodplain: importance of species- and location-specific characteristics. Archives of Environmental Contamination and Toxicology, 52, 603–613.
Wuana, R. A., & Okieimen, F. E. (2011). Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. International Scholarly Research Network Ecology, 2011, 1–20.
Acknowledgments
We thank USDS-Georgia Wildlife Services personnel for allowing access to the specimens they collected.
Funding
Funding for lab analyses was provided by a Valdosta State University faculty research seed grant to JML and GKB-F, and an equipment grant to GKB-F for the atomic absorption spectrophotometer.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hough, S.E., Lockhart, J.M., Loughry, W.J. et al. Comparative metal analysis in a species assemblage of mammals from the Southeastern United States. Environ Monit Assess 192, 306 (2020). https://doi.org/10.1007/s10661-020-08259-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-020-08259-5