Abstract
Manganese and iron were found at high concentrations (3.61 mg/L and 19.8 mg/L, respectively) in the water of the Rio Doce after the dams of Fundão and Santarém broke in Mariana/MG (Brazil). These same metals were found in fish and crustacean muscle (15 mg/kg and 8 mg/kg wet weight, respectively) in the specimens collected near the Rio Doce’s outfall. Due to the variation in Mn concentration found in the lower Rio Doce, this study aimed to determine the effects of Mn in Oreochromis niloticus, at the concentrations allowed by CONAMA, and in concentrations found in the Rio Doce after the dams broke. The animals were exposed to the following dissolved concentrations: control group (0.0 mg/L), 0.2; 1.5 and 2.9 mg/L manganese for 96 h. In addition, a positive control was conducted, injecting intraperitoneally with cyclophosphamide (at 25 mg/kg). These exposures caused significant erythrocyte micronucleus formation in the organisms exposed to the highest concentration, as well a significant increase in the DNA damage index of erythrocytes from organisms exposed to 1.5 mg/L and 2.9 mg/L treatments. The glutathione S-transferase enzyme activity also showed a significant increase in the liver of the organisms exposed to 2.9 mg/L. However, catalase activity increased significantly in the gills of the animals exposed to all concentrations of manganese that were tested. Manganese bioconcentrated in greater quantities in the liver than the gills. Thus, manganese causes significant damage to genetic material, generates nuclear abnormalities, activates the body’s detoxification system and can accumulate in animal tissue.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The extensive use of water by humans makes this the resource most used by man (Andreoli and Carneiro 2005; Mitic et al. 2013) but has compromised its quality (Bruchchen et al. 2013). Most contaminants found in the ecosystems are from industrial, agricultural or domestic sources (Cantanhêde et al. 2016; Libânio 2005). In the aquatic environment, dissolved or suspended chemicals may be absorbed by organisms through the contact surface, gastrointestinal tract or gills (Fontaínhas-Fernandes 2005; Saha and Naman 2011). Many organisms have a high potential to bioconcentrate metals from the environment (Repula et al. 2012).
Manganese (Mn) is an abundant metal in natural environments (Bordean et al. 2014). It is a common industrial product and an element from iron ore deposits (Pereira et al. 2008). Mn is an essential metal for some vital functions in microquantities (in humans 2.3 mg/day for adult males and 1.8 mg/day for adult females) (Food and Nutrition Board 2001) such as the synaptic neurotransmission (Duran et al. 2014; Vieira et al. 2012) and is necessary for bone structure and reproduction (Adeyeye et al. 1996). However, Mn can cause neurological disorders (Rocha and Afonso 2012), decrease natural populations, and cause respiratory, metabolic and endocrine changes, thereby altering concentrations of steroid hormones and resulting in losses in the reproductive process such as in concentration of approximately 0.6 mg/L, in Astyanax bimaculatus (Kida 2014). It was also reported that Mn causes oxidative stress (at 8.4 and 16.2 mg/L), mitochondrial dysfunction (at 4.2, 8.4 and 16.2 mg/L), anemia and interferes in carbohydrate metabolism (at 8.4 and 16.2 mg/L) in fish (Rhamdia quelen) (Dolci et al. 2013).
The Rio Doce basin (in Brazil) has a history of intense human occupation and economic activities, which consequently generate pollution from chemical compounds. One of the most common metals that has shown a relevant increase in concentration in sediment is Mn (ranging from 50 mg/kg to 200 mg/kg), possibly due to mining-related activities occurring locally. At the end of 2015, the Fundão and Santarém dams broke, culminating in the leakage of approximately 34 million cubic meters of tailing sludge from iron mining (Brasil 2015; Freitas et al. 2016). This incident will be noted in history and ecosystems as one of the greatest socioenvironmental tragedies in the country. The mud covered 663 km along the Rivers Gualaxo do Norte and Doce, reaching two states, Minas Gerais and Espírito Santo (Porto 2016). According to Carvalho et al. (2017), in August 2016, Mn, similar to other metals, was still at concentrations above the maximum value allowed in the water by CONAMA Resolution 357/05, which is 0.5 mg of Mn/L (Brasil 2005), reaching 1.638 mg/L of Mn dissolved in the water. Similar results were found by Maia (2017) for Mn dissolved in Rio Doce water (concentrations 10 times higher than that allowed by CONAMA Resolution 357/05). Additionally, the Mn concentrations found in fish and crustacean muscle were higher in the specimens collected near the Rio Doce’s outfall, reaching 15 and 8 mg/kg wet weight, respectively (Bianchini 2016).
Given these events, it is crucial to carry out environmental biomonitoring of the impacted area and to perform these monitoring analyses using bioindicators, which are considered essential instruments, (Freitas and Siqueira-Souza 2009); moreover, numerous studies have been conducted around the world (Jovičić et al. 2015; Zhuang et al. 2013). Fish are considered excellent bioindicators because they are capable of reflecting disturbances at different environmental scales due to their biological and ecological characteristics (Jovičić et al. 2015; Lins et al. 2010). The biomarkers are also used to assess biological responses that reflect the toxic effects of chemical substances (Bebianno et al. 2015; Isla-Flores et al. 2013). Among the biomarkers of exposure, the most commonly used in fish are enzymes involved in the detoxification of xenobiotics and their metabolites (Cappello et al. 2013; Klaassen 2001; Lech and Vodicnik 1985; Natalotto et al. 2015; van der Oost et al. 2003), and genotoxicity biomarkers, such as comet assay and micronucleus test (Kendall et al. 2001; Peakall 1994). The combined use of different biomarkers is encouraged to obtain a broader understanding of the responses of the organism to the contaminant, to predict the severity of the damages caused and to establish a cause and effect relationship (Brandão et al. 2011). Many of these biomarkers are initially tested in laboratory assays, where exposure experiments are performed on test organisms for different toxic agents in which changes in biomarker responses are evaluated. In this way, one can ascertain the effects of the substance in isolation or in synergy with other substances; however, under these conditions, physico-chemical parameters are kept constant so that they do not influence the results. Based on the results obtained with the biomarker and bioindicator responses, one can then extrapolate to what may happen in the environment, and thus, in the future, be used and applied to take measures to protect the ecosystem. These laboratory analyses also allow biomarkers to be carried out in environmental biomonitoring studies.
Due to the variation in Mn concentration found in the lower Rio Doce, this study aimed to determine genotoxic, biochemical and bioconcentration effects of Mn in Oreochromis niloticus, at the concentration allowed by CONAMA and in concentrations found in the Rio Doce after the dams broke. This species was used because of its countrywide distribution, including the Rio Doce, and because it can be easily acquired from farmers.
Materials and methods
Acclimatization
A total of 50 O. niloticus juveniles (8.5 ± 1.5 g) were purchased from aquaculture (Guarapari, Espírito Santo) and transported to the Applied Ichthyology Laboratory (LABPEIXE/UVV), where they were stored in a 500 L plastic tank in filtered and dechlorinated water, with constant aeration for 3 months for acclimatization. During this period, 50% of the water in the tank was exchanged once time a week, and the physical–chemical characteristics of the water were monitored six times a week: temperature = 29.73 ± 0.79 °C, dissolved oxygen = 6.48 ± 0.3 mg/L, pH = 6.60 ± 0.34, total ammonia = 0.93 ± 0.37 mg/L, nitrite = 0.44 ± 0.31 mg/L, total hardness = 52.94 ± 3.83 mg CaCO3/L and alkalinity = 8.96 ± 4.61 mg CaCO3/L. These analyses were performed using EcoSense pH 100 A equipment, EcoSense YSI DO 200 and following standard methods (APHA 1989). The acclimatization tanks were maintained under 12 h of light and 12 h of darkness. The animals were fed daily with commercial feed with 55% protein. The use of these animals in the present experiment was approved by the Committee on Ethics, Bioethics and Animal Welfare (CEUA – UVV) according to protocol No. 371–2016.
Experimental design
After the acclimatization period, 32 specimens (21.75 ± 8.2 g and 11.88 ± 2.3 cm) were placed in an individual glass aquarium (6 L), which contained dechlorinated water with constant aeration, where they remained for 24 h before the start of the experiments. The specimens were divided into treatments with different dissolved concentrations of Mn: (1) 0.0 mg of Mn/L (no contaminant – control group); (2) 0.2 mg Mn/L; (3) 1.5 mg Mn/L and (4) 2.9 mg Mn/L (n = 8 for each treatment). In addition, a group of fish injected with cyclophosphamide (CPM), a well-known genotoxic drug, was used as positive control. For this procedure, fish were sedated with 0.1 g/L benzocaine solution, weighed, and injected with CPM (at 25 mg/kg), and then transferred to a glass aquarium containing dechlorinated water in the same conditions as the control group. At the beginning of the experiments (0 h), the contaminant was carefully added to water of each test aquarium, and then fish remained under these conditions for 96 h. To achieve the dissolved Mn concentrations tested (0.2, 1.5 and 2.9 mg/L), the following volumes of MnCl2.4H2O were added in the glass aquariums (6 L): 1, 5 and 10.46 mL, respectively. The MnCl2.4H2O was added to the aquariums by a 2000 mg of Mn/L stock solution. Daily, during this period, possible deaths were observed. Feeding of the animals was suspended 24 h before the fish were transferred to the aquariums.
The choice of 0.2 mg/L Mn for one of the experimental groups was chosen because this level is near to the limit concentration on the definition of Freshwater Class 2 provided in CONAMA (Conselho Nacional do Meio Ambiente/National Environment Council) Resolution no. 357/05 (0.5 mg/L); 1.5 mg/L Mn was selected based on previous study that shows the dissolved concentration of 1.638 mg of Mn/L in the Rio Doce water (Carvalho et al. 2017); and 2.9 mg/L Mn was included because it simulates the large Mn concentrations present in the environment (Segura et al. 2016). Mn chloride analytical reagent (AR) (MnCl2.4H2O) was purchased from Sigma-Aldrich®.
Analyses of physico-chemical water parameters (temperature, dissolved oxygen, pH, total ammonia, nitrite, total hardness, and alkalinity) were performed at the initial time (0 h), just after the water contamination, and at the end of the experiment (96 h) (Table 1).
After the experiment, the animals were sedated with 0.1 g/L benzocaine solution. Blood collection was performed via caudal puncture using pre-heparinized syringes for the micronucleus test and comet assay. Liver, muscle and gills were also removed after a cervical section euthanasia procedure. Half of the liver, gill and muscle samples were stored in a freezer at −80 °C for biochemical analysis, and the other half of samples of all these tissues were also stored at −20 °C for metal quantification.
Genotoxic analysis
The micronucleus test was performed using blood drawn on slides according to Grisolia et al. (2005). Two slides were prepared for each animal (8 animals per treatment, for a total of 16 slides for each treatment). The dried blood material was fixed with methanol for 30 min, stained with 5% Giemsa solution for 40 min, and washed and dried at room temperature. The micronuclei were identified following the criteria proposed by Fenech et al. (2003), and the mean frequency of micronuclei (‰) present in erythrocytes was calculated for each individual.
The comet assay was performed by the alkaline method, and staining with silver nitrate following the methodology of Tice et al. (2000) with modifications and was performed using two slides per individual. The slides were precoated with 1.5% agarose, and the blood material diluted in RPMI solution was mixed with low melting agarose at 37 °C. After being dried, the slides were placed in lysis solution (2.5 M NaCl, 100 mM EDTA and 10 mM Tris, pH 10.0–10.5), 1% Triton X-100 and 10% DMSO in a refrigerator and sheltered from light. The slides were then placed in electrophoresis cell and immersed in electrophoretic buffer for 20 min, followed by electrophoretic run at 25 V and 300 mA for 15 min. The slides were neutralized with TRIS buffer and stained with silver nitrate. The comets formed were observed under an optical microscope at 40-fold magnification, and 100 cells per slide were counted. The damage was classified from 0 (undamaged) to 4 (maximum damage) according to the shape and size of the tail, where the tail size is proportional to the number of DNA fragments (Collins 2004).
Biochemical analysis
The animal tissues were stored in a freezer (Ultra Freezer CL 120 −80 °C) until the period of analysis. For analyses of glutathione S-transferase (GST), catalase (CAT) and total proteins, the tissues were homogenized with phosphate buffer (pH 7.0) and centrifuged (13,000 rpm) for 30 min at 4 °C to obtain the supernatants.
The absolute activity of the GST enzyme was estimated using phosphate buffer (pH 7.0), 1 mM 1-chloro-2,4-dinitrobenzene (CDNB) and 1 mM GSH as substrate, for 1 min, at 10-s intervals (Habig et al. 1974). The GST activity was calculated from the absorbance obtained using the Absorbance Microplate Reader SpectraMax 190, at the wavelength of 340 nm. The results are expressed as U/mg of protein/min. The CAT enzyme activity was evaluated by the continuous observation of the decrease of the hydrogen peroxide (H2O2) concentration, for 1 min, at 10-s intervals. The reaction medium was used as reaction medium 10 mM H2O2, 1 M TRIS HCl and 5 mM EDTA (Aebi 1984), and absorbance reading was performed using 10 μL of the sample with 690 μL of the reaction medium in a spectrophotometer Biospectro SP-220, at wavelength of 240 nm. The results are expressed in μmol H2O2 metabolized/min/mg of protein. The concentration of total proteins in the liver and gill was determined by the Bradford method Bradford (1976), and the samples were assayed in Absorbance Microplate Reader SpectraMax 190, at the wavelength of 595 nm. The analysis of total proteins was necessary to calculate the enzymatic activities. In all biochemical analyses, two replicates were made for each animal (eight animals per treatment).
Mn bioconcentration
The bioconcentration of Mn was performed according to Veronez et al. (2016), with adaptations. Water samples were collected from each aquarium soon after the addition of the contaminant (0 h) and at the end of the experimental time (96 h). For quantification of the metal dissolved in water, the samples (n = 8 per treatment) were acidified with 10% of the total volume of the samples with nitric acid (65%), and before reading, the samples were filtered with pre-cleaned and non-sterile 13 mm Analitica filters with 0.45 μm pores. For the quantification of metal present in the tissues, the wet weight of the tissues was first measured. Half of liver, muscle and gill samples (n = 8 per treatment of each tissue) were stored in falcon tubes (15 mL), where 2 mL of nitric acid (65%) was added for digestion. Then, the falcon tubes were incubated in an ultrasonic bath to accelerate the digestion process.
The concentration of the Mn in the digested samples and dissolved in the water was determined by atomic absorption spectrophotometry (detection limit = 0.297 µg of Mn/L; quantification limit = 0.899 µg of Mn/L) in the graphite mode (Analítica, ASS ICE 3300) by the Laboratory of Applied Ichthyology (LABPEIXE), University of Vila Velha (UVV). The results are expressed as μg/g (wet weight).
The results obtained for the reference material were evaluated using ERM-BB422 (European Reference Materials®) fish tissue for Mn analysis. The sample of this certified reference material was treated and analyzed in the same way as the samples of the biological material collected and evaluated in the present study, as previously described. The certified value for the reference material was 0.368 ± 0.028 mg/kg, and the measured value was 0.341 mg/kg, with a recuperation of 92.66%.
Statistical analysis
Normality of the results was tested using the Shapiro–Wilk test, and the data were then compared between the treatments. Differences were detected by a one-way analysis of variance (ANOVA), followed by the Tukey post-test for multiple comparisons, and the control (without addition of Mn) and positive control groups were compared by t-test. Linear regression was performed between the Mn accumulation in the evaluated tissues (muscle, liver and gills) and the individual’s weight. Sigma Plot 12.0 software was used for the analysis. Significant differences were defined when p < 0.05.
Results
Water physico-chemical parameters
The values were expressed by the mean ± standard deviation of two measurements of all aquariums: at the beginning (0 h) and at the end of experiment (96 h). The values obtained from the physico-chemical parameters in the water of the aquarium tests are presented in Table 1. The parameters evaluated did not show differences among the treatments, indicating the maintenance of the quality of the water.
The concentrations of the Mn dissolved in the water measured by spectrophotometry were 0.2 mg/L, 1.5 mg/L and 2.9 mg/L at the beginning of the experiment (0 h). At the end (96 h), the measurements were 0.1 mg/L, 0.3 mg/L and 0.7 mg/L. This decrease in the concentration of bioavailable metal after the experimental period is probably due to fish bioconcentration of the metal.
During the experiment, there was no mortality in any specimen.
Genotoxic analysis
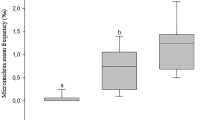
The exposure of individuals to Mn caused the formation of micronuclei in the erythrocytes; however, there was a significant (p < 0.001) incidence of micronuclei (1.2‰), only at the 2.9 mg/L concentration, compared with the control group (0.13‰) and to the other treatments (Fig. 1a). In 0.2 mg/L and 1.5 mg/L concentrations, and the following micronucleus frequency means were obtained: 0.31‰ and 0.56‰, respectively. In the positive control (25 mg/kg of CPM), the incidence of micronuclei was 2.3‰.
a Mean frequency of micronucleated erythrocytes (‰) and (p < 0.001). b DNA damage index (DI) (p < 0.001) in erythrocytes of Oreochromis niloticus (n = 8) exposed to different concentrations of manganese and a positive control group (Cyclophosphamide at 25 mg/kg) for 96 h. The results are expressed as the mean and standard deviation. Different letters indicate significant differences (p ≤ 0.05) between the treatments (ANOVA test). Asterisk marks (*) indicate significant differences between positive control and control groups (t test) (t = 12. 167; df = 14; p < 0.001)
For the DNA damage index, there was a significant (p < 0.001) increase in erythrocytes from fish exposed to Mn at 2.9 mg/L concentration, resulting in the highest incidence of DNA damage (Fig. 1b). In 2.9 mg/L treatment, most of the cell fragments were classified as class 3 (severe damage) or 4 (very severe damage) compared with organisms exposed to other concentrations. This incidence of damage in classes 3 and 4 reflects the higher toxicity and capacity to cause damage to the genetic material of 2.9 mg of Mn/L, whereas the other concentrations caused minor injuries.
Biochemical analysis
The GST activity showed a significant (p < 0.001) increase in the livers of the exposed organisms only at the highest concentration (2.9 mg/L) compared with the other treatments (0.2 mg/L and 1.5 mg/L) and the control group, indicating the activation of this enzyme at high concentrations of Mn (Fig. 2a). The different treatments resulted in no significant difference in CAT activity in the liver of O. niloticus individuals (Fig. 2b).
a Activity of glutathione S-transferase (p < 0.001) and b catalase (p = 0.358) in the liver; and c glutathione S-transferase (p = 0.228) and d catalase (p = 0.01) in the gills of Oreochromis niloticus (n = 8) exposed to different concentrations of manganese. The results are expressed as the mean and standard deviation. Different letters indicate significant differences (p ≤ 0.05) between the treatments
When the gills of O. niloticus individuals were analyzed, GST activity did not change in individuals exposed to Mn when compared with the control group (Fig. 2c). Regarding CAT enzyme activity in the gills, a significant increase (p = 0.01) in activity was observed in all treatments compared with the control group (Fig. 2d).
Mn bioconcentration
Linear regression was performed between the Mn accumulation in the evaluated tissues (muscle, liver and gills) and the individual’s weight but was not significant (liver: r² = 0.148, p = 0418; gills: r² = 0.08, p = 0.648; muscle: r² = 0.123, p = 0.501). The bioconcentration analysis of Mn in the different tissues showed that this metal is liable to bioconcentrate in O. niloticus tissues. The liver showed high bioconcentration of Mn, followed by the gills and muscle. The treatment groups showed increasing bioconcentration of Mn in the liver compared with the control group, and at 2.9 mg/L and 1.5 mg/L, concentrations presented a significant difference compared with the control group but not compared with each other (Fig. 3a). The 2.9 mg/L treatment showed a mean of 27.97 μg Mn/g wet weight of the sample (liver), and the other treatments had a mean of 3.87 μg/g and 7.68 μg/g in 0.2 mg/L and 1.5 mg/L, respectively.
a Bioconcentration of manganese in the liver (p = 0.011), b gills (p < 0.001) and c muscle (p = 0.067) of Oreochromis niloticus (n = 8) exposed to different concentrations of manganese. The results are expressed as the mean and standard deviation. Different letters indicate significant differences (p ≤ 0.05) between the treatments
In the gills, there was a significant increase (p < 0.001) in Mn bioconcentration only in the individuals exposed to the highest concentration tested (2.9 mg/L treatment) compared with the other treatments and to the control group (Fig. 3b); this group had a mean value of 9.18 μg/g wet weight sample and significantly differed from the other treatments.
Muscle had the lowest bioconcentration of Mn compared with the other tissues (hepatic and gill tissues), presenting mean values of 0.61 μg/g, 0.71 μg/g, 2.13 μg/g and 2.43 μg/g body weight for the control, and 0.2 mg/L, 1.5 mg/L and 2.9 mg/L treatments, respectively. In addition, there was no significant difference between the different concentrations of Mn used (Fig. 3c).
Discussion
According to a 2016 report, after the accident in the Rio Doce, the concentration of Mn presents a gradient where the highest values are found at the outfall of the river (Bianchini 2016), so we conducted this study to evaluate the effects of this metal on fish. We observed that the exposure to Mn, during 96 h, can induce and increase micronucleus in the erythrocytes of O. niloticus. According to Winter et al. (2007), Mn can cause chromosomal breaks or changes in the mitotic spindle during the period of cell division, demonstrating its toxic potential. Micronucleus can be formed due to the interaction of physical, chemical or biological agents with structures, such as the mitotic spindle and the centromere, thereby promoting changes in the mitotic apparatus and resulting in failure of chromosome segregation (Souza and Fontanetti 2006). The results obtained in this work at the 2.9 mg/L concentration are supported by the literature demonstrating that Mn causes DNA damage and interferes with the fidelity of genetic replication, both in laboratory experiments and in environmental biomonitoring analyses (Costa and Aschner 2014; Duarte et al. 2012; Falfushynska et al. 2011). Falfushynska et al. (2011) showed that Carassius auratus gibelio individuals exposed to 1.7 mg/L Mn for 14 days had a significant incidence of micronucleus (5‰), and a significant result (4‰) for the same species exposed to 0.05 mg/L of copper for 14 days was also observed. Duarte et al. (2012) also showed a significant result (1.56 ‰) studying Oreochromis niloticus from a polluted lagoon at Espírito Santo, Brazil (where the Mn concentration was 0.052 mg/L).
As in the micronucleus test, the DNA damage index analysis, performed using the comet assay, showed a significant increase in organisms exposed to the 1.5 mg/L and 2.9 mg/L treatments. This test assesses damages with pre-mutagenic potential, as it is these injuries that can be repaired in the cells. The results showed that Mn was able to cause significant damage to the DNA of erythrocytes of O. niloticus following acute exposure; thus confirming its potential to generate damage to the genetic material in the analyzed cells. The integrity and stability of the genetic material are essential for cellular balance, the passage of complete genetic information to the daughter cells and for the survival of the organism (Scalon et al. 2010). These functions may have been impaired in the O. niloticus specimens exposed to the highest concentrations of Mn tested in this work. Various pollutants such as metals can act directly on organisms or through free oxygen radicals, generating degenerative processes and causing genotoxic effects (Halliwell and Gutteridge 1985). Our results agree with those obtained by Matsumoto et al. (2006), who analyzed the genotoxicity of Mn and the index of damage to the genetic material of O. niloticus in Córrego dos Bagres, Franca – SP, and by Ahmed et al. (2011), who exposed Oreochromis mossambicus individuals to arsenic for 96 h and observed a significant increase in DNA damage. In addition, as in the present study, the increase in the damage to genetic material was shown to be dose dependent, with the highest concentration tested resulting in an increased damage index. The researchers also demonstrated that metals other than Mn can cause DNA damage.
Toxicological bioassays with samples collected in the Rio Doce (Brazil) soon after the accident in November of 2015 indicated cytotoxic potential and DNA damage, which was shown by the damage index induced by exposing Human Hepatocellular Carcinoma epithelial cells (HepG2 cells) to the Rio Doce water samples (Segura et al. 2016). In a laboratory experiment where individuals of Geophagus brasiliensis, another species of cichlid, were exposed to water from the Rio Doce before and after the break of the Fundão and Santarém, Mariana/MG dams, results showed a significant increase in the frequency of micronucleus (4‰ before the break and 4.5‰ after the break) and of the damage index (200 before the break and 160 after the break), demonstrating that even before the accident, Rio Doce water already had genotoxic potential (Gomes et al. 2018). Together with the Escobar (2015) data, these results confirm that the Rio Doce was already polluted, but the mining tailings contributed with pollutants harmful to the local fauna.
The presence of toxic agents in the aquatic environment can affect the synthesis of detoxification enzymes such as GST, and produce ROS at the same time (Sreejai and Jaya 2010). These changes, in turn, activate expression of a gene (CYP1A) linked to the production of enzymes of the antioxidant process, such as CAT, to decrease the concentration of reactive oxygen species (ROS) within animal cells (Sheehan et al. 2007). In the present work, GST activation was observed only in the hepatic tissues of the O. niloticus specimens exposed to the highest concentration of Mn, whereas CAT showed a significant increase in activity in the gill tissues of the organisms exposed to all Mn concentrations. According to Padmini and Usha Rani (2009), the enzymatic activity can be increased or inhibited by exposure to metals, depending on the concentration and the period of exposure. Exposure to metals may increase liver GST activity (Guilherme et al. 2008), as shown in the present study, and in GST activity in the gills, as shown in similar results that by Dautremepuits et al. 2009. This activation of the detoxification system of the organism occurs due to the functions of various enzymes, which neutralize toxic compounds and convert them to hydrophilic compounds, thus facilitating their excretion from the organism. CAT is the primary excretion enzyme of hydrogen peroxide; therefore, when its activity is low, the availability of hydrogen peroxide in the organism is higher (Cerqueira and Fernandes 2002; Monteiro et al. 2009). In this study, the enzyme activity was higher in the hepatic than gill tissues. Similar results were obtained by Atli and Canli (2007), who exposed O. niloticus individuals to different concentrations (5, 10 and 20 µM) of cadmium, copper, zinc and lead for 14 days and observed that the liver is the organ most capable of combating oxidative stress and the organ with the highest activity of antioxidant enzymes such as CAT. Together with the action of superoxide dismutase (SOD), CAT represents a first defense system of the organism against oxidative stress (Qu et al. 2014).
In an aquatic environment, when exposed to high levels of metals, fish can absorb these bioavailable contaminants through the gills or skin or ingest them in contaminated food and water. These metals are carried by the bloodstream and sent to various organs (van der Putte and Pärt 1982). The accumulation of metals may vary according to the physiological differences and position of the tissue in the body of the fish (Kotze 1997). The accumulation of Mn by fish also depends on the availability of the metal in the environment, the water parameters and the individual characteristics and conditions of each organism (Bervoets and Blust 2003).
The liver had a mean concentration of all treatments (0.2 mg/L, 1.5 mg/L and 2.9 mg/L) of 21.27 μg/g wet weight, followed by 4.49 and 1.77 μg/g body weight for gills and muscle, respectively. According to Niemic et al. (2015), the fish liver is characterized by a high ability to bioconcentrate Mn and therefore is widely used in Mn pollution monitoring biomonitoring. The presence of essential metals, such as Mn, is common in the fish liver due to its role in metabolism in the animal (Zhao et al. 2012). However, the accumulation of Mn in the gills is directly linked to the process of water absorption, since this organ is in constant contact with the aquatic environment due to the respiratory process (Hernroth et al. 2004). However, contrary to what was found in other studies (Badr et al. 2014; Gabriel et al. 2013), in the present work, a higher concentration of Mn was found in the liver than the gills. In Badr et al. (2014), tilapia (O. niloticus) collected in the Nile River were analyzed, and Mn accumulation in the gills was 5.61 μg/g wet weight and that in the liver was 1.25 μg/g wet weight. In the present study, muscle had low accumulation of Mn, similar to the results found in other studies, such as Badr et al. (2014), since muscle is not a typical tissue for significant biotransformation and bioconcentration of metals (Elnabris et al. 2013). The presence of lower concentrations of Mn in the muscle is important because the muscle is an edible part of the animal and is widely consumed throughout the world (Heath 1991; Iranshani et al. 2011). Mn concentration 5.13 μg/g was found in Paralichthys sp. and 2.08 μg/g in Balistes capriscus muscles; these were animals collected in the outfall of the Doce River after the break dams (Bianchini 2016). The analyses carried out in the present study showed that the metal was concentrated more in the inedible portions than in the fish’s edible tissues, corroborating the results obtained by Iranshani et al. (2011), Steenkamp et al. (1994) also shows that the highest Mn tissue concentrations found in freshwater crabs (Potamonautes warreni) from polluted sites were found in the carapace (587 µg/g wet weight) instead of in the muscle (15 µg/g wet weight).
According to Ye et al. (2009), high concentrations of Mn in water result in bioconcentration of this metal in tissues of various organisms, in addition to causing changes in sodium balance, reduced calcium and phosphorus absorption, caused alterations in carbohydrate metabolism and impaired immune functions of the animal. Additionally, at high concentrations, this metal may still cause changes in synaptic processes and the central nervous system because Mn ions cross the blood–brain barrier. Thus, exposure to high concentrations of Mn causes harmful effects in organisms. In the present study, genotoxic and biochemical alterations were also observed in O. niloticus specimens. In general, activities such as mining and related activities or impacts imply changes in the environment such as impairment of water quality (Oliveira et al. 2015). Some studies have pointed to a risk of extinction of some species typical of the river, besides the alteration of the entire trophic chain and will continue to cause impacts on local biodiversity in the short, medium and long term (Espindola et al. 2016), including loss of biodiversity, ecosystem compromise and loss of ecosystem services (Garcia et al. 2016).
Conclusion
After carrying out the analyses, we found that Mn at high concentrations, such as 2.9 mg/L, causes significant genotoxic damage, promoting a significant formation of erythrocyte micronuclei and causing substantial damage to the genetic material of erythrocytes. The exposure of O. niloticus organisms to Mn probably stimulated the activation of the GST and CAT enzymes, which detoxify the fish organism and can be accumulated in greater quantities in the liver, followed by the gills and muscle. This fact is important because tilapia is economically valuable. The authors consider it necessary to carry out future studies evaluating the quality of the Rio Doce sediment, as well as the synergistic effects of the metals found in greater concentrations in this environment.
References
Adeyeye EI, Akinyugha NJ, Fesobi ME, Tenabi VO (1996) Determination of some metals in Clarias gariepinus (Cuvier and Vallenciennes), Cyprinus carpio (L.) and Oreochromis niloticus (L.) fishes in a polyculture freshwater pond and their environments. Aquaculture 147:205–214
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ahmed MdK, Habibullah-Al-Mamum Md, Hossain MA, Arif M, Parvin E, Akter MA, Khan MS, Islam MdM (2011) Assessing the genotoxic potentials of arsenic in tilapia (Oreochromis mossambicus) using alkaline comet assay and micronucleus test. Chemosphere 84(1):143–149
Andreoli CV, Carneiro C (2005) Gestão integrada de mananciais de abastecimento eutrofizados. Eng Sanit Ambient 10(2):95
APHA - American Public Health Association, American Water Works Association, and Water Pollution Control Federation (1989) Standard Methods for the Examination of Waste, Sewage, and Industrial Wastewater, 17th edition. APHA, Washington, DC
Atli G, Canli M (2007) Enzymatic responses to metal exposures in freshwater fish Oreochromis niloticus. Comp Biochem Physiol C Toxicol Pharmacol 145(2):282–287
Badr AM, Mahana NA, Eissa A (2014) Assessment of heavy metal levels in water and their toxicity in some tissues of Nile Tilapia (Oreochromis niloticus) in river Nile basin at Greater Cairo, Egypt. Global. Veterinaria 13(4):432–443
Bebianno MJ, Pereira CG, Reya F, Cravo A, Duarte D, D’Errico G, Regoli F (2015) Integrated approach to assess ecosystem health in harbor areas. Sci Total Environ 514:92–107
Bervoets L, Blust R (2003) Metal concentrations in water, sediment and gudgeon (Gobio gobio) from a pollution gradient: relationship with fish condition factor. Environ Pollut 126:9–19
Bianchini A (2016) Avaliação do impacto da lama/pluma Samarco sobre os ambientes costeiros e marinhos (ES e BA) com ênfase nas Unidades de Conservação. 1ª Expedição do Navio de Pesquisa Soloncy Moura do CEPSUL/ICMBio. Universidade Federal do Rio Grande – FURG
Bordean DM, Niica DV, Harmanescu M, Banatean-Dunea I, Gergen II (2014) Soil manganese enrichment from industrial inputs: a gastropod perspective. PLoS ONE 9(1):1–8
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of proteine-dye binding. Anal Biochem 72:248–254
Brandão FP, Pereira JL, Gonçalves F, Nunes B (2011) The impact of paracetamol on selected biomarkers of the Mollusc Species Corbicula fluminea. Environ Toxicol 29:74–83
Brasil (2005) Resolução CONAMA n° 357/05, de 17 de março de 2005. Conselho Nacional de Meio Ambiente
Brasil (2015) Laudo Técnico Preliminar: impactos ambientais decorrentes do desastre envolvendo o rompimento da barragem de Fundão, em Mariana, Minas Gerais. In: Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis – IBAMA. Minas Gerais
Bruchchen LM, Silva PS, Silveira FZ, Defaveri TM, Pich CT, Geremias R (2013) Avaliação da toxicidade das águas do Rio Cricíuma (Cricíuma, Santa Catarina, Brasil), utilizando parâmetros físico-químicos e abordagens ecotoxicológicas. Ecotoxicol Environ Contam 8(2):23–30
Cantanhêde SM, Castro GS, Pereira NJ, Campos JSP, Silva J, Tchaicka L, Neta RNFC, Junior JRST, Santos DMS (2016) Evaluation of environmental quality of two estuaries in Ilha do Maranhão, Brazil, using histological and genotoxic biomarkers in Centropomus undecimalis (Pisces, Centropomidae). Environ Sci Pollut Res 23:21058–21069
Cappello T, Maisano M, D’Agata A, Natalotto A, Mauceri A, Fasulo S (2013) Effects of environmental pollution in caged mussels (Mytilus galloprovincialis). Mar Environ Res 91:52–60
Carvalho MS, Moreira RM, Ribeiro KD, Almeida AM (2017) Concentração de metais no rio Doce em Mariana, Minas Gerais, Brasil. Acta Bras 1(3):37–41
Cerqueira CC, Fernandes MN (2002) Gill tissue recovery after copper exposure and blood parameter responses in the tropical fish Prochilodus scrofa. Ecotoxicol Environ Saf 52:83–91
Collins AR (2004) The comet assay for DNA damage and repair. Mol Biotechnol 26:249–261
Costa L, Aschner M (2014) Manganese in health and disease. Royal Society of Chemestry, Cambridge
Dautremepuits C, Marcogliese DJ, Gendron AD, Fournier M (2009) Gill and kidney antioxidant processes and innate immune system responses of yellow perch (Perca flavescens) exposed to different contaminants in the St. Lawrence River, Canada. Sci Total Environ 407:1055–1064
Dolci GS, Dias VT, Roversi K, Pase CS, Segat HJ, Teixeira AM, Benvegnu DM, Trevizol F, Barcelos RCS, Riffel APK, Nunes MAG, Dressler VL, Flores EMM, Baldisserotto B, Buerger ME (2013) Moderate hypoxia is able to minimize the manganese-induced toxicity in tissues of silver catfish (Rhandia quelen). Ecotoxicol Environ Saf 91:103–109
Duarte ID, Dias MC, David JAO, Matsumoto ST (2012) A qualidade da água da Lagoa Jacuném (Espírito Santo, Brasil) em relação a aspectos genotóxicos e mutagênicos, mensurados respectivamente pelo ensaio do cometa e teste do micronúcleo em peixes da espécie Oreochromis niloticus. Rev Bras De Biociências 10(2):211–219
Duran A, Tuzen M, Soylak M (2014) Assessment of trace metal concentrations in muscle tissue of certain commercially available fish species from Kayseri, Turkey. Environ Monit Assess 186(7):4619–4628
Elnabris KJ, Muzyed SK, El- Ashgar NM (2013) Heavy metal concentrations in some commercially important fishes and their contribution to heavy metals exposure in Palestinian people of Gaza Strip (Palestine). J Assoc Arab Univ Basic Appl Sci 13(1):44–51
Escobar H (2015) Mud tsunami wreaks ecological havoc in Brazil. Science 350(6265):1138–1139
Espindola HS, Campos RBF, Lamounier KCC, Silva RS (2016) Desastre da Samarco no Brasil: desafios para a conservação da biodiversidade. Froteiras J Social Technol Environ Sci 5(3):72–100. https://doi.org/10.21664/2238-8869.2016v5i3.p72-100
Falfushynska HI, Gnatyshyna LL, Stoliar OB, Nam YK (2011) Various responses to copper and manganese exposure of Carassius auratus gibelio from two populations. Comp Biochem Physiol C Toxicol Pharmacol 154(3):242–253
Fenech M, Chang WP, Kirsch-Volders M, Holland N, Bonassi S, Zeiger E (2003) HUMN project: detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutat Res 534:65–75
Fontaínhas-Fernandes A (2005) The use of biomarkers in aquatic toxicology studies. Revista Portuguesa de. Zootecnia 12(1):67–86
Food and Nutrition Board (2001) Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. National Academy Press, Washington DC
Freitas CM, Silva MA, Menezes FC (2016) O desastre na barragem de mineração da Samarco – fratura exposta dos limites do Brasil na redução de riscos de desastres. Cienc Cult 68(3):25–30
Freitas CEC, Siqueira-Souza FK (2009) O uso de peixes como bioindicador ambiental em áreas de várzea da bacia amazônica. Revista Agrogeoambiental Agosto: 39–45
Gabriel D, Riffel APK, Finamor IA, Saccol EMH, Ourique GM, Goulart LO, Kochhann D, Cunha MA, Garcia LO, Pavanato MA, Val AL, Baldisserotto B, Llesuy SF (2013) Effects of subchronic manganese chloride exposure on Tambaqui (Colossoma macropomum) tissues: oxidative stress and antioxidant defenses. Arch Environ Contam Toxicol 64(4):659–667
Garcia LC, Ribeiro DB, Roque FO, Ochoa-Quintero JM, Laurance WF (2016) Brazil’s worst mining disaster: corporations must be compelled to pay the actual environmental costs. Ecol Appl. https://doi.org/10.1002/eap.1461
Grisolia CK, Oliveira ABB, Bonfim H, Klautau-Guimarães MN (2005) Genotoxicity evaluation of domestic sewage in a municipal wastewater treatment plant. Genet Mol Biol 28(2):334–338
Gomes LC, Chippari-Gomes AR, Miranda TO, Pereira TM, Merçon J, Davel VC, Barbosa BV, Pereira ACH, Frossard A, Ramos JPL (2018) Genotoxicity effects on Geophagus brasiliensis fish exposed to Doce River water after the environmental disaster in the city of Mariana, MG, Brazil. Brazilian Journal of Biology (in press)
Guilherme S, Válega M, Pereira ME, Santos MA, Pacheco M (2008) Antioxidant and biotransformation responses in Liza aurata under environmental mercury exposure-relationship with mercury accumulation and implications for public health. Mar Poll Bull 56:845–859
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem 249(22):7130–7139
Halliwell B, Gutteridge JMC (1985) Free radicals in biology and medicine. Clarendon, Oxford
Heath AG (1991) Water pollution and fish physiology. Lewis Publishers, Florida
Hernroth B, Baden SP, Holm K, André T, Söderhäll I (2004) Manganese induced imune suppression of the lobster. Nephrops Nor Aquat Toxicol 70(3):223–231
Iranshani F, Faramarzi M, Kiaalvandi S, Jalaee MH, Dehghan M (2011) Effectiveness of Edta in mobilizing of the contaminant metal ions, especially cadmium from tissue of Angel Fish (Pterophyllum scalare schultze) subjected to chronic poisoning with cadmium acetate. American-Eurasian. J Agric Environ Sci 11(4):519–527
Isla-Flores H, Gómez-Oliván LM, Galar-Martínez M, Colín-Cruz A, Neri-Cruz N, García-Medina S (2013) Diclofenac-induced oxidative stress in brain, liver, gill and blood of common carp (Cyprinus carpio). Ecotoxicol Environ Saf 92:32–38
Jovičić K, Nikolić DM, Višnjić-Jeftić Ž, Đikanović V, Skorić S, Stefanović SM, Lenhardt M, Hegediš A, Krpo-Ćetković J, Jarić I (2015) Mapping differential elemental accumulation in fish tissues: assessment of metal and trace element concentrations in wels catfish (Silurus glanis) from the Danube River by ICP-MS. Environ Sci Pollut Res 22(5):3820–3927
Kendall RJ, Anderson TA, Baker RJ, Bens CM, Carr JA, Chiodo LA, Cobb GP, Dickerson RL, Dixon KR, Frame LT, Hooper MJ, Martin CF, McMurry ST, Patino R, Smith EE, Theodorakis CW (2001) Environmental ecotoxicology. In: Klaassen CD (ed) Casarett and Doull’s toxicology: the basic science of poisons, 6th edn. Mcgraw- Hill, New York, NY, p 236
Kida BMS (2014) Influência do pH ácido, alumínio e manganês na fisiologia reprodutiva em machos de Astyanax bimaculatus (Characiformes: Characidae). Dissertação, Universidade de São Paulo
Klaassen CD (2001) Casarett; Doull’s toxicology: the basic science of poisons, 6th edn. McGraw-Hill, p 133–224
Kotze PJ (1997) Aspects of water quality, metal contamination of sediment and fish in the Olifants river, Mpumalanga. Dissertation, Rand Afrikaans University
Lech JJ, Vodicnik MJ (1985) Biotransformation. In: Rand GM, Petrocelli SR (eds) Fundamentals of aquatic toxicology – methods and application. Hemisphere Publishing Corporation, New York, NY, USA, p 526–557
Libânio PAC (2005) A dimensão da qualidade de água: avaliação da relação entre indicadores sociais, de disponibilidade hídrica, de saneamento e de saúde pública. Eng Sanit Ambient 10(3):219–228
Lins JAPN, Kirschnik PG, Queiroz VS, Cirio SM (2010) Uso de peixes como biomarcadores para o monitoramento ambiental aquático. Ciênc Ambient 8:469–484
Maia FF (2017) Elementos traços em sedimentos e qualidade da água de rios afetados pelo rompimento da Barragem de Fundão, em Mariana, MG. Dissertação de Mestrado, Universidade Federal de Viçosa, Viçosa, Minas Gerais, p 44
Matsumoto ST, Mantovani MS, Malaguttii MIA, Dias AL, Fonseca IC, Marin-Morales MA (2006) Genotoxicity and mutagenicity of water contaminated with tannery effluents, as evaluated by the micronucleus test and comet assay using the fish Oreochromis niloticus and chromosome aberrations in onion root-tips. Genet Mol Biol 29(1):148–158
Mitic SS, Pavlovic AN, Tosic SB, Stojanovic BT, Mitic MN, Stojkovic MB (2013) Elemental composition of various apples cultivars grown in Serbia. Asian J Chem 25:6027–6032
Monteiro SM, Rocha E, Mancera JM, Fontaínhas-Fernandes A, Souza M (2009) A stereological study of copper toxicity in gills of Oreochromis niloticus. Ecotoxicol Environ Saf 72:213–223
Natalotto A, Sureda A, Maisano M, Spanò N, Mauceri A, Deudero S (2015) Biomarkers of environmental stress in gills of Pinna nobilis (Linnaeus 1758) from Balearic Island. Ecotoxicol Environ Saf 122:9–16
Niemic M, Wisniowska–Kielian B (2015) Manganese accumulation in selected links of food chain of aquatic ecosystems. J Elem 20(4):945–956
Oliveira HSP, França SCA, Rocha EJP (2015) Atividades de mineração e avaliação de metais em água superficial, sedimento de fundo e peixes no rio tapajós. In: Vieira ICG, Jardim MAG, Rocha EJP (eds) Amazônia em Tempo: estudos climáticos e socioambientais. Universidade Federal do Pará, Belém, p 195–221
Padmini E, Usha Rani M (2009) Evaluation of oxidative stress biomarkers in hepatocytes of grey mullet inhabiting natural and polluted estuaries. Sci Total Environ 407:4533–4541
Peakall DB (1994) The role of biomarkers in environmental assessment (1). Ecotoxicology 3(3):157–160
Pereira AA, van Hattun B, Brower A, van Bodegom PM, Rezende CE, Salomons W (2008) Effects of iron-ore mining and processing on metal bioavailability in a tropical coastal lagoon. J Soils Sediment 8:239–252
Porto MFS (2016) A tragédia da mineração e do desenvolvimento no Brasil: desafios para a saúde coletiva. Cad Saúde Pública 32(2):1–3
Qu R, Feng M, Wang X, Qin L, Wang C, Wang Z, Wang L (2014) Metal accumulation and oxidative stress biomarkers in liver of freshwater fish Carassius auratus following in vivo exposure to waterborne zinc under different pH values. Aquat Toxicol 150:9–16
Repula CMM, Campos BK, Ganzarolli EM, Lopes MC, Quináia SP (2012) Biomonitoramento de Cr e Pb em peixes de água doce. Quím Nova 35(5):905–909
Rocha RA, Afonso JC (2012) Manganês. Quím Nova Esc 34(2):103–105
Saha N, Naman MR (2011) Concentration of selected toxic metals in groundwater and some cereals grown in Shibganj area of Chapai Nawabganj, Rajshani, Bangladesh. Curr Sci 101(3):427–431
Scalon MCS, Rechenmacher C, Siebel AM, Kayser ML, Rodrigues MT, Maluf SW, Rodrigues MAS, da Silva LB (2010) Evaluation of Sinos River water genotoxicity using the comet assay in fish. Braz J Biol 70:1217–1222
Segura FB, Nunes EA, Paniz FP, Paulelli ACC, Rodrigues GB, Braga GUL, Filho WRP, Junior FB, Cerchiaro G, Silva FF, Batista BL (2016) Potential risks of the residue from Samarco’s mine dam burst (Bento Rodrigues, Brazil). Environ Pollut 218:813–825
Souza TS, Fontanetti CS (2006) Micronucleus test and observation of nuclear alterations in erythrocytes of Nile tilapia exposed to waters affected by refinery efluente. Mutat Res 605:87–93
Sheehan D, Tyther R, Dowling V, McDonagh B (2007) Redoxproteomics – a route to identifi cation of damaged proteins. In: Mothersill C, Mosse I, Seymour C (eds) Multiple stressors: a challenge for the future. NATO Science for Peace and Security Series, Amsterdam, p 295–308
Sreejai R, Jaya DS (2010) Studies in lipid peroxidation and antioxidants in fishes exposed to hydrogen sulfide. Toxicol Int 17(2):71–77
Steenkamp VE, du Preez HH, Schoonbee HJ, van Eeden PH (1994) Bioaccumulation of manganese in selected tissues of freshwater crab, Potamonautes warreni (Calman), from industrial and mine-polluted freshwater ecosystems. Hydrobiologia 288:137–150
Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyame Y, Rojas E, Ryu JC, Sasaki YF (2000) Single cell gell/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 35:206–221
van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149
van der Putte I, Pärt P (1982) Oxygen and chromium transfer in perfused gills of rainbow trout (Salmo gairdneri) exposed to hexavalent chromium at two different pH levels. Aquat Toxicol 2:31–45
Veronez AC, Salla RV, Baroni VD, Barcarolli IF, Bianchini A, dos Reis Martinez CB, Chipari-Gomes AR (2016) Genetic and biochemical effects induced by iron ore, Fe and Mn exposure in tadpoles of the bullfrog Lithobates catesbeianus. Aquat Toxicol 174:101–108
Vieira MC, Torrontera R, Córdoba F, Canalejo A (2012) Acute toxicity of manganese in goldfish Carassius aratus is associated with oxidative stress and organ specific oxidant responses. Ecotoxicol Environ Saf 78:212–217
Winter MJ, Ellis LCJ, Hutchinson TH (2007) Formation of micronuclei in erythrocytes of the fathead minnow (Pimephales promelas) after acute treatment with mitomycin C or cyclophosphamide. Mutat Res 629:89–99
Ye CX, Tian LX, Yang HJ, Liang JJ, Niu J, Liu YJ (2009) Growth performance and tissue mineral content of juvenile grouper (Epinephelus coioides) fed diets supplemented with various levels of manganese. Aquacult Nutr 15:608–614
Zhao S, Feng C, Quan W, Chen X, Niu J, Shen Z (2012) Role of living environments in the accumulation characteristics of heavy metals in fishes and crabs in the Yangtze River Estuary, China. Mar Pollut Bull 64(6):1163–1171
Zhuang P, Li Z, McBride MB, Zou B, Wang G (2013) Health risk assessment for consumption of fish originating from ponds near Dabaoshan mine, South China. Environ Sci Pollut Res 20:5844–5854
Acknowledgements
This work was supported by Espírito Santo State Scientific Research Foundation (FAPES), grants #61902861/13. The authors also would like to gratefully acknowledge the FAPES for providing a graduate stipend for the student, Larissa Souza Passos, and post-graduate stipends for the students, Taciana Onesorge Miranda Lopes, Julia Merçon, Dandara Silva Cabral and Lívia Sperandio Caetano. The authors also thank the University of Vila Velha for providing a graduate stipend for the student, Gabriel Carvalho Coppo and the Coordination of Improvement of Higher Level Personnel (CAPES) for providing a post-graduate stipend for the student, Tatiana Miura Pereira.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The use of these animals in the present experiment was approved by the Committee on Ethics, Bioethics and Animal Welfare (CEUA - UVV) according to protocol no. 371–2016.
Rights and permissions
About this article
Cite this article
Coppo, G.C., Passos, L.S., Lopes, T.O.M. et al. Genotoxic, biochemical and bioconcentration effects of manganese on Oreochromis niloticus (Cichlidae). Ecotoxicology 27, 1150–1160 (2018). https://doi.org/10.1007/s10646-018-1970-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-018-1970-0