Abstract
The study aims to assess the seasonal variation in distribution, source identification, and risk of 20 polycyclic aromatic hydrocarbons (20 PAHs) in the sediment of the Ikpoba River, south-south Nigeria. The PAHs were extracted in an ultrasonic bath with a mixture of n-hexane and dichloromethane (1:1 v/v). The extract was cleaned by silica-alumina gel mixed with anhydrous Na2SO4 in a chromatography column, eluted by n-hexane, and analysed by gas chromatography-mass spectrometry. The range of the average PAHs in mg.kg-dw was 0.15 (Nap)-0.54 (Acy) and 0.13 (D.al.P)-0.99 (Acy) in wet and dry periods correspondingly, indicating an increase in concentration from wet to dry period. However, the rings of the average concentration of the PAHs show 6 and 3 rings to be the highest values during the wet and dry seasons, respectively. Based on the human health risk analysis, the hazard quotient (HQ) and hazard index (HI), and carcinogenic risk indices showed low non-carcinogenic and carcinogenic risk for both seasons. The ecological risk analysis showed the mean effect range median quotient (mERMQ) recorded a medium–low effect on the biota of the locations, except in AS3 during the wet season and also in WS8 and WS9 during the dry season. The minimum value of the toxic equivalent quotient (TEQ) was > 0.2 mg/kg, which indicated a recommendation for the clean-up of the Ikpoba River. The isomer ratio and the principal component analysis (PCA) revealed the sources of the PAHs to be majorly combustion, followed by pyrolytic and petrogenic sources for both seasons.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs) are one among the many persistent organic pollutants (POPs) presented to the environment through the continuous use of petroleum products, combustion of biomass and coal, forest fires, etc. (Lawal, 2017). The ring structure of the PAHs makes them susceptible to chemical and biochemical transformation, which leads to their toxicity in the environment. PAHs are known to have harmful toxicity, mutagenicity, carcinogenicity, and biological effects. Cancer is the major health peril of PAHs but has also been linked with cardiovascular diseases and poor fetal growth (Kumar et al., 2016).
The PAHs that are mostly of environmental concern due to their hazardous nature include the 2 rings PAHs (naphthalene), 3 rings PAHs (acenaphthene, acenaphthylene, fluorene, phenanthrene, anthracene), 4ings PAHs (pyrene, chrysene, benzo (a) pyrene, dibenzo (ah) anthracene), 5 rings PAHs (benzo (bjk) fluorene, benzo (e) pyrene, benzo (a)pyrene), and 6 rings (Indeno(123cd) pyrene, dibenzo (ghi) perylene, dibenzo (al) pyrene, dibenzo (ai) pyrene, and dibenzo (ah) pyrene). The lower molecular PAHs (LMPAHs) made up of 2 and 3 rings are more soluble in water, while the higher molecular PAHs (HMPAHs) with 4 rings and above are less soluble in water (Lawal, 2017). The LMPAH’s solubility in the aquatic system compared to the HMPAHs has made the LMPAHs easily available and accessible to the biotic community of the aquatic ecosystem (Cai et al., 2017).
The PAHs get into the urban aquatic system through waste discharge, stratosphere deposition, oil spillage, and surface runoff. They undergo chemical and biochemical reactions and settle on sediment. Sediment and soil are well-known sinks for pollutants such as PAHs. They are strongly held onto inorganic or organic particles and pulled down to the bottom sediment of an aquatic system (Zhao et al., 2021). These contaminants strongly attract organic carbon, making sedimentary organic carbon a very good sink for PAHs (Inam et al., 2018). These PAHs adsorbed on sediment are not easily decomposed by bacteria in anoxic environments. They are released into the aquatic system in a very conducive condition, which threatens the biotic community and the health of humans through bioaccumulation in the food chain at the trophic level (Dudhagara et al., 2016). These PAHs can get to humans through various pathways, such as oral and dermal routes (Cai et al., 2017). These PAHs are influenced by a lot of Physicochemical parameters. The TOC and other physical and chemical parameters are known to influence the distribution of PAHs within the ecosystem. A lot of research has been conducted that reveals the interaction/correlation of EC, pH, NO3, and PO4 with PAHs through multivariate statistical analysis (Cai et al., 2021; Peng et al., 2016; Sun et al., 2017).
The Ikpoba River is an important aquatic system in the Benin metropolis as the river is the source of the Okhoro dam, built to supply treated water to some parts of the Benin metropolis. The Ikpoba slope, Teboga (Ikpoba Okha local government), and Okhoro (Egor local government) communities close to the river use it for domestic purposes, irrigation, fishing, and also as a means of transportation. The location of this body of water over the years has made it prone to a high level of anthropogenic pollution from human activities due to the population explosion as a result of urbanization, industrialization, and intensive agriculture practice within the interior. These activities, over time, have released abundant recalcitrant, ubiquitous persistent organic pollutants (PAHs) into the river (Lawal, 2017). The social, economic, political, and proximity of the Ikpoba river to the various catchment areas has made it an environmental concern.
A lot of environmental research to determine the perilous pollutants within the Ikpoba River has been conducted, with little attention to persistent organic pollutants (POPs). This has made it imperative to study the distribution, source, and risk associated with the PAH-contaminated sediments of the Ikpoba River. The research applied statistical tools such as descriptive statistics, Pearson correlation, principal component analysis, and isomer ratio to analyse the distribution and sources of these PAHs. Furthermore, the sediment quality guidelines (SQGs), mean effect range median quotient (mERMQ), and toxicity equivalency quotient (TEQ) were applied to determine the pollutants’ effects on the biota of the aquatic ecosystem. The PAHs health risks to a human were investigated using the daily average dose (DAD), hazard quotient (HQ), hazard index (HI), and carcinogenic risk (CR) indices (Ambade et al., 2021; Lin et al., 2018; Sun et al., 2018). This research aims to assess the seasonal variation in distribution, source apportionment, and risk of 20PAHs-contaminated sediment of the Ikpoba River, south-south Nigeria.
Materials and methods
Study area
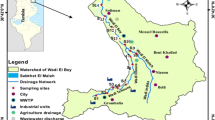
The Ikpoba River is an important aquatic system located at longitude 6° 13° 00° N and latitude 5° 46° 00° E in Edo state (Fig. 1). The river takes its source from the Ishan Plateau and flows in the southwest direction in a sharply carved valley and sandy regions to Edo and links to the Ossiomo River. The river flows across a rainforest with soil rich in organic matter. The river is the source of the popular Okhoro dam, which serves about 30% of the population. The Ikpoba river is a fourth-order stream, and the body of water is situated within the rainforest belt of Benin, the south-south region of Nigeria. The river extended from Egor to Ikpoba Okha local government areas. The local government areas (LGAs) have a population of about 374,671, and 301,447 inhabitants in Egor and Ikpoba Okha, respectively. The Egor and Ikpoba Okha LGAs occupied a total of 93 km2, and 862 km2 individually. The Ikpoba slope, Teboga in Ikpoba LGA, and Okhoro in Egor LGA are communities with proximity to the Ikpoba river and brimming with anthropogenic activities. Okhoro is a small town upstream of the Ikpoba River with agricultural activities, wood charcoal production, and automobile repairs workshops; Teboga is a small town midstream of the Ikpoba River with agricultural activities, engine oil and gasoline market, and automobile repair workshops; and Ikpoba is a larger town downstream of the Ikpoba River known for, micro-chemical industries, mechanical workshops, wood charcoal production, and large community waste site. These communities have similar geographical features. Their average temperature is 28° C, and their relative humidity is 67%. The regions have two major seasons, the wet and dry seasons. The regions experience longer wet seasons and fewer months of the dry period. The highest rainfall in this region occurs during August and September (99.8 in.), while the highest dry period is experienced between February and March (88 °F).
Sample collection
Intensive monthly sampling was carried out during the wet and dry seasons, in which 8 months of sampling were applied (4 months for each season). The sediment samples were collected at three locations: Okhoro (6° 22′ 43″N. 5° 38′ 28″E), Teboga (6° 22’N. 5° 39’E), and Ikpoba (6° 21′N. 5° 39′E) communities. The locations were further stratified into nine catchment areas which included: stations AS1, AS2, AS3 in Okhoro, CS4, CS5, CS6 in Teboga, WS7, WS8, and WS9 in Ikpoba. However, stations in a particular location were 100 m apart. About three sampling sites make a station and sediment samples were collected from each of the sampling sites at different depths of 0–5 cm, 5–10 cm, and 5–15 cm with the aid of a stainless-steel grabber. The samples at different depths from the same sampling sites (sampling sites of each station were 25 m apart) were mixed immediately on-site for homogeneity. A total of 81 samples were collected from the field, and after a homogenous mixture, the sum of 27 samples was made available monthly. The samples were placed in a polythene bag and transported at 4 °C to be air-dried (below room temperature at about 15 °C and low humidity to curtail volatilization of PAHs) in an air conditioner and dehumidifier dark room laboratory. The air-dried sample was taken to the GC–MS laboratory in a glass jar to be extracted and made ready for GC.MS analysis.
Extraction
The air-dried sediment was sieved with a 60-mesh standard sieve, and 2 g of the sieved sediment was mixed with 2 g of anhydrous sodium sulphate to remove any trace of moisture. The moisture-free sediment was transferred into an ultrasonic bath and extracted with 25 ml of a mixture of n-hexane and dichloromethane (1:1 v/v) for about 30 min (this procedure was repeated twice). The extract was reduced to about 2 ml with the aid of a rotary evaporator. The 2 ml extract was cleaned by passing it through a chromatography column packed with silica-alumina gel mixed with anhydrous Na2SO4. The column was eluted with 15 ml of n-hexane to remove non-PAH compounds, followed by a 35 ml mixture of n-hexane and dichloromethane (1:1 v/v). The solution containing PAHs was concentrated to about 2 ml, ready for GC–MS analysis.
GC–MS analysis
The eluent containing PAHs was analysed by gas chromatography-mass spectrometry (Agilent 7890A/5975 C) by Agilent Technologies Inc., USA using the capillary column (DB-5MS, 0.25 mm × 0.25 µm × 30 m) produced by J & K Scientific, San Jose, CA, USA. High-purity (99.999%) helium gas with a flow rate of 1.0 ml/min was used as the carrier. About 1 µl was inserted through an automatic sampling system and at 250 °C, 240 °C, and 280 °C for the inlet, ion source, and transmission respectively. The oven temperature was operated at 80 °C/2 min and was later accelerated by 15 °C after every 1 min until it got to 215 °C/min. The oven was further increased at every 6 °C/min and got to 280 °C for 1 min, and it was further accelerated at 10 °C/min and got 300 °C and left for 5 min. The detector of the GC–MS was run in an electron power mode (70 eV) and at a temperature of 240 °C for the ionic source. The PAHs were detected with the aid of a selected ion mode. The concentrations of the PAHs are given as the dry mass of the sample.
Quality control
All the reagents were of analytical grade, and a standard mixture of PAHs (Ultra Scientific, North Kingstown, USA) was used in the analysis. After the triplicate analysis of the group of samples, a blank sample was analysed to monitor the interference obtained during sample analysis. The concentration of the analyte present in the blank was less than 6% of the minimum concentration of the background signal. The limit of quantification expressed as a signal-to-noise ratio multiplied by ten was also studied. The quantitative minimum value of PAHs in the aquatic sediment was obtained by adding 5 g of the sediment (dry weight) with the PAHs standard. The result of the LOQ of the PAHs was 0.013–0.092 ng/g, while the percentage recovery of the PAHs mixture and the internal standard compounds was 74.60–114.7%, and 85.50–106.60% respectively. Furthermore, the acceptable R2 values of the PAHs with a range of 0.991–0.995 was obtained from the calibration curve (plotting the peak area against the PAHs concentrations).
Ecological risk of PAHs
Mean effect range median quotient
The mean effect range median quotient (\(mERMQ\)) is an index used to quantify the probability of the substantial ecological risk effect of the studied PAHs on the ecosystem (Zhang et al., 2019).
where C is the concentration and n is the number of contaminants within the sampling stations/sites. The ecological risk effects in the ecosystem were classified into four categories of \(mERMQ\) such as high (\(mERMQ\) >1.5), medium–high (\(1.5<mERMQ\) > 0.51), medium–low (0.5 <\(mERMQ\) > 0.11) and low (\(mERMQ\) < 0.11) (Zhang et al., 2019).
The toxicity equivalency quotient
The toxicity equivalency quotient (TEQ) measures the toxicity of the individual pollutants of study by using their individual chemical component toxic equivalency factor (TEF) which is comparative to the most toxic PAHs (B.a.P) (Halfadji et al., 2021). The total toxicity of the contaminants can be computed using Eq. 3 (Tu et al., 2018).
where TEF is the toxic equivalency factor and C is the concentration of the pollutants (mg/kg). The D.ah.A and B.a. P have a TEF of 5 and 1 respectively; B.a.A, B.bjk.F, and I. l23cd.P have a TEF of 0.1; Ant, B.ghi. Py, and Chr have a TEF of 0.01; Ace, Acy, Flt, Flu, Nap, Phe, and Pyr have a TEF of 0.001 (Tu et al., 2018).
Potential health risk
The potential risk of the studied PAHs to humans was also estimated using the average daily dose, hazard quotient, hazard index, and carcinogenic risk indices as shown in Eqs. 4, 5, 6, and 7 respectively (Liu et al., 2019).
where the DAD dermal is the daily average dose through the dermal pathway in mg.kg−1.day−1, CS is the concentration, CF is the contamination factor (1 × 10–6 mg.kg−1), SA is the Area of dermal contact with sediment (5000 cm2), AF is the soil adsorption coefficient of dermal (0.04 mg.cm−2), ABS is the contaminants adsorption coefficient of dermal (0.1 no unit), EF is the exposure frequency (365 days/year), ED is the exposure duration (30 years), BW is adult body weight (70 kg), AT is the average life span (25,550 days). The RfD (0.04 mg.kg−1. day−1) developed for naphthalene was applied in calculating the hazard quotient, SFO is the oral slope factor (7.3 mg−1.kg−1.day−1) of B.a.P (Duodu et al., 2017). The accumulative effects of PAHs are of greater environmental concern compared with the individual effects of PAHs, as these contaminants exist as complex mixtures in the environment. Based on the latter, the sum of the HQ is calculated to obtain the HI value of the contaminants. The HI minimum threshold limit for the studied contaminants is 1 (one), in which any HI value greater than one is intolerable (Duodu et al., 2017).
Sediment quality guidelines
The SQGs are tools used by environmental scientists to evaluate polluted sediment sites and their effect on benthic organisms. The quality of the sediment can be evaluated by comparing the concentration of the pollutants with the SQGs which include the effects range low/effect range median (ERL/ERM). The effect range median (ERM) and effect range low (ERL) are applied to analyse the affiliation between sediment contamination and the lethal response (Duodu et al., 2017).
Statistical analysis
The data obtained for the analysed contaminants were subjected to various statistical analyses such as descriptive statistics, statistical testing, and inferential statistics. Descriptive statistics were conducted to determine the mean, standard deviation, and minimum and maximum values. The inferential statistics included Pearson correlation analysis and principal component analysis. The Pearson correlation (PA) was applied at 2 tails to determine the links among the PAHs. In addition, the principal component analysis (PCA) was also computed using the varimax rotation at Eigen value > 1 to detect the relationship between the PAHs in the sediment. The statistical analysis was conducted with the aid of IBM SPSS statistics 25, manufactured in IL, USA.
Results
Distribution of PAHs and physicochemical parameters
Figures 2, 3, and 4 show the distribution of the PAHs in the Ikpoba River contaminated sampling location/stations during the wet and dry seasons. The distribution was described based on individual PAH concentrations, ring classifications, and the sum of the concentrations of the PAHs, higher molecular (HMPAHs), and lower molecular PAHs (LMPAHs) in each sampling station.
During the wet season, the PAHs distribution in the Okhoro location (Figs. 2, 3, and 4) showed that 2 rings < 3 rings < 4 rings < 5 rings < 6rings in AS1 and AS3, while the 2 rings < 6 rings < 4 rings < 5 rings < 3 rings in AS3. The minimum and maximum concentrations of PAHs during this period were 0.0 l mg/kg-dw of D.ah.A and 2.04 mg/kg-dw of Acy respectively both in the AS3 station. However, during the dry season, the PAHs distribution in the Okhoro location was detailed in the following ascending order: 2 rings < 3 rings < 6 rings < 5 rings < 4 rings, 2 rings < 6 rings < 4 rings < 3 rings < 5 rings, 2 rings < 6 rings < 5 rings < 4 rings < 3 rings for AS1, AS2, and AS3 respectively. The minimum and maximum concentrations of the analysed pollutants were 2.07 mg/kg-dw of Acy and 0.03 mg/kg-dw of B.ghi.Py tentatively and both concentrations were domiciled in AS3. The high concentration of Acy in the Okhoro location may be a result of the wood charcoal production within this location and the high concentration of mechanical workshops (Huang et al., 2012).
The distribution of PAHs (Figs. 2, 3, and 4) in the Teboga location during the wet period were in the following order: 2 rings < 5 rings < 3 rings < 4 rings < 6 rings in CS4, while 2 rings < 3 rings < 5 rings < 4 rings < 6 rings in CS5, and CS6. However, 0.13 mg/kg-dw of Nap in CS5 and 0.47 mg/kg-dw for both I.123 cd.P in CS4 and B.a.P in CS5 was the minimum and maximum concentrations of PAHs in the Teboga location during this aforementioned period. Furthermore, the distribution profile of PAHs during the dry period showed that 2 rings < 5 rings < 4 rings < 6 rings < 3 rings in CS4, CS5, and CS6. In addition, the minimum and maximum concentrations were 0.11 mg/kg-dw of D.ah.P in CS5 and CS6, and 0.98 mg/kg-dw of Acy in CS4. The major sources of I.123 cd.P and B.a.P in Teboga may be from oil and gasoline, while Acy may be from the vehicle exhaust from the mechanical workshops domiciled in the Teboga location (Daso et al., 2016; Szewczyńska et al., 2017). It was further observed in the Teboga location that B.ghi.F was below the detection limit for both seasons of sampling and analysis. The aforementioned observation may be a result of the non or inconsistent and insignificant release of B.bjk.F from anthropogenic and natural activities within the stations in Teboga or the concerned contaminant was freshly released at the time of sampling.
The distribution profile of PAHs (Figs. 2, 3, and 4) across the Ikpoba location during the wet period in the ascending order followed: 2 ring < 6 rings < 4 rings < 5 rings < 3 rings in WS7, 2 rings < 3 rings < 5 rings < 6 rings < 4 rings in WS8, and 2 rings < 3 rings < 5 rings < 4 rings < 6 rings in WS9. Moreover, the minimum and maximum concentrations of the PAHs contaminants in Ikpoba during this season were 0.01 mg/kg-dw of Acy in WS7 and 1.56 mg/kg-dw of I.123 cd.P in WS8 correspondingly. However, the distribution of the PAHs during the dry period in the ascending order followed: 2 rings < 5 rings < 6 rings < 4 rings < 3 rings in WS7, 2 rings < 6 rings < 3 rings < 5 rings < 4 rings in WS8, and 2 rings < 6 rings < 5 rings < 4 rings < 3 rings. The concentration range was 0.01 mg/kg-dw of D.al.P-2.07 mg/kg-dw of Acy and were both located in the WS9 station. The Ikpoba location is home to the urban waste site and paint industries and the Acy, I.123 cd.P, and D.al.P may be derived primarily from the urban waste site and aforementioned industries (Fazeli et al., 2019; Suman et al., 2016).
Furthermore, the 6 rings of PAHs were more prominent in the wet season compared to others, while the 3 rings were more prominent than others during the dry period. It was also observed that the 3 rings of PAHs have the largest total concentration in both seasons, while the 2 rings were the lowest in all seasons. Based on the study of the individual PAHs contaminants and their high concentration, the 3 rings Acy were the most prominent contaminants across the sampling stations (AS1-WS9) of the Ikpoba River contaminated sediment. It was discovered to have the highest concentration in AS1 and WS7 during the wet season, and also in AS3, CS4, and WS9 during the dry period.
The distribution of ∑20 PAHs (Fig. 4) across the stations (AS1-WS9) of the stations of the Ikpoba River contaminated sediment in ascending order follows WS8 < AS1 < WS9 < AS2 < CS6 < CS4 CS5 < WS7 < AS3 and AS2 < AS1 < WS8 < CS6 < CS5 < CS4 < AS3 < WS9 < WS7 during the wet and dry seasons respectively. The minimum and maximum concentration of ∑20 PAHs during the wet period was 3.86–5.7 mg/kg-dw, while during the dry season, we had 4.98–9.26 mg/kg-dw. The ∑20 PAHs in the dry season were all higher than the ∑20 PAHs in the wet period in all the sampling stations. The high ∑20 PAHs in WS7 may be due to the presence and the burning of the urban waste site in this station, as waste tends to release a large concentration of different PAHs into the aquatic system (Sarti et al., 2017).
Furthermore, the distribution of the ∑HMPAHs (Fig. 5a and b) across the sampling sites during the wet and dry seasons in the ascending order follows AS3 < WS8 < WS7 < WS9 < CS4 < CS6 ≤ AS1 < AS2 < CS5 and AS2 < AS3 < CS5 < CS4 < CS6 < WS7 < AS1 < WS9 < WS8 respectively, while the ∑LMPAHs (Fig. 5a and b) through the sampling stations in the ascending order follows: AS2 < AS1 < WS8 < WS9 < CS5 < CS6 < CS4 < WS7 < AS3 and AS1 < AS2 < WS8 < CS6 < CS5 < CS4 < WS9 < AS3 < WS7 during wet and dry period correspondingly. However, the sum of the HMPAHs across the sampling stations was higher than the sum of the LMPAHs for both seasons, except in WS7 during the dry season. This may be due to the low solubility of HMPAHs in water and their strong affinity for sediment, which makes them less accessible in water and strongly adsorbed on the sediment (Abdel-Shafy & Mansour, 2016).
The distribution of the TOC (Fig. 6a and b) in ascending order during the wet season was as follows: AS2 < AS1 < CS5 < CS4 < WS8 < CS6 < AS3 < WS7 < WS9, while during the dry season, it was as follows: AS1 < AS3 < CS4 < CS5 < CS6 < WS7 < AS3 < WS8 < WS9.The pH had minimum and maximum values of 6.90–7.49 and 6.4–7.77 during the wet and dry seasons, respectively. These variations in pH values may be justified by the inconsistency in the quantity of effluent discharged into the aquatic system, as chemical contaminants influence pH values (Ibrahim et al., 2020). However, the distribution of the pH in the ascending order is as follows: CS5 < CS6 < CS4 < WS7 < AS1 < AS2 < AS3 < WS8 < WS9 and CS6 < CS5 < CS4 < WS7 < WS8 < WS9 < AS1 < AS3 < AS2 during the wet and dry seasons correspondingly. The pH range value was 6.96–7.55 and 6.77–7.45 during wet and dry seasons correspondingly.
Furthermore, the dispersion of PO4 through the stations revealed its minimum and maximum values to be 0.85–1.65 and 1.00–1.65 during the wet and dry seasons, respectively. The increase in phosphate content during the dry period may be due to an increase in poor agricultural practices, runoff from urban areas, lawns, leaking septic systems, and discharges from sewage treatment plants (Fang et al., 2020; Isiuku & Enyoh, 2020). The distribution of PO4 across the station during the wet period in ascending order follows: AS < AS2 < AS3 < CS4 < SC6 < WS7 < WS9 < WS8 < CS5, while during the dry period, in ascending order as follows: AS1 < AS3 < CS4 < WS7 < CS6 < AS2 < CS5 < WS8 < WS9.The minimum and maximum values of 12.00–15.32 and 11.2–15.01 for the wet and dry periods were for NO3. However, this physicochemical parameter distribution across the sampling station during the wet season revealed the NO3 present in AS1 < AS2 < AS3 < CS4 < CS5 < WS7 < CS6 < WS9 < WS8, while during the dry period, it was observed that the NO3 present in AS1 < AS2 < AS3 < CS4 < WS9 < CS5 < WS7 < WS8 < CS6. In addition, the range of the EC distributed across the sampling station during the wet and dry seasons was 67.50–75.20 mS/cm and 63.20–70.55 mS/cm. During the wet season, the EC distribution across the sampling stations revealed EC in AS3 < AS2 < WS7 < CS4 < CS6 < WS9 < CS5 < WS8, while during the dry season, the following was observed: AS3 < CS4 < AS1 < WS8 < AS2 WS9 < CS6 < WS7.
Source apportionment
Table S1 showed the application of isomer ratios of PAHs in Okhoro, Teboga, and Ikpoba sampling locations of the Ikpoba river to discover the sources of the PAHs within the studied locations /stations. The isomer ratio Phe/ant was < 10, and this was observed across the stations for both seasons and showed the pollutant source to be pyrolytic (Das et al., 2020). The isomer ratio Flt (Flt + Pyr) was > 0.35 and was observed for both seasons across the stations except in AS1, whose isomer ratio Flt (Flt + Pyr was < 0.2, which disclosed the station’s pollutant source to be combustion, except for AS1, which had a petroleum source. However, the isomer ratio Ant (Ant + Phe) was > 0.5 in all stations, except CS1 during the wet period. This ratio supported the source of PAHs to be from the combustion of coal for all stations, except CS1, which had its PAHs from petroleum combustion because the isomer ratio Ant (Ant + Phe) was in the range of 0.4–0.5. The isomer ratio Ant (Ant + Phe) < 0.4 during the dry period described the PAHs source found in AS2-AS3-CS4-CS5-WS7-WS8-WS9 stations to be petrogenic, while PAHs in AS1 and CS6 were from the combustion of petroleum and coal, respectively(Liao et al., 2017).
Furthermore, the isomer ratio B.a.P/B.ghi.P across the sampling station during the wet period revealed the following: The source of PAHs in AS3-WS8-WS9 was the combustion of gasoline because the isomer ratio B.a.P/B.ghi.P value of the pollutants across the aforementioned stations was < than 0.6. However, PAHs in AS1-AS2-CS4-CS5-CS6-WS7 had a non-gasoline origin because the isomer ratio B.a.P/B.ghi.P was > 0.66. Moreover, during the dry period, the PAHs in all the stations were from the combustion of gasoline because the isomer ratio B.a.P/B.ghi.P was < 0.6 (Han et al., 2019).
The isomer ratio B.a.A/(B.a.A + Chr) in all the sampling stations during the wet and dry seasons was greater than 0.35, which revealed the PAHs’ source to be majorly combustion (Han et al., 2020). During the wet period, however, the isomer ratio I.123 cd.P/(I.123 cd.P + B.ghi.P) was greater than 0.5 in all stations except AS3 (0.04) and WS8 (0.04). This revealed the source of the pollutants to be the combustion of coal, wood, and biomass except for AS3 and WS8, which derived their pollutants from petroleum combustion. Furthermore, the source of PAHs in AS1-CS6 was the combustion of coal, wood, and biomass as the isomer ratio I.123 cd.P/(I.123 cd.P + B.ghi.P) during the dry period was > 0.5 (Yao et al., 2020). However, the PAHs from WS7-WS9 were from the combustion of petroleum, as the isomer ratio I.123 cd.P/(I.123 cd.P + B.ghi.P) was within the range of 0.2–0.5 (Han et al., 2020).
Additionally, the source of PAHs based on the isomer ratio Flt/Pyr during the wet period in AS1-AS2-AS3-VS4-WS7-WS8-WS9 was petrogenic because the isomer ratio value was > 1.0, while the source of PAHs during the same season in CS5 and CS6 was pyrolytic as the value of the isomer ratio was < than 1.0 Cao et al., 2020). However, during the dry, the source of PAHs based on the isomer ratio Flt/Pyr was pyrolytic except for CS5-CS6-WS8, which had their source of PAHs from petrogenic activities (Ma et al., 2020).
The ratio of LMPAHs/HMPAHs in all the sampling sites during the wet and dry seasons was < 1.0 which revealed the pollutant source to be the incomplete combustion of gasoline for both seasons (Han et al., 2021). This isomer ratio method showed PAHs contaminants in the sediment of the aquatic system to be mainly from the combustion of either biomass, coal, wood, or petroleum, followed by pyrolytic, and petrogenic sources in both seasons.
Multivariate statistical analysis of PAHs
Person correlation
Tables (S2a and S2b) showed the Pearson correlation analysis results among the PAHs, physio-chemical parameters, and between PAHs and physiochemical parameters during the wet and dry seasons. It revealed positive and negative correlations at 0.01 and 0.05 confidence levels.
The abovementioned tables showed positive correlations among PAHs pollutants, such as Flu’s positive correlation with Phe, Pyr, B.a.A, B.e.P, Ant, and Flt during the wet season, among many others (see Tables S2a and S2b). The studied PAHs with a high positive correlation may originate from the same source (Emoyan et al., 2020). Furthermore, previous work has shown a tendency for a positive correlation between PAHs compounds with similar origins (Froehner et al., 2018; Hou et al., 2019).
The wet season correlation analysis also revealed the interaction between PAHs pollutants and the analysed physicochemical parameters. This included D.ah.P’s positive correlation with TOC; EC’s positive correlation with PO4 and NO3; and PO4’s positive correlation with NO3. However, a positive interaction of a PAHs pollutant with some physicochemical parameters was observed during the dry period, such as the positive correlation of B.a.P with D.ah.A and TOC, while B.e.P correlated with D.ah.P and pH, and D.ah.A had a positive correlation with TOC and pH, while B.ghi.Py correlated with NO3 positively, while B.a.A correlated with B.e.P, and pH. Positive correlations were also observed among the physicochemical parameters, such as pH correlation with N03, EC correlation with PO4, and PO4 correlation with NO3.
However, the significant positive correlation of D.ah.p with TOC during the wet season and with D.ah.A and B.a.A during the dry season may likely influence the distribution behavior and toxicity of these PAHs. Previous research has recorded a positive correlation between PAHs with TOC (Sun et al., 2017, 2021). In addition, the positive correlation between B.e.P, D.ah.A, and pH during the dry season may increase the concentration of these pollutants within the ecosystem as this can occur at low pH (Minkina et al., 2019). The low pH may be responsible for the low anoxic degradation proportion in sediment, which may be responsible for the accumulation of organic matter (Minkina et al., 2019). As previously reported accumulated organic matter may increase PAH concentrations within the ecosystem (Ukalska-Jaruga et al., 2019). Also, recent research showed PAHs to impede NO3- increase in the sediment/soil (Urakawa et al., 2019). The positive correlation of B.ghi.Py with NO3 may reduce the concentration of nitrate within some sampling areas in which the contact occurred. However, the positive correlation between pH and nitrate may influence the acidity of the sediment as a high nitrate level may lower the pH of the sediment/soil of the ecosystem (Glass & Silverstein, 1998). Positive correlations between EC and phosphate were observed. Phosphate may become significant under anoxic conditions with nitrate present (Šorm et al., 1996). Furthermore, phosphate nitrate positive correlations and relationships may constitute an aquatic nuisance as they favor algae growth, which may lead to the eutrophication of the systems (Fang et al., 2020).
The negative correlation among PAHs may be a result of diverse/ nonpoint sources of the pollutants, while the negative correlation between PAHs and physicochemical parameters may be caused by the non-interaction of the particular physicochemical parameters on the PAHs (Cao et al., 2020). The negative correlation among PAHs was observed when Acy, had a negative correlation with Ant, B,e. P, B.ghi.P, and D.ai.P during the wet season, and Nap negatively correlated with B.a.A and B.e.P during the dry season, This is one among many others in Tables (S2a and S2b).
We also observed in the wet period a negative correlation between PAHs and physicochemical parameters, as D.ah.A negatively correlated with EC and NO3, while D.ah.P negatively correlated with TOC. However, during the dry season, the following was observed: the Ace negative correlation with B.a.A, B.e.P, D.ah.A and pH; Flt negative correlation with B.a.A, B.e.P, D.ah.A and pH; B.a.A negative correlation with NO3; B.ghi.Py negative correlation with NO3, D.al.P negative correlation with pH, and D.ai.P negative correlation with pH. In addition, D.ah.A negatively correlated with EC and NO3, while D.ah.P negatively correlated with TOC. However, this particular season showed a negative correlation between physicochemical parameters, such as the negative correlation between pH and NO3.
Furthermore, it was observed that some of the pollutants never correlated among themselves or correlated with the physicochemical parameters during both seasons. This has also been noticed in previous research. This may be a result of the numerous origins of PAHs or that the PAHs were recently released into the environment (Emoyan et al., 2020). The correlation between PAHs and physiochemical parameters was more pronounced during the dry season compared to the wet season. Positive correlations among PAHs, PAHs, and physicochemical parameters were observed in both seasons compared to negative correlations.
Principal component analysis
Tables (S2a and S2b) showed the principal component analysis (PCA) of PAHs in sediment during wet and dry seasons. The principal component analysis (PCA) of PAHs in the sediment was computed, and the first five components with Eigenvalues greater than one were pulled out and analysed. The five extracted Eigenvalues were responsible for 100% of the variance. Based on the component matrix examination of the PCA during the wet season, it was discovered that Ant- Pyr- B.bjk.F- B.e.P- B.a.P- D.al.P-pH- EC- NO3 -PO4 were all coupled in PC1 at 33.90% variance. Whereas the PCA of the PAHs data obtained for the dry season revealed that the Acy- Phe-Flt-B.c.Phe,- D.ah.A- B.ghi.Py- TOC- NO3-PO4 were coupled in PC1 at 42.02% variance. The presence of TOC may influence the presence of PAHs in PC1 during the dry season as it might likely increase their distribution within the sampling sites/ecosystem (Cai et al., 2019). The interaction between NO3 and PO4 was also observed as in the case of PC1 during both seasons, which may likely favor the algae bloom (Fang et al., 2020). The algae bloom may be hindered to an extent as the presence of PAHs tends to hinder nitrate increase within the ecosystem (Jiang et al., 2017). However, the sources of these PAHs coupled to PC1 during dry and wet seasons favor pyrolytic origin.
The PC2 factor revealed an interlink of B.ghi.Py-B.a.A- I.123 cd.P- D.ai.P- D.ah.P at 24.33% variance during the wet season and Ace-Flu- B.ghi.P- B.e.F- D.al.P- EC at 21.37% variance during the dry season. The ratio, I (123-cd) p/I (123-cd) P + B (ghi) P, and also the B.a.A in PC2 of the wet season increases the probability of the PAHs deriving their source from the combustion of biomass, coal, and gasoline, while the origins of PC2 of the dry season are not well defined, but they may likely come from multiple sources. However, in the PC3 of the wet season, the Acy, Phe, and TOC were coupled at 19.74% variance, while in the PC3 of the dry season, the Nap- Ant- Pyr- Chr, -I.123 cd.P were coupled at 19.39% variance. The TOC of PC3 during the wet season may be responsible for the distribution and increase in the presence of Acy and Phe in the wet season, and their source may be pyrolytic.
The origin of PAHs in the PC3 of the dry season may be from multiple sources which have a high tendency to be petrogenic and pyrolytic in origin. Furthermore, Ace- Flu-B.c.Phe,-D.ah.A were associated with PC4 during the wet season, whereas B.a.A- D.ai.P- D.al.P,- pH was associated with PC4 during the dry season at 15.11% and 9.92%, respectively. The PAHs in PC4 during the dry season may be easily broken down by microorganisms if there is an increase in the linked pH (Moscoso et al., 2012). It was further revealed that Nap and Chr were coupled to PC5 at a 6.93% variance during the wet season, whereas there were no PAHs coupled to PC5 during the dry season at a 7.30% variance.
Moreover, the rotated component matrix revealed that Ant, Pyr-B.bjk.F-B.e.P- B.a.P- TOC- pH- EC- NO3- PO4 at 30.63% variance were coupled in PC1 for the wet period, and Acy- B.c.Phe- D.ah.A- TOC- pH were coupled in PC1 for the dry season at 24.51% variance. During the wet season, all physicochemical parameters influenced the PAHs coupled to PC1 whereas during the dry season, only TOC and pH influenced the PAHs coupled to it. It was discovered during the wet period that the PC1 of the rotated component matrix was slightly similar to the PC1 of the component matrix. However, D.al.P in PC1 of the component matrix was substituted by TOC in PC1 of the rotated component matrix. This showed that the TOC probably has more influence on the fate of Ant- Pyr- B.e.P-B.a.P compared to D.ah.A. The dry season PC1 of the rotated matrix was also similar to that of the component matrix except for the absence of Phe and Flt.
The PC2 for the wet season of the rotated component matrix coupled I.123cd.P- B.ghi.P- D.ai.P- D.ah.P at 24.27% variance, while the PC2 for the dry season coupled Flu-B.bjk.F- D.al.P- PO4 at 21.37% variance. The PAHs coupled to PC2 of the rotated component matrix for the dry season had a strong interaction with PO4, while the wet season does not. The PC2 of the rotated component matrix only differs from the PC2 of the component matrix for the wet season due to the absence of B.a.A in the PC2 rotated component matrix, while for the dry season, it was noticed that Flu and D.al.P were common in both the PC2 of the rotated and component matrix. Also, the PAHs of the rotated component matrix are more influenced by PO4 than the component matrix itself, which is more influenced by the EC.
Furthermore, the Acy- Ace- Flu-Phe-B.a.A-D.ah.A was coupled with the PC3 rotated component matrix at 16.83%. The addition of B.a.A to the factor increases the probability of the origin being from the combustion of biomass and gasoline. However, Flt-B.c.Phe-D.al.P was coupled with PC4 at 15.79% and Nap-Chr was coupled with PC5 at 12.49%. The PAHs coupled to PC4 have a higher tendency to have a pyrolytic origin than those in PC5, which may be from petroleum combustion.
Descriptive statistics
Table 1 shows the descriptive statistics of the PAHs (mg/kg-dw) and physiochemical parameters of the contaminated sediment of the Ikpoba River during the wet and dry seasons. This included the assessment of the maximum, minimum, mean, standard deviation, percentage increase, and decrease of average concentrations of the studied PAHs (mg/kg-dw) and physicochemical parameters from wet to dry periods.
The following values were observed for the descriptive statistics of the concentration of PAHs contaminants distributed across the three locations and nine stations of the Ikpoba river ecosystem. During the wet season, it was noticed that 4.90 mg/kg-dw, 3.55 mg/kg-dw, and 1.35 mg/kg-dw were recorded for the average ∑20 PAHs, ∑HMPAHs, and ∑LMPAHs respectively, while during the dry period, it was noticed that 6.95 mg/kg-dw, 4.18 mg/kg-dw, and 2.77 mg/kg-dw were values for the average ∑20 PAHs, ∑HMPAHs, and LMPAHs respectively. The average concentration of the PAHs pollutant rings in the studied sediment of the river in the ascending order showed that the 2 rings < 4 rings < 5 rings < 3 rings < 6 rings and 2 rings < 6 rings < 5 rings < 4 rings < 3 rings for both wet and dry periods, correspondingly. This further disclosed that the 6 rings were higher during the wet season, while the 3 rings were higher during the dry season. This may be a result of the decrease in the anthropogenic and natural sources of the 6 rings and an increase of 3 rings PAHs from the wet to the dry period (Ambade et al., 2020,).
The percentage increase of the average concentration of PAHs contaminants from wet to dry season was observed in 2 rings, 3 rings, 4 rings (except Chr), 5 rings (except B.bjk.F), and only I.123 cd.P of the 6 rings, an average of ∑PAHs, ∑HMPAHs, and ∑LMPAHs while the percentage decrease from wet to dry period was noticed in Chr, B.bjk.F, and all 6 rings except I.123 cd.P. However, according to Table 1 the range of the average PAHs (mean PAHs) in mg.kg-dw was 0.15 (Nap)-0.54 (Acy) and 0.13 (D.al.P)-0.99 (Acy) in wet and dry periods correspondingly, which further supported an increase in concentration from wet to dry period and the environmental importance of Acy in Ikpoba River. Furthermore, the increase of PAHs concentration from the wet to dry period may be as a result of one or the combination of the following: a surge in the release of PAHs congener from the origin in the dry period, and the dry period upsurge in evaporation of water in the aquatic system that may cause an increase in the concentration of aquatic ecosystem contents. This situation was also observed in the source apportionment and risk assessment of PAHs in the sediment of the Brisbane River Australia. However, the low concentration of PAHs in Brisbane River during the wet period was adjudged to be caused by sediment mixing and dilution (Duodu et al., 2017). Nevertheless, the case was different in the Erjen River in Taiwan as the concentration of PAHs was higher during the wet than the dry period. However, the reason for the case in Erjen River was adjudged to be the high deposition of PAHs contaminated dust on the river during the wet period ((Wang et al., 2015). Also, Table 2 compared the mean of PAHs obtained in the sediment of the Ikpoba River to those obtained in the sediment around the world. The mean of the contaminated sediment in the Ikpoba River, in Nigeria, was higher than those detected in the Ovia River in Nigeria and Xinxian River in China and others (Duodu et al., 2017; Tongo et al., 2017; Wang et al., 2015).
The average total organic compound (TOC) value was 40.16 mg/kg-dw and 46.79 mg/kg-dw during the wet and dry seasons individually. An increase in the average TOC from the wet to dry season was also observed. The TOC has a significant impact on the distribution of the PAHs contaminant within the ecosystems. The percentage increase of some PAHs from the wet to dry season may be influenced by the high presence of TOC (Naz et al., 2016). The average concentration of most PAHs in the river sediment was discovered to be higher during the dry season compared to the wet season. This could be due to the 16.50% increase in TOC during the dry season, as TOC influences the fate of PAHs within the environment. The migration of PAHs downward to be adsorbed by sediment may have been very active as an increase in TOC has been known to activate this process. Furthermore, it was also assumed that the high concentration during the dry season may be due to a reduction in the quantity of water in the aquatic system from an increase in evaporation (Cai et al., 2019; Wu et al., 2022).
The average pH value of the contaminated sediment was 7.10 and 7.19 during the wet and dry seasons, respectively. There was a 1.27% slight increase in the average pH value from the wet to dry season. Furthermore, high pH improves the soil’s enzymatic state and aids in the breakdown of PAHs by microorganisms (Méndez García & García de Llasera, 2021). This may be responsible for the percentage decrease of certain PAHs during the dry season. During the wet and dry seasons, the average EC was 70.86 mS/cm and 67.72 mS/cm, respectively, indicating a 4.43% percentage decrease. This decrease in EC showed an increase in the purity of the water as an inorganic impurity in water tends to increase EC (Shrestha & Basnet, 2018). However, this decrease in EC/impurity during the dry period may be a result of a reduction in various runoff (which carries along different inorganic pollutants) into the water body due to a lack of rainfall (Gaur et al., 2022).
The average concentration of PO4 was 1.30 mg/kg-dw and 1.43 mg/kg-dw during the wet and dry seasons, respectively, while the average concentration of NO3 was 14.06 mg/kg-dw and 13.70 mg/kg-dw during the wet and dry seasons, respectively. The average phosphate concentration increased by 10.0% from the wet to dry season, while the average nitrate concentration decreased by 2.56%. The average concentration of nitrate and phosphate for both seasons was above the MAC (10 mg/kg and 0.05 mg/kg, respectively) recommended by USEPA. Both physicochemical parameters are thus potential threats because they can cause eutrophication (Isiuku & Enyoh, 2020).
The sediment quality guideline value (Table 1) was used to assess the quality of the sediment in both seasons. The average PAHs during the wet season as reflected in the descriptive statistic table revealed that the average value of Acy, Ace, Flu, B.bjk.F, and meanPAHs were > ERL but < ERM, while during the dry period, it was observed that the average value of Nap, Ace, Flu, Phe, B.a.A, B.a.P, and ∑meanPAHs were > ERL and < ERM. These PAHs may cause mild harmful effects on the biota of the aquatic system as they have values that are between ERL and ERM within their respective seasons.
Furthermore, the average values of Nap, Phe, Ant, Flt, Pyr, Chr, B.a.P, B.ghi.Py, and the average value of Ant, Flt, Pyr, B.bjk.F, and B.ghi.P were all < ERL during the wet and dry seasons respectively, except B.a.A, which had an average value equal to ERL during the wet period. Furthermore, the average values of Nap, Phe, Ant, Flt, Pyr, Chr, B.a.P, B.ghi.Py, and the average value of Ant, Flt, Pyr, B.bjk.F, and B.ghi.P were all < ERL during the wet and dry seasons respectively, except B.a.A, which had an average value equal to ERL during the wet period. These PAHs may not be associated with an adverse biological effect on the biota within the ecosystem. However, it was only D.ah.A during the wet period, Acy, and D.ah.A during the dry period that had an average value greater than ERM. The average value of D.ah.A (in both seasons) and Acy (dry season only) have a high tendency to have toxic effects on the biota of the aquatic system.
Ecological and human health risk assessment
According to Table 3 of mERMQ, it was noticed during the wet season that all the locations/stations recorded a medium–low effect (0.5 < mERMQ > 0.11) of PAHs contaminants on the ecosystem except station AS3 (of the Okhoro location). The maximum and minimum values of mERMQ during the wet season were observed in AS3 and WS8, respectively. However, it was also noticed during the dry period that the concentration of the contaminant within the majority of the stations had medium–low effects on the aquatic system, except for stations WS7 and WS9, which revealed medium–high effects of the concentration of PAHs on the aquatic system. Also, the maximum and minimum values of mERMQ during the dry season were observed at stations WS7 and AS2 correspondingly.
Furthermore, the maximum value of mERMQ value in AS3 during the wet season might be due to the tremendous increase in the concentration of Acy, while during the dry season, the large value of mERMQ observed in stations WS8 and WS9 may also be due to the large concentration of both Acy and Flu in WS8 and Acy only in WS9. However, it was observed that Acy has become of environmental importance/concern in the aquatic system of the Ikpoba River. The sum of mERMQ by adding the sampling stations that make up a location was also studied and compared with the level or degree of contamination effects. The ∑mERMQ across the sampling locations during the wet in ascending order showed that Ikpoba a < Teboga < Okhoro. The ∑mERMQ in all the sampling locations during the wet period was of medium–high value (1.5 < mERMQ > 0.50), whereas, ∑mERMQ of contaminants during the dry period was as follows: Okhoro < Teboga < Ikpoba. The ∑mERMQ of contaminants in all the AS and CS sampling locations during the dry seasons was lower than 1.5 but was greater than 0.51, which indicated medium–high effects of the studied PAHs on the ecosystem.
During the wet season, it was noticed that all the locations/stations recorded a medium–low effect (0.5 < mERMQ > 0.11) of PAHs contaminants on the ecosystem except station AS3 (of the Oredo location). The maximum and minimum values of mERMQ during the wet season were observed in AS3 and WS8, respectively. However, it was also noticed during the dry period that the concentration of the contaminant within the majority of the stations had medium–low effects on the aquatic system, except for stations WS7 and WS9, which had medium–high effects of PAH concentration on the aquatic system.
Also, the maximum and minimum values of mERMQ during the dry season were observed in sampling sites WS7 and AS2 correspondingly. Furthermore, the maximum value of mERMQ value in AS3 during the wet season might be due to the tremendous increase in the concentration of Acy, while during the dry season, the large value of mERMQ observed in sampling sites WS8 and WS9 may also be due to the large concentration of both Acy and Flu in WS8 and Acy only in WS9.
However, it was observed that Acy has become of environmental importance and concern in the aquatic system of the Ikpoba River. The sum of mERMQ by adding the sampling stations that make up a location was also studied and compared with the level or degree of contamination effects. The ∑mERMQ across the sampling locations during the wet in ascending order showed that Ikpoba < Teboga < Okhoro. The ∑mERMQ in all the sampling locations during the wet period was of medium–high value (1.5 < mERMQ > 0.50), whereas, ∑mERMQ of contaminants during the dry period were as follows: Okhoro < Teboga < Ikpoba. The ∑mERMQ of contaminants in all the AS and CS sampling locations during the dry seasons was lower than 1.5 but was greater than 0.51, which indicated medium–high effects of the studied PAHs on the ecosystem. However, the ∑mERMQ of contaminants in WS exhibited a high PAH effect on the ecosystem of the WS location. The average mERMQ of the studied PAHs in each location was quantified by adding the three-sampling stations together to make up a location. It was discovered that the average mERMQ across the studied locations during the wet period demonstrated a medium–low effect of PAHs on the ecosystem of the locations. Moreover, medium–low PAH effects were observed in AS and CS locations, while medium–high PAH effects were noticed in WS during the dry season.
According to Tables S4 (a) and (b), the TEQ of the studied contaminants across the nine stations during the wet season was higher than 0.2 mg.kg-1, in which the maximum value was 3.74 E + 01 in the AS3 station, and the minimum was 2.26E-01 in the WS8 station. Furthermore, the TEQ analyses of the dry season reveal TEQ values across the nine stations to be higher than 0.2 mg.kg−1. The maximum value of TEQ was 1.83E + 03 in the AS1 station and the minimum was 7.97E-01 in the AS3 station. In addition, the average TEQ value during wet seasons in Okhoro, Teboga, and Ikpoba locations was 2.32E + 01, 1.33E + 01, and 7.80E-01, respectively, while the average TEQ during the dry season in Okhoro, Teboga, and Ikpoba locations was 6.17E + 02, 1.45E + 01, and 2.28E + 01, respectively. The minimum value of TEQ and average TEQ across the studied stations/locations were higher than 0.2 mg.kg−1, which is a criterion for clean-up recommendation (Parra et al., 2020).
All the values of DAD through dermal (DADdermal) (Table 4) were lower than the RfD of B.A.P. This has also been observed in previous research (Duodu et al., 2017; Froehner et al., 2018; Isiuku & Enyoh, 2020). Despite the low values, Acy was a potential human health risk. It was noticed that the maximum DADdermal value of the Acy during the wet and dry seasons was 2.50E-7 and 2.53E-7, respectively, while the average DAD value of the Acy was 4.73E-8 during the wet season and 6.34E-6 during the dry season. Furthermore, they had higher values during the dry period compared to the wet season (which may be deduced to be a result of the difference in concentration). These Acy DADdermal values were greater than the maximum and average DADdermal values of every other PAHs. The HQ and HI (which represent the overall non-carcinogenic toxic risk) values of the PAHs during the wet and dry seasons were all less than 1. The maximum and average values of HQ for Acy during the wet season were 6.26E-6 and 1.66E-6, respectively, while during the dry season, the maximum and average HQ values were 6.43E-6 and 2.42E-6, respectively, and these HQ values < 1 indicated low non-carcinogenic toxic effects. The HI values were also less than a unit value for both seasons and showed the accumulated non-carcinogenic risk to a human to be very low. The carcinogenic risk (CR) (Table 5) has a threshold limit of 1 × 10–6, and a CR value above this limit indicates a potential carcinogenic risk. All the calculated CR values of all the studied PAHs were all lower than 1 × 10–6 and may not likely result in carcinogenic risk. However, the Acy CR values for both seasons revealed that Acy is a more potential carcinogenic risk in the future if the Acy increase in the environment continues unabated.
Conclusion
The majority of pollutants and physiochemical parameters increased in concentration from the wet to the dry season. Based on the isomer ratio and PCA, the major source of the studied PAHs was the combustion of biomass, wood, coal, and petroleum for both seasons. However, the positive correlation among PAHs, PAHs, and physicochemical parameters was strong and more for both seasons compared to the negative correlation, which supported a common source for the majority of the pollutants. The DADdermal, HQ, HI, and CR values of the contaminants revealed the DAD values to be lower than B.a.P, while the HQ and HI values were lower than 1, which showed no accumulative toxic effect on individual health. In addition, the CR values of all the studied contaminants were lower than 1 × 10–6, except Acy a potential carcinogenic that may be responsible for about a maximum of 183 and an average of 886 in every 1000,000 persons during wet and dry seasons respectively to have cancer in their lifetime. The SQGs revealed Acy, Ace, Flu B.bjk.F, and ∑meanPAHs and Ant, Flt, Pyr, B.bjk.F, and B.ghi.P during the wet and dry seasons respectively to have a mild toxic effect on the biota, while Nap, Phe, and Ant, Flt, Pyr, Chr, B.a.P, B.ghi.Py, and Ant, Flt, Pyr, B.bjk.F, and B.ghi.Py during the wet and dry seasons showed zero effect on the biota. The average mERMQ revealed the effects of PAHs contaminants in all locations to be medium–low, except for the Ikpoba location, which had medium–high effects of PAHs on its biota. The value of TEQ across the locations was higher than the threshold limit, which indicated a high recommendation for environmental clean-up of the aquatic ecosystem.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
Abdel-Shafy, H. I., & Mansour, M. S. M. (2016). A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egyptian Journal of Petroleum, 25, 107–123. https://doi.org/10.1016/J.EJPE.2015.03.011
Ambade, B., Sethi, S. S., Kumar, A., Sankar, T. K., & Kurwadkar, S. (2020). Health risk assessment, composition, and distribution of polycyclic aromatic hydrocarbons (PAHs) in drinking water of Southern Jharkhand, East India. Archives of Environmental Contamination and Toxicology, 80(1), 120–133. https://doi.org/10.1007/S00244-020-00779-Y
Ambade, B., Sethi, S. S., Giri, B., Biswas, J. K., & Bauddh, K. (2021). Characterization, behavior, and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in the estuary sediments. Bulletin of Environmental Contamination and Toxicology, 108(2), 243–252. https://doi.org/10.1007/S00128-021-03393-3
Brown, G., & Maher, W. (1992). The occurrence, distribution and sources of polycycic aromatic hydrocarbons in the sediments of the Georges River estuary, Australia. Organic Geochemistry, 18, 657–668. https://doi.org/10.1016/0146-6380(92)90091-B
Cai, C. Y., Li, J. Y., Wu, D., Wang, X. L., Tsang, D. C. W., Li, X. D., Sun, J. T., Zhu, L. Z., Shen, H. Z., Tao, S., & Liu, W. X. (2017). Spatial distribution, emission source and health risk of parent PAHs and derivatives in surface soils from the Yangtze River Delta, eastern China. Chemosphere, 178, 301–308. https://doi.org/10.1016/J.CHEMOSPHERE.2017.03.057
Cai, T., Ding, Y., Zhang, Z., Wang, X., Wang, T., Ren, Y., & Dong, Y. (2019). Effects of total organic carbon content and leaching water volume on migration behavior of polycyclic aromatic hydrocarbons in soils by column leaching tests. Environmental Pollution, 254, 112981. https://doi.org/10.1016/J.ENVPOL.2019.112981
Cai, P., Cai, G., Chen, X., Li, S., & Zhao, L. (2021). The concentration distribution and biohazard assessment of heavy metal elements in surface sediments from the continental shelf of Hainan Island. Marine Pollution Bulletin, 166, 112254. https://doi.org/10.1016/J.MARPOLBUL.2021.112254
Cao, Y., Lin, C., Zhang, X., Liu, X., He, M., & Ouyang, W. (2020). Distribution, source, and ecological risks of polycyclic aromatic hydrocarbons in Lake Qinghai, China. Environmental Pollution, 266, 115401. https://doi.org/10.1016/J.ENVPOL.2020.115401
Das, S., Aria, A., Cheng, J. O., Souissi, S., Hwang, J. S., & Ko, F. C. (2020). Occurrence and distribution of anthropogenic persistent organic pollutants in coastal sediments and mud shrimps from the wetland of central Taiwan. PLoS One, 15, e0227367. https://doi.org/10.1371/JOURNAL.PONE.0227367
Daso, A. P., Akortia, E., & Okonkwo, J. O. (2016). Concentration profiles, source apportionment and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in dumpsite soils from Agbogbloshie e-waste dismantling site, Accra, Ghana. Environmental Science and Pollution Research, 23(11), 10883–10894. https://doi.org/10.1007/S11356-016-6311-3
Dudhagara, D. R., Rajpara, R. K., Bhatt, J. K., Gosai, H. B., Sachaniya, B. K., & Dave, B. P. (2016). Distribution, sources and ecological risk assessment of PAHs in historically contaminated surface sediments at Bhavnagar coast, Gujarat, India. Environmental Pollution, 213, 338–346. https://doi.org/10.1016/J.ENVPOL.2016.02.030
Duodu, G. O., Ogogo, K. N., Mummullage, S., Harden, F., Goonetilleke, A., & Ayoko, G. A. (2017). Source apportionment and risk assessment of PAHs in Brisbane River sediment, Australia. Ecological Indicators, 73, 784–799. https://doi.org/10.1016/J.ECOLIND.2016.10.038
Emoyan, O. O., Onocha, E. O., & Tesi, G. O. (2020). Concentration assessment and source evaluation of 16 priority polycyclic aromatic hydrocarbons in soils from selected vehicle-parks in southern Nigeria. Scientific African, 7. https://doi.org/10.1016/J.SCIAF.2020.E00296
Fang, L., Zeng, W., Xu, L., & Huang, L. Z. (2020). Green rusts as a new solution to sequester and stabilize phosphate in sediments under anoxic conditions and their implication for eutrophication control. Chemical Engineering Journal, 388, 124198. https://doi.org/10.1016/J.CEJ.2020.124198
Fazeli, G., Karbassi, A., Khoramnejadian, S., & Nasrabadi, T. (2019). Evaluation of urban soil pollution: A combined approach of toxic metals and polycyclic aromatic hydrocarbons (PAHs). International Journal of Environmental Research, 13(5), 801–811. https://doi.org/10.1007/S41742-019-00206-8
Feng, J., Xi, N., Zhang, F., Zhao, J., Hu, P., & Sun, J. (2015). Distributions and potential sources of polycyclic aromatic hydrocarbons in surface sediments from an emerging industrial city (Xinxiang). Environmental Monitoring and Assessment, 188(1), 1–14. https://doi.org/10.1007/S10661-015-5060-Y
Froehner, S., Rizzi, J., Vieira, L. M., & Sanez, J. (2018). PAHs in water, sediment and biota in an area with port activities. Archives of Environmental Contamination and Toxicology, 75(2), 236–246. https://doi.org/10.1007/S00244-018-0538-6
Gaur, N., Sarkar, A., Dutta, D., Gogoi, B. J., Dubey, R., & Dwivedi, S. K. (2022). Evaluation of water quality index and geochemical characteristics of surfacewater from Tawang India. Scientific Reports, 12(1), 1–26. https://doi.org/10.1038/s41598-022-14760-3
Glass, C., & Silverstein, J. (1998). Denitrification kinetics of high nitrate concentration water: PH effect on inhibition and nitrite accumulation. Water Research, 32, 831–839. https://doi.org/10.1016/S0043-1354(97)00260-1
Halfadji, A., Naous, M., Bettiche, F., & Touabet, A. (2021). Human health assessment of sixteen priority polycyclic aromatic hydrocarbons in contaminated soils of Northwestern Algeria. Journal of Health & Pollution, 11, 1–13. https://doi.org/10.5696/2156-9614-11.31.210914/469467
Han, J., Liang, Y., Zhao, B., Wang, Y., Xing, F., & Qin, L. (2019). Polycyclic aromatic hydrocarbon (PAHs) geographical distribution in China and their source, risk assessment analysis. Environmental Pollution, 251, 312–327. https://doi.org/10.1016/J.ENVPOL.2019.05.022
Han, B., Liu, A., He, S., Li, Q., & Zheng, L. (2020). Composition, content, source, and risk assessment of PAHs in intertidal sediment in Shilaoren Bay, Qingdao, China. Marine Pollution Bulletin, 159, 111499. https://doi.org/10.1016/J.MARPOLBUL.2020.111499
Han, B., Liu, A., Gong, J., Li, Q., He, X., Zhao, J., & Zheng, L. (2021). Spatial distribution, source analysis, and ecological risk assessment of polycyclic aromatic hydrocarbons (PAHs) in the sediments from rivers emptying into Jiaozhou Bay, China. Marine Pollution Bulletin, 168, 112394. https://doi.org/10.1016/J.MARPOLBUL.2021.112394
Hou, S., Zheng, N., Tang, L., Ji, X., Li, Y., & Hua, X. (2019). Pollution characteristics, sources, and health risk assessment of human exposure to Cu, Zn, Cd and Pb pollution in urban street dust across China between 2009 and 2018. Environment International, 128, 430–437. https://doi.org/10.1016/J.ENVINT.2019.04.046
Huang, W., Wang, Z., & Yan, W. (2012). Distribution and sources of polycyclic aromatic hydrocarbons (PAHs) in sediments from Zhanjiang Bay and Leizhou Bay, South China. Marine Pollution Bulletin, 64, 1962–1969. https://doi.org/10.1016/J.MARPOLBUL.2012.05.023
Ibrahim, H., Aniyikaye, R., Ezekiel-Adewoyin, D. T., Osunde, A. O., & Bala, A. (2020). Effect of different combinations of organic and inorganic nitrogen sources on growth and pod yield of Okra (Abelmoschus esculentus) in Minna, Niger State. 44th Annual Conference proceeding of soil science society of Nigeria.
Inam, E., Etuk, I., Offiong, N. A., Kim, K. W., Kang, S. Y., & Essien, J. (2018). Distribution and ecological risks of polycyclic aromatic hydrocarbons (PAHs) in sediments of different tropical water ecosystems in Niger Delta, Nigeria. Environmental Earth Sciences, 77(5), 1–14. https://doi.org/10.1007/S12665-018-7396-4
Isiuku, B. O., & Enyoh, C. E. (2020). Pollution and health risks assessment of nitrate and phosphate concentrations in water bodies in South Eastern, Nigeria. Environmental Advances, 2, 100018. https://doi.org/10.1016/J.ENVADV.2020.100018
Jiang, S., Xie, F., Lu, H., Liu, J., & Yan, C. (2017). Response of low-molecular-weight organic acids in mangrove root exudates to exposure of polycyclic aromatic hydrocarbons. Environmental Science and Pollution Research, 24, 12484–12493. https://doi.org/10.1007/S11356-017-8845-4
Keshavarzifard, M., Zakaria, M. P., ShauHwai, T., MohamatYusuff, F. F., Mustafa, S., Vaezzadeh, V., Magam, S. M., Masood, N., Alkhadher, S. A. A., & Abootalebi-Jahromi, F. (2014). Baseline distributions and sources of Polycyclic Aromatic Hydrocarbons (PAHs) in the surface sediments from the Prai and Malacca Rivers, Peninsular Malaysia. Marine Pollution Bulletin, 88, 366–372. https://doi.org/10.1016/J.MARPOLBUL.2014.08.014
Kumar, V., Kothiyal, N. C., Saruchi, Vikas, P., & Sharma, R. (2016). Sources, distribution, and health effect of carcinogenic polycyclic aromatic hydrocarbons (PAHs) – current knowledge and future directions. 4, 302–321. https://doi.org/10.1080/22243682.2016.1230475
Lawal, A. T. (2017). Polycyclic aromatic hydrocarbons. A review. 3. https://doi.org/10.1080/23311843.2017.1339841. http://www.editorialmanager.com/cogentenv
Liao, J., Chen, J., Ru, X., Chen, J., Wu, H., & Wei, C. (2017). Heavy metals in river surface sediments affected with multiple pollution sources, South China: Distribution, enrichment and source apportionment. Journal of Geochemical Exploration, 176, 9–19. https://doi.org/10.1016/J.GEXPLO.2016.08.013
Lin, F., Han, B., Ding, Y., Li, Q., Gao, W., & Zheng, L. (2018). Distribution characteristics, sources, and ecological risk assessment of polycyclic aromatic hydrocarbons in sediments from the Qinhuangdao coastal wetland, China. Marine Pollution Bulletin, 127, 788–793. https://doi.org/10.1016/J.MARPOLBUL.2017.09.054
Liu, J., Zhang, J., Zhan, C., Liu, H., Zhang, L., Hu, T., Xing, X., & Qu, C. (2019). Polycyclic Aromatic Hydrocarbons (PAHs) in Urban Street Dust of Huanggang, Central China: Status, Sources and Human Health Risk Assessment. Aerosol and Air Quality Research, 19, 221–223. https://doi.org/10.4209/AAQR.2018.02.0048
Ma, X., Wan, H., Zhou, J., Luo, D., Huang, T., Yang, H., & Huang, C. (2020). Sediment record of polycyclic aromatic hydrocarbons in Dianchi lake, southwest China: Influence of energy structure changes and economic development. Chemosphere, 248, 126015. https://doi.org/10.1016/J.CHEMOSPHERE.2020.126015
Méndez García, M., & García de Llasera, M. P. (2021). A review on the enzymes and metabolites identified by mass spectrometry from bacteria and microalgae involved in the degradation of high molecular weight PAHs. Science of the Total Environment, 797. https://doi.org/10.1016/J.SCITOTENV.2021.149035
Minkina, T., Sushkova, S., Konstantinova, E., Kumar Yadav, B., Mandzhieva, S., Konstantinov, A., Khoroshavin, V., Nazarenko, O., & Antonenko, E. (2019). Polycyclic aromatic hydrocarbons in urban soils within the different land use: A case study of Tyumen, Russia. 40, 1251–1265. https://doi.org/10.1080/10406638.2018.1540997
Moscoso, F., Teijiz, I., Deive, F. J., & Sanromán, M. A. (2012). Efficient PAHs biodegradation by a bacterial consortium at flask and bioreactor scale. Bioresource Technology, 119, 270–276. https://doi.org/10.1016/J.BIORTECH.2012.05.095
Naz, A., Chowdhury, A., Mishra, B. K., Gupta, S. K. (2016). Metal pollution in water environment and the associated human health risk from drinking water: A case study of Sukinda chromite mine, India. 22, 1433–1455. https://doi.org/10.1080/10807039.2016.1185355
Parra, Y. J., Oloyede, O. O., Pereira, G. M., de Almeida Lima, P. H. A., da Silva Caumo, S. E., Morenikeji, O. A., & de Castro Vasconcellos, P. (2020). Polycyclic aromatic hydrocarbons in soils and sediments in Southwest Nigeria. Environmental Pollution, 259, 113732. https://doi.org/10.1016/J.ENVPOL.2019.113732
Peng, N., Li, Y., Liu, Z., Liu, T., & Gai, C. (2016). Emission, distribution and toxicity of polycyclic aromatic hydrocarbons (PAHs) during municipal solid waste (MSW) and coal co-combustion. Science of the Total Environment, 565, 1201–1207. https://doi.org/10.1016/J.SCITOTENV.2016.05.188
Sarti, E., Pasti, L., Scaroni, I., Casali, P., Cavazzini, A., & Rossi, M. (2017). Determination of n-alkanes, PAHs and nitro-PAHs in PM2.5 and PM1 sampled in the surroundings of a municipal waste incinerator. Atmospheric Environment, 149, 12–23. https://doi.org/10.1016/J.ATMOSENV.2016.11.016
Shrestha, A. K., & Basnet, N. (2018). The correlation and regression analysis of physicochemical parameters of river water for the evaluation of percentage contribution to electrical conductivity. Journal of Chemistry. https://doi.org/10.1155/2018/8369613
Šorm, R., Bortone, G., Saltarelli, R., Jeníček, P., Wanner, J., & Tilche, A. (1996). Phosphate uptake under anoxic conditions and fixed-film nitrification in nutrient removal activated sludge system. Water Research, 30, 1573–1584. https://doi.org/10.1016/0043-1354(95)00331-2
Suman, S., Sinha, A., & Tarafdar, A. (2016). Polycyclic aromatic hydrocarbons (PAHs) concentration levels, pattern, source identification and soil toxicity assessment in urban traffic soil of Dhanbad, India. Science of the Total Environment, 545–546, 353–360. https://doi.org/10.1016/J.SCITOTENV.2015.12.061
Sun, Z., Liu, J., Zhuo, S., Chen, Y., Zhang, Y., Shen, H., Yun, X., Shen, G., Liu, W., Zeng, E. Y., & Tao, S. (2017). Occurrence and geographic distribution of polycyclic aromatic hydrocarbons in agricultural soils in eastern China. Environmental Science and Pollution Research, 24(13), 12168–12175. https://doi.org/10.1007/S11356-017-8838-3
Sun, R., Sun, Y., Li, Q. X., Zheng, X., Luo, X., & Mai, B. (2018). Polycyclic aromatic hydrocarbons in sediments and marine organisms: Implications of anthropogenic effects on the coastal environment. Science of the Total Environment, 640–641, 264–272. https://doi.org/10.1016/J.SCITOTENV.2018.05.320
Sun, Y., Zhang, R., Ma, R., Zhou, H., Zhang, F., Guo, G., Li, H., & Lü, C. (2021). Distribution, sources, and ecological risk assessment of polycyclic aromatic hydrocarbons in the sediments of Daihai Lake in Inner Mongolia, China. Environmental Science and Pollution Research, 28(18), 23123–23132. https://doi.org/10.1007/S11356-021-12349-3
Szewczyńska, M., Dąbrowska, J., & Pyrzyńska, K. (2017). Polycyclic aromatic hydrocarbons in the particles emitted from the diesel and gasoline engines. Polish Journal of Environmental Studies, 26, 801–807. https://doi.org/10.15244/PJOES/64914
Tongo, I., Ezemonye, L., & Akpeh, K. (2017). Levels, distribution and characterization of Polycyclic Aromatic Hydrocarbons (PAHs) in Ovia river, Southern Nigeria. Journal of Environmental Chemical Engineering, 5, 504–512. https://doi.org/10.1016/J.JECE.2016.12.035
Tu, Y. T., Ou, J. H., Tsang, D. C. W., Dong, C. D., Chen, C. W., & Kao, C. M. (2018). Source identification and ecological impact evaluation of PAHs in urban river sediments: A case study in Taiwan. Chemosphere, 194, 666–674. https://doi.org/10.1016/J.CHEMOSPHERE.2017.12.008
Ukalska-Jaruga, A., Smreczak, B., & Klimkowicz-Pawlas, A. (2019). Soil organic matter composition as a factor affecting the accumulation of polycyclic aromatic hydrocarbons. Journal of Soils and Sediments, 19, 1890–1900. https://doi.org/10.1007/S11368-018-2214-X/TABLES/5
Urakawa, H., Rajan, S., Feeney, M. E., Sobecky, P. A., & Mortazavi, B. (2019). Ecological response of nitrification to oil spills and its impact on the nitrogen cycle. Environmental Microbiology, 21, 18–33. https://doi.org/10.1111/1462-2920.14391
Wang, Y. B., Liu, C. W., Kao, Y. H., & Jang, C. S. (2015). Characterization and risk assessment of PAH-contaminated river sediment by using advanced multivariate methods. Science of The Total Environment, 524–525, 63–73. https://doi.org/10.1016/J.SCITOTENV.2015.04.019
Wu, Z., Lin, T., Hu, L., Guo, T., & Guo, Z. (2022). Polycyclic aromatic hydrocarbons in sediment–porewater system from the East China Sea: Occurrence, partitioning, and diffusion. Environmental Research, 209, 112755. https://doi.org/10.1016/J.ENVRES.2022.112755
Yao, X., Wang, K., Wang, Wan, Zhang, T., Wang, Wei, Yang, X., Qian, F., & Li, H. (2020). Reduction of polycyclic aromatic hydrocarbons (PAHs) emission from household coal combustion using ferroferric oxide as a coal burning additive. Chemosphere, 252, 126489. https://doi.org/10.1016/J.CHEMOSPHERE.2020.126489
Zhang, H., Walker, T. R., Davis, E., & Ma, G. (2019). Ecological risk assessment of metals in small craft harbour sediments in Nova Scotia, Canada. Marine Pollution Bulletin, 146, 466–475. https://doi.org/10.1016/J.MARPOLBUL.2019.06.068
Zhao, Z., Gong, X., Zhang, L., Jin, M., Cai, Y., & Wang, X. (2021). Riverine transport and water-sediment exchange of polycyclic aromatic hydrocarbons (PAHs) along the middle-lower Yangtze River, China. Journal of Hazardous Materials, 403, 123973. https://doi.org/10.1016/J.JHAZMAT.2020.123973
Acknowledgements
I wish to acknowledge my fellow technologists in the main campus Chemistry Laboratory of Federal University Dutsin-Ma, Katsina, Nigeria, and my research colleagues in the Analytical Chemistry Laboratory of Ahmadu Bello University Zaria, Nigeria for their immense contribution. I am also very grateful to Okenma Amayan a professional limnologist and botanist for her assistance in the field of sampling and to Uakheme Precious Areguamen for his assistance during the research work and writing of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study: OIA, NNC, CEG, and OJO were involved in the design of the manuscript, choice of methodology, and quality control of the analysis. OIA, ATA, and AE designed the map of the sampling area using GIS and participated in the field and laboratory work. OIA, NNC, CEG, OJO, ATA, and AE participated in the statistical, ecological, and human health risk analysis. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare. All co-authors have seen and agree with the contents of the manuscript and there is no financial interest to report. We certify that the submission is original work and is not under review at any other publication.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Areguamen, O.I., Calvin, N.N., Gimba, C.E. et al. Assessment of seasonal variation in distribution, source identification, and risk of polycyclic aromatic hydrocarbon (PAH)–contaminated sediment of Ikpoba River, South-South Nigeria. Environ Monit Assess 195, 302 (2023). https://doi.org/10.1007/s10661-023-10927-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-10927-1