Abstract
Manganese (Mn) and iron (Fe) are transition metals that are crucial to the appropriate growth, development, function, and maintenance of biological organisms. Because of their chemical similarity, in organisms ranging from bacteria to mammals they share and compete for many protein transporters, such as the divalent metal transporter-1. As such, during conditions of low Fe, abnormal Mn accumulation occurs. Conversely, when Mn concentrations are altered, the homeostasis and deposition of Fe and other transition metals are disrupted. Our lab has undertaken a series of studies in rats involving pregnant dams, neo- and perinatal pups, and adult animals. Animals were exposed to various concentrations of dietary Fe and/or Mn, and protein transporter expression, blood Mn and Fe concentrations, brain transition metal concentrations, and temporal brain deposition patterns were examined. As a result, we have demonstrated the importance of the interdependence of the transport of Mn and Fe, and established brain metal concentrations in several longitudinal studies. The purpose of this review is to examine these studies in their entirety and highlight the importance of monitoring the deposition and accumulation of both Mn and Fe when designing future studies related to either dietary or environmental changes in transition metal levels. Finally, this review will provide information about various transport proteins currently under investigation in the research community related to Fe and Mn regulation and transport.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Biological Importance of Manganese and Iron
The transition metals manganese (Mn) and iron (Fe) are required for proper growth, development, and maintenance of numerous organisms, ranging from bacteria to mammals. Mn, for example, is necessary for the maturation of bones and cartilage (Neilson 2006), increases wound-healing via activation of metal matrix metalloproteinase-2 and -9 (Chebassier et al. 2004), and promotes metabolic activity through its incorporation into pyruvate carboxylase, phosphoenolpyruvate carboxykinase, and arginase (Leach and Harris 1997). While Mn is perhaps best known as a cofactor for the mitochondrially expressed antioxidant protein superoxide dismutase (Miao and St Clair 2009), it is also involved in the production of reactive oxygen species (ROS). Intracellularly, Mn is distributed both in the nuclei and mitochondria (Gunter et al. 2009).
Much like Mn, Fe plays a critical role in normal health and metabolism. Most importantly, however, Fe, incorporated into heme, is required for effective oxygen transport and storage throughout the body (Beard and Dawson 1997). Fe is also an essential cofactor for many of the mitochondrial electron transport chain cytochromes (Mathews et al. 2000). As such, it plays a crucial role in energy production and metabolism. Although accumulation of excess Fe leads to the production of various ROS through Fenton chemistry, Fe, as well as Mn, is an integral structural component of both catalase and peroxidase enzymes, which prevent oxidative stress (Beard and Dawson 1997).
Mn and Fe Transport
Uptake and efflux of both metals must be tightly regulated, as too much or too little of either can result in disease states. Although clinical Mn deficiency is rare, many individuals may not be optimized for Mn levels (Wood 2009). Typically, Mn toxicity results from exposure to greater than 5 μg Mn/m3, which may be found in various industrial settings: welding (Bowler et al. 2006; Park et al. 2007; Sung et al. 2007), metal smelting (Myers et al. 2003b, c), Mn mining (Garcia Avila and Penalver Ballina 1953; Myers et al. 2003a), or battery manufacturing (Bader et al. 1999). Fe overload is also thought to result in or contribute to various disease states, such as hemochromatosis, which affects approximately 1 out of 200 people, primarily from northern European descent (Beaton and Adams 2007). Other disorders, such as thalassemia or chronic liver disease, or environmental factors, such as dietary excess or transfusional overload, may also contribute to high levels of biological Fe (Kohgo et al. 2008). Typically, however, Fe overload is less common than Fe deficiency, which may affect as many as two billion people world wide (WHO 2003) and results in anemic disease states (Jarrah et al. 2007; Skalicky et al. 2006; Toteja et al. 2006; Zhou et al. 2006). As a result, Mn and Fe metabolism, as well as that of numerous other metals, is highly regulated at multiple levels (Jensen et al. 2009; Kaisman-Elbaz et al. 2009; Lee and Beutler 2009; Miyayama et al. 2009; Rondon et al. 2008) to reduce the risk that either too much or too little of the metal poses to normal cellular homeostasis and function. Additionally, the requirements for various metals change during development; thus, many regulatory protein expression patterns vary temporally (Peters et al. 2007; Yang et al. 1997).
Mn and Fe are chemically and structurally similar. For example, both are first-row transition metals with similar atomic masses (Mn: 54.94 amu; Fe: 55.85 amu), radii (Mn: 127 pm; Fe: 125 pm), and electron structure (Mn: [Ar] 3d5, 4s2; Fe: [Ar] 3d6, 4s2). Their d-shell electrons participate in bonding; they exist in multiple oxidation states; they have similar electronegativity (Mn: 1.55; Fe: 1.83); finally, their initial ionization energies are comparable (Mn: 718 kJ/mol; Fe: 763 kJ/mol). Taken together, it is not surprising that they share many biological protein transporters. On the other hand, Mn ions tend to be more stable in aqueous solution, compared to Fe ions. As a result, Mn is less likely to undergo spontaneous redox cycling, even though both metals have been reported to participate in Fenton chemistry (Gregus 2008). Early reports noted that both metals, in addition to others such as copper and zinc, were transported by the divalent metal transporter-1 (DMT-1; also called NRAMP or Slc11A2; Gunshin et al. 1997), and via the transferrin (Tf)/Tf receptor (TfR) system (Au et al. 2008; Burdo et al. 2003; Mims and Prchal 2005; Rouault and Cooperman 2006). As a result of this overlap in transport, it was hypothesized that mutations in any of these protein transporters might affect the accumulation and homeostasis of both metals. This was borne out in studies involving the Belgrade rat and microcytic mouse model systems, where both metals were disrupted following a mutation in DMT-1 (Chua and Morgan 1997; Fleming et al. 1997).
These data suggested that changes in dietary or environmental levels of either Mn or Fe could lead to dysregulation in tissue levels of these important transition metals. The question of differential Mn and Fe regulation took on new significance in the 1980s following the addition of the gasoline additive methylcyclopentadienyl manganese tricarbonyl in the US and Canada (Abbott 1987; Cooper 1984). As a result, we, and others, undertook a series of studies to determine whether increased Mn levels would affect the availability or concentration of either (1) various transporters or (2) brain Fe distribution and accumulation. Conversely, other studies were designed to determine whether Fe deficiency would lead to greater Mn accumulation, as there would be less blood Fe to compete with Mn for these various transporters. This latter question is important considering that approximately one-third of the world’s population suffers from some degree of Fe deficiency or anemia (de Benoist et al. 2008).
Dietary Manipulation Leads to Brain Metal and Protein Dysregulation
Studies from our lab (Garcia et al. 2006) indicated that developing rat pups receiving milk from dams fed a high Mn diet (100 mg Mn/kg chow; control chow has 10 mg Mn/kg chow and 35 mg Fe/kg chow) had decreased hemoglobin and plasma Fe, but increased plasma Tf and total Fe binding capacity. Additionally, brain Mn, chromium, and zinc accumulation was increased, but brain Fe was lower, compared to controls (Garcia et al. 2006; Guilarte and Chen 2007; Guilarte et al. 2006). Since dysregulation of these metals might have resulted from changes in transport protein levels, Western blot analysis was undertaken to examine expression levels of DMT-1 and TfR. Data indicated an up-regulation of these proteins in multiple brain regions, including cerebellum, cortex, hippocampus, midbrain, and striatum (Garcia et al. 2006).
When pregnant and lactating dams were given either a low-Fe (3.5 mg Fe/kg chow and 10 mg Mn/kg chow) or low-Fe/high-Mn chow (3.5 mg Fe/kg chow and 100 mg Mn/kg chow), various biomarkers in nursing pups and dams resembled those observed in Fe-deficient animals: decreased hemoglobin and plasma Fe, but increased plasma Tf and total Fe binding capacity (Garcia et al. 2007). Similar to high-Mn-treated animals, pups from both treatment groups (low-Fe or low-Fe/high-Mn) showed decreased brain Fe, but increased Mn, copper, chromium, zinc, cobalt, and aluminum. As before, Western blot analysis confirmed increases in DMT-1 and TfR, similar to recent data from young pigs (Hansen et al. 2009). These data suggest that deposition and homeostasis of multiple metals, not just Mn and Fe, were disrupted when these transition metal levels are altered. Interestingly, changes in dietary Mn and Fe also resulted in disruption of various neurotransmitters (Anderson et al. 2007a, b, 2008, 2009). Although it appears that the catecholaminergic neurotransmitters were preferentially affected, γ-aminobutyric acid and glutamate also were regionally affected. These neurochemical changes may help explain why dams fed the low-Fe/high-Mn diet also performed poorly in the Morris water task (Fitsanakis et al. 2009).
Our studies in adult animals receiving subchronic injections of low doses of Mn also reinforce the interdependence of Mn and Fe on their transport. Animals fed normal dietary Fe (35 mg Fe/kg chow), while receiving weekly Mn injections (3 mg Mn/kg body mass), were imaged longitudinally using magnetic resonance (MR) technology. While there was no difference in blood Mn levels at the conclusion of the study, brain Mn was significantly elevated compared to controls (Fitsanakis et al. 2008), particularly in the hippocampus (Finkelstein et al. 2008). There were, however, no statistically significant changes in blood or brain Fe levels. If Mn injections remained the same (3 mg Mn/kg body mass) but dietary Fe concentrations were either increased (300 mg Fe/kg chow) or decreased (3.5 mg Fe/kg chow), brain Mn levels exceeded those of Mn-injected animals alone (Fitsanakis et al. 2008). This was true even for brain regions, such as the cortex and striatum, which did not show any increase in Mn deposition when rats were fed control chow. Additionally, brain Fe actually decreased in the cortex, midbrain, and cerebellum of animals receiving either Fe-deficient or Fe-supplemented food (Fitsanakis et al. 2008). Taken together, these data suggest that brain metal regulation is tightly coupled and that concentration and deposition of multiple metals may be disrupted following excess or deficiency.
Non-Invasive Support for Common Transporters
In general, neither Mn nor Fe exists in high concentrations as a free ion in biological systems due to both the presence of water (forming hydration shells) and the numerous proteins available to incorporate these metals. This is not to say, however, that some fraction is not in a ‘free’ state. Using R1 and R2 rate constants from T1- and T2-weighted, MR imaging data as a measure of brain Mn and Fe, respectively, mathematical models were derived to estimate the amount of both metals in general bound and unbound states. The best fit was obtained when it was assumed that Fe and Mn are (1) potentially competing for the same binding sites, (2) present in some type of unbound state, or (3) binding to other, non-common, protein sites (Zhang et al. 2009b). Most interesting, however, was the fact that the model predicts that Mn and Fe differentially compete with each other in a regionally specific manner.
For example, the modeling data suggest that most of the Fe in the hippocampus of control animals (24.33 mmol−1/kg tissue) would likely be found bound to sites (other Fe) that may not compete with potential Mn binding sites. Another way of stating this is that 2.20 mmol−1 Fe/kg tissue (bound Fe) is predicted to bind to sites that may also compete with Mn for binding. If one examines data from the cortex, it is likely that more Fe will be found bound to non-Mn competing sites (other Fe) rather than to site that might compete with Mn (bound Fe). This points to a potential for regional specificity related to how Mn and Fe may interact with each other and with various proteins to which they may bind (Table 1; for more extensive information concerning the mathematical equations and the statistical analyses used to validate the model from which data in Table 1 are derived, readers are encouraged to see Zhang et al. 2009b).
While the MR data can suggest information about the apparent binding state of the metals, it cannot provide details about the specific proteins to which the metals are bound. As mentioned above, it is well established that both can bind to DMT-1 and Tf. Other transport proteins, such as those described below, exist as well, which facilitate not only the accumulation, but also the extrusion of Mn and Fe from cells and tissues.
Potential Protein Transporter Interactions
Recent reviews have focused on the ability of DMT-1 (Au et al. 2008; Garrick et al. 2003) and the Tf/TfR system (Macedo and de Sousa 2008; Moos 2002) to transport Mn and Fe in an interdependent manner. Additionally, the relationship between ceruloplasmin and Mn and Fe has been known for many years (Jursa and Smith 2009; Murthy et al. 1981). The most recent study, however, suggested roles for metal transport protein-1 (Wang et al. 2008), ferroportin (Aydemir et al. 2009; Ge et al. 2009; Zhang et al. 2009a), hepcidin (De Domenico et al. 2009; Lee and Beutler 2009), and prion protein (PrP) (Brazier et al. 2008; Choi et al. 2006; Kralovicova et al. 2009) in Fe and Mn transport. Currently, the more complex questions relate to the dependency and interactivity of these proteins on and with one another, and the complex regulation of each by Mn and Fe. As the regulatory roles of hepcidin and PrP to Fe and Mn are likely the least understood, these two proteins, and their interactions with other transporters, will be discussed in further detail.
Hepcidin, Ferroportin, and Transferrin/Transferrin Receptor
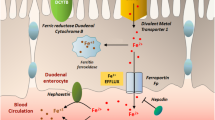
Hepcidin is a 25-amino acid hormone that is synthesized in the liver (Rossi 2005). It is known that translation of hepcidin is directly related to Fe levels: increased Fe facilitates hepcidin synthesis, whereas conditions of anemia or Fe deficiency result from decreased hepcidin synthesis. Ultimately, however, it is the interaction of hepcidin with either the Fe-extrusion protein, ferroportin, found, for example, on duodenal enterocytes and macrophages or with DMT-1 (Mena et al. 2006, 2008) that helps maintain Fe homeostasis (De Domenico et al. 2005). Typically, enterocytes and macrophages store or release Fe as plasma levels increase or decrease, respectively. Currently, however, more is known about hepcidin’s interaction with ferroportin and Tf/TfR.
Under the conditions of low plasma Fe, ferroportin increases the release of Fe from these storage cells so that Fe can be transported throughout the body by the Tf/TfR system. Thus, interactions of these three biomolecules are intimately related in the following manner (Fig. 1a): under conditions of high plasma Fe (or high concentrations of the Fe–Tf complex), hepcidin translation is increased; hepcidin binds to ferroportin, causing the membrane-bound ferroportin to be internalized and degraded (De Domenico et al. 2007, 2009); loss of ferroportin leads to decreased Fe extrusion and lower concentrations of plasma Fe available for binging to Tf; and lowered amounts of Fe–Tf complex result in decreased Fe transport throughout the organism. The converse is true when either plasma Fe or Fe–Tf concentrations are low (Fig. 1b). Interestingly, mice with a mutation in the hemochromatosis gene hfe, which is required for normal synthesis of hepcidin, show dysregulation of mitochondrial Fe, Mn, copper, and zinc levels (Jouihan et al. 2008), demonstrating the importance and interrelationship of Fe and Mn homeostasis. Additionally, the hfe gene product, HFE, is thought to compete with Tf for binding of the TfR (Schmidt et al. 2008).
Prion Protein
Although PrP is typically most noted for its role in the etiology of transmissible spongiform encephalopathies (Caramelli et al. 2006; Caughey and Chesebro 1997), it is also a known metal-binding protein that is concentrated in synapses (Pocchiari et al. 2009; Singh et al. 2009b). For example, ‘normal’ or cellular PrP (PrPC) bound to the membrane of cerebellar neurons appears to aid in copper uptake in vitro (Brown 1999) and in vivo (Brown et al. 1997). Additionally, PrPC has an affinity for nickel, zinc, and Mn (Brown et al. 2000), and has antioxidant properties similar to that of superoxide dismutase (Brown et al. 1997, 1999).
Recent data suggest that PrPC appears to regulate neuronal Fe uptake and transport (Singh et al. 2009a, b, c). While it had been postulated that the presence of Fe and other redox active compounds catalyzed the conversion of PrPC to PrPSC (the pathological variant), it appears more likely now that PrPC interacts with the Tf/TfR system to transport Fe to ferritin for further storage (Fig. 2). When Singh et al. (2009c) transfected human neuroblastoma cells with PrPC, cells with increased amounts of PrPC also demonstrated increased intracellular Fe with a concomitant decrease in Tf and TfR expression. Levels of ferritin were increased, however, suggesting that the cells were appropriately up-regulating the transcription and translation of that Fe storage protein. The authors hypothesize that PrPC may be involved in intracellular Fe transport through enhancing the binding of Fe to Tf, or facilitating the endocytosis of the Fe–Tf/TfR complex (Singh et al. 2009c), although such speculation was beyond the scope of their studies. Furthermore, loss of PrPC, either through knocking out the gene or PrPC conversion to PrPSC, leads to Fe deficiency (Singh et al. 2009a, b). Considering that PrPC (1) aids in the transport of Fe, (2) binds Mn and other divalent metals, and (3) may interact directly with Tf, it seems reasonable to consider this novel protein among the various metal transporter candidates more often cited in the literature.
Protein–Metal Interactions
Our longitudinal MR studies (Finkelstein et al. 2008; Fitsanakis et al. 2008; Zhang et al. 2009b) involving manipulation of both Mn and Fe suggest that there is either (1) differential competition between Mn and Fe for similar proteins or (2) differential expression of various metal transport proteins. In reality, it is likely a combination of the two. The data (Table 1), however, do provide insight as to predictions for putative concentrations of each metal competitively bound to a transport protein. Furthermore, the data from our model may be useful in suggesting concentrations of Mn or Fe that may be bound to a protein specific for a respective metal, or the metal in its unbound state.
Close examination of the results suggests that for most brain regions, Mn treatment, regardless of the dietary Fe levels, should lead to an increase in brain Mn levels bound to competitive and shared Fe binding sites. Should this hold, the amount of Fe bound to these competitive sites would also significantly decrease. Interestingly, the amount of Fe in other forms (i.e., bound to non-competitive, Fe-specific proteins) also decreases. On the one hand, this could suggest that Mn successfully out-competes Fe for these common Fe-binding sites. These data may also point to the exquisite role Fe plays in regulating proteins that also bind Mn. The latter hypothesis is supported by research related to the importance of the Fe responsive element and Fe regulatory protein pathways (Muckenthaler et al. 2008).
Conclusions
Scientists have identified many proteins involved in the up-take, storage, regulation, and efflux of transition metals, particularly Fe and Mn. In order to understand the primary role of these metals in various organisms, many studies, including those from our lab, have examined biological consequences of altering only Fe or Mn. Questions related to the specific protein(s) involved in the disruption of metal homeostasis, however, were beyond the scope of our non-invasive imaging studies. As we and others have demonstrated, the transport and regulation of these metals appears to be intimately intertwined. It is also likely that manipulation of a single metal will lead to disruption of numerous other metals (Garcia et al. 2006; Liu et al. 2001; Malhotra et al. 1984; Sakai et al. 2004). Thus, knowledge of metal metabolism, regulation, and toxicity will be greatly improved as distribution and accumulation patterns of multiple metals, as well as the binding status of multiple transport proteins, are examined in future studies.
References
Abbott PJ (1987) Methylcyclopentadienyl manganese tricarbonyl (MMT) in petrol: the toxicological issues. Sci Total Environ 67:247–255
Anderson JG, Cooney PT, Erikson KM (2007a) Brain manganese accumulation is inversely related to gamma-amino butyric acid uptake in male and female rats. Toxicol Sci 95:188–195
Anderson JG, Cooney PT, Erikson KM (2007b) Inhibition of DAT function attenuates manganese accumulation in the globus pallidus. Environ Toxicol Pharmacol 23:179–184
Anderson JG, Fordahl SC, Cooney PT, Weaver TL, Colyer CL, Erikson KM (2008) Manganese exposure alters extracellular GABA, GABA receptor and transporter protein and mRNA levels in the developing rat brain. Neurotoxicology 29:1044–1053
Anderson JG, Fordahl SC, Cooney PT, Weaver TL, Colyer CL, Erikson KM (2009) Extracellular norepinephrine, norepinephrine receptor and transporter protein and mRNA levels are differentially altered in the developing rat brain due to dietary iron deficiency and manganese exposure. Brain Res 1281:1–14
Au C, Benedetto A, Aschner M (2008) Manganese transport in eukaryotes: the role of DMT1. Neurotoxicology 29:569–576
Aydemir F, Jenkitkasemwong S, Gulec S, Knutson MD (2009) Iron loading increases ferroportin heterogeneous nuclear RNA and mRNA levels in murine J774 macrophages. J Nutr 139:434–438
Bader M, Dietz MC, Ihrig A, Triebig G (1999) Biomonitoring of manganese in blood, urine and axillary hair following low-dose exposure during the manufacture of dry cell batteries. Int Arch Occup Environ Health 72:521–527
Beard JL, Dawson HD (1997) Iron. In: O’Dell BL, Sunde RA (eds) Handbook of nutritionally essential minerals. Marcel Dekker, Inc., New York, pp 275–334
Beaton MD, Adams PC (2007) The myths and realities of hemochromatosis. Can J Gastroenterol 21:101–104
Bowler RM, Koller W, Schulz PE (2006) Parkinsonism due to manganism in a welder: neurological and neuropsychological sequelae. Neurotoxicology 27:327–332
Brazier MW, Davies P, Player E, Marken F, Viles JH, Brown DR (2008) Manganese binding to the prion protein. J Biol Chem 283:12831–12839
Brown DR (1999) Prion protein expression aids cellular uptake and veratridine-induced release of copper. J Neurosci Res 58:717–725
Brown DR, Qin K, Herms JW, Madlung A, Manson J, Strome R, Fraser PE, Kruck T, von Bohlen A, Schulz-Schaeffer W, Giese A, Westaway D, Kretzschmar H (1997) The cellular prion protein binds copper in vivo. Nature 390:684–687
Brown DR, Wong BS, Hafiz F, Clive C, Haswell SJ, Jones IM (1999) Normal prion protein has an activity like that of superoxide dismutase. Biochem J 344(Pt 1):1–5
Brown DR, Hafiz F, Glasssmith LL, Wong BS, Jones IM, Clive C, Haswell SJ (2000) Consequences of manganese replacement of copper for prion protein function and proteinase resistance. EMBO J 19:1180–1186
Burdo JR, Antonetti DA, Wolpert EB, Connor JR (2003) Mechanisms and regulation of transferrin and iron transport in a model blood-brain barrier system. Neuroscience 121:883–890
Caramelli M, Ru G, Acutis P, Forloni G (2006) Prion diseases: current understanding of epidemiology and pathogenesis, and therapeutic advances. CNS Drugs 20:15–28
Caughey B, Chesebro B (1997) Prion protein and the transmissible spongiform encephalopathies. Trends Cell Biol 7:56–62
Chebassier N, El Houssein O, Viegas I, Dreno B (2004) In vitro induction of matrix metalloproteinase-2 and matrix metalloproteinase-9 expression in keratinocytes by boron and manganese. Exp Dermatol 13:484–490
Choi CJ, Kanthasamy A, Anantharam V, Kanthasamy AG (2006) Interaction of metals with prion protein: possible role of divalent cations in the pathogenesis of prion diseases. Neurotoxicology 27:777–787
Chua A, Morgan E (1997) Manganese metabolism is impaired in the Belgrade laboratory rat. J Comp Physiol B 167:361–369
Cooper WC (1984) The health implications of increased manganese in the environment resulting from the combustion of fuel additives: a review of the literature. J Toxicol Environ Health 14:23–46
de Benoist B, McLean E, Egli I, Cogswell M (eds) (2008) Worldwide prevalence of anaemia: 1993–2005. World Health Organization, Geneva
De Domenico I, Ward DM, Nemeth E, Vaughn MB, Musci G, Ganz T, Kaplan J (2005) The molecular basis of ferroportin-linked hemochromatosis. Proc Natl Acad Sci USA 102:8955–8960
De Domenico I, Ward DM, Langelier C, Vaughn MB, Nemeth E, Sundquist WI, Ganz T, Musci G, Kaplan J (2007) The molecular mechanism of hepcidin-mediated ferroportin down-regulation. Mol Biol Cell 18:2569–2578
De Domenico I, Lo E, Ward DM, Kaplan J (2009) Hepcidin-induced internalization of ferroportin requires binding and cooperative interaction with Jak2. Proc Natl Acad Sci USA 106:3800–3805
Finkelstein Y, Zhang N, Fitsanakis VA, Avison MJ, Gore JC, Aschner M (2008) Differential deposition of manganese in the rat brain following subchronic exposure to manganese: a T1-weighted magnetic resonance imaging study. Isr Med Assoc J 10:793–798
Fitsanakis VA, Zhang N, Anderson JG, Erikson KM, Avison MJ, Gore JC, Aschner M (2008) Measuring brain manganese and iron accumulation in rats following 14 weeks of low-dose manganese treatment using atomic absorption spectroscopy and magnetic resonance imaging. Toxicol Sci 103:116–124
Fitsanakis VA, Thompson KN, Deery SE, Milatovic D, Shihabi ZK, Erikson KM, Brown RW, Aschner M (2009) A chronic iron-deficient/high-manganese diet in rodents results in increased brain oxidative stress and behavioral deficits in the morris water maze. Neurotox Res 15:167–178
Fleming MD, Trenor CC III, Su MA, Foernzler D, Beier DR, Dietrich WF, Andrews NC (1997) Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat Genet 16:383–386
Garcia Avila M, Penalver Ballina R (1953) Manganese poisoning in the mines of Cuba. Ind Med Surg 22:220–221
Garcia SJ, Gellein K, Syversen T, Aschner M (2006) A manganese-enhanced diet alters brain metals and transporters in the developing rat. Toxicol Sci 92:516–525
Garcia SJ, Gellein K, Syversen T, Aschner M (2007) Iron deficient and manganese supplemented diets alter metals and transporters in the developing rat brain. Toxicol Sci 95:205–214
Garrick M, Dolan K, Horbinski C, Ghio A, Higgins D, Porubcin M, Moore E, Hainsworth L, Umbreit J, Conrad M, Feng L, Lis A, Roth J, Singleton S, Garrick L (2003) DMT1: a mammalian transporter for multiple metals. Biometals 16:41–54
Ge XH, Wang Q, Qian ZM, Zhu L, Du F, Yung WH, Yang L, Ke Y (2009) The iron regulatory hormone hepcidin reduces ferroportin 1 content and iron release in H9C2 cardiomyocytes. J Nutr Biochem 20(11):860–865
Gregus Z (2008) Mechanisms of toxicity. In: Klassen CD (ed) Casarett and Doull’s toxicology: the basic science of poison. McGraw-Hill Medical, New York
Guilarte TR, Chen MK (2007) Manganese inhibits NMDA receptor channel function: implications to psychiatric and cognitive effects. Neurotoxicology 28:1147–1152
Guilarte TR, Chen MK, McGlothan JL, Verina T, Wong DF, Zhou Y, Alexander M, Rohde CA, Syversen T, Decamp E, Koser AJ, Fritz S, Gonczi H, Anderson DW, Schneider JS (2006) Nigrostriatal dopamine system dysfunction and subtle motor deficits in manganese-exposed non-human primates. Exp Neurol 202:381–390
Gunshin H, Mackenzie B, Berger U, Gunshin Y, Romero M, Boron W, Nussberger S, Gollan J, Hediger M (1997) Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388:482–488
Gunter TE, Gavin CE, Gunter KK (2009) The case for manganese interaction with mitochondria. Neurotoxicology 30:727–729
Hansen SL, Trakooljul N, Liu HC, Moeser AJ, Spears JW (2009) Iron transporters are differentially regulated by dietary iron, and modifications are associated with changes in manganese metabolism in young pigs. J Nutr 139:1474–1479
Jarrah SS, Halabi JO, Bond AE, Abegglen J (2007) Iron deficiency anemia (IDA) perceptions and dietary iron intake among young women and pregnant women in Jordan. J Transcult Nurs 18:19–27
Jensen LT, Carroll MC, Hall MD, Harvey CJ, Beese SE, Culotta VC (2009) Down-regulation of a manganese transporter in the face of metal toxicity. Mol Biol Cell 20:2810–2819
Jouihan HA, Cobine PA, Cooksey RC, Hoagland EA, Boudina S, Abel ED, Winge DR, McClain DA (2008) Iron-mediated inhibition of mitochondrial manganese uptake mediates mitochondrial dysfunction in a mouse model of hemochromatosis. Mol Med 14:98–108
Jursa T, Smith DR (2009) Ceruloplasmin alters the tissue disposition and neurotoxicity of manganese, but not its loading onto transferrin. Toxicol Sci 107:182–193
Kaisman-Elbaz T, Sekler I, Fishman D, Karol N, Forberg M, Kahn N, Hershfinkel M, Silverman WF (2009) Cell death induced by zinc and cadmium is mediated by clusterin in cultured mouse seminiferous tubules. J Cell Physiol 220:222–229
Kohgo Y, Ikuta K, Ohtake T, Torimoto Y, Kato J (2008) Body iron metabolism and pathophysiology of iron overload. Int J Hematol 88:7–15
Kralovicova S, Fontaine SN, Alderton A, Alderman J, Ragnarsdottir KV, Collins SJ, Brown DR (2009) The effects of prion protein expression on metal metabolism. Mol Cell Neurosci 41:135–147
Leach R, Harris E (1997) Manganese. In: O’Dell B, Sunde R (eds) Handbook of nutritionally essential minerals. Marcel Dekker, Inc., New York, pp 335–355
Lee PL, Beutler E (2009) Regulation of hepcidin and iron-overload disease. Annu Rev Pathol 4:489–515
Liu NQ, Xu Q, Hou XL, Liu PS, Chai ZF, Zhu L, Zhao ZY, Wang ZH, Li YF (2001) The distribution patterns of trace elements in the brain and erythrocytes in a rat experimental model of iodine deficiency. Brain Res Bull 55:309–312
Macedo MF, de Sousa M (2008) Transferrin and the transferrin receptor: of magic bullets and other concerns. Inflamm Allergy Drug Targets 7:41–52
Malhotra KM, Murthy RC, Srivastava RS, Chandra SV (1984) Concurrent exposure of lead and manganese to iron-deficient rats: effect on lipid peroxidation and contents of some metals in the brain. J Appl Toxicol 4:22–25
Mathews CK, van Holde KE, Ahern KG (eds) (2000) Electron transport, oxidative phosphorylation, and oxygen metabolism. Biochemistry. Pearson Prentice Hall, Upper Saddle River, NJ, pp 522–559
Mena NP, Esparza AL, Nunez MT (2006) Regulation of transepithelial transport of iron by hepcidin. Biol Res 39:191–193
Mena NP, Esparza A, Tapia V, Valdes P, Nunez MT (2008) Hepcidin inhibits apical iron uptake in intestinal cells. Am J Physiol Gastrointest Liver Physiol 294:G192–G198
Miao L, St Clair DK (2009) Regulation of superoxide dismutase genes: implications in disease. Free Radic Biol Med 47(4):344–356
Mims MP, Prchal JT (2005) Divalent metal transporter 1. Hematology 10:339–345
Miyayama T, Suzuki KT, Ogra Y (2009) Copper accumulation and compartmentalization in mouse fibroblast lacking metallothionein and copper chaperone, Atox1. Toxicol Appl Pharmacol 237:205–213
Moos T (2002) Brain iron homeostasis. Dan Med Bull 49:279–301
Muckenthaler MU, Galy B, Hentze MW (2008) Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu Rev Nutr 28:197–213
Murthy RC, Srivastava RS, Chandra SV (1981) Serum ceruloplasmin in manganese-treated rats. Toxicol Lett 7:217–220
Myers JE, teWaterNaude J, Fourie M, Zogoe HB, Naik I, Theodorou P, Tassel H, Daya A, Thompson ML (2003a) Nervous system effects of occupational manganese exposure on South African manganese mineworkers. Neurotoxicology 24:649–656
Myers JE, Thompson ML, Naik I, Theodorou P, Esswein E, Tassell H, Daya A, Renton K, Spies A, Paicker J, Young T, Jeebhay M, Ramushu S, London L, Rees DJ (2003b) The utility of biological monitoring for manganese in ferroalloy smelter workers in South Africa. Neurotoxicology 24:875–883
Myers JE, Thompson ML, Ramushu S, Young T, Jeebhay MF, London L, Esswein E, Renton K, Spies A, Boulle A, Naik I, Iregren A, Rees DJ (2003c) The nervous system effects of occupational exposure on workers in a South African manganese smelter. Neurotoxicology 24:885–894
Neilson F (2006) Boron, manganese, molybdenum and other trace elements. In: Bowman B, Russell R (eds) Present knowledge in nutrition, vol 2. International Life Sciences Institute, Washington, DC
Park JD, Chung YH, Kim CY, Ha CS, Yang SO, Khang HS, Yu IK, Cheong HK, Lee JS, Song CW, Kwon IH, Han JH, Sung JH, Heo JD, Choi BS, Im R, Jeong J, Yu IJ (2007) Comparison of high MRI T1 signals with manganese concentration in brains of cynomolgus monkeys after 8 months of stainless steel welding-fume exposure. Inhal Toxicol 19:965–971
Peters JL, Dufner-Beattie J, Xu W, Geiser J, Lahner B, Salt DE, Andrews GK (2007) Targeting of the mouse Slc39a2 (Zip2) gene reveals highly cell-specific patterns of expression, and unique functions in zinc, iron, and calcium homeostasis. Genesis 45:339–352
Pocchiari M, Poleggi A, Principe S, Graziano S, Cardone F (2009) Genomic and post-genomic analyses of human prion diseases. Genome Med 1:63
Rondon LJ, Rayssiguier Y, Mazur A (2008) Dietary inulin in mice stimulates Mg2+ absorption and modulates TRPM6 and TRPM7 expression in large intestine and kidney. Magnes Res 21:224–231
Rossi E (2005) Hepcidin—the iron regulatory hormone. Clin Biochem Rev 26:47–49
Rouault TA, Cooperman S (2006) Brain iron metabolism. Semin Pediatr Neurol 13:142–148
Sakai T, Miki F, Wariishi M, Yamamoto S (2004) Comparative study of zinc, copper, manganese, and iron concentrations in organs of zinc-deficient rats and rats treated neonatally with l-monosodium glutamate. Biol Trace Elem Res 97:163–182
Schmidt PJ, Toran PT, Giannetti AM, Bjorkman PJ, Andrews NC (2008) The transferrin receptor modulates Hfe-dependent regulation of hepcidin expression. Cell Metab 7:205–214
Singh A, Isaac AO, Luo X, Mohan ML, Cohen ML, Chen F, Kong Q, Bartz J, Singh N (2009a) Abnormal brain iron homeostasis in human and animal prion disorders. PLoS Pathog 5:e1000336
Singh A, Kong Q, Luo X, Petersen RB, Meyerson H, Singh N (2009b) Prion protein (PrP) knock-out mice show altered iron metabolism: a functional role for PrP in iron uptake and transport. PLoS One 4:e6115
Singh A, Mohan ML, Isaac AO, Luo X, Petrak J, Vyoral D, Singh N (2009c) Prion protein modulates cellular iron uptake: a novel function with implications for prion disease pathogenesis. PLoS One 4:e4468
Skalicky A, Meyers AF, Adams WG, Yang Z, Cook JT, Frank DA (2006) Child food insecurity and iron deficiency anemia in low-income infants and toddlers in the United States. Matern Child Health J 10:177–185
Sung JH, Kim CY, Yang SO, Khang HS, Cheong HK, Lee JS, Song CW, Park JD, Han JH, Chung YH, Choi BS, Kwon IH, Cho MH, Yu IJ (2007) Changes in blood manganese concentration and MRI t1 relaxation time during 180 days of stainless steel welding-fume exposure in cynomolgus monkeys. Inhal Toxicol 19:47–55
Toteja GS, Singh P, Dhillon BS, Saxena BN, Ahmed FU, Singh RP, Prakash B, Vijayaraghavan K, Singh Y, Rauf A, Sarma UC, Gandhi S, Behl L, Mukherjee K, Swami SS, Meru V, Chandra P, Chandrawati, Mohan U (2006) Prevalence of anemia among pregnant women and adolescent girls in 16 districts of India. Food Nutr Bull 27:311–315
Wang X, Li GJ, Zheng W (2008) Efflux of iron from the cerebrospinal fluid to the blood at the blood-CSF barrier: effect of manganese exposure. Exp Biol Med 233:1561–1571
WHO (2003) Micronutrient deficiencies: battling iron deficiency anaemia, vol 2003. WHO
Wood RJ (2009) Manganese and birth outcome. Nutr Rev 67:416–420
Yang F, Friedrichs WE, Coalson JJ (1997) Regulation of transferrin gene expression during lung development and injury. Am J Physiol 273:L417–L426
Zhang DL, Hughes RM, Ollivierre-Wilson H, Ghosh MC, Rouault TA (2009a) A ferroportin transcript that lacks an iron-responsive element enables duodenal and erythroid precursor cells to evade translational repression. Cell Metab 9:461–473
Zhang N, Fitsanakis VA, Erikson KM, Aschner M, Avison MJ, Gore JC (2009b) A model for the analysis of competitive relaxation effects of manganese and iron in vivo. NMR Biomed 22:391–404
Zhou SJ, Gibson RA, Crowther CA, Baghurst P, Makrides M (2006) Effect of iron supplementation during pregnancy on the intelligence quotient and behavior of children at 4 y of age: long-term follow-up of a randomized controlled trial. Am J Clin Nutr 83:1112–1117
Acknowledgment
This study was partially supported by NIEHS 015628 (V.A.F.), NIEHS 10563 (M.A.), and DoD W81XWH-05-1-0239 (M.A.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fitsanakis, V.A., Zhang, N., Garcia, S. et al. Manganese (Mn) and Iron (Fe): Interdependency of Transport and Regulation. Neurotox Res 18, 124–131 (2010). https://doi.org/10.1007/s12640-009-9130-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-009-9130-1