Abstract
In this work, we assessed the photo-toxicity of four polycyclic aromatic hydrocarbons (PAHs) to embryos and larvae of the European clam Ruditapes decussatus. The exposure of R. decussatus embryos (24 h) and larvae (96 h) to anthracene, fluoranthene, pyrene and benzo[a]pyrene resulted in reduction of normal D-veliger percentages and high larval mortality, both in darkness and under sunlight conditions. Based on the calculated EC50 and LC50 values, the toxicity of the forementioned PAHs was respectively enhanced 72, 35, 60 and 23 times in the embryotoxicity test and 32, 31, 12 and 61 times in the larval mortality test when exposures were performed under sunlight conditions. Simultaneous exposure to sunlight and these PAHs enhanced their toxicity in comparison to dark conditions. The clam embryos and larvae appear to be environmentally relevant life-stages in assessing the toxic and photo-toxic risk of PAHs that enter the marine environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Polycyclic aromatic hydrocarbons (PAHs) are a large group of widespread organic compounds of high environmental concern. Even though PAHs occur naturally, the highest concentrations are mainly due to human activities that cause a continuous increase in PAH levels of estuarine and marine waters (Kennish 1992; Walker et al. 2001). Direct discharges into the marine environment from point sources such as wastewater treatment plants range from <1 μg/L to over 625 μg/L, whilst concentrations of PAHs in industrial effluents range from undetectable to 4.4 mg/L (Latimer and Zheng 2003). Major sources of PAHs to the marine environment are combustion products and petroleum principally from atmospheric deposition (5 × 104 tones/year) and oil spillage (1.7 × 105 tones/year) (Kennish 1992; Meador 2003). Therefore, it is necessary to study the impact of those pollutants to marine organisms of ecological and commercial relevance.
In general, PAHs do not show extremely high acute toxicity to aquatic life, with the majority of intermediate to high molecular weight PAHs not exhibiting acute toxicity within their water solubility limits (NRCC 1983). However, there is a growing body of evidence to suggest that certain PAHs may pose a greater hazard to aquatic organisms than previously demonstrated, due to their potential to cause photo-induced toxicity (photo-toxicity) when exposed to ultraviolet (UV) light.
PAH phototoxicity has been demonstrated in the laboratory, generally for standard aquatic test species, including algae (Gala and Giesy 1992), duckweed (Huang et al. 1993), oligochaetes (Ankley et al. 1995), chironomids (Hatch and Burton 1999), amphipods (Duan et al. 2000; Hatch and Burton 1999), daphnids (Sasson-Brickson and Burton 1991; Wernersson and Dave 1998), brine shrimp (Diamond et al. 2000), mysid shrimp (Pelletier et al. 1997), mollusk larvae (Pelletier et al. 1997; Saco-Álvarez et al. 2008), fish (Diamond et al. 1995; Saco-Álvarez et al. 2008) and amphibians (Hatch and Burton 1998; Monson et al. 1999).
Here we present data on laboratory studies investigating the acute toxicity and potential photo-toxicity of fluoranthène, anthracène, pyrene and benzo[a]pyrene, under natural sunlight (environmentally realistic UV light levels) and in dark conditions, towards embryo-larval stages of the European clam Ruditapes decussatus. Historically, marine invertebrates have been widely used to monitor the biological effects of contaminants. Furthermore, the Joint Assessment Monitoring Programme (JAMP) has recommended the embryo-larval stages of bivalve as suitable bioassays for assessing water quality in Mediterranean waters (Stagg 1998). To our knowledge, the present work is the first using embryo-larval life-stages of R. decussatus to assess photo-toxicity of PAHs. Ruditapes decussatus is widely distributed along the Tunisian coast. This species is economically important, being exported mostly to Europe (Hamza-Chaffai et al. 2003). Its spawning season, in our region, extends from March to September, but we can obtain gametes throughout the year by conditioning adults in the laboratory (Medhioub et al. 2006). This species is a sedentary filter-feeding marine bivalve, thus satisfying criteria in biomonitoring programs for a good bioindicator of pollution.
Materials and Methods
Adults of R. decussatus collected in pristine sites from natural local populations (Oued Maltine; SE of Tunisia) were transferred to the hatchery of the National Institute of Marine Sciences and Technologies, where they were maintained in aquaria with running natural seawater and fed with the microalgae Isochrysis galbana and Chaetoceros calcitrans) for at least 1 week until the experiments began. Handling conditions of the adult stock were 22.5 ± 0.5°C temperature, 36.5 ± 1.5 ppt salinity, 7.2 ± 1.2 mg l−1 O2 and 7.69 ± 0.13 pH (mean ± SD).
Adult clams were induced to spawn by thermal stimulation (temperature cycles at 18°C and 28°C) in separated beakers with 0.2 μm filtered seawater. Viable gametes from healthy males and females were selected and filtered at 32 μm (sperm) and 100 μm (eggs) to remove impurities.
Experimental concentrations were chosen on the basis of preliminary trials and on data from the literature (Pelletier et al. 1997). Experimental solutions were made by dissolving analytical grade anthracene, fluoranthene, pyrene and benzo[a]pyrene (Sigma–Aldrich, Steinheim, Germany) in HPLC-grade acetone, due to the low solubility of PAHs in seawater (Kennish 1992). Two separate dilution series (five concentrations) were prepared for each PAH, one for testing under sunlight and the second for testing in the dark. Nominal and actual PAH concentrations are shown in Table 1. One control was used in each exposure consisting of artificial seawater (ASW) prepared as in Zaroogian et al. (1969). The experimental concentrations were obtained by diluting the stock solutions in ASW. During this dilution, equal amounts of acetone (less than 200 μl l−1), found not to be toxic in preliminary tests, were added to each experimental beaker with PAHs solutions. All glassware was acid-washed (HNO3 10 % vol.) and rinsed with acetone and distilled water before the experiments.
Test solutions intended for chemical analysis were collected from the experimental vials at the beginning of the tests. The test solutions were poured into a separatory funnel and PAHs were extracted with dichloromethane (USEPA 1980). After substitution of the solvent by acetonitrile, the concentration of the PAHs was determined by HPLC (C 20 AT, provided by Shimadzu Co. Tokyo, Japan) coupled with fluorimetric detection. The samples or standard solutions were directly injected onto a PAH–C18 column (20 cm–4.0 mm) at 30°C. Isocratic elution was performed with a mobile phase of acetonitrile/water (60/40, v/v) at a flow rate of 1.0 mL/min. The recoveries in the extraction method were about 94 % for the measured compounds.
For the embryotoxicity test, the fertilized eggs (1 ml ca. 400 eggs/ml), obtained by in vitro fertilization, were transferred to 15 ml vials containing PAH solutions that were incubated under two light conditions (natural sunlight and dark) at ambient temperature (22°C) for 24 h, until the second larval stage (D-veliger) was attained. After incubation, a few drops of 40 % buffered formalin were added, and the percentage of normal D-hatched larvae was recorded. A larva was considered malformed when some developmental defect was observed (embryos that had not reached the D-veliger stage or D-veliger larvae with convex hinge, incomplete shell or protruding mantle). The EC50 was defined as the PAH concentration that resulted in a 50 % reduction in the number of normal embryos. The physico-chemical conditions of the experiments were 32 ± 0.15 ppt salinity, 6.92 ± 0.40 mg l−1 O2 and 7.75 ± 0.15 pH (mean ± std).
In the larval mortality test, fertilized eggs were filtered through a 20 μm mesh nylon net and then washed gently three times with filtered seawater (1 μm). They were then resuspended in ASW at 23°C. After 24 h, the swimming veliger larvae (D-larvae stage) obtained were re-suspended in a 2 l glass beakers (approximately 2*104 larvae), containing 1.8 l of PAH solutions or ASW (control). Larvae were also incubated under two light conditions (natural sunlight and dark) at ambient temperature 24°C and during 96 h with 12/12 h photoperiod. Veliger larvae were fed daily with phytoplankton (Isochrysis tahiti and C. calcitrans). The number of dead larvae was then assessed under a microscope at the end of the experiment, and the percentage of larval mortality was calculated using the following formula (% Mortality = 100*dead larvae/total number of larvae). All experiments were conducted in five replicates.
Ultraviolet light levels in natural sunlight were measured at different times of the day. UVA levels ranged from 970 to 2,300 μW/cm2 with an average level of 1,655 ± 101 μW/cm2. UVB levels ranged from 140 to 512 μW/cm2 with an average level of 345 ± 87 μW/cm2. Thus, the ultraviolet light levels in the natural sunlight exposures were three to four times greater than those in previous studies using artificial UV light exposures (Pelletier et al. 1997).
Statistical analyses were conducted using the SPSS version 17.0 statistical software (IBM Corporation, USA). Differences between treatments were tested for significance by means of one-way analysis of variance (ANOVA). When differences among groups were significant the Dunnett’s test was employed to compare the control group and each of the experimental groups. The EC10, EC50 and LC50 and their 95 % confidence intervals (95CI) were calculated according to the Probit method after normalizing data to the mean control response using Abbot’s formula (Emmens 1948). For analysis, data were first arcsine-transformed to achieve normality (Hayes 1991).
Results and Discussion
Results from the analytical chemistry applied for checking the nominal PAH concentrations in seawater at the beginning of the experiment are shown in Table 1. Measured concentrations were within 11–18 % of the nominal concentrations. In general, no significant modifications of nominal values of the PAHs concentrations were shown.
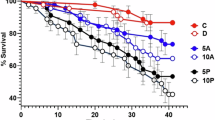
Following 24 h of exposure of R. decussatus embryos to increased concentrations of anthracene, significant (p < 0.05) decrease of the D-veliger larvae percentages were observed in the concentrations of 250 and 2.5 μgl−1 in the dark and under sunlight conditions, respectively (Fig. 1). Under sunlight conditions, the inhibition of the embryonic development exceeded 95 % at 25 μgl−1 of anthracene. The EC10 and EC50 values of this PAH were 27.9 and 361.1 μgl−1 (156.5 and 2,026 nM) in the dark, and 0.48 and 4.97 μgl−1 (2.7 and 27.9 nM) under sunlight conditions, respectively (Table 2).
Percentage of D-veliger larvae after 24 h exposure, in dark (a) and under sunlight (b), of R. decussatus fertilized eggs to different concentrations (μg/l) of anthracene, fluoranthene, pyrene and benzo[a]pyrene. Error bars represent standard deviations. (n = 5, * p < 0.05; ** p < 0.01; *** p < 0.001)
Fluoranthene also showed toxicity to clam embryos by significantly (p < 0.05) reducing the percentages of D-veliger larvae up to 40 %, compared to the control, at concentrations as high as 100 and 2.5 μgl−1 in dark and under sunlight conditions, respectively (Fig. 1).
The 24 h exposure of clam embryos to increasing concentrations of pyrene resulted in a significant (p < 0.05) reductions of D-veliger larvae percentages of 30 and 70 % both at dark and under sunlight conditions from a concentration of 64 and 2.5 μgl−1, respectively. Clam embryonic development was totally inhibited at the highest tested concentration (50 μgl−1) under sunlight conditions (Fig. 1). The calculated EC10 and EC50 values were shown in Table 2. The fourth PAH tested, the benzo[a]pyrene (BaP), was toxic to clam embryos from 5 and 0.25 μgl−1 in dark and under sunlight conditions, respectively, as shown in Fig. 1. Compared to control, the reduction of normal D-veliger larvae percentages, at these concentrations, was up to 40 %. Under sunlight conditions, BaP induced a total inhibition (99 %) of the clam embryonic development at a concentration of 5 μgl−1, yielding EC10 and EC50 values of 0.07 and 0.31 μgl−1 (0.28 and 1.23 nM), respectively.
Based on the EC50 values, the toxicity of the tested PAHs to clam embryos was up to 72, 35, 60 and 23 times higher under sunlight than dark conditions, respectively, for anthracene, fluoranthene, pyrene and benzo[a]pyrene. This indicates a definite phototoxic response of those compounds.
In dark conditions, anthracene, pyrene and benzo[a]pyrene were not ecologically toxic to R. decussatus embryos since their EC50 values (361.1, 113.8, 7.15 μgl−1, respectively) were above their respective seawater solubility limits (45, 130 and 3.8 μgl−1, respectively) (Mackay et al. 1992; Callahan et al. 1979; U.S. Environmental Protection Agency 1982). Conversely, the fluoranthene EC50 in dark condition was lower than its seawater solubility limit (206 μgl−1, U.S. Environmental Protection Agency 1982) which indicates the toxicity of this PAH in the absence of UV light.
After a 96 h exposure period of R. decussatus veliger larvae to increasing concentrations of four PAHs in dark and under sunlight conditions, mortality percentages were recorded and LC50 values were calculated. Anthracene induced significant (p < 0.05) larval mortality at concentrations of 50 (10 %) and 5 μgl−1 (28 %) in dark and under sunlight conditions, respectively. The presence of UV light (sunlight) induced anthracene toxicity, with 100 % mortality occuring at 50 μgl−1 (Fig. 2). The calculated LC50 of anthracene in both conditions are given in Table 2.
In darkness, exposure of larvae to fluoranthene resulted in 18.6 ± 3.2 % of mortality (compared to control) at a concentration of 50 μgl−1, with an LC50 of 285.8 μgl−1. Under sunlight conditions, the lowest anthracene concentration that induced significant mortality (36.5 ± 9.7 %) was 2.5 μgl−1, with an LC50 of 8.75 μgl−1 (Table 2). Pyrene resulted in toxicity to clam larvae at a concentration as low as 64 μgl−1 in dark conditions, reducing the percentage of live veliger larvae by 15 % with respect to controls and yielding an LC50 value of 134.7 μgl−1 (666 nM). The calculated LC50 under sunlight was 10.7 μgl−1 (53 nM). Total mortality occurred at 256 and 50 μgl−1 of pyrene in darkness and under sunlight conditions, respectively (Fig. 2).
On the other hand, benzo[a]pyrene was toxic to larvae only at the two highest concentrations in dark conditions (25 and 50 μgl−1), with an LC50 value of 34.53 μgl−1 (137 nM). Toxicity of BaP was greatly increased under sunlight, and mortality exceeded 80 and 90 % at 1 and 5 μgl−1, respectively, with an LC50 value of 0.56 μgl−1 (2.2 nM).
Significant differences between experiments carried out in the dark and under sunlight were observed for all tested PAHs. According to LC50 values, the toxicity under sunlight was 32, 31, 12 and 61 times higher, respectively for anthracene, fluoranthene, pyrene and benzo[a]pyrene. In this experiment the tested PAHs, excepting fluoranthene, were considered not ecologically toxic in darkness, since calculated LC50s were above their respective seawater solubility limits. Based on EC50 and LC50 values (molarities basis), the tested PAHs were ranked in order of higher toxicity as following: benzo[a]pyrene > pyrene > fluoranthene > anthracene.
The values of EC50 (24 h embryonic development) obtained under sunlight (with UV) were about 72, 35, 60 and 23 times higher than in the dark condition, respectively for anthracene, fluoranthene, pyrene and benzo[a]pyrene. For larvae, toxicity increased 32, 31, 12 and 61 times, respectively, for these same PAHs with sunlight exposure. In both bioassays, results indicated that the toxicity of the four PAHs to the clam R. decussatus was enhanced in the presence of UV light.
Toxicity enhancement seems to be time-dependent. In the present study, anthracene seems to be the most sensitive of the four PAHs after 24 h of exposure. Contrarily, when exposure time was 96 h, benzo[a]pyrene showed the higher sensitivity to photo-induced enhancement. The order of toxic strength, based on EC50 values (nM), both in dark and under sunlight conditions, was benzo[a]pyrene > pyrene > fluoranthene > anthracene.
Comparison of our results with previous research is not straightforward since studies on the effects of PAHs to aquatic organisms carried out in dark and under sunlight conditions, as those in the natural habitats of early embryonic stages of marine invertebrates, are scarce.
A comprehensive literature search has returned only a few studies where exposure to PAHs was conducted in the dark. For instance, Bellas et al. (2008) investigated the photo-induced toxicity of 6 PAHs to sea-urchin (Paracentrotus lividus) larvae and mussel (Mytillus galloprovincialis) embryos. In their mussel embryo tests, they reported EC50 values of >640 and 657 nM for pyrene and >1,250 and 263 nM for fluoranthene under dark and fluorescent light conditions, respectively. Bellas and Thor (2007) also evaluated the toxicity of fluoranthene and pyrene on survival, egg production and recruitment of the copepod Acartia tonsa. EC50 values ranged between 385 and 824 nM for fluoranthene and between 295 and 306 nM for pyrene under fluorescent light (no UV). In the present work, R. decussatus yielded a lower degree of toxicity in the dark (EC50 values of 562.7 and 444 nM for fluoranthene and pyrene, respectively). In general, the clam species that we tested seems to be more sensitive to PAHs than mussels and less sensitive than copepods. In contrast, Kagan et al. (1985) did not find toxicity of fluoranthene and pyrene to embryos and larvae of freshwater organisms (brine shrimp Artemia salina, water flea Daphnia magna, mosquito Aedes aegypti, leopard frog Rana pipiens, and fish Pimephales promelas) when incubations were conducted in the dark. This is in agreement with previous studies suggesting higher sensitivities of marine than freshwater invertebrates to PAHs (Hutchinson et al. 1998; Leung et al. 2001; Robinson 1999).
In the present study, only fluoranthene was ecologically toxic in the dark since EC50 and LC50 values were below its maximum aqueous solubility. On the other hand pyrene, fluoranthene and benzo[a]pyrene did not cause a 50 % decrease in the biological responses below the maximum aqueous solubility. Pelletier et al. (1997) reported 48-h EC50s for embryos and 96-h LC50s for juveniles of the dwarf surf clam, Mulinia lateralis of 4,260 and >13,300 μgl−1 (anthracene), 58.8 and 3,310 μgl−1 (fluoranthene) and >9,454 and >11,900 μgl−1 (pyrene) respectively. These EC50 and LC50 values suggest that for both anthracene and pyrene there is no toxicity to embryonic M. lateralis, as the EC50 values are above the seawater solubility limits of these PAHs. However, fluoranthene does exhibit toxicity to developing M. lateralis embryos in a dissolved state. The low aqueous solubility of most PAHs is an important factor to take into account in an assessment of the risk of these compounds in the marine environment, since their bioavailability and therefore the maximum lipid concentration attained in the organisms is constrained by their aqueous solubility (Di Toro and McGrath 2000).
A growing body of evidence suggests that toxicity of some intermediate molecular weight PAHs may be enhanced in the presence of ultraviolet (UV) light. Although photo-induced toxicity of PAHs has been demonstrated in many studies with freshwater organisms since the early 1980s (reviewed by Arfsten et al. 1996), the first report of photo-toxicity to marine species is relatively recent (Pelletier et al. 1997). In that study, toxicity of anthracene, fluoranthene and pyrene to larvae and juveniles of the bivalve M. lateralis and the mysid Mysidopsis bahia was 12 to >50,000 times higher under UV light than under fluorescent light. Likewise, Spehar et al. (1999) attained a 21–1880 times increase in fluoranthene toxicity under UV light to several freshwater and marine species compared to incubation under fluorescent light. Lyons et al. (2002) also found that, under fluorescent light, benzo[a]pyrene and pyrene provoked 50 % impairment on the embryonic development of the pacific oyster Crassostrea gigas at approximately 2.5 and 100 μgl−1 respectively, whilst under UV light 1 and 5 μgl−1 caused a 100 % inhibition of the embryonic development. Peachey (2005) reported significantly higher toxicity of fluoranthene and pyrene to larvae of three crustaceans (Libnia dubia, Menippe adina and Panopeus herbstii) under UV light; however, this study used very high UV light regimes (UVA: 1455–4,914 μWcm−2, UVB: 196–581 μWcm−2) in comparison with realistic UV light intensities measured in aquatic habitats (Barron et al. 2000), and therefore its ecological relevance is questionable.
The forementioned studies and previous evaluations of PAH photo-toxicity with aquatic organisms only compared toxicity under fluorescent and artificial UV light exposure, and did not consider PAH toxicity in the dark. Indeed, care must be taken regarding the interpretation of photo-enhanced toxicity of PAHs under UV light in laboratory toxicity tests, since UV light itself may cause damage to marine organisms, and particularly to the sensitive early life-stages (Browman et al. 2000; Kuhn et al. 2000).
The present work reports acute effects of PAHs on the early life-stages of a marine invertebrate of ecological and commercial relevance. Although these effects were detected at levels above the reported typical concentrations in coastal areas and therefore little risk is predicted to occur, a higher level of risk may occur in PAH-polluted estuaries. Moreover, photo-activation of PAHs at naturally relevant levels may increase the risk of those compounds in the marine environment.
References
Ankley GT, Erickson RJ, Phipps GJ, Mattson VR, Kosian PA, Sheedy BR, Cox JS (1995) Effects of light intensity on the phototoxicity of flouranthene to a benthic invertebrate. Environ Sci Technol 29:2828–2833
Arfsten DP, Schaeffer DJ, Mulveny DC (1996) The effects of near ultraviolet radiation on the toxic effects of polycyclic aromatic hydrocarbons in animals and plants: a review. Ecotox Environ Saf 33:1–24
Barron MG, Little EE, Calfee R, Diamond S (2000) Quantifying solar spectral irradiance in aquatic habitats for the assessment of photoenhanced toxicity. Environ Toxicol Chem 19:920–925
Bellas J, Thor P (2007) Effects of selected PAHs on reproduction and survival of the calanoid copepod Acartia tonsa. Ecotoxicology 16:465–474
Bellas J, Saco-Álvarez L, Nieto Ó, Beiras R (2008) Ecotoxicological evaluation of polycyclic aromatic hydrocarbons using marine invertebrate embryo–larval bioassays. Mar Pollut Bull 57:493–502
Browman HI, Alonso Rodriguez C, Béland F, Cullen JJ, Davis RF, Kouwenberg JHM, Kuhn PS, McArthur B, Runge JA, St-Pierre JF, Vetter RD (2000) Impact of ultraviolet radiation on marine crustacean zooplankton and ichthyoplankton: a synthesis of results from the estuary and Gulf of St. Lawrence, Canada. Mar Ecol Prog Ser 199:293–311
Callahan MA et al (1979) Water-related environmental fate of 129 priority pollutants: halogenated aliphatic hydrocarbons, halogenated ethers, monocyclic aromatics, phthalate esters, polycyclic aromatic hydrocarbons, nitrosamines, and miscellaneous compounds, vol II, EPA 440/4-79-029. U.S. Environmental Protection Agency, Washington, DC
Di Toro DM, McGrath JA (2000) Technical basis for narcotic chemicals and polycyclic aromatic hydrocarbon criteria. II. Mixtures and sediments. Environ Toxicol Chem 19:1971–1982
Diamond SA, Oris JT, Guttman SI (1995) Adaptation of fluoranthrene exposure in a laboratory population of fathead minnows. Environ Toxicol Chem 14:1393–1400
Diamond SA, Mount DR, Burkhard LP, Ankley GT, Makynen EA, Leonard EN (2000) Effect of irradiance spectra on the photoinduced toxicity of three polycyclic aromatic hydrocarbons. Environ Toxicol Chem 19:1389–1396
Duan Y, Guttman SI, Oris JT, Huang X, Burton GA (2000) Genotype and toxicity relationships among Hyalella azteca: II. Acute exposure to fluoranthrene-contaminated sediment. Environ Toxicol Chem 19:1422–1426
Emmens CW (1948) Principles of biological assay. Chapman and Hall Ltd., London
Gala WR, Giesy JP (1992) Photo-induced toxicity of anthracene to the green alga, Selenastrum capricornutum. Arch Environ Contam Toxicol 23:316–323
Hamza-Chaffai A, Pellerin J, Amiard JC (2003) Health assessment of a marine bivalves Ruditapes decussatus from the Gulf of Gabès (Tunisia). Environ Int 28:609–617
Hatch AC, Burton GA Jr (1998) Effects of photoenhanced toxicity of fluoranthene on amphibian embryos and larvae. Environ Toxicol Chem 17:1777–1785
Hatch AC, Burton GA Jr (1999) Photo-induced toxicity of PAHs to Hyalella azteca and Chironomus tentans: effects of mixtures and behavior. Environ Pollut 106:157–167
Hayes WJ (1991) Dosage and other factors influencing toxicity. In: Hayes WJ, Laws ER (eds) Handbook of pesticide toxicology, vol 1. Academic Press, San Diego, CA, pp 39–105
Huang X, Dixon DG, Greenburg BM (1993) Impacts of UV radiation and photomodification on the toxicity of PAHs to the higher plant Lemna gibba (duckweed). Environ Toxicol Chem 12:1067–1077
Hutchinson TT, Scholz N, Guhl W (1998) Analysis of the ECETOC aquatic toxicity (EAT) database IV: comparative toxicity of chemical substances to freshwater versus saltwater organisms. Chemosphere 36:143–153
Kagan J, Kagan ED, Kagan IA, Kagan PA, Quigley S (1985) The phototoxicity of non-carcinogenic polycyclic aromatic hydrocarbons in aquatic organisms. Chemosphere 14:1829–1834
Kennish MJ (1992) Ecology of estuaries: anthropogenic effects. CRC Press, Boca Raton, FL
Kuhn PS, Browman HI, Davis RF, Cullen JJ, McArthur BL (2000) Modeling the effects of ultraviolet radiation on embryos of Calanus finmarchicus and Atlantic cod (Gadus morhua) in a mixing environment. Limnol Oceanogr 45:1797–1806
Latimer JS, Zheng J (2003) The sources, transport, and fate of PAHs in the marine environment. In: Douben PE (ed) PAHs: an ecotoxicological perspective. Wiley, London, pp 9–33
Leung KMY, Morritt D, Wheeler JR, Whitehouse P, Sorokin N, Toy R, Holt M, Crane M (2001) Can saltwater toxicity be predicted from freshwater data? Mar Poll Bull 42:1007–1013
Lyons BP, Pascoe CK, McFadzen IRB (2002) Phototoxicity of pyrene and benzo(a)pyrene to embryo–larval stages of the pacific oyster Crassostrea gigas. Mar Environ Res 54:627–631
Mackay D, Shiu WY, Ma KC (1992) Illustrated handbook of physical–chemical properties and environmental fate for organic chemicals, vol 2. Lewis, Chelsea, MI, USA
Meador JP (2003) Bioaccumulation of PAHs in marine invertebrates. In: Douben PE (ed) PAHs: an ecotoxicological perspective. Wiley, London, pp 147–171
Medhioub MN, Lymayem Y, Fathallah S, Abed MM, Medhioub A (2006) Cycle of artificial breeding of the clam Ruditapes decussatus (mollusk bivalve). Act of 13th scientific journey of agricol research—Hammamet, 14 & 15 December
Monson PD, Call DJ, Cox DA, Liber K, Ankley GT (1999) Photoinduced toxicity of fluoranthene to northern leopard frogs (Rana pipiens). Environ Toxicol Chem 18:308–312
NRCC (1983) Polycyclic aromatic hydrocarbons in the aquatic environment: formation, sources, fate and effects on aquatic biota. NRC associate committee on the scientific criteria for environmental quality, National Research Council of Canada, NRCC Publication No. 18981, Ottawa, 209 pp
Peachey RBJ (2005) The synergism between hydrocarbons pollutants and UV radiation: a potential link between coastal pollution and larval mortality. J Exp Mar Biol Ecol 315:103–114
Pelletier MC, Burguess RM, Ho KT, Kuhn A, McKinney RA, Ryba SA (1997) Phototoxicity of individual polycyclic aromatic hydrocarbons and petroleum to marine invertebrate larvae and juveniles. Environ Toxicol Chem 16:2190–2199
Robinson PW (1999) The toxicity of pesticides and organics to mysid shrimps can be predicted from Daphnia spp. Water Res 33:1545–1549
Saco-Álvarez L, Bellas J, Nieto Ó, Bayona JM, Albaigés J, Beiras R (2008) Toxicity and phototoxicity of water-accommodated fraction obtained from Prestige fuel oil and Marine fuel oil evaluated by marine bioassays. Sci Total Environ 394:275–282
Sasson-Brickson G, Burton GA Jr (1991) In situ and laboratory sediment toxicity testing with Ceriodaphnia dubia. Environ Toxicol Chem 10:201–207
Spehar RL, Poucher S, Brooke LT, Hansen DJ, Champlin D, Cox DA (1999) Comparative toxicity of fluoranthene to freshwater and saltwater species under fluorescent and ultraviolet light. Arch Environ Contam Toxicol 37:496–502
Stagg RM (1998) The development of an international programme for monitoring the biological effects of contaminants in the OSPAR convention area. Mar Environ Res 46:307–313
USEPA (1980) Test methods for evaluating solid waste: physical/chemical methods. The official EPA, first ed., EPA SW-846, US Environmental Protection Agency, Washington, DC
Walker CH, Hopkin SP, Sibly RM, Peakall DB (2001) Principles of ecotoxicology, 2nd edn. Taylor & Francis, London
Wernersson A, Dave G (1998) Effects of different protective agents on the phototoxicity of fluoranthene to Daphnia magna. Comp Biochem Physiol C 120:373–381
Zaroogian GE, Pesh G, Morrison G (1969) Formulation of an artificial sea-water medium suitable for oyster larvae development. American Zool 9:1144
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fathallah, S., Medhioub, M.N. & Kraiem, M.M. Photo-induced Toxicity of Four Polycyclic Aromatic Hydrocarbons (PAHs) to Embryos and Larvae of the Carpet Shell Clam Ruditapes decussatus . Bull Environ Contam Toxicol 88, 1001–1008 (2012). https://doi.org/10.1007/s00128-012-0603-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-012-0603-1