Abstract
Major tailings dam failures have occurred recently around the world and resulted in severe environmental impacts, such as metal contamination. Manganese is a metal highly associated with mining activities, largely detected in mining dam collapses. This metal is considered necessary for different organisms, but it can be toxic and cause oxidative stress and genetic damage in fishes. In this study, we investigated the toxic effects of manganese on Astyanax lacustris, by exposing the fish individually to different concentrations of this metal (2.11, 5.00, and 10.43 mg/L) for 96 h. To assess the effects of manganese, we used biochemical biomarkers (glutathione S-transferase, catalase, and acetylcholinesterase enzyme activity) and the manganese bioaccumulation in different tissues (liver and gills). The obtained data showed that only at concentrations of 5.00 mg/L and 10.43 mg/L the activity of glutathione S-transferase differed significantly. Additionally, the acetylcholinesterase activity in the brain tissue was inhibited. The highest level of manganese bioaccumulation was observed in the liver and branchial tissue. Overall, we concluded that high concentrations of manganese may cause physiological changes in Astyanax lacustris.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Various ecosystems naturally contain metals due to geological activity, but they can enter aquatic environments through anthropogenic activities such as wastewater, industrial effluents, agricultural activities, and mining (Anandkumar et al. 2018; Zhong et al. 2018). Because mining byproducts like metals are prevalent in mining tailings dams, they represent a high risk to aquatic environments (Zhang et al. 2018). Major tailings dam ruptures have occurred recently around the world and resulted in severe environmental impacts and human deaths (Islam and Murakami 2021). In aquatic ecosystems, metals are considered important pollutants due to their environmental persistence and potential for toxicity and bioaccumulation (Zhong et al. 2018). Manganese (Mn) is one of the metals associated with mining activities and dam collapses (Segura et al. 2016; Passos et al. 2021). The maximum concentration of manganese allowed by Brazilian legislation (CONAMA 357/05) in freshwater environments is 0.5 mg/L (Brasil 2005). The tailings that contaminated the Doce River in 2015 contained an average Mn concentration of 433 ± 110 mg/kg (Queiroz et al. 2018). The concentration of total and dissolved manganese in the water varied temporally after contamination, but Mn concentrations of up to 1638 mg/L were reported in 2016 in the Rio Doce (Carvalho et al. 2017). Although Mn is considered a necessary for protein transport and neurological functions (Fitsanakis et al. 2010), in high concentrations it can be toxic and induce oxidative stress, affect enzymatic activity, and cause genetic damages and behavioral changes in fishes (Cavas 2011; Qu et al. 2014; Tuzuki et al. 2017; Passos et al. 2021; Coppo et al. 2018; Marinho et al. 2019; Marins et al. 2019; Gnocchi et al. 2023), Mn also accumulate in animal tissues (Gabriel et al. 2013; Anandkumar et al. 2018; Coppo et al. 2018).
A. lacustris (Characidae) is an abundant freshwater fish species with a short life cycle, easy capture, handling, and adaptability in the laboratory, which facilitates its use in bioassays (Stevanato and Ostrensky 2018). This fish species is commonly found in freshwater bodies in the Neotropics, including surrounding rivers affected by the dam rupture in Mariana, Brazil in November 2015 (Eschmeyer 2013; Salvador et al. 2018). A. lacustris play an important ecological role in freshwater ecosystems as the main prey of diverse carnivorous fishes (Garutti 2003) and are broadly consumed and used as live bait by riverine human populations (Fonseca et al. 2017; Súarez et al. 2017). In this study, we evaluate the potential effects of manganese on A. lacustris, by analyzing biochemical biomarkers (GST, CAT, and AChE), as well as the bioaccumulation of manganese in liver and gills tissues. We hypothesize that the presence of manganese affects the enzymatic activities of GST, CAT, and AChE, and accumulate in the hepatic and branchial tissues of A. lacustris.
Material and methods
Acclimatization
Juveniles of A. lacustris (3.04 g ± 0.87 and 5.95 cm ± 0.60) were obtained from local breeding facility in Itarana, Espírito Santo State, SE Brazil, and transported to the Laboratory of Applied Ichthyology (LabPeixe/UVV). In the lab, they acclimated for 30 days in a 310 L polyethylene tank with 12:12 h (dark/light) photoperiod and continuous aeration. They were fed thrice daily with Propescado (Nutriave Alimentos Ltda., 45% crude protein, extruded). Weekly, 70% tank water was changed, and daily bottom water was siphoned. Water parameters were monitored using a YSI 85 multiparameter: dissolved oxygen (5.87 mg/L ± 0.50), pH (7.7 ± 0.6), temperature (23.9 °C ± 0.5), and conductivity (137.4 μS/cm ± 12.5). Alkalinity (13.44 mg/L CaCO3 ± 4.75) and hardness (59.63 mg/L CaCO3 ± 10.43) were analyzed following APHA (2005)’s protocols. Fish fasted for 24 h before transferring to test aquariums. Animal use and experimental procedures were previously approved by the animal ethics committee (CEUA UVV - 508/2018).
Toxicity bioassay
After acclimatization, 24 fish were randomly transferred to 6 L glass aquariums for 48 h of acclimation. After that, animals were exposed to different Mn concentrations for 96 h. The fish were divided into four treatments with different nominal manganese concentrations: (i) Control (C - university’s standard supply water); (ii) 3.33 mg/L Mn; (iii) 6.65 mg/L Mn; and (iv) 13.33 mg/L Mn, based on results from previous studies (Segura et al. 2016; Carvalho et al. 2017; Coppo et al. 2018; Passos et al. 2021). Each group had six individually exposed fish in the aquariums. Manganese was introduced in the water using a stock solution (Manganese chloride tetrahydrate, 2 g/L; Sigma - M3634). Environmental conditions matched the acclimation period: dissolved oxygen (5.87 mg/L ± 0.50), pH (7.4 ± 0.5), temperature (23.93 °C ± 0.60), conductivity (119.96 μS/cm ± 18.80), alkalinity (13.01 mg/L CaCO3 ± 4.42), and hardness (64.78 mg/L CaCO3 ± 10.31). After 96 h of exposure, fish were anesthetized (0.2 g/L Benzocaine) and euthanized by cervical section (Winkaler et al. 2007). Gills, liver, and brain were frozen at −80 °C for biochemical and accumulation analysis.
For Mn bioaccumulation in liver and gills, samples were digested in a microwave oven (Ethos UP - Milestone) at 1000 W and 200 °C. Mn concentrations in the digested samples and water were quantified using an Atomic Absorption Spectrophotometer (Thermo Scientific, AAS ICE 3500) and expressed as mg/kg for tissues and mg/L for water. To validate the method, blanks were prepared following the same sample procedure using fish tissue reference material (ERM-BB422™). The highest purity reagents were used throughout the digestion process and solutions were prepared using ultrapure water with a resistivity of 18.2 MΩ obtained from a Millipore system. Quality assurance and quality control (QA/QC) testing analyses were performed to monitor the reliability of the methods. A calibration curve (analytical curve) was performed, and the R-squared of standard regression was 0.999, the recovery of 118%, the limit of quantification (LQ) was 4.8 µg/L, and the limit of detection (LD) of 1.6 µg/L.
Gill and brain samples were homogenized in a phosphate buffer, and total tissue protein was assessed following Bradford (1976)’s method. Glutathione S-transferase (GST) and catalase (CAT) activities in the gills were quantified following the procedures described by Habig et al. (1974), Habig and Jakoby (1981), and Aebi (1984). Acetylcholinesterase (AChE) activity in brain tissue was measured according to Ellman et al. (1961).
Statistical analysis
Data normality was assessed with the Shapiro–Wilk test. One-way analysis of variance (ANOVA) was utilized for treatment comparisons, followed by Tukey’s post-hoc test for multiple comparisons. Furthermore, the NOEC (no observed effect) and LOEC (lowest observed effect concentration) were determined to assess risk based on significant biomarkers. NOEC represented the highest Mn concentration without significant change compared to the control (ANOVA followed by Dunnett test), while LOEC was the lowest concentration with a significant effect compared to the control. Statistical significance was determined at p ≤ 0.05. Data are presented as mean ± standard error. All statistical analyses were conducted using SigmaPlot 12.5.
Results
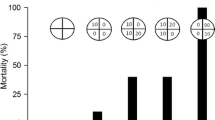
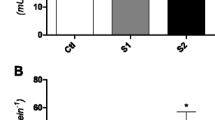
During exposure, Mn dissolved concentrations in water were 0.24 mg/L ± 0.02 (control), 2.11 mg/L ± 0.62 (3.33 mg/L group), 5.00 mg/L ± 0.96 (6.65 mg/L group), and 10.43 mg/L ± 1.21 (13.33 mg/L group). Manganese accumulation in liver tissue indicated a significant difference only in the 10.43 mg/L group compared to the control (p = 0.03; Fig. 1A). However, all exposed groups exhibited a significant Mn increase in gill tissue compared to the control (p ≤ 0.001) (Fig. 1B).
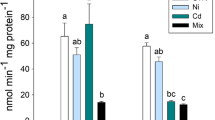
Results for the GST enzyme revealed increased activity in gill tissue for fish exposed to 10.43 mg/L compared to 5.00 mg/L (p = 0.007; Fig. 2A). CAT activity did not exhibit significant differences among treatments (p = 0.07; Fig. 2B). AChE activity in the brain tissue of exposed fish was inhibited at high manganese concentrations (5.00 and 10.43 mg/L; p = 0.002; Fig. 2C).
Enzymatic activity in different tissues of A. lacustris exposed to different manganese concentrations (2.11, 5.00, and 10.43 mg/L) for 96 h. (A) Glutathione S-transferase (GST) activity in the gills; (B) Catalase (CAT) activity in the gills; and (C) Acetylcholinesterase (AChE) activity in the brain tissue. Different letters indicate statistical significative differences (p ≤ 0.05). Results are expressed as mean ± standard error
NOEC and LOEC results show that even at the lowest Mn concentration (2.11 mg/L), gill tissue already accumulates this metal. These results also indicate that at 5.00 mg/L of Mn, changes in AChE enzymatic activity in the brain tissue occur, highlighting the potential toxicity of Mn to fish in short-term exposure (96 h) (Table 1).
Discussion
The increased manganese accumulation in gill and liver tissues may result from the higher metal ion availability in water at elevated metal concentrations, facilitating greater tissue accumulation. Additionally, this heightened Mn accumulation at higher concentrations is influenced by the equilibrium between fish absorption capacity and clearance (Anandkumar et al. 2018). The gill tissue, being a thin and extensive surface directly in contact with water, is more susceptible to metal-induced damage than other tissues (Gabriel et al. 2013). Assessing metal toxicity in fish often utilizes gill tissue, as it is metabolically active and considered the first line of defense against environmental toxins due to its direct exposure to the surroundings (Javed et al. 2015). Moreover, metal accumulation may vary between tissues due to physiological differences and body position (Kraal et al. 1995). The liver plays a key role in metal metabolism, contributing to detoxification. Consequently, high metal concentrations are typically observed in the liver, making it a valuable indicator for assessing the effects of exposure to various metal concentrations, including manganese (Siscar et al. 2014). Similarly to our findings, previous studies have reported significant manganese concentrations in the liver tissue of different fish species exposed to varying Mn concentrations in laboratory experiments (Coppo et al. 2018; Passos et al. 2021; Gnocchi et al. 2023) and samples collected from natural environments (Badr et al. 2014).
In the gill tissue of fish exposed to 10.43 mg/L, GST activity was higher than in those exposed to 5.00 mg/L (p = 0.007). This enzyme activation may indicate a response to detoxify the excess manganese in the gills. Conversely, at 5.00 mg/L, lower enzymatic activity suggests reduced production of reactive oxygen species (ROS). Antioxidant system enzymes can exhibit varying activity levels depending on the stressor type and concentration (Javed et al. 2015). Catalase did not show significant differences in activity among different exposure concentrations, suggesting that its metabolic pathway was not activated. Gabriel et al. (2013) investigated the antioxidant system responses in Colossoma macropomum fish exposed to 3.88 mg/L of manganese for 96 h and also did not observed changes in GST branchial activity or CAT hepatic activity. This implies that there is no consistent pattern of antioxidant activity in tissues of fish exposed to metals. Similarly, Tuzuki et al. (2017) reported no differences in GST activity in Centropomus parallelus exposed to manganese (3.18 mg/L of Mn2+) for 96 h at two different temperatures (24 and 27 °C), suggesting that Mn2+ metabolism occurs via an alternative biotransformation pathway.
Additionally, the responses of the antioxidant defense system may vary depending on the specific tissue being evaluated (Gabriel et al. 2013). For instance, Coppo et al. (2018) observed alterations in GST activity in the liver tissue of Oreochromis niloticus exposed to different manganese concentrations (0.2, 1.5, and 2.9 mg/L), while GST activity in the gill tissue did not differ among treatments. This aligns with our findings, highlighting that enzyme activity can be either activated or inhibited based on the tissue analyzed.
In the brain of fish exposed to manganese, AChE activity demonstrated significant inhibition at higher metal concentrations (p = 0.002). This suggests that elevated environmental metal concentrations may act as inhibitors of AChE activity, potentially disrupting the transmission of nervous impulses in organisms. The inhibition of AChE by metals occurs because these compounds can bind to the functional groups of proteins, compromising catalytic activity and resulting in the loss of enzymatic function (Haverroth et al. 2015). AChE plays a key role in acetylcholine (ACh) hydrolysis at cholinergic synapses, ensuring the intermittent nature of nervous impulses responsible for neuronal communication (Lopes et al. 2019; Araújo et al. 2016).
The inhibition of AChE activity due to metal exposure, as observed in this study, can lead to the excessive accumulation of the neurotransmitter ACh in the synaptic cleft. This accumulation promotes the hyperstimulation of postsynaptic receptors, potentially causing various issues, including cholinergic syndrome and even the death of the individual (Lopes et al. 2019). De Lima et al. (2013) also observed the inhibition of acetylcholinesterase enzymatic activity in Danio rerio after exposure to various metals such as copper, iron, and cadmium. Our results indicate that metals can disrupt brain physiology, inducing changes in AChE enzymatic activity because metals can bind to functional protein groups, compromising their catalytic activity and resulting in the loss of AChE function.
NOEC and LOEC results reveal that manganese accumulation in gill tissue is observable even at the lowest concentration used (2.11 mg/L). A similar pattern of Mn accumulation in gill tissue was observed by Coppo et al. (2018), in which O. niloticus specimens were exposed to the lowest manganese concentration of 2.9 mg/L. This suggests that even at relatively low concentrations, manganese can accumulate in animal tissues, representing environmental risks to fish.
Moreover, the NOEC and LOEC results indicate that even at the concentration of 5.00 mg/L of Mn, there are already noticeable changes in the enzymatic activity of AChE in the brain tissue, highlighting the potential toxicity of Mn to fish in a short-term exposure (96 h). Our findings suggest that alterations in enzymatic activity can serve as valuable biomarkers in the context of environmental manganese contamination. The need for studies that document the effects of manganese on fish is essential, given their crucial role in various trophic chains. Living organisms can exhibit adverse effects due to pollutants present in the ecosystem through a range of behaviors (Wieczerzak et al. 2016).
Conclusion
Our results showed that manganese, even as an essential metal on different physiological functions, in high concentrations can alter biochemical parameters of A. lacustris. Furthermore, our results showed that even under acute exposure manganese can accumulate in different tissues (liver and gills) of this fish depending on environmental concentrations, but chronic exposures may lead to long-term impacts, such as changes in the growth and reproduction rates, and survival of this species. Additionally, we highlight the suitability of A. lacustris as a bioindicator for impacts caused by manganese on the resident biota of the aquatic ecosystem.
References
Aebi H (1984) Catalase in vitro. Method Enzymol 105(2):121–126
Anandkumar A, Nagarajan R, Prabakaran K et al (2018) Human health risk assessment and bioaccumulation of trace metals in fish species collected from the Miri coast, Sarawak, Borneo. Mar Pollut Bull 133:655–663. https://doi.org/10.1016/j.marpolbul.2018.06.033
Araújo MCde, Assis CRD, Silva LC et al (2016) Brain acetylcholinesterase of jaguar cichlid (Parachromis managuensis): from physicochemical and kinetic properties to its potential as biomarker of pesticides and metal ions. Aquat Toxicol 177:182–189. https://doi.org/10.1016/j.aquatox.2016.05.019
Badr AM, Mahana NA, Eissa A (2014) Assessment of heavy metal levels in water and their toxicity in some tissues of Nile Tilapia (Oreochromis niloticus) in river Nile basin at Greater Cairo, Egypt. Global. Veterinaria 13(4):432–443
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1-2):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Brasil (2005) Resolução CONAMA n° 357/05, de 17 de março de 2005. Conselho Nacional de Meio Ambiente. Brasilia, Brazil
Carvalho MS, Moreira RM, Ribeiro KD, Almeida AM (2017) Concentração de metais no rio Doce em Mariana, Minas Gerais, Brasil. Acta Bras 1(3):37–41. https://doi.org/10.22571/Actabra13201758
Cavas T (2011) In vivo genotoxicity evaluation of atrazine and atrazine-based herbicide on fish Carassius auratus using the micronucleus test and the comet assay. Food Chem Toxicol 49(6):1431–1435. https://doi.org/10.1016/j.fct.2011.03.038
Coppo GC, Passos LS, Lopes TOM et al (2018) Genotoxic, biochemical and bioconcentration effects of manganese on Oreochromis niloticus (Cichlidae). Ecotoxicology 27:1150–1160. https://doi.org/10.1007/s10646-018-1970-0
Ellman GL, Courtney KD, Andres VJ, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–90
Eschmeyer WN (2013) Catalog of fishes. Eletronic version. California Academy Sciences. California, USA
Fitsanakis VA, Zhang N, Garcia S, Aschner M (2010) Manganese (Mn) and iron (Fe): interdependency of transport and regulation. Neurotox Res 18:124–131. https://doi.org/10.1007/s12640-009-9130-1
Fonseca T, Costa-Pierce BA, Valenti WC (2017) Lambari aquaculture as a means for the sustainable development of rural communities in Brazil. Rev Fish Sci Aquac 25(4):316–330. https://doi.org/10.1080/23308249.2017.1320647
Gabriel D, Paula A, Isabela KR et al (2013) Effects of subchronic manganese chloride exposure on Tambaqui (Colossoma macropomum) tissues: oxidative stress and antioxidant defenses. Arch Environ Contam Toxicol 64:659–667. https://doi.org/10.1007/s00244-012-9854-4
Garutti V (2003) Psicultura Ecológica. UNESP,São Paulo
Gnocchi KG, Boldrini-França J, Passos LS et al (2023) Multiple biomarkers response of Astyanax lacustris (Teleostei: Characidae) exposed to manganese and temperature increase. Environ Toxicol Pharmacol 100:104124. https://doi.org/10.1016/j.etap.2023.104124
Habig WH, Jakoby MJ (1981) Assays for differentiation of glutathione-s-transferases. Methods Enzimol 77:398–405
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem 249(22):7130–7139
Haverroth GMB, Welang C, Mocelin RN et al (2015) Copper acutely impairs behavioral function and muscle acetylcholinesterase activity in zebrafish (Danio rerio). Ecotoxicol Environ Saf 122:440–447. https://doi.org/10.1016/j.ecoenv.2015.09.012
Islam K, Murakami S (2021) Global-scale impact analysis of mine tailings dam failures: 1915–2020. Glob Environ Change 70:102361. https://doi.org/10.1016/j.gloenvcha.2021.102361
Javed M, Usmani N, Ahmad I, Ahmad M (2015) Studies on the oxidative stress and gill histopathology in Channa punctatus of the canal receiving heavy metal-loaded effluent of Kasimpur Thermal Power Plant. Environ Monit Assess 187:4179. https://doi.org/10.1007/s10661-014-4179-6
Kraal MH, Kraak MHS, De Groot CJ, Davids C (1995) Uptake and tissue distribution of dietary and aqueous cadmium by Carp (Cyprinus carpio). Ecotoxicol Environ Saf 31:179–183
De Lima D, Roque GM, De Almeida EA (2013) In vitro and in vivo inhibition of acetylcholinesterase and carboxylesterase by metals in zebrafish (Danio rerio). Mar Environ Res 91:45–51. https://doi.org/10.1016/j.marenvres.2012.11.005
Lopes DFC, Assis CRDde, Sant’Anna MCSde et al (2019) Brain acetylcholinesterase of three perciformes: From the characterization to the in vitro effect of metal ions and pesticides. Ecotoxicol Environ Saf 173:494–503. https://doi.org/10.1016/j.ecoenv.2019.02.047
Marinho CS, Matias MVF, Brandão IGF et al (2019) Characterization and kinetic study of the brain and muscle acetylcholinesterase from Danio rerio. Comp Biochem Physiol Part - C Toxicol Pharmacol 222:11–18. https://doi.org/10.1016/j.cbpc.2019.04.005
Marins K, Lazzarotto LMV, Boschetti G et al (2019) Iron and manganese present in underground water promote biochemical, genotoxic, and behavioral alterations in zebrafish (Danio rerio). Environ Sci Pollut Res 26:23555–23570. https://doi.org/10.1007/s11356-019-05621-0
Passos LS, Coppo GC, Pereira TM et al (2021) Do manganese and iron in association cause biochemical and genotoxic changes in Oreochromis Niloticus (Teleostei: Cichlidae)? Bull Environ Contam Toxicol 108:708–715. https://doi.org/10.1007/s00128-021-03382-6
Qu R, Feng M, Wang X et al (2014) Metal accumulation and oxidative stress biomarkers in liver of freshwater fish Carassius auratus following in vivo exposure to waterborne zinc under different pH values. Aquat Toxicol 150:9–16. https://doi.org/10.1016/j.aquatox.2014.02.008
Queiroz HM, Nóbrega GN, Ferreira TO, Almeida LS, Romero TB, Santaella ST, Bernardino AF, Otero XL (2018) The Samarco mine tailing disaster: A possible time-bomb for heavy metals contamination? Sci Total Envrion 637-638:498–506. https://doi.org/10.1016/j.scitotenv.2018.04.370
Salvador GN, Frederico RG, Pessali TC et al (2018) Length-weight relationship of 21 fish species from Rio Doce River basin, Minas Gerais, Brazil. J Appl Ichthyol 34(5):1198–1201. https://doi.org/10.1111/jai.13734
Segura FR, Nunes EA, Paniz FP et al (2016) Potential risks of the residue from Samarco’s mine dam burst (Bento Rodrigues, Brazil). Environ Pollut 218:813–825. https://doi.org/10.1016/j.envpol.2016.08.005
Siscar R, Koenig S, Torreblanca A, Solé M (2014) The role of metallothionein and selenium in metal detoxification in the liver of deep-sea fish from the NW Mediterranean Sea. Sci Total Environ 466-467:898–905. https://doi.org/10.1016/j.scitotenv.2013.07.081
Stevanato DJ, Ostrensky A (2018) Ontogenetic development of tetra Astyanax lacustris (Characiformes: Characidae). Neotrop Ichthyol 16(2):e170073. https://doi.org/10.1590/1982-0224-20170073
Súarez YR, Silva EA, Viana LF (2017) Reproductive biology of Astyanax lacustris (Characiformes: Characidae) in the southern Pantanal floodplain, upper Paraguay River basin, Brazil. Environ Biol Fish 100:775–783. https://doi.org/10.1007/s10641-017-0604-3
Tuzuki BLL, Delunardo FAC, Ribeiro LN et al (2017) Effects of manganese on fat snook Centropomus parallelus (Carangaria: Centropomidae) exposed to different temperatures. Neotrop Ichthyol 15(4):e170054. https://doi.org/10.1590/1982-0224-20170054
Wieczerzak M, Namieśnik J, Kudłak B (2016) Bioassays as one of the green chemistry tools for assessing environmental quality: a review. Environ Int 94:341–361. https://doi.org/10.1016/j.envint.2016.05.017
Winkaler EU, Santos TRM, Machado-Neto JG, Martinez CBR (2007) Acute lethal and sublethal effects of neem leaf extract on the neotropical freshwater fish Prochilodus lineatus. Comp Biochem Physiol Part - C Toxicol Pharmacol 142(2):236–244. https://doi.org/10.1016/j.cbpc.2006.12.009
Zhang L, Zhao B, Xu G, Guan Y (2018) Characterizing fluvial heavy metal pollutions under different rainfall conditions: Implication for aquatic environment protection. Sci Total Environ 635:1495–1506. https://doi.org/10.1016/j.scitotenv.2018.04.211
Zhong W, Zhang Y, Wu Z et al (2018) Health risk assessment of heavy metals in freshwater fish in the central and eastern North China. Ecotoxicol Environ Saf 15:343–349. https://doi.org/10.1016/j.ecoenv.2018.03.048
Author contributions
All authors contributed to the study conception and design. Funding acquisition, material providing, and supervision was done by Adriana Regina Chippari-Gomes. Material preparation, data collection, and analysis were performed by Karla Giavarini Gnocchi, Larissa Souza Passos, Tatiana Miura Pereira, Letícia Alves de Souza, Barbara Chisté Teixeira. Statistical analysis was conducted by Karla Giavarini Gnnochi, Larissa Souza Passos, and Gabriel Carvalho Coppo. The first draft of the manuscript was written by Karla Giavarini Gnocchi, Larissa Souza Passos, and Gabriel Carvalho Coppo, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was funded by the São Paulo State Research Foundation (FAPESP), Brazil [grant number 2021/14239–1].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gnocchi, K.G., Passos, L.S., Pereira, T.M. et al. Biochemical changes and bioaccumulation of manganese in Astyanax lacustris (Teleostei: Characidae). Ecotoxicology 33, 677–682 (2024). https://doi.org/10.1007/s10646-024-02765-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-024-02765-9