Abstract
The coastal lagoons of the Gulf of California support important traditional fisheries and mollusc cultures (generally oysters) and receive important volumes of agricultural, industrial and urban effluents, consumption of the oysters could pose risk to human health. The concentrations of arsenic (As), cadmium (Cd), copper (Cu), iron (Fe), lead (Pb), and zinc (Zn) in the oysters Saccostrea palmula and Crassostrea corteziensis, from four coastal lagoons (Altata, AL; Macapule, ML; Navachiste, NL; El Colorado, ECL) in the Southeast Gulf of California, were seasonally evaluated (summer 2019–spring 2020). The order of magnitude of potentially toxic elements concentrations in the soft tissue in both oyster species and at all sites was Zn > Fe > Cu > As > Cd > Pb. Cadmium, Cu, Pb, and Zn exceeded the maximum permissible limits in more than one sampling site. The highest concentrations (mg kg–1, wet weight) of As (4.2 ± 1.1, spring) and Cd (3.3 ± 0.7, autumn) were registered in S. palmula et al. and NL sampling sites, respectively. Crassostrea corteziensis presented higher levels of Cu (40.5 ± 6.7, spring), Pb (2.0 ± 0.4, spring), and Zn (96.9 ± 20.4, spring) in ECL and Fe (62.2 ± 25.4, autumn) in ML. The hazard quotient (HQ) values exceeded the safe level of 1 for Cd in S. palmula and C. corteziensis in NL for children (~ 16 kg weight). In addition, in children, the hazard index (HI) values in both species of oysters ranged from 0.7 to 2.1 and 0.6 to 1.9, respectively. On the other hand, the intake of the studied elements through the consumption of oysters would not induce adverse effects to human health (men and women weighing 70 and 60 kg, respectively); HQ and HI values were < 1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Potentially toxic elements (PTEs), as well as microplastics and agrochemicals, constitute the groups of anthropogenic pollutants with the greatest impact on environment and human health (Alamri et al., 2021; García-Hernández et al., 2021; Manisalidis et al., 2020). Most of the living organisms need small amounts of essential metals, such as copper (Cu), iron (Fe), and zinc (Zn), to perform their metabolic, biochemical, and physiological functions; however, when a certain concentration level is exceeded, the essential metals become toxic (Singh et al., 2011).
In the last decades, several coastal lagoons of Gulf of California have become a matter of growing environmental concern for Mexico, due to increased PTEs contamination and possible risks to fish and seafood consumers (Páez-Osuna et al., 2017). Arsenic (As), cadmium (Cd), Cu, Fe, lead (Pb) and Zn, accumulate in the tissues of aquatic organisms at concentrations higher than in water, may be biomagnified in the food chain and cause physiological impairment at higher trophic levels and in human consumers (Sujitha et al., 2019). Due to natural processes such as geological weathering, PTEs reach the environment at low concentrations (Henry et al., 2003; Murray & Busty, 2015); however, anthropogenic sources like mining, manufacturing, urbanization, aquaculture, and agriculture, among others, have contributed to increase their levels in sediments, water, and tissues of aquatic organisms (Jara-Marini et al., 2020).
Specifically, bivalve mollusks accumulate PTEs from food, water, and sediments to concentrations that may exceed those of their environment (Chan et al., 2021; Jonathan et al., 2017; Sepúlveda et al., 2020). Due to their characteristics including filter feeding, cosmopolitan distribution, sessile/sedentary life, abundance, longevity, year–round availability, easy sampling, and identification (Zhou et al., 2008), bivalves have been widely used as biomonitors. In this way, some mussel and oyster species have been successfully used worldwide for several decades in environmental monitoring programs (Otchere, 2019; Yap et al., 2021; Zuykov et al., 2013). Related studies indicate the PTEs high persistence in low trophic levels organisms (mussels, clams, and oysters) along the Southeast (SE) coast of the Gulf of California, which are commonly consumed raw, representing a human health risk (Frías-Espericueta et al., 2008; Ruelas-Inzunza & Páez-Osuna, 2008).
The oysters from present study, Saccostrea palmula (Carpenter, 1857) and Crassostrea corteziensis (Hertlein, 1951), are distributed along the Pacific coast from Mexico to Peru; they are native to the Northwest of Mexico (Lodeiros et al., 2020). Additionaly, oysters are widely utilized for human consumption and has important commercial value. At the same time, these oysters are used as a biomonitors of PTEs in coastal lagoons in the SE Gulf of California (Osuna-Martínez et al., 2011; Ruiz-Fernández et al., 2018); however, results vary with time, location, and species of oyster, making necessary to monitor its PTEs levels continuously. Therefore, the aim of the present study was to analyze the content of As, Cd, Cu, Fe, Pb, and Zn in the oysters S. palmula and C. corteziensis from four coastal lagoons of SE Gulf of California, and to assess the human health risk from its consumption.
Materials and methods
Study area
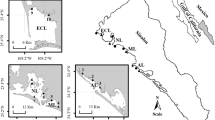
The present study was performed in ten sampling sites of four coastal lagoons of the SE Gulf of California (Mexico): Altata (AL, three sites: AL1, AL2, and AL3), Macapule (ML, two sites: ML4 and ML5), Navachiste (NL, three sites: NL6, NL7, and NL8), and El Colorado (ECL, two sites: ECL9 and ECL10) (Fig. 1). The specimens of the oysters S. palmula and C. corteziensis (Fig. 1S) did not coexist at the sampling sites; therefore, S. palmula (72.1 ± 2.7 mm average size, n = 2520) and C. corteziensis (73.8 ± 4.4 mm average size, n = 3780) were hand–collected from the mangrove roots at six and nine sampling sites, respectively. Samplings were carried out at the end of each season (summer–autumn 2019 to winter–spring 2020).
The geographical coordinates of each sampling site were recorded using a handheld global positioning system (Magellan Garmin Explorist 310). In addition, water physicochemical parameters were obtained: temperature (°C) and dissolved oxygen (DO) were measured using an oxymeter (YSI, 55/12 FT, Yellow Springs, OH, USA); for salinity, a precision refractometer (Amago, S / Mill) was used, whereas the pH was measured with a potentiometer (Hanna Instruments, Woonsocket, RI, USA) (Rodríguez-Quiroz et al., 2016). A PVC tube (5 cm diameter and 2 L capacity) fitted with an end plunger seal was used to sample the water column to determine the organic matter (OM), inorganic matter (IM), and chlorophyll a (Cl–a). The amounts of OM and MI were defined with the gravimetric method described by APHA (1995), which consists of the weight increase experienced by a Whatman GF/F filter (0.7 μm pore size) after vacuum filtration of a sample that is subsequently dried at different temperatures. The quantification of Cl–a was carried out with the spectrophotometric technique described by Strickland and Parsons (1972).
The lagoons are surrounded by four mangrove species (unevenly distributed): red mangrove (Rhizophorae mangle), buttonwood mangrove (Conocarpus erectus), white mangrove (Laguncularia racemosa), and black mangrove (Avicennia germinans), and keep a permanent connection to the Gulf of California through one or two mouths that create a marine environment most of the year (Páez-Osuna & Osuna-Martínez, 2015). The climate in these regions is warm–semidry, with an annual mean temperature of 24 °C and precipitations between 300 and 600 mm per year, with rains during the summer (Muñoz-Sevilla et al., 2017).
The AL (8800 ha) receives directly the discharges of the untreated sewage system from Culiacán and Navolato Municipalities (905,265 and 154,352 inhabitants, respectively) and the highly technified agriculture waste from the Culiacán valley (116,409 ha). There are other human activities, such as rapidly developing of tourism, traditional fishing, semi–intensive shrimp farms (17,511 ha), and broiler chicken production (57,624,911 chicken/year) (Frías-Espericueta et al., 2008; Sepúlveda et al., 2020). The ML and NL (3800 and 14,000 ha, respectively) are protected natural areas (RAMSAR sites) of the Gulf of California. However, they are strongly impacted by the effluents from shrimp farms (18,735 ha), wastewater from Guasave’s city (295,353 inhabitants), and agricultural waters (119,994 ha). Other relevant human activities are traditional and industrial fishing and broiler chicken production (77,785 chicken/year) (Jonathan et al., 2017). The ECL (11,500 ha) is mainly impacted by a highly technical agricultural area (189,064 ha), municipal waste (449,215 inhabitants), traditional fishing, shrimp farms (12,639 ha), and the pork industry (94,422 pig/year) (INEGI, 2015; Páez-Osuna & Osuna-Martínez, 2015; CESASIN, 2019; SIAP, 2019).
Sampling and cleaning of oysters
Seasonal samplings for each site consist of 105 wild oysters (75 for PTEs analysis and 30 for condition index, CI), with a size range of 60 to 90 mm to avoid the variability in the metallic contents due to size differences. However, the variation of the soft tissue weight of the oysters could not be limited, due to the presence of different gonadal phases at each season (Góngora-Gómez et al., 2020). After sampling, oysters were placed in polythene bags and transported in ice to the laboratory. Then, they were cleaned with a plastic bristle brush and a stainless steel knife with tap water, to remove excess sediment, sticky shells, epifauna, and mangrove debris (Frías-Espericueta et al., 2018). Once cleaned, the oysters were measured (mm) and CI was determined using Eq. (1) proposed by Walne and Mann (1975):
Analytical work
Sample processing was performed according to MESL (1997). The oyster tissue samples were freeze–dried (Labconco, Kansas City, MO, USA) at low temperature (− 85 °C) and high vacuum (0.035 mBar), ground in a Teflon mortar, homogenized by quartering, and the water content (WC, average of 81.7 ± 2.1%) was determined. The wet and dry weights of the samples were recorded using a digital analytical balance (Adam Equipment, Milton Keynes, UK). Portions of ~ 1.0 ± 0.1 g were pre–digested overnight at room temperature with 10 mL of HNO3 (trace metal grade), in PTFE vessels (Savillex, 60 mL capacity). Digestion was performed on a heating plate (Barnstead) for 4 h at 120 °C; subsequently, each sample was diluted with deionized water to a final volume of 50 mL.
Potentially toxic elements analysis and quality control
The concentrations of Cd, Cu, Fe, Pb, and Zn were analyzed using flame atomic absorption spectrophotometry (Perkin–Elmer, Inc., Waltham, MA, USA, PinAAcle 900 T); As was assessed using hydride generation (Perkin–Elmer, Inc., Analyst 100). The samples were analyzed in duplicate, the accuracy of the analytical method was evaluated with the standard reference material DOLT–5® (National Research Council Canada). Additionally, one blank and standard reference material was included per each set of 10 samples. Mean recoveries values were: As = 106.3 ± 3.9%, Cd = 93.6 ± 2.7%, Cu = 93.9 ± 3.1%, Fe = 91.2 ± 1.6%, Pb = 95.9 ± 6.4%, and Zn = 93.9 ± 3.3%. The variation coefficient was below 7%. The detection limits were 0.02, 0.11, 0.32, 0.45, 0.19, and 0.11 mg kg–1 for As, Cd, Cu, Fe, Pb, and Zn, respectively (Table 1S). Blanks were used to check contamination and all materials were acid–washed (Moody & Lindstrom, 1977). The final concentrations are expressed as mg kg–1 on a wet weight basis (w/w).
Human health risk assessment
The hazard quotient (HQ) was used to determine the risk to human health; it was calculated with Eq. (2) proposed by Newman and Unger (2002):
where C = mean PTEs concentration (mg kg–1) in oyster samples on a w/w; I = weekly mean consumption of oysters (0.4 kg year–1 = 7.5 g week–1) (CONAPESCA, 2017); and BW = mean body weight in the general population or subpopulation (70 kg for men, 60 kg for women, and 16 kg for five year–old children). The oral reference dose (RfD) values were as follows (mg kg–1 body weight day–1): As (0.0003), Cd (0.001), Zn (0.3) (integrated risk information system; EPA, 2020), Cu (0.04) (risk–based concentration table; EPA, 2000), Fe (0.7) (provisional peer–reviewed toxicity values; EPA, 2006), and Pb (0.0125) (interim reference level; FDA, 2019). Because of the lack of information for total As, the RfD data of inorganic arsenic (iAs) was used (considering 1% as iAs in oysters, Bergés-Tiznado et al., 2013). Values of HQ ≤ 1 suggest that adverse health effects are unlikely.
Cancer risk (CR) is the probability of an individual developing any type of cancer (internal organs and skin) (EPA, 2013). CR was calculated only for As, using the following Eq. (3):
where CDI is the chronic daily intake averaged over the 70 years in mg kg–1 body weight day–1, and the slope factor (SF) for As is 1.5 mg kg–1 body weight day–1 (EPA, 2017).
The hazard index (HI) was used to assess the overall potential non–carcinogenic health risk posed by more than one PTEs. For this study, the HI was calculated as the sum of the HQ for each studied PTEs (HI < 1 indicated no risks to human health) (Newman & Unger, 2002).
Statistical analysis
The mean and standard deviation (SD) of data sets were estimated by sampling site and season (mean ± SD). All data sets passed the assumptions of normality (Kolmogorov–Smirnov) and homoscedasticity (Bartlett). Comparisons of the biometric data of oysters and average concentrations of PTEs among the lagoons, sampling sites, and seasons were performed using multiple comparison of means (ANOVA). The Pearson's rank (rp) order correlations were applied to test the correspondence of the water parameters (temperature, DO, salinity, pH, OM, IM, and Cl–a), oyster sizes, CI, and the As, Cd, Cu, Fe, Pb and Zn levels in oysters from the four coastal lagoons. Finally, the significant differences of PTEs in S. palmula and C. corteziensis were detected with the Student's t test for independent variables. The significance level was α = 0.05 for all statistical analyses (Zar, 2010), which were performed using the STATISTICA 7 software package (StatSoft, Tulsa, OK, USA).
Results and discussion
The lowest temperature, DO, salinity, pH, OM, IM, and Cl–a values were found in ECL10 (20.8 °C, autumn), ML5 (4.2 mg L–1, spring), ML4 (15.0 ‰, winter), AL3 (7.1, summer), ML4 (5.0 mg L–1, autumn), NL8 (13.3 mg L–1, autumn), and AL2 (1.6 mg m–3, autumn), respectively; and the highest values in ECL10 (32.9 °C, summer), NL6 (8.7 mg L–1, winter), ECL10 (43.0 ‰, spring), NL6 (8.0, spring), AL2 (16.2 mg L–1, autumn), ECL10 (58.7 mg L–1, summer), and NL6 (9.3 mg m–3, winter), respectively. With the exception of OM and IM, the rest of the physicochemical parameters are within the intervals previously reported in the four sampled coastal lagoons (Góngora-Gómez et al., 2020; Rodríguez-Quiroz et al., 2016). The differences found in OM and IM could be explained by the residence time of the water and the intensity of the tides (Takasu et al., 2020), concentration of particulates (Middelburg & Herman, 2007), use of molecules in the first trophic levels (Hope et al., 2020), and contributions of anthropogenic activities (Canuel & Hardison, 2016), among other factors. The seasonal mean values of the physicochemical parameters obtained in all lagoons (Table 1) were within the optimal range for oyster growth (Chávez-Villalba, 2014; Mazón-Suástegui, 1996).
Size, CI, and WC of oysters are shown in Table 2. The lowest size values of S. palmula (66.7 ± 4.8 mm, summer) and C. corteziensis (68.1 ± 3.4 mm, summer) corresponded to AL1 and NL8 and the highest values to ECL9 (77.1 ± 3.7 mm, winter) and AL2 (78.3 ± 6.1 mm, winter). The annual average size of S. palmula (72.1 ± 2.7 mm) and C. corteziensis (73.8 ± 4.4 mm) did not show significant differences (p ˃ 0.05) among lagoons because they were selected so that size would not be a limiting factor as a source of variation (Sepúlveda et al., 2020). The average CI in S. palmula (63.1 ± 16.7) and C. corteziensis (64.4 ± 17.1) exhibited the highest peak in winter for all sampling sites, suggesting a reproductive event associated with food availability, the onset of gonadal maturation (autumn), and advanced maturation (winter) (Góngora-Gómez et al., 2020). While the lowest CI was obtained in spring (p < 0.05), when spawning began (Góngora-Gómez et al., 2020) and, therefore, the weight of their tissues was reduced. The CI can be used to assess the physiological activity of bivalves (reproduction, growth, etc.) and to establish the quality of the product for commercial purposes (Lucas & Beninger, 1985). The oysters S. palmula (75.8 ± 19.9) and C. corteziensis (73.0 ± 14.1) presented the highest CI values in NL8 and ML4, respectively, indicating a better health status (Walne & Mann, 1975). The obtained CI were higher than those documented by Góngora-Gómez et al. (2020) for C. corteziensis in the same lagoons, which could be partially explained by the amount and availability of food (Cl–a, OM, IM) given by the prevailing environmental conditions at different times (Muñoz-Sevilla et al., 2017; Rodríguez-Quiroz et al., 2016).
The PTEs concentrations in oysters varied seasonally and among the sampling sites. It is documented that variations in the concentration of the PTEs are related to factors such as the seasonal cycle of oyster reproduction and growth of the others, as well as the patterns of mean annual temperature (García-Rico et al., 2010; Rebelo et al., 2003). For instance, Zn showed the highest seasonal variation, with intervals of 21.35–134.21 and 23.16–122.19 μg g−1 in S. palmula and C. corteziensis, respectively. The lowest Zn levels were recorded in summer 2019 and increased in spring 2020 in both oyster species; subsequently, the level of this metal increased in autumn 2019, and its maximum level was reached in winter 2020 (p < 0.05). Similar variations were observed for the rest of the PTEs studies (Table 3; Fig. 2) coinciding with reports in oysters from other countries (Rajeshkumar et al., 2018; Rebelo et al., 2003) and from the same coastal lagoons (Góngora-Gómez et al., 2017). In fact, Góngora-Gómez et al. (2018) observed similar variation patterns of As, Cu, and Pb in the pen shell Atrina maura from ML. All these authors explained such PTEs variations not only to the reproductive cycle of bivalves, but also, to higher influx of agricultural waste, sewage and sludge by heavy rainfall and floods.
Mean annual concentrations of potentially toxic elements (mg kg–1 w/w) from the oysters S. palmula (white bars) and C. corteziensis (gray bars) collected in the four coastal lagoons (AL Altata lagoon; ML Macapule lagoon; NL Navachiste lagoon; ECL El Colorado lagoon) of the Southeast Gulf of California (Mexico). A arsenic (As); B cadmium (Cd); C copper (Cu); D iron (Fe); E lead (Pb); F zinc (Zn). The dotted lines indicate the maximum permissible limits. Columns with different superscript letters denote significant differences (p < 0.05) between sampling site and oyster species
The order of magnitude of the PTEs concentrations in both oysters was Zn > Fe > Cu > As > Cd > Pb (Table 3; Fig. 2). Similar results were documented by Páez-Osuna and Osuna-Martínez (2015) (Zn > Cu > Cd > Pb) and Frías-Espericueta et al. (2009) (Zn > Cu > Pb > Cd) for the same oyster species in the area, and Góngora-Gómez et al. (2017) (Zn > Cu > Cd > Pb > As) for C. gigas cultivated in ML. Since Zn, Fe, and Cu are essential elements to perform metabolic, biochemical, and physiological functions (Singh et al., 2011), they are usually found in high concentrations in bivalves tissues than other PTEs; however, at high concentrations, they can be harmful to the health of the oyster and the consumers (Chan et al., 2021). The influence of abiotic and biotic factors, such as species, genetics, size, age, reproductive cycle, locality, date of sampling, and sources of PTEs (natural and anthropogenic), among others (García-Rico et al., 2010; Kato et al., 2020), could explain the results.
Research regarding PTEs in oysters S. palmula and C. corteziensis has focused on Cd, Cu, Pb, and Zn, because these elements frequently exceed the regulations, however, other metals have acquired relevance due to their anthropogenic contribution in the area. For example, As found in oysters could be explained by the emissions and drainage water from mining waste, groundwater, agricultural, poultry, and pig wastes from the rivers to the estuaries (García-Rico et al., 2019). In Mexico, the metalloid (e.g., roxarsone: C6AsNH6O6, parsanilic acid: C6H8AsNO3, and derivatives) is used as feed additives for poultry and swine to increase the rate of weight gain and to treat and prevent diseases (Osuna-Martínez et al., 2021). However, Bergés-Tiznado et al. (2013) documented that the greatest contribution of As to the Sinaloa lagoons is due to runoff from rivers and atmospheric deposition.
Although the concentration of Cd found in the Gulf of California has been attributed to the presence of natural phosphorite deposits (Méndez et al., 2006), this metal is also transported from the agricultural and urban drainage basin located in the surroundings of the lagoons (Frías-Espericueta et al., 2009; Jonathan et al., 2017). Góngora-Gómez et al., (2017, 2018) concluded that the presence of this element in farmed C. gigas and wild pen shell, A. maura, is related to the contributions of intense anthropogenic activities in the region (wastewater, agrochemicals used for agricultural practices, and shrimp farming), which increase in the rainy season.
The concentrations of Cu and Zn found in S. palmula and C. corteziensis may be explained by the intense agricultural and aquaculture activities that take place in Northwest Mexico, whose productions has increased over time (Jara-Marini et al., 2020). Each year, this region demands high amounts of Cu and Zn–based chemicals (agrochemicals, food additives, antibiotics, and antifouling paints) for the production of crops and maintenance of aquaculture farms (Frías-Espericueta et al., 2009; Osuna-Martínez et al., 2011).
On other hand, Pb is associated with the large influx of aquaculture farms in the region, as well as the intense transport of vessels used in local tourism, and artisanal and commercial fishing, due to vessel engine emissions (Luoma & Rainbow, 2005). Probably, the most important Pb source is caused by fuels (gasoline) that were used until the 2000’s, whose residues are still considered as an important risk factor for both human health and the environment (Frías-Espericueta et al., 2010; Páez-Osuna et al., 2002). Lead concentrations may also be associated with its release from sediments where it accumulated constantly in previous years (Zhong et al., 2021).
Some of the PTEs levels exceeded the national and international maximum permissible limits (MPL; expressed in mg kg–1 w/w). For example, the highest levels of Cd (2 mg kg–1; NOM, 2009), Cu (30 mg kg–1; MFR, 1985), Pb (1 mg kg–1; NOM, 2009), and Zn (100 mg kg–1; WHO, 1982) in both species of oysters exceeded the permissible limit in at least one of the lagoons. With the exception of As (p < 0.05), the metal concentrations in S. palmula and C. corteziensis varied among the coastal lagoons. The As and Zn average levels were below permisible limits (80 and 100 mg kg–1; NOM, 2009 and WHO, 1982, respectively), but Zn variation (SD) in S. palmula exceeded the limit at AL2, NL8 and ECL9, and C. corteziensis at ECL9 (Fig. 2). The mean Pb values for most coastal lagoons were below the international MPL (1.7 mg kg–1; FDA, 2003), but exceeded the MPL reported for Mexico (1 mg kg–1; NOM, 2009). The average levels of Cu did not exceed the MPL of Malaysia (30 mg kg–1; MFR, 1985) in most sites, but they were very close or exceeded the established MPL of WHO (10 mg kg–1; 1982) (Fig. 2). For Fe, there are not established MPL yet.
When the concentration of PTEs exceeds the MPL of sanitary standards, consumption must be restricted, or the oysters must be depurated (Wang & Wang, 2014). Although its contribution is limited, for greater security, depuration of this bivalve species in the region would represent a mandatory practice before being offered to the consumers.
In general, Cd, Cu, Fe, Pb, and Zn levels found in S. palmula and C. corteziensis (sampled from 2019 to 2020) were comparable with previously recorded levels. However, it can be observed that the concentration of some PTEs in these oysters from the coastal lagoons of Sinaloa has increased (Table 4). For instance, the annual average concentration of As in S. palmula (3.7 ± 0.9 mg kg–1) and C. corteziensis (3.7 ± 0.8 mg kg–1) found in this study is higher than that reported in 2008–2009 for wild populations of C. corteziensis from seven coastal lagoons of Sinaloa (1.5 ± 0.3 mg kg–1) and Nayarit (0.8 ± 0.1 mg kg–1) (Bergés-Tiznado et al., 2013). The concentration of Cd in C. corteziensis from AL has increased from 0.70 mg kg–1 recorded between 1988 and 1991 (Páez-Osuna et al., 1993) to 1.3 mg kg–1 nine years later (Ruelas-Inzunza & Páez-Osuna, 2008). Góngora-Gómez et al. (2017) reported Cd levels (2.4 ± 0.7 mg kg–1) in C. gigas cultivated in LM, being higher than the level obtained in the present study for S. palmula and C. corteziensis (1.3 ± 0.2 and 1.0 ± 0.2 mg kg–1, respectively). The mean concentrations of Cu in the cultured oyster, C. gigas, during the last decade in the ML has been: 9.2 ± 4.6 mg kg–1 in 2011 (Góngora-Gómez et al., 2017), 11.4 ± 5.7 mg kg–1 in 2012 (Jonathan et al., 2017), and 18.1 ± 4.8 mg kg–1 in 2014 (Muñoz-Sevilla et al., 2017), which indicates a Cu increase to the habitat of this bivalve species.
In contrast, the Zn concentration found in this study is lower than previous reports. For example, Zn level in S. palmula and C. corteziensis from AL was 130.8 and 169.7 mg kg–1, respectively (Páez-Osuna et al., 1993), which, later, decreased to 72.8 and 60.2 mg kg–1, for both species in this study. However, comparisons should be made with caution due to the complexity and variation of the samplings (the influence of size, reproductive stage and physiological condition of oysters; Burioli et al., 2017).
Although both oyster species coexist in the same roots of mangroves from the sampled lagoons, S. palmula bioaccumulated higher concentrations of Cd (t = 3.05, p = 0.02 in ML5), Cu (t = 4.41, p = 0.00 and t = 2.81, p = 0.03 in NL6 and NL8, respectively), and Zn (t = 2.96, p = 0.02 in NL6) than C. corteziensis, but less Fe (t = − 4.16, p = 0.00, t = − 2.61, p = 0.04 and t = − 2.58, p = 0.04 in AL2, NL6, and ECL10, respectively) (Table 2S). The levels of As and Pb in both species did not present significant differences (p > 0.05) (Table 2S). The results demonstrated that each oyster species have the ability to accumulate significantly different concentrations of the same PTEs due to internal and external factors (Suami et al., 2019). Páez-Osuna et al. (1991) documented higher Fe bioaccumulation in C. corteziensis compared to S. palmula, associated to its physiological condition, meanwhile Páez-Osuna and Osuna-Martínez (2015) reported that the soft tissue of S. palmula contained concentrations of metals consistently higher than those of C. corteziensis, but the correlation analyses did not show significant differences (p < 0.05).
Pearson's rank correlations for PTEs, water parameters, size, and CI showed significant correlations among them (Table 5). The highest positive (rp = 0.99, p = 0.00) and negative (rp = − 0.95, p = 0.01) correlations were obtained in C. corteziensis for Cu/Zn and Zn/temperature et al., meanwhile, the lowest correlations (rp = 0.71, p = 0.04 in AL and rp = − 0.75, p = 0.03 in NL) were observed in the same oyster species for Cd/Cu and Zn/IM, respectively. With exception of the oysters from NL, Cd, Cu, and Zn exhibited correlation among them (rp = 0.71, p = 0.04 and rp = 0.98, p = 0.01). There are reports that indicate a consistent association between PTEs groups in bivalves established by a common source (Góngora-Gómez et al., 2018). In the present work, significant correlations were found between Cd, Cu, and Zn, which may be due to the fact that these metals are commonly used in anthropogenic activities (Muñoz-Sevilla et al., 2017). Di-Marzio et al. (2019) listed the main anthropogenic sources of PTEs in Latin America. These authors mentioned that Cu is used in the manufacturing, electrical, electronic, and chemical industries (Rodríguez-Heredia, 2017), whereas Cd is incorporated in the coating of metallic objects to protect them from corrosion in the industry of rechargeable batteries, wiring, and PVC pipes (Caviedes-Rubio et al., 2015). Zinc is used in the manufacture of metals and tires (Di-Marzio et al., 2019). Specifically, in Northern Sinaloa, all these PTEs are used in the manufacture of industrial agrochemicals (mainly phosphorous–based) (Raven & Loeppert, 1997; Otero et al., 2005; Sabiha-Javied et al., 2009; Rodríguez-Ortíz et al., 2014), which could be incorporated to urban waste or wastewater that are discharged into the coastal lagoons without prior treatment (Frías-Espericueta et al., 2010).
Table 3S shows the annual mean concentrations of PTEs in oysters from the four coastal lagoons at SE Gulf of California used in the risk assessment calculations. The HQ, HI, and CR for As, Cd, Cu, Fe, Pb, and Zn in both oyster species are displayed in Table 6. The HQ values exceeded the safe level of 1 for Cd in S. palmula and C. corteziensis in NL6 for the 16 kg children. In addition, in children, the HI values in both species of oysters ranged from 0.7 to 2.1 and 0.6 to 1.9, respectively. On the other hand, the intake of the studied elements through the consumption of oysters would not induce adverse effects to human health (men and women weighing 70 and 60 kg, respectively); HQ and HI values were < 1. In Mexico, oyster consupmtion is low (0.4 kg year–1 = 7.5 g week–1; CONAPESCA, 2017) (these value needs to be re–evaluated); however, regional consumption at coastal communities may be high due to the frequent seafood consumption (Frías-Espericueta et al., 2018; Ruiz-Fernández et al., 2018).
The HI values in S. palmula and C. corteziensis ranged from 0.1 to 2.1 and 0.1 to 1.9, respectively. In particular, Cd in S. palmula (57.6%) and C. corteziensis (53.1%) was the metal that contributed the most to the calculated HI value, followed by Cu (14.9%), Pb (13.8%), Zn (8.1%), As (4.2%), and Fe (1.2%) for S. palmula, and Pb (17.3%), Cu (13.8%), Zn (7.7%), As (5.3%), and Fe (2.5%) for C. corteziensis.
Only iAs has been proven as human carcinogen. The CR ranged from 5.0 × 10–6 to 2.9 × 10–5 and 5.4 × 10–6 to 2.7 × 10–5 in S. palmula and C. corteziensis, respectively, and do not present health risks (EPA, 2013). For regulatory purposes, EPA (2013) considers that a CR less than 10–6 is unlikely to trigger health problems; values between 10–6 and 10–4 are considered within an acceptable range or tolerable risk, whereas values above 10–4 are high enough to induce carcinogenic effects. The CR values in this study were between 10–6 and 10–5, which suggests that the intake of As would not induce carcinogenic effects due to the consumption of sampled oysters. However, it is important to take into account that the CR of As can increase through the consumption of other food items such as fish, rice, and red meat (Gundert-Remy et al., 2015).
Conclusions
All the studied PTEs were found in S. palmula and C. corteziensis from the four coastal lagoons sampled in Sinaloa, Mexico, during one year; some of them, exceeding the MPL of national and international regulations. Levels of Cd, Cu, Pb, and Zn exceeded the MPL in more than one sampling site, which deserves special attention. The aforementioned confirms the strong influence of intense anthropogenic activity in the region that impacts these oysters over time. More studies determining the concentration of PTEs in S. palmula and C. corteziensis in this area must be carried out, periodically. Depuration strategies to clean oysters before consumption, should be also implemented to reduce PTEs loads. Since oysters are a popular dietary item in the SE Gulf of California, which is preferably consumed raw, it is important to establish a continuous PTEs monitoring program to determine their possible risk to human health.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Alamri, D. A., Al-Solaimani, S. G., Abohassan, R. A., Rinklebe, J., & Shaheen, S. M. (2021). Assessment of water contamination by potentially toxic elements in mangrove lagoons of the Red Sea, Saudi Arabia. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-021-00956-5
APHA (American Public Health Association). (1995). Standard methods for the examination of water and wastewater. American Public Health Association. Washington, D.C.
Bergés-Tiznado, M. E., Páez-Osuna, F., Notti, A., & Regoli, F. (2013). Biomonitoring of arsenic through mangrove oyster (Crassostrea corteziensis Hertlein, 1951) from coastal lagoons (SE Gulf of California): Occurrence of arsenobetaine and other arseno-compounds. Environmental Monitoring and Assessment, 185, 7459–7468. https://doi.org/10.1007/s10661-013-3112-8
Burioli, E. A. V., Squadrone, S., Stella, C., Foglini, C., Abete, M. C., & Prearo, M. (2017). Trace element occurrence in the Pacific oyster Crassostrea gigas from coastal marine ecosystems in Italy. Chemosphere, 187, 248–260. https://doi.org/10.1016/j.chemosphere.2017.08.102
Canuel, E. A., & Hardison, A. K. (2016). Sources, ages, and alteration of organic matter in estuaries. Annual Review of Marine Science, 8, 409–434. https://doi.org/10.1146/annurev-marine-122414-034058
Caviedes-Rubio, D. I., Muñoz-Calderón, R. A., Perdomo-Gualtero, A., Rodríguez-Acosta, D., & Sandoval-Rojas, I. J. (2015). Tratamientos para la remoción de metales pesados comúnmente presentes en aguas residuales industriales Una Revisión. Ingeniería y Región, 13(1), 73–90.
CESASIN (Comité Estatal de Sanidad Acuícola de Sinaloa). (2019). Programa de sanidad en crustáceos. https://cesasin.mx/programacrustaceos/. Accessed 6 June 2020.
Chan, W. S., Routh, J., Luo, C., Dario, M., Miao, Y., Luo, D., & Wei, L. (2021). Metal accumulations in aquatic organisms and health risks in an acid mine-affected site in South China. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-021-00923-0
Chávez-Villalba, J. (2014). Culture of the oyster Crassostrea gigas: Analysis of 40 years of activities in Mexico. Hidrobiológica, 24(3), 175–190.
CONAPESCA (Comisión Nacional de Acuacultura y Pesca). (2017). Anuario estadístico de acuacultura y pesca. http://www.Anuario-Estadistico-de-Acuacultura-y-Pesca-2013. Accessed 6 June 2020.
Di-Marzio, A., Lambertucci, S. A., Garcia-Fernandez, A. J., & Martínez-López, E. (2019). From Mexico to the Beagle Channel: a review of metal and metalloid pollution studies on wildlife species in Latin America. Environmental Research, 176, 108462. https://doi.org/10.1016/j.envres.2019.04.029
EPA (United States Environmental Protection Agency). (2000). Risk-based concentration table. EPA, Washington, DC. https://www.epa.gov/risk/risk-assessment-guidance. Accessed 12 January 2021.
EPA (United States Environmental Protection Agency), 2006. Provisional Peer-Reviewed Toxicity Values for Iron and Compounds. EPA, Washington, DC. https://cfpub.epa.gov/ncea/pprtv/recordisplay.cfm?deid=338968. Accessed 15 December 2020.
EPA (United States Environmental Protection Agency), 2013. Regional screening levels (RSL) for chemical contaminants at superfund sites. EPA, Washington, DC. https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables. Accessed 20 April 2020.
EPA (United States Environmental Protection Agency). (2017). Arsenic, inorganic (CASRN 7440–38–2). Integrated Risk Information System. https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?&substance_nmbr=278. Accessed 22 May 2020.
EPA (United States Environmental Protection Agency). (2020). Basic Information about the Integrated Risk Information System. https://www.epa.gov/iris/basicinformation-about-integrated-risk-information-system. Accessed 20 November 2020.
FDA (United States Food and Drug Administration). (2003). National Shellfish Sanitation Program (NSSP). https://www.fda.gov/food/federalstate-food-programs/national-shellfish-sanitation-program-nssp. Accessed 27 April 2021.
FDA (United States Food and Drug Administration). (2019). Lead in Food, Foodwares, and Dietary Supplements. https://www.fda.gov/food/conversations-experts-food-topics/what-fda-doing-protect-consumers-toxic-metals-foods. Accessed 20 November 2020.
Frías-Espericueta, M. G., Osuna-López, J. I., Voltolina, D., López-López, G., Izaguirre-Fierro, G., & Muy-Rangel, M. D. (2008). The metal content of bivalve mollusks of a coastal lagoon of NW Mexico. Bulletin of Environmental Contamination and Toxicology, 80, 90–92. https://doi.org/10.1007/s00128-007-9322-4
Frías-Espericueta, M. G., Osuna-López, J. I., Bañuelos-Vargas, I., López-López, G., Muy-Rangel, M. D., Izaguirre-Fierro, G., et al. (2009). Cadmium, copper, lead and zinc contents of the mangrove oyster, Crassostrea corteziensis, of seven coastal lagoons of NW Mexico. Bulletin of Environmental Contamination and Toxicology, 83, 595–599. https://doi.org/10.1007/s00128-009-9828-z
Frías-Espericueta, M. G., Osuna-López, J. I., Izaguirre-Fierro, G., Aguilar-Juárez, M., & Voltolina, D. (2010). Cadmio y plomo en organismos de importancia comercial de la zona costera de Sinaloa, México: 20 años de estudios. CICIMAR Oceánides, 25(2), 121–134.
Frías-Espericueta, M. G., Vargas-Jiménez, A., Ruelas-Inzunza, J., Osuna-López, I., Aguilar-Juárez, M., Bautista-Covarrubias, J. C., & Voltolina, D. (2018). Total mercury in the mangrove oyster Crassostrea corteziensis of the subtropical Urías lagoon (NW Mexico). Turkish Journal of Fisheries and Aquatic Sciences, 18, 853–858. https://doi.org/10.4194/1303-2712-v18_7_03
García-Hernández, J., Leyva-Morales, J. B., Bastidas-Bastidas, P., Leyva-García, G. N., Valdez-Torres, J. B., Aguilar-Zarate, G., & Betancourt-Lozano, M. (2021). A comparison of pesticide residues in soils from two highly technified agricultural valleys in northwestern Mexico. Journal of Environmental Science and Health, Part B. https://doi.org/10.1080/03601234.2021.1918977
García-Rico, L., Tejeda-Valenzuela, L., & Burgos-Hernández, A. (2010). Seasonal variations in the concentrations of metals in Crassostrea corteziensis from Sonora, Mexico. Bulletin of Environmental Contamination and Toxicology, 85, 209–213. https://doi.org/10.1007/s00128-010-0055-4
García-Rico, L., Meza-Figueroa, D., Gandolfi, A. J., Ibañez del Rivero, C., Martínez-Cinco, M. A., & Meza-Montenegro, M. M. (2019). Health risk assessment and urinary excretion of children exposed to arsenic through drinking water and soils in Sonora Mexico. Biological Trace Element Research, 187(1), 9–21. https://doi.org/10.1007/s12011-018-1347-5
Góngora-Gómez, A. M., García-Ulloa, M., Muñoz-Sevilla, N. P., Domínguez-Orozco, A. L., Villanueva-Fonseca, B. P., Hernández-Sepúlveda, J. A., & Ortega-Izaguirre, R. (2017). Heavy-metal contents in oysters (Crassostrea gigas) cultivated on the Southeast coast of the Gulf of California Mexico. Hidrobiológica, 27(2), 219–227.
Góngora-Gómez, A. M., Domínguez-Orozco, A. L., Villanueva-Fonseca, B. P., Muñoz-Sevilla, N. P., & García-Ulloa, M. (2018). Seasonal levels of heavy metals in soft tissue and muscle of the pen shell Atrina maura (Sowerby, 1835) (Bivalvia: Pinnidae) from a farm in the Southeast coast of the Gulf of California Mexico. Revista Internacional De Contaminación Ambiental, 34(1), 57–68. https://doi.org/10.20937/RICA.2018.34.01.05
Góngora-Gómez, A. M., Sepúlveda, C. H., Verdugo-Escobar, H. A., Astorga-Castro, O., Rodríguez-González, H., Domínguez-Orozco, A. L., et al. (2020). Gonadal maturity of Crassostrea corteziensis cultivated in the Gulf of California. Latin American Journal of Aquatic Research, 48(3), 381–395. https://doi.org/10.3856/vol48-issue3-fulltext-2422
Gundert-Remy, U., Damm, G., Foth, H., Freyberger, A., Gebel, T., Golka, K., et al. (2015). High exposure to inorganic arsenic by food: The need for risk reduction. Archives of Toxicology, 89(12), 2219–2227. https://doi.org/10.1007/s00204-015-1627-1
Henry, C. D., McDowell, F. W., & Silver, L. T. (2003). Geology and geochronology of granitic batholithic complex, Sinaloa, Mexico: Implications for cordilleran magmatism and tectonics. In S. E. Johnson, S. R. Paterson, J. M. Fletcher, G. H. Girty, D. L. Kimbrough, & A. Martín-Barajas (Eds.), Tectonic evolution of northwestern México and the Southwestern USA (pp. 237–273). Geological Society of America Special Papers.
Hope, J. A., Hewitt, J., Pilditch, C. A., Savage, C., & Thrush, S. F. (2020). Effect of nutrient enrichment and turbidity on interactions between mycrophytonethos and a key nibalve: Implications for higher trophic levels. Frontiers in Marine Science, 7, 695. https://doi.org/10.3389/fmars.2020.00695
INEGI (Instituto Nacional de Estadística y Geografía). (2015). Número de habitantes por municipios de Sinaloa. http://cuentame.inegi.org.mx/monografias/informacion/sin/poblacion/. Accessed 7 June 2020.
Jara-Marini, M. E., Molina-García, A., Martínez-Durazo, A., & Páez-Osuna, F. (2020). Trace metal trophic transference and biomagnification in a semiarid coastal lagoon impacted by agriculture and shrimp aquaculture. Environmental Science and Pollution Research, 27, 5323–5336. https://doi.org/10.1007/s11356-019-06788-2
Jonathan, M. P., Muñoz-Sevilla, N. P., Góngora-Gómez, A. M., Luna-Varela, R. G., Sujitha, S. B., Escobedo-Urías, D. C., et al. (2017). Bioaccumulation of trace metals in farmed pacific oysters Crassostrea gigas from SW Gulf of California coast, Mexico. Chemosphere, 187, 311–319. https://doi.org/10.1016/j.chemosphere.2017.08.098
Kato, L. S., Gomes-Ferraria, R., Meirelles-Leiteb, J. V., & Conte-Junior, C. A. (2020). Arsenic in shellfish: A systematic review of its dynamics and potential health risks. Marine Pollution Bulletin, 161, 111693. https://doi.org/10.1016/j.marpolbul.2020.111693
Lodeiros, C., Valentich-Scott, P., Chávez-Villalba, J., Mazón-Suástegui, J. M., & Grijalva-Chon, J. M. (2020). Tropical and subtropical ostreidae of the American Pacific: Taxonomy, biology, ecology, and genetics. Journal of Shellfish Research, 39(2), 181–206. https://doi.org/10.2983/035.039.0202
Lucas, A., & Beninger, P. (1985). The use of physiological condition indices in marine bivalve aquaculture. Aquaculture, 44, 187–200.
Luoma, S. N., & Rainbow, P. S. (2005). Why is metal bioaccumulation so variable? Biodynamics as a unifying concept. Environmental Science & Technology, 39(7), 1921–1931. https://doi.org/10.1021/es048947e
Manisalidis, I., Stavropoulou, E., Stavropoulos, A., & Bezirtzoglou, E. (2020). Environmental and health impacts of air pollution: A review. Frontiers in Public Health, 8, 14. https://doi.org/10.3389/fpubh.2020.00014
Mazón-Suástegui, J. M. (1996). Cultivo de ostión japonés Crassostrea gigas. In M. Casas-Valdez & G. Ponce-Díaz (Eds.), Estudio del Potencial Pesquero y Acuícola de Baja California Sur (pp. 625–650). Editorial CIBNOR.
Méndez, L., Palacios, E., Acosta, B., Monsalvo-Spencer, P., & Álvarez-Castañeda, T. (2006). Heavy metals in the clam Megapitaria squalida collected from wild and phosphorite mine-impacted sites in Baja California, Mexico: Considerations for human health effects. Biological Trace Element Research, 110, 275–287.
MESL (Marine Environmental Studies Laboratory). (1997). Standard operating procedures. International Atomic Energy Agency, Agency Monaco.
MFR (Malaysia Food Regulation). (1985). Malaysian law on food and drugs. Malaysian Law Publisher, Kuala Lumpur.
Middelburg, J. J., & Herman, P. M. J. (2007). Organic matter processing in tidal estuaries. Marine Chemistry, 106(1–2), 127–147. https://doi.org/10.1016/j.marchem.2006.02.007
Moody, J. R., & Lindstrom, R. M. (1977). Selection and cleaning of plastic containers for storage of trace elements samples. Analytical Chemistry, 49(14), 2264–2267. https://doi.org/10.1021/ac50022a039
Muñoz-Sevilla, N. P., Villanueva-Fonseca, B. P., Góngora-Gómez, A. M., García-Ulloa, M., Domínguez-Orozco, A. L., Ortega-Izaguirre, R., & Campos-Villegas, L. E. (2017). Heavy metal concentrations in diploid and triploid oysters (Crassostrea gigas) from three farms on the north-central coast of Sinaloa Mexico. Environmental Monitoring and Assessment, 189, 536. https://doi.org/10.1007/s10661-017-6223-9
Murray, B. P., & Busty, C. J. (2015). Epithermal mineralization controlled by synextensional magmatism in the Guzapares mining district of the Sierra Madre Occidental silicic large igneous province, Mexico. Journal of South American Earth Sciences, 58, 54–71. https://doi.org/10.1016/j.jsames.2014.12.009
Newman, M. C., & Unger, M. A. (2002). Fundamentals of Ecotoxicology. Lewis Publishers.
NOM (Norma Oficial Mexicana). (2009). Productos y servicios. Productos de la pesca frescos, refrigerados, congelados y procesados. Especificaciones sanitarias y métodos de prueba. NOM-242-SSA1-2009. Diario Oficial de la Federación, México, D.F.
Osuna-Martínez, C. C., Páez-Osuna, F., & Alonso-Rodríguez, R. (2011). Cadmium, copper, lead and zinc in cultured oysters under two contrasting climatic conditions in coastal lagoons from SE Gulf of California, Mexico. Bulletin of Environmental Contamination and Toxicology, 87, 272–275. https://doi.org/10.1007/s00128-011-0355-3
Osuna-Martínez, C. C., Armienta, M. A., Bergés-Tiznado, M. E., & Páez-Osuna, F. (2021). Arsenic in waters, soils, sediments, and biota from Mexico: An environmental review. Science of the Total Environment, 752, 142062. https://doi.org/10.1016/j.scitotenv.2020.142062
Otchere, F. A. (2019). A 50-year review on heavy metal pollution in the environment: Bivalves as bio-monitors. African Journal of Environmental Science and Technology, 13(6), 220–227. https://doi.org/10.5897/AJEST2018.2597
Otero, N., Vitoria, L., Soler, A., & Canals, A. (2005). Fertiliser characterisation: Major, trace and rare earth elements. Applied Geochemistry, 20(8), 1473–1488.
Páez-Osuna, F., Zazueta-Padilla, H. M., & Izaguirre-Fierro, G. (1991). Trace metals in bivalves from Navachiste lagoon Mexico. Marine Pollution Bulletin, 22(6), 305–307.
Páez-Osuna, F., Osuna-López, J. M., Izaguirre-Fierro, G., & Zazueta-Padilla, H. M. (1993). Heavy metals in oysters from a subtropical coastal lagoon associated with an agricultural drainage basin. Bulletin of Environmental Contamination and Toxicology, 50, 696–702.
Páez-Osuna, F., Ruíz-Fernández, A. C., Botello, A. V., Ponce-Vélez, G., Osuna-López, J. I., Frías-Espericueta, M. G., et al. (2002). Concentrations of selected trace metals (Cu, Pb, Zn), organochlorines (PCBs, HCB) and total PAHs in mangrove oysters from the Pacific Coast of Mexico: An overview. Marine Pollution Bulletin, 44, 1303–1308. https://doi.org/10.1016/s0025-326x(02)00172-8
Páez-Osuna, F., & Osuna-Martínez, C. C. (2015). Bioavailability of cadmium, copper, mercury, lead, and zinc in subtropical coastal lagoons from the Southeast Gulf of California using mangrove oysters (Crassostrea corteziensis and Crassostrea palmula). Archives of Environmental Contamination and Toxicology, 68, 305–316. https://doi.org/10.1007/s00244-014-0118-3
Páez-Osuna, F., Álvarez-Borrego, S., Ruiz-Fernández, A. C., García-Hernández, J., Jara-Marini, M. E., Bergés-Tiznado, M. E., et al. (2017). Environmental status of the Gulf of California: A pollution review. Earth-Science Reviews, 166, 181–205. https://doi.org/10.1016/j.earscirev.2017.01.014
Rajeshkumar, S., Liu, Y., Zhang, X., Ravikumar, B., Bai, G., & Li, X. (2018). Studies on seasonal pollution of heavy metals in water, sediment, fish and oyster from the Meiliang Bay of Taihu Lake in China. Chemosphere, 191, 626–638. https://doi.org/10.1016/j.chemosphere.2017.10.078
Raven, K. P., & Loeppert, R. H. (1997). Trace element composition of fertilizers and soil amendments. Journal of Environmental Quality, 26, 551–557. https://doi.org/10.2134/jeq1997.00472425002600020028x
Rebelo, M. F., & Reboucas do Amaral, M. C., & Pfeiffer, W. C. (2003). High Zn and Cd accumulation in the oyster Crassostrea rhizophorae, and its relevance as a sentinel species. Marine Pollution Bulletin, 46, 1341–1358.
Rodríguez-Heredia, D. (2017). Occupational poisoning due to heavy metals. MEDISAN, 21(12), 3372.
Rodríguez-Ortiz, J. C., Alcalá-Jáuregui, J. A., Hernández-Montoya, A., Rodríguez-Fuentes, H., Ruiz-Espinoza, F. H., García-Hernández, J. L., & Díaz-Flores, P. E. (2014). Trace elements in fertilizers and manure used in organic and conventional agriculture. Revista Mexicana De Ciencias Agrícolas, 5(4), 695–701.
Rodríguez-Quiroz, G., García-Ulloa, M., Domínguez-Orozco, A. L., Valenzuela-Hernández, T. N., Nava-Pérez, E., & Góngora-Gómez, A. M. (2016). Relación del crecimiento, condición y supervivencia del ostión del Pacífico Crassostrea gigas y las variables ambientales, cultivado en suspensión en el sistema lagunar Navachiste-Macapule, Sinaloa, México. Revista De Biología Marina y Oceanografía, 51(3), 541–551. https://doi.org/10.4067/S0718-19572016000300006
Ruelas-Inzunza, J. R., & Páez-Osuna, F. (2008). Trophic distribution of Cd, Pb, and Zn in a food web from Altata-Ensenada del Pabellón subtropical lagoon, SE Gulf of California. Archives of Environmental Contamination and Toxicology, 54, 584–596. https://doi.org/10.1007/s00244-007-9075-4
Ruiz-Fernández, A. C., Wu, R., Lau, T. C., Pérez-Bernal, L. H., Sanchez-Cabeza, J. A., & Chiu, J. M. Y. (2018). A comparative study on metal contamination in Estero de Urias lagoon, Gulf of California, using oysters, mussels and artificial mussels: Implications on pollution monitoring and public health risk. Environmental Pollution, 243, 197–205. https://doi.org/10.1016/j.envpol.2018.08.047
Sabiha-Javied, T., Mehmood, M., Chaudhry, M., Tufail, M., & Irfan, N. (2009). Heavy metal pollution from phosphate rock used for the production of fertilizer in Pakistan. Microchemical Journal, 91(1), 94–99. https://doi.org/10.1016/j.microc.2008.08.009
Sepúlveda, C. H., Góngora-Gómez, A. M., Pérez-Álvarez, S., Rodríguez-González, H., Muñoz-Sevilla, N. P., Villanueva-Fonseca, B. P., et al. (2020). Trace metals in two wild populations of the squalid callista clam (Megapitaria squalida) in the Southeastern Gulf of California Mexico. Revista Internacional De Contaminación Ambiental, 36(3), 667–676. https://doi.org/10.20937/RICA.53565
SIAP (Servicio de Información Agroalimentaria y Pesquera). (2019). Anuario Estadístico de la Producción Agrícola. https://nube.siap.gob.mx/cierreagricola/. Accessed 6 June 2020.
Singh, R., Gautam, N., Mishra, A., & Gupta, R. (2011). Heavy metals and living systems: An overview. Indian Journal of Pharmacology, 43(3), 246–253. https://doi.org/10.4103/0253-7613.81505
Strickland, J. D., & Parsons, T. R. (1972). A practical handbook for the seawater analysis. Bulletin of Fisheries Research Board of Canada.
Suami, R. B., Al Salah, D. M. M., Kabala, C. D., Otamonga, J. P., Mulaji, C. K., Mpiana, P. T., & Poté, J. W. (2019). Assessment of metal concentrations in oysters and shrimp from Atlantic Coast of the Democratic Republic of the Congo. Heliyon, 5(12), e03049. https://doi.org/10.1016/j.heliyon.2019.e03049
Sujitha, S. B., Jonathan, M. P., Aurioles-Gamboa, D., Campos-Villegas, L. E., Bohórquez-Herrera, J., & Hernández-Camacho, C. J. (2019). Trace elements in marine organisms of Magdalena Bay, Pacific coast of Mexico: Bioaccumulation in a pristine environment. Environmental Geochemical and Health, 41(3), 1075–1089. https://doi.org/10.1007/s10653-018-0198-5
Takasu, H., Uchino, K., & Mori, K. (2020). Dissolved and particulate organic matter dynamics relative to sediment resuspension induced by the tidal cycle in minotidal estuaries, Kyushu Japan. Water, 12(9), 2561. https://doi.org/10.3390/w12092561
Walne, P. R., & Mann, R. (1975). Growth and biochemical composition in Ostrea edulis and Crassostrea gigas. In H. Barnes (Ed.), Proceedings of the 9th European Marine Biological Symposium (pp. 587–607). Aberdeen University Press.
Wang, L., & Wang, W. X. (2014). Depuration of metals by the green-colored oyster Crassostrea sikamea. Environmental Toxicology and Chemistry, 33(10), 2379–2385. https://doi.org/10.1002/etc.2695
WHO (World Health Organization). (1982). Toxicological evaluation of certain food additives and contaminants. Joint FAO/WHO expert committee on food additives. WHO food additives. WHO, Geneva.
Yap, C. K., Sharifinia, M., Cheng, W. H., Al-Shami, S. A., Wong, K. W., & Al-Mutairi, K. A. (2021). A commentary on the use of bivalve mollusks in monitoring metal pollution levels. International Journal of Environmental Research and Public Health, 18, 3386. https://doi.org/10.3390/ijerph18073386
Zar, J. (2010). Biostatistical analysis (5th ed.). Prentice Hall Pearson.
Zhong, G., Liu, Y., & Tang, Y. (2021). Oyster shell powder for Pb(II) immobilization in both aquatic and sediment environments. Environmental Geochemistry and Health, 43(5), 1891–1902. https://doi.org/10.1007/s10653-020-00768-z
Zhou, Q., Zhang, J., Fu, J., Shi, J., & Jiang, G. (2008). Biomonitoring: An appealing tool for assessment of metal pollution in the aquatic ecosystem. Analytica Chimica Acta, 606(2), 135–150. https://doi.org/10.1016/j.aca.2007.11.018
Zuykov, M., Pelletier, E., & Harper, D. A. T. (2013). Bivalve mollusks in metal pollution studies: From bioaccumulation to biomonitoring. Chemosphere, 93(2), 201–208. https://doi.org/10.1016/j.chemosphere.2013.05.001
Acknowledgements
The authors thank the support from Instituto Politécnico Nacional (IPN), through Secretaría de Investigación y Posgrado, Estímulo al Desempeño de los Investigadores, and Comisión de Operaciones y Fomento de Actividades Académicas, and also are grateful with the Sistema Nacional de Investigadores (SNI–CONACyT). Carlos Humberto Sepúlveda and Maria Isabel Sotelo-Gonzalez thank CONACyT for the research fellowship. Thanks to Ingrid Helena Mascher Gramlich for English editing of the manuscript.
Funding
This work was funded by the Secretaría de Investigación y Posgrado (SIP) of the Instituto Politécnico Nacional (IPN) through the projects: SIP 20200526 “Incidencia estacional de nematodos y Perkinsus marinus de la Almeja venus Chione undatella de bahía de La Paz y del Puerto Minero de Santa Rosalía, Baja California Sur, México” and SIP 20200527 “Morfometría y fisiología de la Almeja venus Chione undatella de bahía de La Paz y del Puerto Minero de Santa Rosalía, Baja California Sur, México”.
Author information
Authors and Affiliations
Contributions
All authors contributed to this work. CHS: investigation, sampling, methodology, resources, validation, formal analysis, writing – original draft. MISG: sampling, methodology, writing – review and editing. CCOM: conceptualization, resources, formal analysis, visualization, writing. MGFE: resources, visualization, writing – review and editing. RSC: visualization, writing – review and editing. MEBT: conceptualization, funding acquisition, resources, investigation, formal analysis, writing – review and editing. AMGG: visualization, resources, sampling, methodology. MGU: conceptualization, funding acquisition, project administration, resources, investigation, formal analysis, writing – review and editing.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sepúlveda, C.H., Sotelo-Gonzalez, M.I., Osuna-Martínez, C.C. et al. Biomonitoring of potentially toxic elements through oysters (Saccostrea palmula and Crassostrea corteziensis) from coastal lagoons of Southeast Gulf of California, Mexico: health risk assessment. Environ Geochem Health 45, 2329–2348 (2023). https://doi.org/10.1007/s10653-022-01347-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-022-01347-0