Abstract
Aquatic ecosystems have been suffering deleterious effects due to the development of different economic activities. Metal(loid)s are one of the most persistent chemicals in environmental reservoirs, and may produce adverse effects on different organisms. Since fishes have been largely used in studies of metal(loid)s exposure, tilapia and largemouth bass were collected in three ecosystems from the Yaqui River Basin to measure the concentrations of metal(loid)s (chromium (Cr), copper (Cu), iron (Fe), manganese (Mn), nickel (Ni), zinc (Zn) arsenic (As), mercury (Hg), and selenium (Se)) and some biomarkers (somatic indices, metallothionein expression and histopathological analysis) in tissues of both species. Metal(loid) concentrations varied seasonally among ecosystems in tissues of both species. The elements varied seasonally and spatially in tissues of both species, with a general distribution of liver > gills > gonads. Also, biomarkers showed variations indicative that the fish species were exposed to different environmental stressor conditions. The highest values of some biomarkers were in largemouth bass, possibly due to differences in their biological characteristics, mainly feeding habits. The multivariate analysis showed positive associations between metal(loid)s and biomarkers, which are usually associated to the use of these elements in metabolic and/or regulatory physiological processes. Both fish species presented histological damage at different levels, from SI types (changes that are reversible for organ structure) to SII types (changes that are more severe but may be repairable). Taken together, the results from this study suggest that the Yaqui River Basin is moderately impacted by metals and metalloids.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aquatic ecosystems have been targeted by distinct anthropogenic activities that have resulted in deleterious consequences for their overall health and the organisms that reside within them (Huang et al. 2022). Anthropogenically derived effluents that are released by activities, such as mining and agriculture, contaminate aquatic ecosystems, with metal(loid)s being some of the most persistent chemicals (Rainbow and Luoma 2011; Wood 2011). Metal(loid)s accumulate in water, sediments, soils, and biota, and may suffer chemical speciation and biological transformations through the food web, depending on biotic and abiotic conditions (Rainbow and Luoma 2011; Mackay et al. 2018). Organisms may develop several adverse effects to metal(loid)s when the threshold concentration is exceeded, from physiological responses to chronic disease (Mackay et al. 2018). Metal(loid) contamination of aquatic ecosystems is a serious threat to wildlife, including fishes that occupy different levels in food webs (Wood 2011; Ali and Khan 2018; Chua et al. 2018).
Since fishes have different feeding habits, they have been used efficiently as sentinel species for assessing contamination of aquatic ecosystems around the world (Wepener et al. 2011; Yi et al. 2017; Hatice and Turgay, 2020; El-Agri et al. 2022). Their biological characteristics as wide distribution, abundance, ease of capture and management, and longevity, make them suitable to study xenobiotic exposure (Chatterjee, et al. 2018; Marie, 2020; Thoré et al. 2021).
Biomarkers have been used in several studies since their approval by the United States National Research Council in 1987 (El-Agri et al. 2022), and act at different levels of biological organization to assess the health status of a population or an ecosystem (Mussali-Galante et al. 2013; Fahd et al. 2020; Delfino-Vieira et al. 2022). Thus, the effects of xenobiotics, such as metal(loid)s, could be determined at a specific level using biomarkers and appropriate sentinel species (Stentiford et al. 2003; Sanchez and Porcher 2009; El Agri et al. 2022).
The tilapia (Oreochromis niloticus) and largemouth bass (Micropterus salmoides) are widely distributed in many freshwater ecosystems around the world (Hinck et al. 2008; Dwivedi et al. 2015; Prabu et al. 2019; Hussein et al. 2020). These species are used in aquaculture with high commercial values, acceptable meat quality, and high protein content. Both species play major ecological roles within their respective ecosystems as benthic and pelagic species, and are able to survive in a wide range of environmental conditions (Sepúlveda et al. 2003; Mehinto et al. 2014; Dwivedi et al. 2015; Abdel-Khalek et al. 2016).
The Yaqui River Basin is one of the most important ecoregions in northwest Mexico that provide ecological, social, and economic services. The ecosystems embrace numerous endemic, migratory, and introduced species, such as tilapia and largemouth bass (Martínez-Durazo et al. 2020). It also provides water for human consumption and multiple economic activities (Gortáres-Moroyoqui et al. 2011; McCullough and Matson 2016). Previous studies have documented metal(loid) contamination in the basin in soils, sediments, and fishes (Meza-Figueroa et al. 2009; De la O-Villanueva et al., 2013; Meza-Montenegro et al. 2012; Martínez-Durazo et al. 2020). Considering that elevated levels of metals were reported in tilapia and largemouth bass from Yaqui River Basin, the aim of this study was to investigate if the concentrations of metal(loid)s were associated with biomarkers in gills, gonads and liver of both species, from three ecosystems of this basin.
Material and methods
Study area
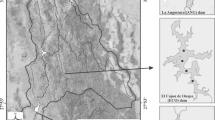
The Yaqui River basin is located in northeastern Mexico (26°10′–30°40′ N and 106°80′–111°40′ W) spanning an area of 71,452 km2 (Fig. 1). The predominant climate is semi-arid with a range of annual average precipitation of 366.1–600.8 mm. There are some reservoirs in the basin that provide water for human consumption and productive activities. Three dams were chosen based on their predominant anthropogenic activities. First, La Angostura (ANG; 1040 m meters above mean sea level (MAMSL)) dam is situated in the Nacozari copper mining district, which is the second most extensive mining area in the region. Second, El Cajon de Onapa (ECO; 556 m MAMSL) dam is situated on the Sahuaripa River, a small tributary of the Yaqui River, where agriculture, fish aquaculture and cattle raising at small scale are the principal economic activities. Third, El Oviachic (OVI; 58 m MAMSL) dam is situated at the end of basin and near to Yaqui Valley, one of the most extensive agricultural areas in the northwest of Mexico (CONAGUA 2020).
Field survey and fish collection
Fishes were sampled during the dry and rainy seasons of 2018 at the three dams of the Yaqui River Basin. Approximately 30 individuals of each species (tilapia O. niloticus, and largemouth bass M. salmoides) were collected at ANG, ECO, and OVI dams during the two seasons, which is enough to achieve biomarker responses according to Gagnon and Hodson (2012). Fish sampling was performed using commercial 4-cm gill nets with the help of local fishers. Total fish length and weight, as well as liver and gonad weights, were recorded using an icthyometer (Aquatic Biotechnology, model IK2R) and a digital balance (Ohaus, Scout Pro 6000, 6000 ± 0.001 g) in order to calculate Condition Factor (CF), Hepatosomatic Index (HSI), Gonadosomatic Index (GSI), and Histopathological Alterations Index (HAI) (Azevedo et al. 2009; García-Gasca et al. 2016; Banday et al. 2020).
Specimens were identified and sacrificed by brain spiking according to the guidelines for the use of fishes in research (American Fisheries Society 2014). Immediately after dissection, small sections of liver tissues were transferred into RNA-later solution (Thermo Fisher Scientific, Waltham, USA) to preserve RNA integrity for further gene expression analysis (Vehniäinen et al. 2019). Similarly, a fraction of each liver, gonad, gills, and muscle, were collected, washed with distilled water, and preserved in 4% buffered formalin (Mustafa 2020). Also, liver, gonads, stomach, gills, and muscle were dissected in the field, placed in labeled sterile polyethylene bags, iced, and transported to the laboratory, and frozen at −20 °C for processing for metal analysis. The fish carcass of each specimen was placed in labeled sterile polyethylene bags, transported on ice, to the laboratory, and frozen at −20 °C for morphometric and meristic measurements (García-Gasca et al. 2016).
Determination of somatic indices
The somatic indices were calculated using the total length and weight of each fish, in order to evaluate the overall fish health (Banday et al. 2020). The CF, HSI and GSI were determined according to Nash et al. (2006), Azevedo et al. (2009) and García-Gasca et al. (2016):
Metallothionein gene expression
Total RNA was isolated from liver tissue using Tripure (Roche Applied Science, Penzberg, Alemania) reagent according to manufacturer’s instructions, and RNA purity and concentration were measured using NanoDrop™ (NanoDrop-ONE, Thermo Scientific). Turbo DNA-free kit™ (Invitrogen, Thermo Fischer Scientific) was used to eliminate genomic DNA from the RNA. DNA-free RNA was reverse transcribed into complementary DNA (cDNA) using M-MLV Reverse Transcriptase (Promega, Mexico), and random hexamers (Invitrogen, Thermo Fischer Scientific) in a 20 μL reaction containing 1046.56 ± 923.14 ng µL−1 for tilapia and 1562.82 ± 1073.35 ng µL−1 for largemouth bass, for total RNA.
Metallothionein (mt) gene expression in tilapia and largemouth bass was used as biomarker of metal(loid)s contamination, using the Eukaryotic Translation Elongation Factor 1 Alpha 1 (eEf1a) as the reference gene (Girgis et al. 2019; Ma et al. 2019; Wang et al. 2014). The amplification of mt and eEf1a for tilapia and largemouth bass were performed using the primers shown in the Table 1. Primers for mt were designed using the Primer-BLAST software (Ye et al. 2012). For eEf1a, primers were synthesized according to sequences reported by Ma et al. (2019) and Wang et al. (2015). PCR conditions were set as follows: 1 cycle at 94 °C for 5 min; 35 cycles at 94 °C for 30 s, 60 °C for 30 s, 72 °C for 30 s, and a final elongation cycle was added at 72 °C for 5 min, rendering products for mt of 141 pb for tilapia and 158 pb for largemouth bass. The eEf1a rendered a product of 250 and 156 bp for tilapia and largemouth bass, respectively (Ma et al. 2019; Wang et al. 2015). Melting curve analysis was performed at 87 °C to assess target amplification specificity. Values of threshold cycle (Ct) were obtained using Quant Studio Real-Time PCR Software V1.2 system software. Ct values from target genes of tilapia and largemouth bass were normalized with the eEf1a gene, and the relative level of expression of mt was determined according to Pfaffl (2004).
Histological analysis
For histopathological analysis, gills and liver tissues were washed with distilled water and transferred to 70% ethanol (Joshi and Sreekumar 2015). Tissues were dehydrated in ascending concentrations of ethanol and embedded in paraffin (Stentiford et al. 2003). Paraffin blocks were cut into 5-μm thick sections with a rotatory microtome (Slee Cut 5062, Carl Zeiss, Germany) and stained with Hematoxylin and Eosin (Javed et al., 2017). The slides were analyzed using light microscopy (Leica DM 1000, Leica Microsystems, Switzerland) with a camera (Leica CH-9435, Leica Microsystems, Switzerland), and an image-capture system (Motic Image Plus 2.0, China).
The Histopathological Alterations Index (HAI) for the gills was calculated according to Cerqueira and Fernandes (2002), and for the liver according to Camargo and Martinez (2007); both indices are based on the type, location and severity of the lesion. Gill lesions were classified into six groups: epithelium lifting, hypertrophy of mucous membrane cells, deformation of secondary lamellar structure, aneurysm of lamella, lamella fusion, and loss of gill cell structure. Liver lesions were classified in four groups: pycnosis, vacuolization, inflammation and necrosis. The lesions were classified into three progressive stages (S) based on the possibility that the normal structure of the organ would be restored after environmental improvement, according to Bernet et al. (1999), Ali and Khan (2018) and Chua et al. (2018): SI, changes that are reversible in which the normal organ structure can be recovered; SII, changes that are more severe and affect the organ function but may be repairable after environmental improvement; and SIII, changes in which the restoration of organ structure is not possible even after environmental improvement. The HAI was calculated from the sum of the lesion types within each of the three stages multiplied by the stage index according to Ali and Khan (2018) and Chua et al. (2018).
Metal analysis
Gill, gonad, and liver tissues from tilapia and largemouth bass were freeze-dried (LABCONCO Model 77530; −48 °C and 32 × 10−3 mbar for 48 h) and manually grounded (Teflon mortar for 10 min). The powdered tissues were digested in microwave (MARS-X) in one step: 5 mL of concentrated HNO3 were added to 0.30 ± 0.003 g of tissue and heated to 100 °C for 5 min, to 120 °C for 5 min, and to 140 °C for 10 min (Jara-Marini et al. 2020). The quantification of Cr, Cu, Fe, Mn, Ni, and Zn were determined with air–acetylene (for Cu, Fe, Mn, Ni, and Zn) and nitrous oxide–acetylene (Cr) flames, using the SpectrAA-240 FS Absorption Atomic Spectrophotometer (AAS, Varian, Mulgrave, Australia). The determination of As, Hg, and Se were performed using AAS coupled to hydride and vapor generator (Varian VGA 77).
Standard reference material (DORM-4 Fish protein from the National Research Council of Canada) and blanks were used as controls. Certified values for all elements were better than 15%, with recovery percentages of: Cr = 90.75%, Cu = 96.60%, Fe = 101.55%, Mn = 88.70%, Ni = 91.80%, and Zn = 103.30%, As = 85.90%, Hg = 90.55% and Se = 86.20%. The detection limits were estimated (μg g−1) as Cr = 0.035, Cu = 0.026, Fe = 0.041, Mn = 0.024, Ni = 0.036, and Zn = 0.030; for the rest of elements (ng g−1) were As = 0.41, Hg = 0.34 and Se = 0.38. The metal(loid) concentrations are reported as dry weights.
Data analysis
Metal(loid) results are represented as mean ± standard deviation (n from 5 to 90, see Tables 2 and 3). For comparison of somatic indices (FC, HSI and GSI), mt gene expression, and liver metal concentrations, a one-way ANOVA was used to compare differences of means among sampling sites, at the level of α = 0.05. To calculate the biomarker responses between sites and to compare the impact of metal(oid)s contamination, the integrated biomarker response (IBR) was calculated according to Beliaeff and Burgeot (2002) and Sanchez et al. (2013). Correlation between hepatic somatic indices, mt expression and metal(loid) concentrations in fish between seasons were evaluated using the Pearson correlation analysis. Multiple linear regression analysis was calculated to explain relationships between metals (predictors) and, FC, GSI, and mt for tilapia, and FC, HSI, and mt for largemouth bass (responses). Canonical Correspondence analysis was used to evaluate the relationship between metal(oid)s and biomarker responses in liver tissue. To evaluate the effect of the sites and seasons on the distribution of metal(oids) in liver samples, and which Site-Season combination differed from each other, the distance-based permutational multivariate analysis of variance (PERMANOVA) was used. Two one-way ANOVA designs were conducted, the first one compared the metal(oid) concentrations in tilapia liver, whereas the second design was used to evaluate differences in metal(oid) distribution in largemouth bass liver; both, comparing all the Site-Season levels. All the statistical analyses were performed using Minitab 17 R v. 3.6.1 (R Core Development Team 2017), and PRIMER 7 + PERMANOVA (PRIMER-E Ltd., Devon, UK) software.
Results
Metal(oid) concentrations were higher, in orders of magnitude, in tilapia than in the largemouth bass. Both species accumulate high concentrations of metals like Cu, Cr, Fe, Zn (Tables 2 and 3), and followed a decreased order by ecosystems (ECO > ANG > OVI). The results of Cu, Cr, Fe, Mn, Ni, Zn were previously reported in Martinez-Durazo et al. (2020).
Values for condition factor (CF), hepatosomatic index (HSI), and gonadosomatic index (GSI) for tilapia and largemouth bass are shown in Figs. 2–4. In general, the highest values of CF, HSI, and GSI were recorded in largemouth bass compared with tilapia. Biomarker responses in both species followed a similar tendency but different magnitude. In tilapia, metal concentrations did not alter CF, GSI, and HSI (Figs. 2–4); however, mt gene expression and HAI were significantly altered by metals (Fe, Cu, Zn, Cr) (Fig. 5a, b). The largemouth bass followed a similar biomarker response pattern but not in the same order of magnitude, for instance, CF, GSI, and HSI did not show a significant (p < 0.05) response in the IBR analysis, whereas mt gene expression and HAI showed significant differences (p < 0.05) in largemouth bass in the different ecosystems.
Condition factor (CF) in female and male tilapia (Oreochromis niloticus) and largemouth bass collected from three ecosystems (La Angostura, ANG; El Cajon de Onapa, ECO; and El Oviachic, OVI) of the Yaqui River Basin in northwestern of Mexico. Different letters indicate statistical differences (lower case for ecosystems and upper case for seasons) at α = 0.05
Hepatosomatic indexes (HSI) in female and male tilapia (Oreochromis niloticus) and largemouth bass (Micropterus salmoides) collected from three ecosystems (La Angostura, ANG; El Cajon de Onapa, ECO; and El Oviachic, OVI) of the Yaqui River Basin in northwestern of Mexico. Different letters indicate statistical differences (lower case for ecosystems and upper case for seasons) at α = 0.05
Gonadosomatic index (GSI) in in female and male tilapia (Oreochromis niloticus) and largemouth bass (Micropterus salmoides) collected from three ecosystems (La Angostura, ANG; El Cajon de Onapa, ECO; and El Oviachic, OVI) of the Yaqui River Basin in northwestern of Mexico. Different letters indicate statistical differences (lower case for ecosystems and upper case for seasons) at α = 0.05
Canonical correspondence analysis of metal(loid) concentrations and biological indexes in tilapia (Oreochromis nilocticus, grouped by metals (a) and organisms (b)) and largemouth bass (Micropterus salmoides, grouped by metals (c) and organisms (d)) from three ecosystems (La Angostura, ANG; El Cajon de Onapa, ECO; and El Oviachic, OVI) of the Yaqui River Basin in northwestern of Mexico
Although the overall mt gene expression in both fish species was not significant (p > 0.05) among seasons (Fig. 6), relative mt expression in liver of tilapia and largemouth bass showed significant differences (p < 0.05) between the three ecosystems (Fig. 6). Tilapia mt gene expression followed an order by ecosystems ECO > ANG > OVI. The relative expression of mt in largemouth bass showed the highest (p < 0.05) values in fish collected in OVI, and ANG.
Metallotionein (mt) gene expression in the livers of in tilapia (Oreochromis niloticus) and largemouth bass (Micropterus salmoides) collected from three ecosystems (La Angostura, ANG; El Cajon de Onapa, ECO; and El Oviachic, OVI) of the Yaqui River Basin in northwestern of Mexico. Different letters indicate statistical differences (lower case for ecosystems and upper case for seasons) at α = 0.05
Histology analysis showed different kinds of damage in gill and liver tissues of the tilapia and largemouth bass (Figs. 7 and 8, respectively). In general, the tissues of both species in the three ecosystems showed reversible lesions, SI types (changes that are reversible for organ structure) to SII types (changes that are more severe but may be repairable), according to Bernet et al. (1999) and Poleksić and Mitrović-Tutundžić (1994). The main lesions in gills were lamellar fusion (principal and secondary lamellas), deformation of the secondary lamella, epithelial lifting, hypertrophy of mucous membrane cells, lamellar aneurysms, and loss of gill cell structure (Fig. 7). Similarly, livers analyzed in both species from three dams presented different types of damages. The most common lesions determined in tilapia and largemouth bass were pycnosis, vacuolization, inflammation, and necrosis (Fig. 8).
Histology of the gills from tilapia (Oreochromis niloticus) and largemouth bass (Micropterus salmoides) collected from three ecosystems (La Angostura, ANG; El Cajon de Onapa, ECO; and El Oviachic, OVI) of the Yaqui River Basin in northwestern of Mexico. Tissue without damages (Control, A) and tissues with damage [tilapia from ANG (a), ECO (b), and OVI (c); largemouth bass from ANG (d), ECO (e), and OVI (f)]. Damage: 1. Epithelium lifting. 2. Hypertrophy of mucous membrane cells. 3. Deformation of secondary lamellar structure. 4. Aneurysm of lamella. Arrowhead: lamella fusion. *Loss of gill cell structure
Histology of livers from tilapia (Oreochromis niloticus) and largemouth bass (Micropterus salmoides) collected from three ecosystems (La Angostura, ANG; El Cajon de Onapa, ECO; and El Oviachic, OVI) of the Yaqui River Basin in northwestern of Mexico. Tissue without damages (Control, A) and tissues with damages [tilapia from ANG (a), ECO (b), and OVI (c); largemouth bass from ANG (d), ECO (e), and OVI (f)]. Damage: 1. Pycnosis. 2. Vacuolization. Arrowhead: inflammation. n: necrosis
Histological damage in the tissues of both fish species was also determined semi-quantitatively with the HAI (Fig. 9). The ranges of mean HAI values in the livers of tilapia varied from 66.5 to 88.5% in the dry season and from 65.0 to 77.0% in the rainy season. The highest HAI values in the livers of tilapia during the dry season were observed in the ANG and OVI ecosystems, while the HAI values in the rainy season were similar between ecosystems (p > 0.05). Similarly, the ranges of mean HAI values in the gills of tilapia varied from 17.0 to 40.0% in the dry season and from 22.0 to 38.5% in the rainy season. The highest mean HAI values in the gills of tilapia were found in the ANG ecosystem in both seasons (HAI = 40.0% and 38.5% in the dry and rainy seasons, respectively).
In the largemouth bass, the ranges of mean HAI values in the liver varied from 29.0 to 88.0% in the dry season and from 29.0 to 88.5% in the rainy season (Fig. 9). The highest (p < 0.05) mean HAI values in the liver during the dry season were found in the ECO (HAI = 85.0%) and OVI (HAI = 88.5%) ecosystems, while the highest mean HAI value was found in the OVI ecosystem in the rainy season (HAI = 85.2%). The ANG and ECO ecosystems showed similar HAI values. The mean HAI values in the gills of largemouth bass ranged from 30.5 to 59.5% in the dry season and from 59.0 to 79.5% in the rainy season. During the dry season, the highest (p < 0.05) mean HAI values were found in the ECO (59.7%) and OVI (59.8%) ecosystems, while the highest values were found in ANG (76.5%) and OVI (79.7%) ecosystems in the rainy season. The IBR analysis revealed that the biomarker response agreed with that of the impacts of metals in the different ecosystems and followed a decreasing pattern with regard to the manifestation of biological endpoints (ECO > ANG > OVI). In addition, there were significant differences (p < 0.05) in IBR between species and between ecosystems (Figs. 10 and 11).
The results of the Pearson correlation analysis indicated that relationships between metal(loid) concentrations and biological indexes were present. For tilapia, no correlation was found between HSI and the metal(loid) concentrations, although the GSI was significantly and positively correlated with the Zn concentration (p = 0.007, r = 0.484). The CF was significantly correlated with the Fe (p = 0.034, r = 0.367), Ni (p = 0.008, r = 0.474), and Hg (p = 0.000, r = −0.631) concentrations. In largemouth bass, a significant and negative correlation was found between HSI and the Fe concentration (p = 0.005, r = −0.539), while significant correlations were also found between GSI and the Cu concentration (p = 0.052, r = 0.386) and CF and the Hg (p = 0.000, r = 0.841) and Se (p = 0.000, r = 0.859) concentrations.
The results of the CCA also revealed associations among biological indices and metal(loid) concentrations. The first two axes of CCA for tilapia represented 95% of the variability (65% in the first axis and 30% in the second axis). The biological responses were mainly distributed among three quadrants of the CCA (Fig. 5b). Also, in tilapia, the CF was canonically correlated with the Fe, Hg and Se concentrations, and the GSI was canonically correlated with the Cr, Cu, Mn, Ni, and Zn concentrations, whereas the HSI was not canonically correlated with any metal(loid) concentration. The results of the multiple regression analysis corroborated these associations with the following correlation equations for FC = 0.15 + 0.07 Fe − 0.34 Hg (r = 0.51, p < 0.05) and GSI = −0.37 − 0.09 Mn + 0.22 Ni + 0.21 Zn + 0.48 Hg (r = 0.41, p < 0.05).
In the case of largemouth bass, the two main axes of the CCA represented 96% of the variance (65% in the first axis and 45% in the second axis; Fig. 5c, d). The FC was canonically correlated with the Fe and Ni concentrations, and to a lesser extent with the Cr, Cu, and Mn concentrations. The HSI was canonically correlated with the As, Cr, Cu, Mn, and Ni concentrations and to a lesser degree with the Zn and Hg concentrations. The GSI was not canonically correlated or related with any metal(loid) concentration given the results of the CCA and multiple regression analysis. The multiple regression analysis corroborated these associations with the following correlation equations for FC = 0.0316 − 0.0502 Fe − 0.1586 Cr + 0.2942 As + 0.4158 Se + 0.2531 Hg (r = 0.97, p < 0.05) and HSI = 1.48 + 0.77 Ni − 0.65 Cu − 0.63 Zn − 1.20 As + 2.37 Se − 0.76 Hg (r = 0.36, p < 0.05). In tilapia, the results of the CCA indicated positive correlations between mt expression in the liver and the As and Fe concentrations, while the multiple regression analysis resulted in the following correlation equation of mt = −1.185 + 0.27 Fe + 0.24 Zn + 0.29 As + 0.46 Hg (r = 0.22, p < 0.05). In largemouth bass, the CCA showed significant correlations between mt expression and the As, Cu, Fe, Mn, Ni and Se concentrations, which were confirmed by the results of the multiple regression analysis with the following correlation equation of mt = −3.17 + 1.04 Mn − 0.74 Ni + 0.80 Cu + 0.76 Zn + 1.67 As (r = 0.32, p < 0.05).
In the case of IBR, the results of the PERMANOVA indicated differences between biomarker responses and metal(oid) concentrations in both species (pseudo-F = 65.59, p = 0.001; pseudo-F = 58.15, p = 0.001 for tilapia and largemouth bass, respectively). Tilapia showed higher IBR values than those of the largemouth bass, with the highest IBR found in the ECO ecosystem (Fig. 10). The main metal(oid)s recorded in the ECO ecosystem for tilapia were Fe, Cu, Zn, and Cr [Similarity percentages (SIMPER; similitude, contribution): Fe (23.8%, 25.5%), Cu (19.2%, 20.5%), Zn (12.5%, 13.4%), and Cr (11.5%, 12.3%)]. In largemouth bass, the main metal(oid)s recorded were Fe, Cu, Zn, and Cr [SIMPER (similitude, contribution): Fe (27.0%, 27.9%), Zn (15.5%, 16.0%), Cr (11.6%, 12.0%), and Cu (11.1%, 11.5%); Tables 4–6]. The results of the IBR analysis of the metal(oid)s were higher in tilapia than in largemouth bass. Biomarker responses in both species followed similar tendencies but of different magnitudes. In tilapia, the CF, GSI, and HSI were not altered by metal concentrations (Fig. 10). On the other hand, mt gene expression and HAI were significantly altered by these elements. The IBR analysis in largemouth bass did not show a significant (p < 0.05) response for CF, GSI, or HIS (Fig. 11), while mt gene expression and HAI showed significant differences (p < 0.05) among the three ecosystems.
Discussion
The Yaqui River Basin is an important ecoregion in northwestern Mexico and is particularly important for the state of Sonora, as it supplies water for human consumption and various economic activities are conducted along its margins (Arreola-Lizarraga et al. 2020). In the upper part of the watershed, extensive mining projects dominate the activities of the region, and most of the wastewater from these activities ends up in the watershed in the absence of any regulations (Meza-Figueroa et al. 2009; De la O-Villanueva et al. 2013; Martínez-Durazo et al. 2020). In the central portion of the watershed, subsistence and artisanal activities are the most important economic occupations, and the region has not been notably altered by anthropogenic activities; however, important natural mineralization and metal(oid) contaminants are of special concern in this area (Gortáres-Moroyoqui et al. 2011). Finally, the lower portion of the basin, known as the Yaqui Valley, serves as one of the most important agriculture zones in Mexico, and untreated wastewater effluents are discharged from agricultural activities (Ochoa-Contreras et al. 2021). Environmental monitoring research in this zone is scarce, which is particularly concerning due to the ecological, social, cultural, and economic importance of the region.
Due to the different anthropogenic activities of the region, this study aimed to establish associations between metal(oid) concentrations and biomarkers (biological and histological indexes) in tilapia and largemouth bass, both of which are resident species in the reservoirs of the Yaqui River Basin. Several biotic and abiotic factors are known to drive accumulation of contaminants in aquatic environments. In addition, fish species can incorporate metals and metalloids through respiratory and/or gastrointestinal processes, which produce differential distributions of these compounds among different tissues (Rainbow and Luoma 2011; Mackay et al. 2018). The incorporation rates in fish gills are strongly affected by the amounts of dissolved metal(loid)s, while the bioavailable fractions of these elements in food items are incorporated through the digestive tract (Rainbow and Luoma 2011; Lall and Kaushik, 2021). Given that incorporated metal(loid)s are metabolized in the liver, several biomarkers are used to measure their levels in liver tissue (Corredor-Santamaría et al. 2021). In this study, the concentrations of metal(loid)s in the tissues of tilapia and largemouth bass presented differences in accumulation as well as seasonal variability among the three ecosystems. Differential metal accumulation in fish tissues depends on the function of each organ (Łuczyńska et al., 2018), and thus the liver accumulates notably greater levels of these elements than those in other organs, followed by the levels in the gills and gonads (Martinez-Durazo et al. 2020).
In this study, tilapia showed higher concentrations of metal(loid)s than largemouth bass, which could be explained by the feeding habits of each species. In an earlier study, Leung et al. (2014) reported similar accumulation of metal(loid)s, which were also higher in tilapia than in largemouth bass. Yi et al. (2017) reported that fishes living in middle-lower layers or fishes feeding on benthos accumulated higher concentrations of metal(loid)s when compared with fishes living in the pelagic zone. Usually, benthic fish are more exposed to metal(loid)s because these elements are deposited on sediments and are linked to fine particles (fractions <63 μm; Suresha et al. 2012). However, other authors explain that pelagic predatory fishes maybe more exposed to these elements because of the variety of prey and may accumulate higher levels of metal(loid)s than benthic fishes (Gu et al. 2016; Yi et al., 2017). However, the accumulation of essential elements (in this study Cu, Cr, Fe, Mn, Ni, Se, and Zn) in different tissues and fish species may be related to their physiological demand in specific organs according to biological characteristics (such as sex, maturity, nutritional status, and age) (Leung et al. 2014; Łuczyńska et al. 2018).
Fish gills are the main sites for physiological functions such as acid-base balance, gas exchange, ion pumps, and waste excretion. Metal(loid)s are bioreactive with the gills, increasing the incorporation rates with dissolved ion levels in the water, and therefore gills are sensitive to metal(loid) accumulation. The toxicity of these elements may produce interference in gill functions, mainly gas exchange and ion pumps (Wood 2011).
The gonads are sensitive to endocrine disruptors. Some studies in which fish were exposed to metal(loid)s have shown that accumulation in the gonads resulted in reductions in the production and viability of gametes. Further, fertilization rate, vitellogenin storage, and embryonic development in fish may be adversely affected by metal contamination (Jezierska et al. 2009; Paschoalini et al. 2019). Our data showed similar accumulation of metal(loid)s in tilapia and largemouth bass, except Zn, that accumulated at higher levels in largemouth bass. The levels of Cu, Fe, Mn, Ni, and Zn in gonads in both fish species were higher than those reported by Oğuz and Yeltekin (2014) in Tarek (Alburnus tarichi); the authors explain that gonadal abnormalities may derive from the accumulation of Zn and Cu; moreover, the combination of metals with other chemicals present in the aquatic environment might have led to those abnormalities.
Somatic indices have been widely used in environmental risk assessments with different fish species (Kroon et al. 2017). Previous studies have measured somatic indices in largemouth bass and tilapia to assess metal contamination in aquatic ecosystems (Gehringer et al. 2013; Girgis et al. 2019). The metal accumulation results in this study for tilapia agree with those of Shaw and Handy (2006). In that study, fish were exposed to various levels of Cu in food, and differences in metal accumulation among tissues were observed, with Cu accumulation being higher in exposed specimens than in control specimens. However, the HSI, which is an indicator of hepatomegaly, was similar between exposed and non-exposed organisms. In a different study with tilapia exposed to metals and persistent organic pollutants (Ibor et al. 2017), HSI values were found to be lower in exposed organisms (0.3–0.9) than in control fish (no exposure, 0.9–5.7); moreover, males showed higher HSI values than females. In contrast, another study with Tilapia mossambica, Al-Ghais (2013) reported that higher HSI values were observed in organisms exposed to sewage effluents (with elevated levels of contaminants including metals) than non-exposed organisms. In that study, HSI values were also higher in exposed organisms compared to those of organisms exposed to sewage effluents that were then cleared in fresh water for 6 weeks. In a bioassay with tilapia exposed to Cd, Cu, and a mixture of both elements, El-Serafy et al. (2013) reported that the CF, HSI and GSI were significantly lower in the exposed specimens with respect to the values of the control fish. These outcomes were then considered to be adverse effects due to metal exposure.
In a study with largemouth bass conducted by Orlando et al. (1999), significant differences in the HSI were reported between organisms collected from a contaminated site in Florida (0.57 ± 0.02) and those collected from a reference site (0.85 ± 0.03) in the same state. Similarly, Friedmann et al. (2002) reported significant variations in the HSI in largemouth bass collected from sites with different degrees of Hg contamination, with values ranging from 0.81 ± 0.04 in organisms from the most contaminated site to 1.16 ± 0.09 in organisms from the least contaminated site. The study showed that CF and GSI were not associated with an Hg gradient of contamination, as similar values were detected among sites. In a bioassay with largemouth bass fingerlings exposed to contaminated water and sediments with effluents from the paper industry, the highest HSI and CF values were observed in non-exposed organisms that had been maintained in carbon-dechlorinated tap water, while organisms exposed to contaminated effluents showed lower values (Baer et al. 2009). These lower HSI and CF values were attributed to the elevated amounts of contaminants present in the ecosystem. A study with yellow perch from three lakes with different degrees of metal contamination showed different results, with the highest HSI and CF values found in organisms from the most contaminated lakes, while the GSI values were higher in organisms from lakes with lower levels of contamination (Pyle et al. 2005).
In a study with various contaminants and biological indexes in eight basins of rivers in the Unites States, the variations in the accumulation of 15 metal(loid)s in whole-body composite samples of largemouth bass Micropterus spp. were reported by Hinck et al. (2008). Slight variations in CF, HSI, and GSI were determined between females and males from the different ecosystems, in spite of differences in the accumulation of some elements. However, some significant positive correlations between biological indexes and some metals were found. Positive associations between metal(loid)s and biomarkers are usually associated to the use of these elements in physiological processes, either for metabolic processes or in regulatory routes (Rainbow and Luoma 2011; Huang et al 2022).
Changes in GSI are mostly determined by variations in yolk concentration during different oocyte stages in females, and the size and mobility of sperm in males; thus, it provides information about maturation and seasonal patterns in gonad development (McPherson et al. 2011; Flores et al. 2015). In this study we determined differences in GSI values among seasons and ecosystems, with the highest values in largemouth bass. GSI correlated with Cr, Cu, Mn, Ni, and Zn in tilapia, which may be explained by their physiological use in oocyte development.
The expression of metallothionein (mt) is a biomarker frequently used in studies of metal(oid) exposure, both in field and laboratory studies. Because mt is an intracellular protein involved in regulation and detoxification of metal(loids), the exposure to these elements is a common inducer of its synthesis (Vašák and Hasler 2000). The exposure of O. niloticus to different levels of methyl mercury chloride for 60 days increased the relative expression of mt from 0.5 to 10%, in respect to non-exposed organisms (Alam et al. 2021). Similarly, the exposure of farmed tilapia to Cu and Pb induced the expression of mt (Girgis et al. 2019). The expression of mt is usually different among target tissues, as was reported in tilapia O. niloticus injected with different concentrations of metals (Cheung et al. 2004); all of the metal ions tested induced high levels of hepatic and gill mt, contrasting with the low renal mt levels. High hepatic mt levels were also induced by the exposure to various metal ions in O. mossambicus and O. aureus (Cheung et al. 2005). In another study with tilapia O. niloticus fingerlings exposed to silver nanoparticles, Thummabancha et al. (2016) reported that the expression in liver and spleen decreased from 0.3 to 0.7 times, while the expression of mt in kidney increased 2–3 times.
The expression of mt as biomarker of exposure to metal(loid)s has also been used in the largemouth bass, in which mt expression was proportional to Hg levels in fish collected in the Quachita River, USA (Schlenk et al. 1995); the concentrations of Hg were associated with predatory habits. Gehringer et al. (2013) reported the expression of mt in kidney and liver of largemouth bass collected in the San Joaquín River, California, which is an area contaminated by mining activities; gene expression was significantly higher in organisms with elevated concentrations of Cu, Zn, Mn, Al, and Ni. In contrast, Mehinto et al. (2014) reported that adults of largemouth bass did not show increased hepatic mt levels after intraperitoneal exposure to Cd (20 µg CdCl2/Kg) (Vašák and Hasler 2000). However, a study with the African catfish Clarias gariepinus reported an increase of mt expression in wild specimens collected in sites contaminated with Zn and Cu (M’kandawire et al. 2017).
Our data showed variations of mt expression in tilapia between seasons and ecosystems (ECO > ANG > OVI during the dry season and ANG > ECO > OVI during the rainy season), with significant increments in expression from dry to rainy seasons only in the ANG dam. Multivariate analysis indicated positive correlations between mt expression in tilapia and Fe, Zn, As and Hg. The higher expression of mt in ECO agrees with higher levels of Fe and Hg in this ecosystem during the dry season, contrasting with the levels of Cu, Mn, Zn, and As.
Our data also showed seasonal variations of mt expression in largemouth bass among ecosystems, presenting higher mt expression in the OVI dam during the dry season and in the ANG dam during the rainy season. Multivariate analysis indicated correlations between mt expression in largemouth bass and As, Cu, Fe, Mn and Ni, which did not correspond with levels of these elements (except for Mn) found in OVI in the dry season, and neither corresponded with the levels of any element found in ANG in the rainy season. The expression of mt in largemouth bass in the ecosystems varied between seasons (mean range of 0.85–1.56 during dry season, and of 0.94–1.34 during rainy season), consistent with variations in the levels of metal(loid)s. The levels of contamination with metal(loid)s of the ecosystems of this study were variable. Multivariate analysis showed correlations between mt expression in tilapia with Fe, Zn, As, and Hg, and mt expression in largemouth bass with As, Cu, Fe, Mn, Ni, and Se. However, mt can be induced by a variety of environmental and physiological factors, such as oxygen reactive species, water temperature and glucocorticoids, which can be present in waterbodies and affect mt gene expression. (Vašák and Hasler 2000; Cheung et al., 2005; Wang et al. 2014). In our studies, fish were captured in dams of the Yaqui River Basin in the absence of any fishing restrictions and as a result were subjected to a variety of pollutants, including metal(oid)s.
In this study, the histology of the gills and liver of tilapia and largemouth bass revealed reversible lesions. The HAI in both tissues showed similar values among ecosystems in each season, with the highest values in ANG for tilapia and in ECO and OVI for largemouth bass. Similar results were found by Abdel-Khalek (2015) in tilapia from two sites in the Nile River in Egypt with different degrees of metal contamination. The authors reported evidence ranging from reversible histological changes to severe tissue damage in gills. The histology of the livers of tilapia in a freshwater ecosystem of Saudi Arabia contaminated with various metals reported vacuolization, necrosis, and sinusoidal dilation associated with different types of anthropogenic discharges (Mahboob et al. 2020). Tilapia fingerlings presented gill damage (including hyperplasia, detachment of the branchial epithelium, edema, deformations of the secondary lamella, and total fusion of the lamellae) due to acute exposure to Cu (Alkobaby and El-Wahed 2017). Wepener et al. (2011) reported reversible damage in tissues from the Orange River mudfish (Labeo capensis) that were considered functional in terms of structure; however, some reported alterations, such as fusion of the primary lamellae, that could lead to adverse health effects. Studies with largemouth bass exposed to metal(loid)s have also reported histological damage. The most frequent histopathological lesions found in the livers of wild fish exposed to As and Hg were vacuolated hepatocytes, focal necrosis, and granulomas (Hatice and Turgay 2020). Gehringer et al. (2013) reported major histological damage in juvenile organisms due to exposure to MeHg. Moreover, juvenile fish collected from areas highly contaminated with Hg showed histological damage.
The results presented in this study are limited to some responses and their associations with metal and metalloid concentrations; however, wild fish populations are exposed to complex mixtures of compounds. In addition, environmental physical and chemical factors were not measured in this study. All these factors interact in complex networks making it impossible to establish causal relationships. In addition, fish responses vary depending on age, sex, food availability, and developmental stage. In other words, field studies like this are an approximation to understand ecosystem health but by no means establish causality. Nevertheless, associations between metal and metalloid concentrations and biological responses measured in wild fish populations provide insights into environmental impacts of anthropogenic origin. According to our results, the biomarkers measured in the two fish species provided useful information regarding the status of the three ecosystems of the Yaqui River Basin. As a whole, the system seems to be moderately impacted by metals and metalloids. Surprisingly, ECO, which sustains small-scale agriculture, fish aquaculture, and cattle raising, seems to be the most impacted of the three ecosystems in this study.
Conclusions
This study examined metal(loid) accumulation in tilapia and largemouth bass and associations among biomarkers from three ecosystems of the Yaqui River Basin. Our results show that tilapia can incorporate higher concentrations of metal(oid)s, with a clear and more consistent biomarker response compared to that of largemouth bass, which showed a comparatively milder response to the adverse effects of metal(oid)s. There are several other biotic and abiotic factors that were not measured in this study that affect biomarker responses in wild fish populations, which may have influenced the results. However, based on our data, the three ecosystems seem to be moderately impacted by metals and metalloids, with the ECO dam being the most impacted ecosystem in this study.
Data availability
Data are available from the corresponding upon reasonable request.
References
Abdel-Khalek AA (2015) Risk assessment, bioaccumulation of metals and histopathological alterations in Nile tilapia (Oreochromis niloticus) facing degraded aquatic conditions. Bull Environ Contam Toxicol 94:77–83. https://doi.org/10.1007/s00128-014-1400-9
Abdel-Khalek AA, Elhaddad E, Mamdouh S, Saed Marie MA (2016) Assessment of metal pollution around Sabal drainage in River Nile and its impacts on bioaccumulation level, metals correlation and human risk hazard using Oreochromis niloticus as a bioindicator. Turkish J Fish Aquat Sci 16:227–239. https://doi.org/10.4194/1303-2712-v16_2_02
Alam RTM, Abu Zeid EH, Khalifa BA, Arisha AH, Reda RM (2021) Dietary exposure to methyl mercury chloride induces alterations in hematology, biochemical parameters, and mRNA expression of antioxidant enzymes and metallothionein in Nile tilapia. Environ Sci Pollut Res 28:31391–31402. https://doi.org/10.1007/s11356-021-13014-5
Al-Ghais SM (2013) Acetylcholinesterase, glutathione and hepatosomatic index as potential biomarkers of sewage pollution and depuration in fish. Mar Pollut Bull 74(1):183–186. https://doi.org/10.1016/j.marpolbul.2013.07.005
Ali H, Khan E (2018) Bioaccumulation of non-essential hazardous heavy metals and metalloids in freshwater fish: Risk to human health. Environ Chem Lett 16:903–917. https://doi.org/10.1007/s10311-018-0734-7
Alkobaby AI, El-Wahed RKA (2017) The acute toxicity of copper to nile tilapia (Oreochromis niloticus) fingerlings and its effects on gill and liver histology. J Aquac Res Development 8(1):2–6. https://doi.org/10.4172/2155-9546.1000465
American Fisheries Society (2014) Guidelines for the use of fishes Joint committee of the American Fisheries Society, the American Institute of Fishery Research Biologists, and the American Society of Ichthyologists and Herpetologists. Bethesda, Maryland. p 104. https://fisheries.org/docs/wp/Guidelines-for-Use-of-Fishes.pdf
Arreola-Lizarraga JA, Padilla-Arredondo G, Cruz-García LM, Ruiz-Ruiz TM, Polanco-Mizquez EI (2020). Analysis of a socio-ecological system: Coastal zone of the yaqui indigenous community (NW México). In: Socio-ecological studies in natural protected areas linking community development and conservation in Mexico. https://doi.org/10.1007/978-3-030-47264-1
Azevedo JS, Serafim A, Company R, Braga ES, Fávaro DI, Bebianno MJ (2009) Biomarkers of exposure to metal contamination and lipid peroxidation in the benthic fish Cathorops spixii from two estuaries in South America. Brazil Ecotox 18(8):577–586. https://doi.org/10.1007/s10646-009-0370-x
Baer KN, Bankston CR, Mosadeghi S, Schlenk D (2009) The effects of pulp and paper mill effluent on physiological and hematological endpoints in fingerling largemouth bass (Micropterus salmoides). Drug Chem Toxicol 32(1):59–67. https://doi.org/10.1080/01480540802432643
Banday UZ, Swaleh SB, Usmani N (2020) Heavy metal toxicity has an immunomodulatory effect on metallothionein and glutathione peroxidase gene expression in Cyprinus carpio inhabiting a wetland lake and a culture pond. Chemosphere 251:1–9. https://doi.org/10.1016/j.chemosphere.2020.126311
Beliaeff B, Burgeot T (2002) Integrated biomarker response: a useful tool for ecological risk assessment. Environ Toxicol Chem 21:1316–1322
Bernet D, Schmidt H, Meier W, Burkhardt-Holm P, Wahli T (1999) Histopathology in fish: Proposal for a protocol to assess aquatic pollution. J Fish Dis 22(1):25–34. https://doi.org/10.1046/j.1365-2761.1999.00134.x
Camargo MMP, Martinez CBR (2007) Histopathology of gills, kidney and liver of a neotropical fish caged in an urban stream. Neotrop Ichthyol 5:327–336
Cerqueira CCC, Fernandes MN (2002) Gill tissue recovery after copper exposure and blood parameter responses in the tropical fish, Prochilodus scrofa. Ecotoxicol Environ Saf 52:83–91. https://doi.org/10.1006/eesa.2002.2164
Chatterjee N, Gim J, Choi J (2018) Epigenetic profiling to environmental stressors in model and non-model organisms: Ecotoxicology perspective. Environ Health Toxicol 33(3):e2018015. https://doi.org/10.5620/eht.e2018015
Cheung APL, Lam THJ, Chan KM (2004) Regulation of tilapia metallothionein gene expression by heavy metal ions. Mar Environ Res 58:389–394. https://doi.org/10.1016/j.marenvres.2004.03.084
Cheung APL, Lim Lam VK, Chan KM (2005) Tilapia metallothionein genes: PCR-cloning and gene expression studies. Biochim Biophys Act 1731:191–201. https://doi.org/10.1016/j.bbaexp.2005.09.006
CONAGUA (2020) Comisión Nacional del Agua. Normales Climatológicas por Estado. https://smn.conagua.gob.mx/tools/RESOURCES/%0AMensuales/son/00026068.TXT. Accessed 6 Dec 2020
Corredor-Santamaría W, Mora-Solarte DA, Arbeli Z, Navas JM, Velasco-Santamaría YM (2021) Liver biomarkers response of the neotropical fish Aequidens metae to environmental stressors associated with the oil industry. Heliyon 7(7):1–8. https://doi.org/10.1016/j.heliyon.2021.e07458
Chua EM, Flint N, Wilson SP, Vink S (2018) Potential for biomonitoring metals and metalloids using fish condition and tissue analysis in an agricultural and coal mining region. Chemosphere 202:598–608. https://doi.org/10.1016/j.chemosphere.2018.03.080
De la O-Villanueva M, Meza-Figueroa D, Maier RM, Moreno D, Gómez-Alvarez A, Del Río-Salas R, Mendívil H, Montijo A (2013) Erosive processes in the Presa I mine dam at Nacozari de Garcia, Sonora, and their effect in the dispersion of pollutants. Bol Soc Geol Mex 65:27–38
Delfino-Vieira CE, Aparecida-Marques J, Gonçalves-da-Silva N, Ziviani-Bevitório L, Dornelles-Zebral Y, Maraschi AC, Rutz-Costa S, Gomes-Costa P, Medeiros-Damasceno E, Monteiro-Pirovani JC, Vale-Oliveira M, Marques-Souza M, Gaspar-Martins CM, Bianchini A, Zomer-Sandrini J (2022) Ecotoxicological impacts of the Fundão dam failure in freshwater fish community: Metal bioaccumulation, biochemical, genetic and histopathological effects. Sci Total Environ 832:154878. https://doi.org/10.1016/j.scitotenv.2022.154878
Dwivedi AC, Tiwari A, Mayank P (2015) Seasonal determination of heavy metals in muscle, gill and liver tissues of Nile tilapia, Oreochromis niloticus (Linnaeus, 1758) from the from the tributary of the Ganga River, India. Zool Ecol 25(2):166–171. https://doi.org/10.1080/21658005.2015.1020012
El-Agri AM, Emam MA, Gaber HS, Hassan EA, Hamdy SM (2022) Integrated use of biomarkers to assess the impact of heavy metal pollution on Solea aegyptiaca fish in Lake Qarun. Environ Sci Eur 34:74. https://doi.org/10.1186/s12302-022-00656-6
El-Serafy SS, Zowai ME, Abdel-Hameid NAH, Awwad MH, Omar EH (2013) Effect of dietborne metals on body indices of nile tilapia (Oreochromis niloticus) with emphasis on protein pattern. Egypt J Exp Biol (Zool) 9(1):15–24
Fahd F, Veitch B, Khan F (2020) Risk assessment of Arctic aquatic species using ecotoxicological biomarkers and Bayesian network. Mar Pollut Bull 156:1–11. https://doi.org/10.1016/j.marpolbul.2020.111212
Flores A, Wiff R, Díaz E (2015) Using the gonadosomatic index to estimate the maturity ogive: Application to Chilean hake (Merluccius gayi gayi). ICES Mar Sci Symp 72(2):508–514. https://doi.org/10.1093/icesjms/fsu155
Friedmann AS, Kimble CE, MacLatchy DL, Stansley W, Washuta EJ (2002) Effect of mercury on general and reproductive health of largemouth bass (Micropterus salmoides) from three lakes in New Jersey. Ecotoxicol Environ Saf 52(2):117–122. https://doi.org/10.1006/eesa.2002.2165
Gagnon MM, Hodson PV (2012) Field studies using fish biomarkers – How many fish are enough. Mar Pollut Bull 64:2871–2876. https://doi.org/10.1016/j.marpolbul.2012.08.016
García-Gasca A, Ríos-Sicairos J, Hernández-Cornejo R, Cunha I, Gutiérrez JN, Plascencia-González H, De la Parra LMG, Abad-Rosales S, Betancourt-Lozano M (2016) The white mullet (Mugil curema) as biological indicator to assess environmental stress in tropical coastal lagoons. Environ Monit Assess 188(668):1–15. https://doi.org/10.1007/s10661-016-5714-4
Gehringer DB, Finkelstein ME, Coale KH, Stephenson M, Geller JB (2013) Assessing mercury exposure and biomarkers in largemouth bass (Micropterus salmoides) from a contaminated river system in California. AECT 64(3):484–493. https://doi.org/10.1007/s00244-012-9838-4
Girgis SM, Mabrouk DM, Hanna MI, Abd ElRaouf A (2019) Seasonal assessment of some heavy metal pollution and metallothionein gene expression in cultured Oreochromis niloticus. Bull Natl Res Cent 43(1):1–8. https://doi.org/10.1186/s42269-019-0167-x
Gortáres-Moroyoqui P, Castro-Espinosa L, Naranjo JE, Karpiscak MM, Freitas RJ, Gerba CP (2011) Microbiological water quality in a large irrigation system: El Valle del Yaqui, Sonora México. J Environ Sci Health A Tox Hazard Subst Environ Eng 46:1708–1712. https://doi.org/10.1080/10934529.2011.623968
Gu YG, Lin Q, Huang HH, Wang LG, Ning JJ, Du FY (2016) Heavy metals in fish tissues/stomach contents in four marine wild commercially valuable fish species from the western continental shelf of South China Sea. Mar Pollut Bull 114:1125–1129. https://doi.org/10.1016/j.marpolbul.2016.10.040
Hatice D, Turgay Ş (2020) A morpho-histopathological study in the digestive tract of three fish species influenced with heavy metal pollution. Chemosphere. 242:1–8. https://doi.org/10.1016/j.chemosphere.2019.125212
Hinck JE, Schmitt CJ, Ellersieck MR, Tillitt DE (2008) Relations between and among contaminant concentrations and biomarkers in black bass (Micropterus spp.) and common carp (Cyprinus carpio) from large U.S. rivers, 1995–2004. J Environ Monit 10:1499–1518. https://doi.org/10.1039/b811011e
Huang X, Xu J, Liu B, Guan X, Li J (2022) Assessment of aquatic ecosystem health with Indices of Biotic Integrity (IBIs) in the Ganjiang River System. China Water 278(14):1–17. https://doi.org/10.3390/w14030278
Hussein GHG, Chen M, Qi PP, Cui QK, Yu Y, Hu WH, Tian Y, Fan QX, Gao ZX, Feng MW, Shen ZG (2020) Aquaculture industry development, annual price analysis and out-of-season spawning in largemouth bass Micropterus salmoides. Aquaculture 519:734901. https://doi.org/10.1016/j.aquaculture.2019.734901
Ibor OR, Adeogun AO, Chukwuka AV, Arukwe A (2017) Gross pathology, physiological and toxicological responses in relation to metals and persistent organic pollutants (POPs) burden in tilapia species from Ogun River, Nigeria. Mar Environ Res 129:245–257. https://doi.org/10.1016/j.marenvres.2017.06.003
Jara-Marini ME, Molina-García A, Martínez-Durazo A, Páez-Osuna F (2020) Trace metal trophic transference and biomagnification in a semiarid coastal lagoon impacted by agriculture and shrimp aquaculture. Environ Sci Pollut Res 27:5323–5336. https://doi.org/10.1007/s11356-019-06788-2
Javed M, Ahmad MI, Usmani N, Ahmad M (2017) Multiple biomarker responses (serum biochemistry, oxidative stress, genotoxicity and histopathology) in Channa punctatus exposed to heavy metal loaded waste water. Sci Rep 7(1):1–11. https://doi.org/10.1038/s41598-017-01749-6
Jezierska B, Lugowska K, Witeska M (2009) The effects of heavy metals on embryonic development of fish (a review). Fish Physiol Biochem 35:625–640. https://doi.org/10.1007/s10695-008-9284-4
Joshi KK, Sreekumar KM (2015) Basics of sample collection, preservation and species identification of finfish. In: Joshi KK, Varsha MS, Sruthy VL, Susan V, Prathyusha PV (eds) Summer school on recent advances in marine biodiversity conservation and management. CMFRI, p 134–137
Kroon F, Streten C, Harries S (2017) A protocol for identifying suitable biomarkers to assess fish health: A systematic review. PLoS One 12(4):e0174762. https://doi.org/10.1371/journal.pone.0174762.
Lall SP, Kaushik SJ (2021) Nutrition and metabolism of minerals in fish. Animals. 11(2711):2–45. https://doi.org/10.3390/ani11092711
Leung HM, Leung AOW, Wang HS, Ma KK, Liang Y, Ho KC, Cheung KC, Tohidi F, Yung KKL (2014) Assessment of heavy metals/metalloid (As, Pb, Cd, Ni, Zn, Cr, Cu, Mn) concentrations in edible fish species tissue in the Pearl River Delta (PRD), China. Mar Pollut Bull 78:235–245. https://doi.org/10.1016/j.marpolbul.2013.10.028
Łuczyńska J, Paszczyk B, Łuczyński MJ (2018) Fish as a bioindicator of heavy metals pollution in aquatic ecosystem of Pluszne Lake, Poland, and risk assessment for consumer’s health. Ecotoxicol Environ Saf 153:60–67. https://doi.org/10.1016/j.ecoenv.2018.01.057
M’kandawire E, Mierek-Adamska A, Stürzenbaum SR, Choongo K, Yabe J, Mwase M, Saasa N, Blindauer CA (2017) Metallothionein fromwild populations of the African catfish Clarias gariepinus: from sequence, protein expression and metal binding properties to transcriptional biomarker of metal pollution. Int J Mol Sci 18:1548. https://doi.org/10.3390/ijms18071548
Ma D, Fan J, Tian Y, Jiang P, Wang J, Zhu H, Bai J (2019) Selection of reference genes for quantitative real-time PCR normalization in largemouth bass Micropterus salmoides fed on alternative diets. J Fish Biol 95(2):393–400. https://doi.org/10.1111/jfb.13991
Mackay D, Celsie AKD, Powell DE, Parnis JM (2018) Bioconcentration, bioaccumulation, biomagnification and trophic magnification: a modelling perspective. Environ Sci Processes Impacts 20:72–85. https://doi.org/10.1039/c7em00485k
Mahboob S, Al-Ghanim KA, Al-Balawi HF, Al-Misned F, Ahmed Z (2020) Toxicological effects of heavy metals on histological alterations in various organs in Nile tilapia (Oreochromis niloticus) from freshwater reservoir. J King Saud Univ Sci 32(1):970–973. https://doi.org/10.1016/j.jksus.2019.07.004
Marie B (2020) Disentangling of the ecotoxicological signal using “omics” analyses, a lesson from the survey of the impact of cyanobacterial proliferations on fishes. Sci Total Environ 736:1–15. https://doi.org/10.1016/j.scitotenv.2020.139701
Martínez-Durazo Á, Cruz-Acevedo E, Betancourt-Lozano M, Jara-Marini ME (2020) Comparative assessment of metal bioaccumulation in tilapia and largemouth bass from three dams of the Yaqui River. Biol Trace Elem Res 199:3112–3125. https://doi.org/10.1007/s12011-020-02425-z
McCullough EB, Matson PA (2016) Evolution of the knowledge system for agricultural development in the Yaqui Valley, Sonora, Mexico. Proc Natl Acad Sci USA 113:4609–4614. https://doi.org/10.1073/pnas.1011602108/-/DCSupplemental
McPherson LR, Ganias K, Marshall CT (2011) Inaccuracies in routinely collected Atlantic herring (Clupea harengus) maturity data and correction using a gonadosomatic index model. J Mar Biolog Assoc 91:1477–1487. https://doi.org/10.1017/S002531541100018X
Mehinto AC, Prucha MS, Colli-Dula RC, Kroll KJ, Lavelle CM, Barber DS, Vulpe CD, Denslow ND (2014) Gene networks and toxicity pathways induced by acute cadmium exposure in adult largemouth bass (Micropterus salmoides). Aquat Toxicol 152:186–194. https://doi.org/10.1016/j.aquatox.2014.04.004
Meza-Figueroa D, Maier RM, de la O-Villanueva M, Gómez-Alvarez A, Moreno-Zazueta A, Rivera J, Campillo A, Grandlic CJ, Anaya R, Palafox-Reyes J (2009) The impact of unconfined mine tailings in residential areas from a mining town in a semi-arid environment: Nacozari, Sonora, Mexico. Chemosphere 77:140–147. https://doi.org/10.1016/j.chemosphere.2009.04.068
Meza-Montenegro MM, Gandolfi AJ, Santana-Alcantar ME, Klimecki WT, Aguilar-Apodaca MG, Del Río-Salas R, De la O-Villanueva M, Gómez-Alvarez A, Mendivil-Quijada H, Valencia M, Meza-Figueroa D (2012) Metals in residential soils and cumulative risk assessment in Yaqui and Mayo agricultural valleys, northern Mexico. Sci Total Environ 433:472–481. https://doi.org/10.1016/j.scitotenv.2012.06.083
Mussali-Galante P, Tovar-Sánchez E, Valverde M, Rojas E, Castillo D (2013) Biomarkers of exposure for assessing environmental metal pollution: from molecules to ecosystems. Rev Int Contam Ambie 29(1):117–140
Mustafa SA (2020) Histopathology and heavy metal bioaccumulation in some tissues of Luciobarbus xanthopterus collected from Tigris River of Baghdad, Iraq. Egyptian J Aquat Res 46(2):123–129. https://doi.org/10.1016/j.ejar.2020.01.004
Nash RDM, Valencia AH, Geffen AJ (2006) The origin of Fulton’s condition factor – Setting the record straight. Fisheries 31(5):236–238
Ochoa-Contreras R, Jara-Marini ME, Sanchez-Cabeza JA, Meza-Figueroa DM, Pérez-Bernal JH, Ruiz-Fernández AC (2021) Anthropogenic and climate induced trace element contaminatio in a water reservoir in northwestern Mexico. Environ Sci Pollut Res 28:16895–16912. https://doi.org/10.1007/s11356-020-11995-3
Oğuz AR, Yeltekin A (2014) Metal Levels in the Liver, Muscle, Gill, Intestine, and Gonad of Lake Van Fish (Chalcalburnus tarichi) with Abnormal Gonad. Biol Trace Elem Res 159:219–223. https://doi.org/10.1007/s12011-014-9980-0
Orlando EF, Denslow ND, Folmar LC, Guillette LJ Jr (1999) A comparison of the reproductive physiology of largemouth bass, Micropterus salmoides, collected from the Escambia and Blackwater Rivers in Florida. Environ Health Perspect 107(3):199–204. https://doi.org/10.1289/ehp.99107199
Paschoalini AL, Savassi LA, Arantes FP, Rizzo E, Bazzoli N (2019) Heavy metals accumulation and endocrine disruption in Prochilodus argenteus from a polluted neotropical river. Ecotox Environ Saf 169:539–550. https://doi.org/10.1016/j.ecoenv.2018.11.047
Pfaffl MW (2004) Quantification strategies in real-time PCR. In: Bustin SA (ed) A-Z of quantitative PCR. International University Line (IUL), p 87–112
Prabu E, Rajagopalsamy CBT, Ahilan B, Jeevagan IJMA, Renuhadevi M (2019) Tilapia – an excellent candidate species for world aquaculture: A review. Annu Res Rev Biol 31(3):1–14. https://doi.org/10.9734/arrb/2019/v31i330052
Pyle GG, Rajotte JW, Couture P (2005) Effects of industrial metals on wild fish populations along a metal contamination gradient. Ecotoxicol Environ Saf 61:287–312. https://doi.org/10.1016/j.ecoenv.2004.09.003
Rainbow PS, Luoma SN (2011) Metal toxicity, uptake and bioaccumulation in aquatic invertebrates–Modelling zinc in crustaceans. Aquat Toxicol 105:455–465. https://doi.org/10.1016/j.aquatox.2011.08.001
R Core Development Team (2017) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 20 Jan 2022
Sanchez W, Burgeot T, Porcher JM (2013) A novel Integrated Biomarker Response calculation based on reference deviation concept. Environ Sci Pollut Res Int 20(5):2721–2725. https://doi.org/10.1007/s11356-012-1359-1
Sanchez W, Porcher JM (2009) Fish biomarkers for environmental monitoring within the Water Framework Directive of the European Union. Trends Analyt Chem 28(2):150–158. https://doi.org/10.1016/j.trac.2008.10.012
Schlenk D, Zhang YS, Nix J (1995) Expression of hepatic metallothionein messenger RNA in feral and caged fish species correlated with muscle mercury levels. Ecotoxicol Environ Saf 31:282–286
Sepúlveda MS, Quinn BP, Denslow ND, Holm SE, Gross TS (2003) Effects of pulp and paper mill effluents on reproductive success of largemouth bass. Environ Toxicol Chem 22(1):205–213
Shaw BJ, Handy RD (2006) Dietary copper exposure and recovery in Nile tilapia, Oreochromis niloticus. Aquat Tox 76(2):111–121. https://doi.org/10.1016/j.aquatox.2005.10.002
Spirito-Santo HM, Ernest-Magnusson W, Zuanon J, Pereira-Mendoza F, Lemes-Landeiro V (2009) Seasonal variation in the composition of fish assemblages in small Amazonian forest streams: Evidence for predictable changes. Freshw Biol 54:536–548. https://doi.org/10.1111/j.1365-2427.2008.02129.x
Stentiford GD, Longshaw M, Lyons BP, Jones G, Green M, Feist SW (2003) Histopathological biomarkers in estuarine fish species for the assessment of biological effects of contaminants. Mar Environ Res 55(2):137–159. https://doi.org/10.1016/S0141-1136(02)00212-X
Suresha G, Sutharsan P, Ramasamy V, Venkatachalapathy R (2012) Assessment of spatial distribution and potential ecological risk of the heavy metals in relation to granulometric contents of Veeranam lake sediments, India. Ecotoxicol Environ Saf 84(1):117–124. https://doi.org/10.1016/j.ecoenv.2012.06.027
Thoré ESJ, Philippe C, Brendonck L, Pinceel T (2021) Towards improved fish tests in ecotoxicology – Efficient chronic and multi-generational testing with the killifish. Chemosphere 273:1–12. https://doi.org/10.1016/j.chemosphere.2021.129697
Thummabancha K, Onparn N, Srisapoome P (2016) Analysis of hematologic alterations, immune responses and metallothionein gene expression in Nile tilapia (Oreochromis niloticus) exposed to silver nanoparticles. J Immunotoxicol 13(6):909–917. https://doi.org/10.1080/1547691X.2016.1242673
Vašák M, Hasler DW (2000) Metallothioneins: New functional and structural insights. Curr Opin Chem Biol 4(2):177–183. https://doi.org/10.1016/s1367-5931(00)00082-x
Vehniäinen ER, Ruusunen M, Vuorinen PJ, Keinänen M, Oikari AOJ, Kukkonen JVK (2019) How to preserve and handle fish liver samples to conserve RNA integrity. Environ Sci Pollut Res 26(17):17204–17213. https://doi.org/10.1007/s11356-019-05033-0
Wang E, Wang K, Chen D, Wang J, He Y, Long B, Yang L, Yang Q, Geng Y, Huang X, Ouyang P, Lai W (2015) Evaluation and selection of appropriate reference genes for real-time quantitative PCR analysis of gene expression in Nile tilapia (Oreochromis niloticus) during vaccination and infection. Int J Mol Sci 16(5):9998–10015. https://doi.org/10.3390/ijms16059998
Wang WC, Mao H, Ma DD, Yang WX (2014) Characteristics, functions, and applications of metallothionein in aquatic vertebrates. Front Mar Sci 1(4):1–12. https://doi.org/10.3389/fmars.2014.00034
Wepener V, Van Dyk C, Bervoets L, O’Brien G, Covaci A, Cloete Y (2011) An assessment of the influence of multiple stressors on the Vaal River, South Africa. Phys Chem Earth 36:949–962. https://doi.org/10.1016/j.pce.2011.07.075
Wood CM (2011) An introduction to metals in fish physiology and toxicology: Basic principles. In: Wood CM, Farrell AP, Brauner CJ (eds) Homeostasis and toxicology of essential metals, fish physiology, 31(A). Elsevier Inc., p 1–51
Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL (2012) Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform 13:134–144
Yi Y, Tang C, Yi T, Yang Z, Zhang S (2017) Health risk assessment of heavy metals in fish and accumulation patterns in food web in the upper Yangtze River, China. Ecotoxicol Environ Saf 145:295–302. https://doi.org/10.1016/j.ecoenv.2017.07.022
Acknowledgements
The authors would like to thank Isabel Ramírez-Hernández, Jorge Cervantes-García, Adolfo Americano, Nicolás Lara-Vázquez, Rodrígo Campa-Molina, and Crisóforo Olaje-Murrieta for their help with fish collection. The authors are grateful to Manuel Lastra-Encinas, Karen Astorga-Cienfuegos, Rubí Hernández-Cornejo, and Christian Mariana Rodriguez Armenta, for their technical support with sample processing, preparation, and laboratory analysis.
Funding
The work was supported by the Centro de Investigación en Alimentación y Desarrollo, A.C. [project number 10368]. Á.M.-D. was supported by a scholarship from Consejo Nacional de Ciencia y Tecnología (CONACYT), México.
Author information
Authors and Affiliations
Contributions
Á.M.-D. and M.E.J.-M.: conceptualization, methodology, data processing, writing—original draft preparation. M.R.-D., S.A.G.-G., M.B.-L. and E.C.-A.: conceptualization, methodology, supervising, writing—reviewing, editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
Mexican Legislation does not have regulations for fish slaughter in the wildlife. However, fish treatments were performed according to the guidelines for the use of fishes in research from the American Fisheries Society, in the way to reduce animal suffering.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Martínez-Durazo, Á., Rivera-Domínguez, M., García-Gasca, S.A. et al. Assessing metal(loid)s concentrations and biomarkers in tilapia (Oreochromis niloticus) and largemouth bass (Micropterus salmoides) of three ecosystems of the Yaqui River Basin, Mexico. Ecotoxicology 32, 166–187 (2023). https://doi.org/10.1007/s10646-023-02620-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-023-02620-3