Abstract
The sources, distribution and risk assessment of polycyclic aromatic hydrocarbons (PAHs) were investigated in the mangrove sediments of Trombay and Vashi, along the Thane Creek, Maharashtra, India, for a period of 6 months. The results showed that the concentration of Ʃ15 PAHs ranged from 902.58 to 1643.60 and from 930.69 to 1158.30 ng g−1 in Trombay and Vashi, respectively. Trombay showed significantly higher PAH concentration (p < 0.05) than Vashi. The four carcinogenic PAHs, (benzo(b)fluorathene, benzo(k)fluorathene, Indeno(1,2,3-cd)pyrene and dibenz(a,h)anthracene) accounted for maximum concentration of the total PAHs. Specific PAH diagnostic indices and the molecular index indicated the presence of both pyrolytic and petrogenic sources with the predominance of pyrolytic origin. A positive correlation (r = 0.736, p < 0.05) existed between the benzo(k)fluorathene level and total PAHs, suggesting the use of this compound as a potential molecular marker for PAH pollution in mangrove sediment. Assessments of potential environmental risks associated with PAHs in this study revealed that the sediment was moderately polluted with high molecular weight PAHs. The study reports the baseline data that can be used for regular monitoring of contamination level considering the heavy industrialization and urbanization along the creek and its coastal region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous contaminants in the environment with known or potential toxic, mutagenic or carcinogenic properties. They are organic compounds which contain typically up to 10 aromatic rings that are produced by high-temperature reactions, such as incomplete combustion and pyrolysis of fossil fuels and other organic materials, and release of petroleum and petroleum products (Cao et al. 2010).
These chemicals are known to enter the near-shore marine environment through the spillage of petroleum, industrial discharge and atmospheric fallouts, shipping activities, storm water drains and urban run-off (Gevao et al. 1998; Cavalcante et al. 2009). PAHs have high hydrophobic properties with relatively stable chemical structure and thus can be found at high concentrations in contaminated coastal marine sediments (LaFlamme and Hites 1978; Ranjan et al. 2012). PAH contamination is a major hazard that is a concern for aquatic life in marine sediments, particularly in areas close to anthropogenic sources (Liu et al. 2009; Veltman et al. 2012).
The solubility of PAHs in water is generally low and decreases as the molecular weight increases; hence, they are rapidly sequestered by suspended and bed sediment. It can, therefore, be considered as a sink for PAH contaminants and serves as the major pathway to remove PAHs from the water body. The inputs of PAHs from human activities such as oil spill, offshore production, transportation and combustion are very significant and pose serious threat to habitats such as mangroves (Corredor et al. 1990; Fathallah et al. 2012). Mangrove ecosystems are noted as important inter-tidal estuarine wetlands along the coastlines of tropical and subtropical regions, which are exposed to anthropogenic contamination by PAHs from tidal water, river water and land-based sources (Tian et al. 2008; Sarkar et al. 2012).
Mumbai City is one of the most heavily populated, industrialized and sixth largest metropolitan region of the world. The creek, known as “Thane Creek” separates the Island City of Mumbai in the west from the mainland in the east and houses industrial areas at a distance of about 25 km. The creek has attracted greater attention of the environmentalists over the last few decades, as Mumbai harbour situated in the west and New Nava Sheva port in the east, handling more than 30 million tons of goods per year, additionally contributing to the pollutants in the creek by way of leakage, spill and corrosion. The mangrove sediment of Thane Creek is prone to the contamination of PAHs due to voluminous release of effluents from a variety of industries including oil refineries and petrochemical complexes, loading and unloading of crude oil and its products at Mumbai Port, and indiscriminate release of untreated or partially treated sewage (Singare et al. 2010).
The contamination, environmental persistence, bioaccumulation and trophic transfer of PAHs in aquatic ecosystem are important in assessing the ecological risk of pollutants (Yan et al. 2009; Sinaei and Mashinchian 2014). The accumulation, distribution, sources and the risk assessment of PAHs in the sediment of Thane Creek have not been investigated. Therefore, the present study was undertaken to determine the concentration, spatial distribution and sources of PAHs in surface sediment of mangrove swamps of Thane Creek.
Materials and methods
Study area
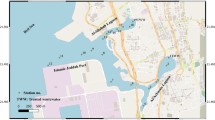
The study was carried out in Thane Creek (72°.55′ to 73°.02′ E and 19°.00′ to 19°.15′ N) near Mumbai City which is a mangrove-fringed tropical coastal ecosystem along the central west coast of India. The mangrove swamp along Trombay (Lat. 19° 00′ N Long. 72° 90′ E) and Vashi (Lat. 19.05° 00′ N Long. 72.59° 00′ E) part of the creek was selected for the study (Fig. 1). This mangrove area is one of the most dynamic and complex with Avicennia as the most dominant species. It is a tide-dominated estuarine mangrove area and is characterized by complex network of tidal creeks, exposed to different elevations at high and low semidiurnal tides. The creek is narrow and shallow in the north where Ulhas River flows into it through a minor connection and is broader and deeper towards the sea. Due to geomorphic head near Thane City, the creek receives negligible fresh water flow from the Ulhas River. Hence, except during monsoon, it is tidally influenced with dominance of neritic waters. However, the present heavy industrialization and urbanization along the creek has resulted in release of effluents in quantities far exceeding the assimilating capacity of the creek (Quadros and Athalye 2012).
Sampling site. T1 (19° 00.323′ N, 72° 55.562′ E), T2 (19° 00.450′ N, 72° 55.761′ E), T3 (19° 00.650′ N, 72° 55.458′ E), T4 (19° 00.788′ N, 72° 55.869′ E), T5 (19° 01.221′ N, 72° 56.114′ E) and VI (19° 03.651′ N, 72° 59.416′ E), V2 (19° 03.599′ N, 72° 59.274′ E), V3 (19° 03.549′ N, 72° 59.444′ E), V4 (19° 03.488′ N, 72° 59.528′ E), V5 (19° 03.651′ N, 72° 59.274′ E)
Sampling
The sediment samples for the analysis of PAHs were collected at five different stations each at Trombay and Vashi mangroves on monthly basis from September 2011 to February 2012 (coordinates recorded using GPS) using Van Veen Grab Sampler. They were packed in labelled aluminium foil and brought in ice box and stored at −20 °C for further analysis. The sediment samples for the analysis of physico-chemical parameters were packed separately in labelled polythene bags and processed in the laboratory.
Extraction and purification of PAHs
Prior to extraction, the sediments were defrosted, dried in an oven at 50 °C overnight, ground with mortar and pestle and passed through a sieve (2000 μm) to remove gravel and other unwanted materials. PAHs were extracted from the sediment samples by using Soxhlet apparatus. Extraction was carried out by mixing 250 ml of n-hexane and dichloromethane (50:50 v/v) for 16 h at 36 °C (UNEP 1992). After 16 h of extraction, the extracted samples were dried up until 1.0 ml volume using rotary evaporator at 40 °C and 151 bs pressure (Wang et al. 2001). Extracts were purified using a 1.0 × 20 cm glass chromatography column filled with 2 g of 100–200 mesh silica gel which was pre-baked at (120 °C for 4 h). On top of the silica gel, about 10 mm activated Cu (activated in concentrated HCl and rinsed with dichloromethane) was packed to remove sulphur from the extract. The extract was added to the column using a glass pipette while the PAHs were eluted with a 25 ml mixture of 25 % DCM and 75 % hexane. The elute sample was collected in a 50-ml pear-shaped flask. After reducing the volume to about 1.0 ml by rotary evaporation, the sample was transferred to an amber glass vial and was further concentrated to 100 μl with N2. The samples were stored at −20 °C until further laboratory analyses. After elution of each sample, the column was cleaned and repacked with new silica gel. All the glasswares were acid-washed, Milli-Q water and solvent rinsed and baked at 120 °C for 24 h before use.
Analysis of PAH
The concentration of PAH compounds was analyzed using a Shimadzu 14-B gas chromatography with a flame ionization detector (GC-FID) and a BP1 (Sl. no. 4289A17) fused silica capillary column (30 m × 0.25 mm id × 0.25 μm film thickness). The oven temperature programme for PAH analyses was from 100 °C (initial time, 4 min) to 120 °C at a rate of 1 °C min−1 and from 120 to 300 °C at a rate of 3 °C min−1 and held at 300 °C for 10 min. The injector and detector temperatures were set at 280 and 300 °C, respectively. Nitrogen was used as the carrier gas. The response factor of individual PAH compounds to the internal standard (phenanthrene-d10, obtained from Sigma, USA) was measured and calculated three times: at the beginning, in the middle, and at the end for each batch of GC injections (10 samples). Identification and quantification analytes were carried out with GC-FID using external standard methods. The chromatogram of 15 PAH standards analysed (naphthalene (N), acenaphthylene (A), acenaphthene (Ac), fluorene (F), phenanthrene (P), anthracene (An), fluoranthene (Fl), pyrene (Py), benzo(a)anthracene (BaA), chrysene (C), benzo(b)fluoranthene (BbF), benzo(k)fluoranthene (BkF), indeno(1,2,3-cd)pyrene (IP), dibenzo(ah)anthracene (DahA), benzo(ghi)perylene (BP).
Physicochemical characteristics of sediment
Sediment pH and electrical conductivity (EC) were measured using pH and EC meters. Total carbon and total nitrogen was analysed using Vario Micro Cube CHNS Elementar analyser from Germany, following manufacture’s manual; C/N ratio was calculated while the sediment texture was analysed by pipette method. Total organic carbon (TOC) was analysed according to the method of Walkley and Black (1934). Total nitrogen and organic carbon were expressed as g C kg−1 dry sediment.
Risk assessment
Comparisons among the concentrations of specific pollutants detected in the sediments and their corresponding sediment quality values (i.e. concentrations below which adverse effects in the marine ecosystem were unlikely) were performed. The levels of risk posed by certain chemicals in the sediments were characterized by the risk quotient which was calculated using the formula according to Dominguez et al. (2010):
The two values were used to calculate risk quotient under best case (RQbcs) and worst case (RQwcs)
Method validation
All samples were spiked with deuterated PAH surrogate standards of p-Terphenyl-d14, Naphthalene-d8 and Phenanthrene-d10 and analysed to determine the general recovery procedure. The average recovery of the samples ranged from 82.78 to 89.64 % (n = 45).
Statistical analysis
Data obtained were analyzed using the statistical package SPSS 17.0 computer program (SPSS Inc. Chicago, Illinois, USA). Differences in PAH concentrations in the stations were subjected to one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test to determine level of significance at 5 % probability level.
Results and discussion
Sediment characteristics
The sediment quality parameters (pH, EC, total organic carbon (TOC), total nitrogen and C/N ratio) are presented in Table 1. The result shows that pH of the sediment ranged from 7.7 to 7.8 and 7.5 to 7.8 at Trombay and Vashi, respectively, indicating a slight neutral to basic mangrove sediment. Positive correlation between concentrations of PAHs and TOC in sediments has been reported (Shi et al. 2007; Charriau et al. 2009); higher TOC in mangrove sediment might make adsorption of less polar organic compounds easier and thus uptaking and preservation of PAHs possible (Zhang et al. (2004). In this study, the total organic carbon varied from 12.90 to 18.64 kg−1 and 13.38 to 21.24 kg−1 at Trombay and Vashi, respectively (Table 1), indicating high TOC in both sampling area. It can be suggested that TOC played a more important role in controlling the distributions of low molecular weight polycyclic aromatic hydrocarbons (LPAHs) than high molecular weight polycyclic aromatic hydrocarbons (HPAHs) in surface sediments of Thane Creek. However, Notar et al. (2001) noted that PAH concentrations tend to be higher in sediments with higher TOC contents due to the high sorption capacity of organic carbon. The higher TOC recorded at the Vashi than the Trombay may be attributed to the fact that Vashi receives discharges of high biological productivity and sedimentation rates than Trombay because of its location than Trombay. The samples at the Vashi are located in the mouth of the Creek. Montero et al. (2013) reported that a water body which receives discharges from biological productivity and high sedimentation rates has high concentrations of TOC and organic matter and identified to be main cause of low to moderate toxicity.
The result of total nitrogen ranged from 0.62 to 1.72 g kg−1 (Trombay) and 0.52 to 1.16 g kg−1 (Vashi). The level of total nitrogen recorded in the study may be attributed to the contamination of microbial structure of mangrove sediment by the PAHs that limit the activities of nitrogen-fixing bacteria. Sun et al. (2012) documented that high concentration of PAH has larger impact on the nitrogen-fixing bacteria than low concentration of PAH in mangrove sediment.
The C/N ratio varied from 9.98 to 23.86 and 14.45 to 28.49 at Trombay and Vashi, respectively. The ratio of C/N was high probably due to low nitrogen content in the sediments, indicating possible contamination of PAHs from anthropogenic sources in mangrove sediment. Higher C/N ratios in the marsh sediments indicate a mixture of vascular plant and marine algal sources, whereas the lower C/N ratios in shelf sediments indicate that organic matter was derived more from phytoplankton (Wang et al. 2014). Furthermore, the variation among chemical properties of the sediment, especially organic carbon and textural properties, might influence the PAH accumulation in the marine sediment.
The textural composition of the sites indicated that total percentage of clay varied from 47.10 to 55.48 % and 47.46 to 61.67 % at Trombay and Vashi, respectively. Sediment contained higher percentage of clay (61.67 %) at Vashi than Tombay. Silt percentage varied equally at both the locations. The percentage of silt ranged from 35.23 to 45.19 % at Tombay and 31.78 to 43.9 7 % at Vashi sediment while the percentage of sand varied from 4.56 to 7.47 % and 6.54 to 8.75 % at Trombay and Vashi, respectively. The overall sediment texture of all the sites was clayey-loamy. Textural differences may be as a result of vigorous estuarine mixing, suspension-resuspension and flocculation-deflocculation processes (Dominguez et al. 2010).
Polycyclic aromatic hydrocarbons
The 16 USEPA priority PAHs have been used as representative of PAHs to evaluate the anthropogenic pollution levels in sediments and other environmental compartments all over the world by a number of researchers (Mai et al. 2001; Agarwal et al. 2006; Qiao et al. 2006). In the present study, 15 USEPA priority PAH compounds were analysed because the Shimadzu 14-B gas chromatography was unable to estimate the remaining PAH. The concentration of total PAH (Σ15 PAHs) varied in the range of 902 to 1643 and 930 to 1158 ng g−1 at Trombay and Vashi, respectively (Tables 2 and 3). According to Boonyatumanond et al. (2006), the distribution of PAHs in surface sediment is controlled by the hydrodynamic conditions of the mangroves, which includes factors such as (1) textural composition and depositional rate of sediments, (2) input rate of PAHs, (3) differential resuspension and redeposition of sedimentary PAHs, (4) vertical mixing due to physical and biological processes, and (5) microbial degradation of PAHs. ANOVA showed significant difference in total PAH (p < 0.05) between sites. Total PAHs in the sediment at Trombay was significantly higher than that at Vashi. Trombay sediment showed minimum (902 ng g−1) as well as maximum (1643.90 ng g−1) concentration of total PAH. The high PAH at Trombay is due to higher harbour activities of sailing of crude oil and the use of fishing boats by the fishermen than at Vashi area. The leakage of petroleum products and engine oil from the boats and ferries may elevate the level and contribute immensely to PAH recorded at Trombay. Similar to our finding, Nasher et al. (2013) recorded high concentration of PAH in Langkwai Island, Malaysia, owing to the intense harbour activities and disposal of engine oil from boat and ferries.
Distribution of individual PAHs
Analysis of variance for individual PAHs showed significant difference among the months, sites and month × site interaction with respect to majority of PAHs. Statistically, there was no significant difference (p > 0.05) in the concentration of fluorene and anthracene between Trombay and Vashi. Trombay recorded 172.24 and 285.61 while that at Vashi was 174.78 and 281.72 for fluorine and anthracene, respectively. Monthwise significant difference (p < 0.05) was observed with respect to benzo(a)anthracene. The month and site interaction was significant with respect to benzo(k)fluorathene. Fluorene in the sediment at Vashi (174.78) was significantly higher (p < 0.05) than that of Trombay (172.24). The monthly mean concentration of benzo(k)fluorathene was found in the range of 118.03 to 732.29 and 418.92 to 507.20 ng g−1 at Trombay and Vashi, respectively (Tables 2 and 3). There was statistically significantly higher (p < 0.05) benzo(k)fluorathene at Trombay (327.79) than Vashi (239.36).
Naphthalene is a typical constituent of refined petroleum and is used as an indicator of petroleum-source PAH contamination (Steinhauer and Boehm 1992). The low naphthalene concentration was observed in the sediments (21.82 to 106.69 and 26.94 to 59.73 ng g−1 at Trombay and Vashi, respectively). The low molecular weight naphthalene in sediment is easily depleted by volatilization from oil, dissolution from sediments and degradation by bacteria within a short period of time (Ho et al. 1999). In our findings, acenaphthylene, acenaphthene, fluorene, phenanthrene, anthracene, fluorathene and pyrene were found in mangrove sediment at both sites during the study period. The presence of the above PAHs may be attributed to the availability of diesel fuel in the mangrove sediment, as reported by Wang et al. (2009) who noted that the predominance of phenanthrene, fluoranthene and pyrene is characteristic of diesel fuel.
PAHs are primarily products of incomplete combustion processes and comprise two to six fused aromatic rings. The low molecular weight (two and three rings) PAHs have a significant acute toxicity, whereas some of the higher molecular weight PAHs are carcinogenic (Neff 1979; Witt 1995). The average concentration of four carcinogenic PAHs (benzo(b)fluorathene, benzo(k)fluorathene, indeno (1,2,3-cd)pyrene and dibenz(a,h)anthracene) was found to be 4.04 to 35.88 and 5.41 to 38.77 % of total PAHs at Trombay and Vashi, respectively (Figs. 2 and 3). A positive correlation (r = 0.736, p < 0.05) existed between benzo(k)fluorathene and total PAHs making this compound potential molecular marker for PAH pollution in the sediment. Benzo(k)fluorathene showed an overall similar distribution in all the months at both the sites indicating common sources of origin. The importance of high molecular mass PAHs has been commonly observed in sediments from marine and lacustrine environments (Mostafa et al. 2009). The abundance of high molecular in weight PAHs in sediment samples is more likely to be transported to the sediment bed due to their increased sorption and resistance to degradation (Mirza et al. 2012).
Mean percentage of PAH compounds in the sediments at a Trombay and b Vashi. S September, O October, N November, D December, J January, F February, Naph napthalene, Acenap acenaphthylene, Acenaph acenaphthene, Fluo fluorene, Phena phenanathrene, Anth anthracene, Fluora fluorathene, Pyr pyrene, B(a)anth benzo(a)anthracene, Chry chrysene, B(b)flu benzo(a)fluorathene, B(k)fluo benzo(k)fluorathene, I(1,2,3-c,d)pyr indeneo(1,2,3-c,d)pyrene, D(a,h)anth dibenzo(a,h,anthracene), B(ghi)per benzo(ghi)perylene)
Mean percentage of four carcinogenic PAH compounds from total PAH in the sediments at a Trombay and b Vashi. S September, O October, N November, D December, J January, F February, B(b)flu benzo(a)fluorathene, B(k)fluo benzo(k)fluorathene, I(1,2,3-c,d)pyr indeneo(1,2,3-c,d)pyrene, D(a,h)anth dibenzo(a,h,anthracene)
Sources of PAH
The study of PAH ratio profiles based on its physical-chemical properties have been commonly used to assess the differences between those of pyrolytic and petrogenic origin (Mirza et al. 2012). Isomer index was also used based on the concentration of PAHs at the study sites to assign source of PAHs (Table 4). In these sites, the heterogeneity of compounds and the complexity of the environmental matrix make source identification difficult and more than one source identification method is required to be used to identify the PAH origin (Riccardi et al. 2013).
In this study, the Phe/Ant and Flu/Pyr ratios were lower than 10 for all the months in the study sites indicating pyrolytic origin (Table 5). Budzinski et al. (1997) noted that values greater than 1 are characteristic of pyrolytic origin, whereas values lower than 1 are related to petrogenic. The Phe/Ant ratio is temperature dependent; thus, high-temperature processes, such as combustion of organic matter, generate PAHs characterized by a low Phe/Ant ratio of 10 (Riccardi et al. 2013) whereas the slow maturation of organic matter during catagenesis leads to much higher Phe/Ant values >15 (Baumard et al. 1998). Both pyrolytic and petrogenic sources showed their presence in the mangrove sediment with pyrolytic source being dominant. In general, HPAHs are more abundant over LPAHs in river and marine sediments (Guo et al. 2007; Yan et al. 2009).
Isomer pair ratios A/178 and IPy/(IPy/BPer) have been calculated to show relative distribution of PAHs with regard to their possible source of origin. A/178 pair ratios of the study area also showed petroleum combustion sources (Yunker et al. 2002). The combustion of crude oil was possibly the major source of PAHs in the sediments that have been transported from distal (away from) sources because small-size PAHs are more labile (Hu et al. 2010; Venkatachalapathya et al. 2012).
At Trombay and Vashi, the ratio of IPY/(IPY + BghiP) isomer pair showed the origin from petroleum combustion with value of more than 0.20 (Table 5). Although distinct sources might be inferred from PAH isomer ratios, it is well documented that in urban and industrial areas, PAHs might originate from a variety of sources and are transported by different pathways in the study areas.
Baumard et al. (1998), classified ΣPAH pollution levels into four different categories which included low, moderate, high and very high level when ΣPAHs are 0–100, 100–1000, 1000–5000 and >5000 ng g−1 d.w., respectively. Consistent with our present findings, when compared with a worldwide concentration of PAHs (Table 6), the study sites appear to be contaminated with PAHs in moderate to high level.
Assessment of sediment quality using environmental quality thresholds
The effects range low (ERL) and the effects range median (ERM) values were used for impact assessment on aquatic sediment with a ranking of low to high impact values (Long et al. 1995; Khim et al. 1999). The measured concentrations of PAHs were compared with the existing ERL and ERM values. Results showed that the total PAH concentrations at both the sites (Table 7) were below the ERL of 4000 and ERM of 44,792 ng g−1, indicating moderate pollution of PAHs in the mangrove sediment. Zhao et al. (2014) noted that the ΣPAHs in ≥ ERL and < ERM demonstrate adverse biological toxicity effect that would occur occasionally, confirming that the study areas are moderately polluted with PAHs.
The potential environmental risk associated with PAHs in the study is shown in Table 7. Sediment quality values used in the calculation of risk quotients included ERL and ERM guidelines values from US National Oceanic and Atmospheric Administration (NOAA) [(http://response.restoration.noaa.gov/cpr/sediment/squirt/squirt.html)] (Burton 2002). Only contaminants, for which sediment quality value was available, were assessed in the study. All the RQbcs estimated were below 1 suggesting that pollutants under investigation pose little hazard to the ecosystems but RQwcs was higher than 1 at both the sites, indicating that concentration of these chemicals in the sediments poses a risk to the aquatic environment. RQwcs values revealed that the concentration of PAHs was more than the recommended level at both the sites. This might affect adversely most of the living organisms, and hence, preventive measures should be undertaken to avoid the ecological risks on marine organisms, especially the benthic species. High molecular weight PAHs, recorded in the study, are highly mutagenic and carcinogenic revealing risk to the ecosystem.
Conclusion
Relative to other urbanized coastal areas of the world’s coastal region, Thane Creek, Mumbai can be considered moderately contaminated with PAHs. The presence of high concentration of carcinogenic benzo(k)fluorathrene in the sediment makes this compound as a potential molecular marker for PAH pollution in the sediment. The data provide baseline information on the distribution and potential sources of PAHs in the mangrove sediment at Trombay and Vashi and could help in effective management of the persistent pollutants in the ecosystem. Further investigations are to be carried out to study the accumulation pattern and effect of these compounds in biota in the ecosystem.
References
Agarwal, T., Khillare, P. S., & Shridhar, V. (2006). PAHs contamination in bank sediments of the Yamuna River, Delhi, India. Environmental Monitoring and Assessment, 123, 151–166.
Baumard, P., Budzinski, H., & Garrigues, P. (1998). Polycyclic aromatic hydrocarbons (PAHs) in sediments and mussels of the western Mediterranean Sea. Environmental Toxicology and Chemistry, 17(5), 765–776.
Baumard, P., Budzinski, H., Garrigues, P., Dizer, H., & Hansen, P. D. (1999). Polycyclic aromatic hydrocarbons in recent sediment and mussels (Mytilusedulis) from the Western Baltic Sea: occurrence, bioavailability and seasonal variations. Marine Environmental Research, 47, 17–47.
Boonyatumanond, R., Wattayakorn, G., Togo, A., & Takada, H. (2006). Distribution and origins of polycyclic aromatic hydrocarbons (PAHs) in riverine, estuarine, and marine sediments in Thailand. Marine Pollution Bulletin, 52, 942–956.
Budzinski, H., Jones, I., Bellocq, J., Pierard, C., & Garrigues, P. (1997). Evaluation ofsediment contamination by polycyclic aromatic hydrocarbon in Gironde estuary. Marine Chemistry, 58, 85–97.
Burton, G. A. (2002). Sediment quality criteria in use around the world. The Japanese Society of Limnology, 3, 65–75.
Cao, Z., Liu, J., Luan, Y., Li, Y., Ma, M., Xu, J., et al. (2010). Distribution and ecosystem risk assessment of polycyclic aromatic hydrocarbons in the Luan River, China. Ecotoxicology, 19, 827–837.
Cavalcante, R. M., Sousa, F. W., Nascimento, R. F., Silveira, E. R., & Freire, G. S. S. (2009). The impact of urbanization on tropical mangroves (Fortaleza, Brazil): evidence from PAH distribution insediments. Journal of Environmental Management, 91, 328–335.
Charriau, A., Bodineau, L., Ouddane, B., & Fischer, J. C. (2009). Polycyclic aromatic hydrocarbons and n-alkanes in sediments of the Upper Scheldt River Basin: contamination levels and source apportionment. Journal of Environmental Monitoring, 11(5), 1086–1093.
Corredor, J. E., Morell, J. M., & del Castillo, C. E. (1990). Persistence of spilled crude oil in a tropical intertidal environment. Marine Pollution Bulletin, 21, 385–388.
DomÍnguez, C., Sarkar, S. K., Bhattacharya, A., Chatterjee, M., Bhattacharya, B. D., Jover, E., et al. (2010). Quantification and source identification of polycyclic aromatic hydrocarbons in core sediments from Sundarban Mangrove Wetland, India. Archives of Environmental Contamination and Toxicology, 59, 49–61.
Fathallah, S., Medhioub, M. N., & Kraiem, M. M. (2012). Photo-induced toxicity of four polycyclic aromatic hydrocarbons (PAHs) to embryos and larvae of the carpet shell clam Ruditapesdecussatus. Bulletin of Environmental Contamination and Toxicology, 88, 1001–1008.
Gevao, B., Hamilton-Taylor, J., & Jnes, K. C. (1998). PCB and PAH deposition to and exchange at the air–water interface of a small rural lake in Cumbria, UK. Environmental Pollution, 102, 63–75.
Guo, W., He, M., Yang, Z., Lin, C., Quan, X., & Wang, H. (2007). Distribution of polycyclic aromatic hydrocarbons in water, suspended particulate matter and sediment from Daliao River watershed, China. Chemosphere, 68, 93–104.
Ho, K. A. Y., Patton, L., Latimer, J. S., Pruell, R. J., Pelletier, M., McKinney, R., et al. (1999). The chemistry and toxicity of sediment affected by oilfrom the North Cape spilled into Rhode Island Sound. Marine Pollution Bulletin, 38, 314–323.
Hu, N., Shi, X., Liu, J., Huang, P., Liu, Y., & Liu, Y. (2010). Concentrations and possible sources of PAHs in sediments from Bohai Bay and adjacent shelf. Environmental Earth Sciences, 60, 1771–1782.
Khim, J. S., Kannan, K., Villeneuve, D. L., Koh, C. H., & Giesy, J. P. (1999). Characterization and distribution of trace organic contaminants in sediment from Masan Bay, Korea: instrumental analysis. Environmental Science and Technology, 33, 4199–4205.
Laflamme, R. E., & Hites, R. A. (1978). The global distribution of polycyclic aromatic hydrocarbons in recent sediments. Geochimica et Cosmochimica Acta, 42, 289–303.
Liu, Y., Chen, L., Huang, Q. H., Li, W. Y., Tang, Y. J., & Zhao, J. F. (2009). Source apportionment of polycyclic aromatic hydrocarbons (PAHs) in surface sediments of the Huangpu River, Shanghai, China. Science of the Total Environment, 407, 2931–2938.
Long, E. R., MacDonald, D. D., Smith, S. C., & Calder, F. D. (1995). Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environmental Management, 19, 81–97.
Ma, M., Feng, Z., Guan. C., Ma, Y., Xu, H., & Li, H. (2001). DDT, PAH and PCB in sediments from the Bohai Sea and the Yellow Sea. Marine Pollution Bulletin, 42,132–136.
Macias-Zanmora, J. V., Mendoza-Vega, E., & Villaescusa-Celaya, J. A. (2002). PAHs composition of surface marine sediments: a comparison to potential local sources in TodosSantos Bay, BC, Mexico. Chemosphere, 46, 459–468.
Mai, B., Fu, J., Zhang, G., Lin, Z., Min, Y., Sheng, G., et al. (2001). Polycyclic aromatic hydrocarbons in sediments from the Pearl river and estuary, China: spatial and temporal distribution and sources. Applied Geochemistry, 16, 1429–1445.
Mai, B.X., Fu, J.M., Sheng, G.Y., Kang, Y.H., Lin, Z., Zhang, G., et al. (2002). Chlorinated and polycyclic aromatic hydrocarbons in riverine and estuarine sediments from Pearl River Delta, China. Environmental Pollution, 117, 457–474.
Mirza, R., Faghiri, I., & Abedi, E. (2012). Contamination of polycyclic aromatic hydrocarbons in surface sediments of Khure-Musa Estuarine, Persian Gulf. World Journal of Fish and Marine Sciences, 4(2), 136–141.
Montero, N., Belzunce-Segarra, M. J., Menchaca, I., Garmendia, J. M., Franco, J., Nieto, O., et al. (2013). Integrative sediment assessment at atlanticspanish harbours by means of chemical and ecotoxicological tools. Environmental Monitoring and Assessment, 185, 1305–1318.
Mostafa, A. R., Terry, L. W., Stephen, T. S., & Barakat, A. O. (2009). Distribution and characteristics of polycyclic aromatic hydrocarbons (PAHs) in sediments of Hadhramout coastal area, Gulf of Aden. Marine Systems, 78, 1–8.
Nasher, E., YookHeng, L., Zakaria, Z., & Surif, S. (2013). Assessing the ecological risk of polycyclic aromatic hydrocarbons in sediments at Langkawi Island, Malaysia. The Scientific World Journal. doi:10.1155/2013/858309.
Neff, J. M. (1979). Polycyclic aromatic hydrocarbons in the aquatic environment sources fates and biological effects (p. 262). London: Applied Science Publisher Ltd.
Notar, M., Leskovs, H., & Faganeli, J. (2001). Composition, distribution and sources of polycyclic aromatic hydrocarbons in sediments of gulf of Trieste, Northern Adriatic Sea. Marine Pollution Bulletin, 42, 36–44.
Qiao, M., Wang, C., Huang, S., Wang, D., & Wang, Z. (2005). Composition, sources and toxicological significance of PAHs in the surface sediments of the Meiliang Bay, Taihu Lake, China. Environment International, 32, 28–33.
Qiao, M., Wang, C. X., Huang, S. B., Wang, D. H., & Wang, Z. J. (2006). Composition, sources, and potential toxicological significance of PAHs in the surface sediments of the Meiliang Bay, Taihu Lake, China. Environment International, 32(1), 28–33.
Quadros, G., & Athalye, R. P. (2012). Decline of fish diversity in the anthropogenically polluted Thane creek along the Central West Coast of India. International Research Journal of Biological Sciences, 1(4), 17–21.
Ranjan, R. K., Routh, J., Ramanathan, A. L., & Klump, J. V. (2012). Polycyclic aromatichydrocarbon fingerprints in the Pichavaram mangrove–estuarine sediments, south-eastern India. Organic Geochemistry, 53, 88–94.
Riccardi, C., Di Filippo, P., Pomata, D., Di Basilio, M., Spicaglia, S., & Buiarelli, F. (2013). Identification of hydrocarbon sources in contaminated soils of three industrial areas. Science of the Total Environment, 450, 13–21.
Sarkar, S. K., Binelli, A., Chatterjee, M., Bhattacharya, B. D., Parolini, M., Riva, C., et al. (2012). Distribution and ecosystem risk assessment of polycyclic aromatic hydrocarbons (PAHs) in core sediments of Sundarban Mangrove Wetland, India. Polycyclic Aromatic Compounds, 32(1), 1–26.
Savinov, V. M., Savinova, T. N., Matishov, G. G., Dahle, S., & Naes, K. (2003). Polycyclic aromatic hydrocarbons (PAHs) and organochlorines (OCs) in bottom sediments of the GubaPechenga, Barents Sea, Russia. Science of the Total Environment, 306, 39–56.
Shi, Z., Tao, S., Pan, B., Liu, W. X., & Shen, W. R. (2007). Partitioning and source diagnostics of polycyclic aromatic hydrocarbons in rivers in Tianjin, China. Environmental Pollution, 146(2), 492–500.
Sinaei, M., & Mashinchian, A. (2014). Polycyclic aromatic hydrocarbons in the coastal sea water, the surface sediment and Mudskipper Boleophthalmusdussumieri from coastal areas of the Persian Gulf: source investigation, composition pattern and spatial distribution. Journal of Environmental Health Science and Engineering, 12(59), 1–11.
Singare, P. U., Lokhande, R. S., & Pathak, P. P. (2010). Soil pollution along Kalwa Bridge at Thane Creek of Maharashtra, India. Journal of Environmental Protection, 1, 121–128.
Steinhauer, M. S., & Boehm, P. D. (1992). The composition and distribution of saturated and aromatic hydrocarbons in nearshore sediments, and coastal peat of the Alaskan Beaufort Sea: implications for detecting anthropogenic hydrocarbon input. Marine Environmental Research, 33, 223–253.
Sun, F. L., Wang, Y. S., Sun, C. C., Peng, Y. L., & Deng, C. (2012). Effects of three different PAHs on nitrogen-fixing bacterial diversity in mangrove sediment. Ecotoxicology, 21(6), 1651–1660.
Tian, Y., Luo, Y. R., Zheng, T. L., Cai, L. Z., Cao, X. X., & Yan, C. L. (2008). Contamination and potential biodegradation of polycyclic aromatic hydrocarbons in mangrove sediments of Xiamen, China. Marine Pollution Bulletin, 56, 1184–1191.
Tolun, L. G., Okay, O. S., Gaines, A. F., Tolay, M., Tufekci, H., & Kirati, N. (2001). The pollution status and the toxicity of surface sediments in Yzmit Bay (Marmara Sea), Turkey. Environment International, 26, 163–168.
UNEP (1992) Determination of petroleum hydrocarbons in sediments. Reference methods for marine pollution studies No. 20 Reference methods for marine pollution. 75p
Veltman, K., Huijbregts, M. A. J., Rye, H., & Hertwich, E. G. (2012). Including impacts ofparticulate emissions on marine ecosystems in life cycle assessment: the case of offshore oil and gas production. Integrated Environmental Assessment and Management, 7, 678–686.
Venkatachalapathya, R., Veerasingam, S., & Rajeswari, V. (2012). Distribution and origin ofpetroleum hydrocarbons in Pichavaram mangrove swamp along Tamilnadu coast, Bay of Bengal, India. Geochemistry International, 50, 476–480.
Walkley, J. A., & Black, J. A. (1934). Estimation of organic carbon by the chromic acid titration method. Soil Science, 37, 29–31.
Wang, X. C., Zhang, Y. X., & Chen, R. F. (2001). Distribution and partitioning of Polycyclic Aromatic Hydrocarbons (PAHs) in different size fractions in sediments from Boston Harbor, United States. Marine Pollution Bulletin, 42, 1139–1149.
Wang, L., Yang, Z., Niu, J., & Wang, J. (2009). Characterization, ecological risk assessment and source diagnostics of polycyclic aromatic hydrocarbons in water column of the Yellow River Delta, one of the most plenty biodiversity zones in the world. Journal of Hazardous Materials, 169(1–3), 460–465.
Wang, Z., Liu, Z., Xu, K., Mayer, L. M., Zhang, Z., Kolker, A. S., et al. (2014). Concentrations and sources of polycyclic aromatic hydrocarbons in surface coastal sediments of the northern Gulf of Mexico. Geochemical Transactions, 15(2), 1–12.
Witt, G. (1995). Polycyclic aromatic hydrocarbons in water and sediments of the Baltic Sea. Marine Pollution Bulletin, 31, 237–248.
Wu, Y., Zhang, J., & Zhu, Z. (2003). Polycyclic aromatic hydrocarbons in the sediments of the Yaluhiang Estuary, North China. Marine Pollution Bulletin, 463, 619–625.
Yamashita, N., Kannan, K., Imagawa, T., Villeneuve, D. L., Hashimoto, S., Miyazaki, A., et al. (2000). Vertical profile of polychlorinated-dibenzo-p-dioxins, dibenzofurans, -naphthalenes, -biphenyls, polycyclic aromatic hydrocarbons and alkylphenols in a sediment core from Tokyo Bay, Japan. Environmental Science and Technology, 34, 3560–3567.
Yan, W., Chi, J. S., Wang, Z. Y., Huang, W. X., & Zhang, G. (2009). Spatial and temporaldistribution of polycyclic aromatic hydrocarbons (PAHs) in sedimentsfrom Daya Bay, South China. Environmental Pollution, 30, 1–8.
Yim, U. H., Hong, S. H., & Shim, W. J. (2007). Distribution and characteristics of PAHs in sediments from the marine environment of Korea. Chemosphere, 68, 85–92.
Yunker, M. B., Macdonald, R. W., Vingarzan, R., Mitchell, R. H., Goyette, D., & Stephanie, S. (2002). PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Organic Geochemistry, 33, 489–515.
Zhan, Z. L., Hong, H. S., Zhou, J. L., & Yu, G. (2004). Phase association of polycyclic aromatic hydrocarbons in the Minjang River Estuary, China. Science of the Total Environment, 323, 71–86.
Zhang, J., Cai, L., Yuan, D., & Chen, M. (2004). Distribution and sources of polynuclear aromatic hydrocarbons in Mangrove surficial sediments of Deep Bay, China. Marine Pollution Bulletin, 49, 479–486.
Zhao, S. M., Wang, B., Wang, D. W., Li, X. M., Huang, B., Hu, P., et al. (2014). Environmental behavior of PAHs in Dianchi Lake distributions, sources and risk assessment of polycyclic aromatic hydrocarbons in surface sediments from Dianchi Lake, China. International Journal of Environmental Research, 8(2), 317–328.
Acknowledgments
The authors would like to thank Dr. W. S. Lakra, Director, Central Institute of Fisheries Education, Mumbai and Indian Council of Agriculture Research, New Delhi, India for all the help and financial support in carrying out the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sukhdhane, K.S., Pandey, P.K., Vennila, A. et al. Sources, distribution and risk assessment of polycyclic aromatic hydrocarbons in the mangrove sediments of Thane Creek, Maharashtra, India. Environ Monit Assess 187, 274 (2015). https://doi.org/10.1007/s10661-015-4470-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4470-1