Abstract
We evaluated mercury (Hg) exposure and two biomarkers, metallothionein (MT) gene expression and histopathological alterations in a wild fish species, largemouth bass (Micropterus salmoides), collected from the Sacramento-San Joaquin Delta, CA, a region polluted with Hg from historic mining activities. Hg is highly toxic and can disrupt multiple physiological systems in vertebrate species, including the immune system. Total mercury (THg) concentration in muscle tissue ranged from 0.12 to 0.98 ppm (wet weight) and was not related to body condition (r 2 = 0.005, p = 0.555). Using linear regression analysis, we found a positive relationship between MT gene expression (as determined using quantitative polymerase chain reaction) and copper, zinc, manganese, aluminum, and nickel (decreased to one variable by way of principal component analysis) (r 2 = 0.379, p = 0.044), a negative relationship with selenium (r 2 = 0.487, p = 0.017), and a weak, negative relationship with THg concentrations (r 2 = 0.337, p = 0.061). Juvenile largemouth bass collected from Hg-contaminated areas displayed histopathological features of immunosuppression compared with those collected from less contaminated areas as evidenced by significantly lower macrophage density in kidney and liver tissue (p = 0.018 and 0.020, respectively), greater trematode density in liver tissue (p = 0.014), and a greater number of adult trematodes. Our results suggest that largemouth bass may be experiencing sublethal effects from chronic Hg exposure. Furthermore, our findings illustrate the utility of examining multiple sublethal markers of effect to assess the impacts of contaminant exposure on physiological function in wild species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Mercury (Hg) is a well-known environmental contaminant (National Research Council 2000) with coal-burning power plants and mining activity believed to be the primary sources of anthropogenic pollution (Tchounwou et al. 2003). Hg is particularly problematic in wetlands (Hurley et al. 1995) because wetland sediments are rich in sulfate-reducing bacteria that have been shown to convert inorganic Hg into the highly toxic methylmercury (MeHg) (Compeau and Bartha 1985). One reason for MeHg’s toxicity is that it is readily absorbed (Boudou et al. 1991), sequestered, and bioaccumulated within an individual (Mason et al. 1991) as well as biomagnified upward through food webs (Schwarzbach and Adelsbach 2002; Davis et al. 2008). Hg exposure can cause a wide range of toxic effects (e.g., reproductive impairment, immune disruption, neurological damage) in fish (Hammerschmidt et al. 2002; Berntssen et al. 2004; Sweet and Zelikoff 2001) as well as birds (Finkelstein et al. 2007; Wolfe et al. 1998) that rely on aquatic ecosystems. Moreover, humans who consumed fish from areas known to be contaminated with Hg have also had severe physiological dysfunction (Lebel et al. 1998).
For the past 15 years, the state of California has advised women and children to avoid eating sportfish (e.g., largemouth bass, striped bass) from the Sacramento-San Joaquin Delta (bay-delta) in central California due to increased Hg concentrations in those fish (Office of Environmental Health Hazard Assessment 1999). The bay-delta region is heavily polluted with Hg transported from mining activities during the 1,800s in the foothills of the Sierra Nevada Mountains. Mine tailings were deposited into various tributaries connected to the Sacramento-San Joaquin Delta (delta) (United States Geological Survey 2005) and the biota in this region is known to contain increased MeHg concentrations (Davis et al. 2002, 2008).
Although multiple studies have evaluated the effects of Hg exposure to fish in laboratory settings (Hammerschmidt et al. 2002; Berntssen et al. 2004; Gehringer 2007), there are limited investigations on the sublethal effects of fish in the wild (Adams et al. 1999). Biomarkers assess physiological function and are thus useful for evaluating the effects of contaminants, such as Hg, on wild populations (Finkelstein et al. 2007; Adams et al. 1999). Production of metallothionein (MT), a protein that binds to and sequesters many divalent transition metals [Hg, copper (Cu), cadmium (Cd), and zinc (Zn)], has been used as a molecular biomarker to assess effects to fish exposed to MeHg under laboratory (Gonzalez et al. 2005; Berntssen et al. 2004) and field conditions (Schlenk et al. 1995). In addition, histopathology can be a useful tool to elucidate tissue damage from MeHg exposure, including potential impacts to immune-related parameters, because Hg is well-known to disrupt immune function in vertebrate species, including fish (Sweet and Zelikoff 2001).
We investigated the effects of Hg exposure in largemouth bass (Micropterus salmoides), a high trophic–level sportfish known in the Sacramento-San Joaquin Delta to exceed the human health safety limit for Hg (OEHHA 1999). We evaluated the relationship between Hg concentrations in adult muscle tissue and biological markers of effect, including MT gene expression and various immune-associated histopathological alterations (i.e., macrophage density). A comparison between histopathological alterations in juvenile and adult largemouth bass was also conducted to determine whether juvenile fish may be more sensitive to MeHg exposure, a finding that has been observed in mammals (National Research Council 2000). To better understand the cause for variation in MT gene expression, concentrations of trace metals were analyzed in adult largemouth bass liver samples. Ultimately, we illustrate the utility of examining multiple sublethal markers of effect to assess the impacts of Hg exposure on fish physiological function.
Materials and Methods
Site Selection and Sample Collection
The sampling efforts (Fig. 1, Table 1) took place as part of the Fish Mercury Project, a project funded by California Bay-Delta Authority, with data sharing permitted by San Francisco Estuary Institute. The aims of the Fish Mercury Project are to determine Hg concentrations in fish collected from the Sacramento and San Joaquin watersheds and to provide information required to update fish consumption advisories. Samples were collected in the San Francisco bay-delta complex, which forms the largest estuary (~40,000 ha) on the west coast of the United States and provides essential habitat for many wildlife species. Largemouth bass were selected for this study because of their high trophic level (Moyle 2002), which allows for biomagnification of MeHg. Largemouth bass are abundant and widely distributed throughout the delta (Davis et al. 2008) and were collected during the summer of 2005 and 2006 using boat-based electroshocking methods. Length and weight measurements of adult fish and lengths of juvenile fish were obtained on collection. In 2005, 24 juvenile fish (ages 2–3 years) and 26 adult fish (ages 4–6 years) were collected; in 2006, 43 adult fish (ages 3–8+ years) were collected. Ages were determined from fork length measurements using growth estimates determined by Schaffter (1998). Killing of the fish was performed by delivering a blunt blow to the head (San Jose State University Institutional Animal Care and Use Committee protocol no. 872).
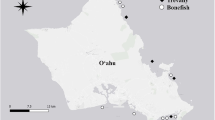
Largemouth bass sample collection sites in 2005 and 2006. Samples collected in 2005 are represented by triangles, and samples collected in 2006 are represented by the letter X. Sites 1 through 15 are northern or eastern delta sites that represent high (mean 0.471 ppm, SE 0.024 ppm [n = 57]) exposure for juvenile largemouth bass. Sites 17 through 21 are central delta sites that represent low (mean 0.218 ppm, SE 0.018 ppm [n = 14]) exposure for juvenile largemouth bass. For sample size and THg concentrations, see Table 1
Samples were collected in 2005 at sites where adult largemouth bass (305–450 mm TL) were expected to have average Hg concentrations of <0.3 or >0.9 ppm (Davis et al. 2008). The Fish Mercury Project sampled fewer sites in 2006; thus, adult fish from all sites in that year were included in this study (Fig. 1). Juvenile fish were collected at sites where historical data (Davis et al. 2008) indicated consistently “low” exposure (mean adult THg concentrations <0.4 ppm) or “high” exposure (mean adult THg concentrations >0.4 ppm) (Fig. 1; Table 1). Low-exposure sites were in the central delta, and high-exposure sites were located in the northern and eastern delta (Fig. 1; Table 1).
MT Expression Analysis
Hepatic MT gene expression was analyzed for 13 adult largemouth bass (Table 2) collected in 2006 using real-time quantitative polymerase chain reaction (qPCR). Liver tissue was collected immediately after killing, and half of the tissue was stored in RNAlater (Invitrogen, Carlsbad, CA, USA) at room temperature and subsequently stored at –20 °C. RNA was extracted from tissue (0.015 g ± 0.001 g) using TRIzol Reagent (Invitrogen Life Technologies, Carlsbad, CA), and sample integrity was determined by assessing the quality of the two ribosomal RNA bands present on a 1.2 % formaldehyde agarose gel (Fig. 2). When these ribosomal RNA bands were bright and discrete, RNA concentrations were determined using a Nanodrop spectrophotometer ND-1000, and RNA (mean = 1556 ng [SE = 94.9 ng]) was DNased (Fisher Scientific, Fair Lawn, NJ) to minimize amplification of genomic DNA. Reverse transcription (Reverse-iT First Strand Synthesis Kit; Abgene, Epsom, UK) was performed to synthesize cDNA from equal mass of RNA. MT expression was standardized against a commonly used reference gene, elongation factor 1α, using the 2−ΔCt method (Livak and Schmittgen 2001) to normalize for sample integrity and technical error.
Largemouth bass MT was isolated using a degenerate forward primer designed for conserved regions of percoideian MT sequences using Primer 3 software (Rozen and Skaletsky 2000) and a 3′-RACE T17 adapter (Hastings et al. 1999) primer (Table 3). The resulting PCR products were cloned using TOPO TA Cloning Kit for Sequencing (Invitrogen) and sequenced (Table 3). Primers for the elongation factor 1α were designed based on highly conserved regions in several perciform and percoideian sequences (Table 3).
The conditions for the qPCR reactions were optimized as follows: MT, elongation factor 1α : 1x Sybr Green Master Mix with Fluorescein (Abgene), 0.5 μM primers, and 30 ng cDNA. Reactions were run in a Bio-Rad iCycler iQ thermocycler in opaque 96-well plates sealed with optical film (Fisher-Thermo Scientific). Cycling parameters for both genes were as follows: initial 95° denaturing step (15 min) followed by 40 cycles of 95 °C (30 s), 55 °C (30 s), and 72 °C (45 s). Determination of the crossing threshold (Ct) was performed automatically in BioRad thermocycler software using cycles 2 through 10 to define baseline fluorescence level. We monitored qPCR over 50 PCR cycles. Thirty nanograms of cDNA was determined to be appropriate for this study because cDNA >60 ng inhibited reactions. PCR products were visualized on a 1.2 % gel to confirm a single PCR product of correct size and little or no primer-dimer. Assessment of genomic DNA contamination in cDNA was examined using 5 ng DNased RNA without reverse-transcription as template, and no-template controls were performed for each PCR experiment (Fig. 2).
Histopathological Analyses
The histopathology of liver, spleen, and kidney tissue were evaluated because these tissues experience high blood flow and are thus potentially exposed to MeHg after dietary intake (Keating et al. 1997). Juvenile and adult largemouth bass were examined to discern differences in sensitivity to MeHg exposure between age classes. Juvenile (n = 24) and adult (n = 71) largemouth bass (Table 2) liver, kidney, and spleen tissues were fixed in 10 % buffered formalin immediately after the fish were killed, and histological slides were prepared and stained with hematoxylin and eosin using standardized techniques (Histo-Tec in Hayward and the Community Hospital of Monterey Peninsula in Monterey [both CA]). One section (sample) was prepared from each tissue per fish and was analyzed under blind conditions. Tissue damage was quantified using multiple histopathological features (Table 4) that were chosen based on ease of identification, presence in our samples, and findings from Adams et al. (1999). Immune parameters included melanomacrophage centers (hereafter “macrophages”), inflammatory events (eosinophilic granulocytes and lymphocytes aggregates), and granulomas.
Hg Analysis
Adult Fish
Total mercury (THg) concentrations were determined in adult largemouth bass muscle tissue by the Marine Pollutions Studies Laboratory at Moss Landing Marine Laboratories with the EPA Method 7473 and a direct mercury analyzer (Milestone DMA-80) as part of a larger assessment by the Fish Mercury Project. Total Hg in muscle tissue was assumed to be representative of the MeHg body burden because >95 % of THg in largemouth bass has been shown to be MeHg (Bloom 1992) and >80 % of THg found in fish tissues have been found in muscle tissue (Berntssen et al. 2004). All concentrations reported are wet-weight concentrations.
Juvenile Fish
Due to budget constraints, juvenile largemouth bass samples were not analyzed for THg but instead were categorized into low MeHg- and high MeHg-exposure groups based on the mean adult THg concentration for all adult largemouth bass collected for each site for the Fish Mercury Project, which included a greater number of individuals than those analyzed for this study (Table 1). Our categorization of juvenile exposure groups is consistent with previous findings that juvenile and adult largemouth bass tissues are known to contain high concentrations of Hg when collected from the northern, southern, and eastern delta tributaries compared with those collected from the central delta (Foe et al. 2003; Davis et al. 2008). Furthermore, a significant positive relationship between the concentrations of THg in adult and juvenile largemouth bass collected from the same location has been previously documented (Melwani et al. 2009).
Trace Metal Analyses
Largemouth bass liver samples were processed and analyzed for trace metals (silver [Ag], aluminum [Al], arsenic [As], Cd, chromium [Cr], Cu, mangenese [Mn], nickel [Ni], lead [Pb], selenium [Se], and Zn) by the California Department of Fish and Game according to United States Environmental Protection Agency (USEPA) method 200.7 on a Perkin-Elmer Sciex Elan 9000 inductively coupled plasma mass spectrophotometer. Standard reference material [Dorm3 (fish protein homogenate) and 2976] and sample (largemouth bass liver) spike recoveries were within 25 % of all elements evaluated.
Data Analysis
All analyses were performed in SYSTAT (11th ed; Systat, San Jose, CA) using parametric tests (simple linear regression, two-sample Student t tests), and statistical significance was set at α ≤0.05. When necessary, data were log transformed to fit parametric model assumptions, or data were evaluated using nonparametric equivalents (Mann–Whitney U test).
General Physiological Assessment
Body condition [100 × (body weight/body length3)] for each adult largemouth bass was compared with THg concentration in muscle tissue using linear regression.
MT Expression Analysis
Adult largemouth bass analyzed for MT gene expression were selected based on THg concentrations (lowest = 0.27 to 0.37 ppm [n = 7] and highest = 0.63 to 0.97 ppm [n = 6]) and sufficient RNA integrity (Fig. 2). To validate that there was no treatment effect on elongation factor 1α expression, the Ct was examined by treatment using two-sample Student t test (p = 0.625). Gene expression of elongation factor 1α was stable (SD = 0.92) as determined using BestKeeper (Pfaffl et al. 2004).
Principal component analysis (PCA) was performed to avoid the confounding effects of colinearity among independent variables (contaminants) (Quinn and Keough 2002). Principal components were used in analyses if their eigenvalues were >1 (Quinn and Keough 2002). Variables with a PC loading of >0.71 or <–0.71 (the correlation coefficient of the relationship between the component and original variable) were determined to be excellent and exceptionally well represented by the PC (Kevin et al. 2000). Because one of the goals of our study was an exploratory investigation of relationships between MT expression and metal exposure to help guide further study, we examined the relationship between each individual metal (THg, Se, Cd, and Pb), or PC1 (Al, Mn, Ni, Cu, and Zn) and MT expression [log(standardized MT expression +1)] using linear regression analysis.
Histopathological Analyses
Histopathological data from largemouth bass samples collected in 2005 and 2006 were combined because preliminary analysis found no difference in the distribution and trends between years. Statistical relationships were only examined for parameters that were detected in ≥66 % of a particular tissue type (Table 4). Histopathological alternations in adult fish were analyzed using linear regression analyses, whereas those in juvenile fish were analyzed using two-sample Student t test.
Although up to 14 statistical analyses were performed to examine the relationship between MeHg exposure (in juvenile fish) or THg concentration (in adult fish) and histopathology; alpha was not adjusted for multiple comparisons because this procedure can result in false rejection of real effects in studies with typical sample sizes (Rothman 1990; Gotelli and Ellison 2004). As such, recent studies that examined relationships between multiple contaminant exposures and biological effects in wildlife did not perform multiple comparison adjustments (Verreault et al. 2004; Finkelstein et al. 2007).
Results and Discussion
Metal Concentrations
Adult largemouth bass THg concentrations ranged from 0.12 to 0.65 ppm in 2005 and 0.27 to 0.97 ppm in 2006, with 72 % of the samples collected exceeding the USEPA screening value of 0.3 ppm (Table 5). However, we found no evidence that body condition was affected by THg concentration (r 2 = 0.005, p = 0.555, linear regression). Previous studies reported measurable toxicity when THg concentrations in muscle tissue were near 1 ppm (Adams et al. 1999; Berntssen et al. 2004), and ~9 % of our samples had THg concentrations between 0.8 and 0.97 ppm (wet weight). The mean THg concentration in adult fish collected from low-exposure sites was 0.290 ppm wet weight (SE = 0.019 [n = 32], range = 0.117–0.494) and 0.755 ppm wet weight (SE = 0.102 [n = 16], range = 0.302–1.587) collected from fish at high-exposure sites (Table 1; Fig. 1), thus confirming our prediction that these sites would fall into low and high categories and validate the assignment of juvenile fish from these sites into low- and high-exposure groups.
The geometric means of several trace metal (Cu, Cd, THg, and Se) concentrations (Table 5) in largemouth bass livers were greater than the national geometric mean concentrations from whole-body tissue analyses in largemouth bass (Schmitt and Brumbaugh 1990), suggesting that the samples in our study represent fish collected from a polluted environment. Because >50 % of liver tissues had Cr, Ag, and As concentrations lower than the detection limit (Table 5), these metals were not examined for a relationship with standardized MT expression. D PCA reduced five (Cu, Zn, Mn, Al, and Ni) of the nine remaining metals into one principal component (PC1) that explained 46 % of the variation. The independent variables [PC1 (Cu, Zn, Mn, Al, and Ni), THg, Se, Pb, and Cd] were evaluated for relationships with MT gene expression using simple linear regression analysis.
MT Expression
Linear regression analysis indicated a significant positive relationship between standardized MT gene expression and PC1 (representing Cu, Zn, Mn, Al, and Ni) (Table 5), a finding supported by previous studies on the relationship between MT gene expression and Cu and Zn exposure (Hamilton and Mehrle 1986). However, unlike previous studies, Cd concentrations were not significantly correlated with MT gene expression and may have been too low in our samples (Table 5) to elicit induction (Hollis et al. 2001). MT expression also showed a significant negative relationship with Se concentration (Table 5). Se, a sulfur analog, can bind to the divalent form of Hg because MeHg is demethylated in the liver; this interaction between Se and Hg may impact MT production (Chmielnicka and Brzeznicka 1978).
Although previous laboratory and field-based studies observed a positive relationship between MT and MeHg (Berntssen et al. 2004; Gonzalez et al. 2005; Schlenk et al. 1995), we observed a weak negative relationship (Table 5). It is noteworthy that adults fish with low THg concentrations (mean = 0.312 ± 0.008 SE ppm [n = 5]) had greater variability of MT gene expression (mean = 0.828 ± 0.287 SE) than those with high THg concentrations (THg mean = 0.797 ± 0.048 SE ppm [n = 6]; MT mean = 0.292 ± 0.0764 SE) (Fig. 3). Furthermore, previous laboratory studies (Berntssen et al. 2004; Gonzalez et al. 2005) administered doses of MeHg to generate significantly greater tissues concentrations (5–13.5 ppm dry weight) than those observed in our study.
Histopathological Analyses
Although some histopathological alternations were significantly related to THg, there was high variability within each category suggesting the influence by other unknown factors (Table 6). We found that for both juvenile and adult fish, MeHg had a negative association with macrophage density as evidenced by significantly lower mean macrophage densities in juvenile kidney and liver tissues from the high THg-exposure group, a trend toward significance in juvenile spleen tissue, and a significant relationship between THg and macrophage density in adult kidney tissue.
Our results also indicate that inflammation in kidney tissue may be influenced by increased Hg exposure because adult fish had significantly lower densities of inflammatory events with increased Hg exposure (r 2 = 0.06, p = 0.041, linear regression), and this relationship was marginally apparent in juvenile fish (p = 0.066, two-sample Student t test). Although the occurrence of granulomas was not significantly related to Hg exposure in adult fish, a greater incidence of granulomas in spleens from juvenile fish in the high- versus low-exposure groups (granulomas in 25 % [n = 4] of low-exposure individuals vs. 60 % [n = 5] of high-exposure individuals) was detected.
Larval trematode density was not significantly associated with Hg concentrations; however, high-exposure juvenile fish with larval trematodes had a greater incidence of mature trematode occurrence than that of low-exposure juvenile fish. Of the low-exposure juvenile fish with larval trematodes present (55 % [n = 11], kidney tissue), 17 % had mature trematodes in kidney tissue, and 17 % had mature trematodes in liver tissue. In contrast, of the high-exposure juvenile fish with larval trematodes present (50 % [n = 8]), 100 % of the individuals had mature trematodes in the kidney, and 50 % had mature trematodes present in the liver. Spleen tissue was not evaluated for trematode presence trends due to an insufficient number of samples collected (low-exposure [n = 4], high-exposure [n = 5]), only some of which had larval trematodes present. These findings indicate that although both exposure groups appear to be equally infected with the parasite, the trematodes may be more successful at reaching adulthood in high-exposure juvenile fish. Furthermore, the high-exposure juvenile fish appear immunosuppressed because they displayed significantly lower densities of macrophages and inflammatory events, which should increase with foreign bodies, despite the increased incidence of mature trematodes. The potential for Hg to disrupt immune function in fish, including at the cellular level, has been well documented (Barst et al. 2011; Sweet and Zelikoff 2001).
Mature trematode density was greater in juvenile liver tissues from the high-exposure group (p = 0.014, two-sample Student t test). This pattern was also consistent in spleen (mature trematodes present in 25 % [n = 4] of low exposed and 60 % [n = 5] of high exposed juvenile fish) and kidney (mature trematodes present in 18 % [n = 11] of low-exposed and 63 % [n = 8] of high-exposed juvenile fish) tissues. Adult largemouth bass had lower densities of larval and mature trematodes in kidney and liver tissues (mean = 0.21 ± 0.05 SE [n = 27]; mean = 0.08 ± 0.02 SE [n = 32], respectively), than juvenile largemouth bass (mean = 0.40 ± 0.16 SE [n = 10]; mean = 0.23 ± 0.13 SE [n = 9], respectively). This trend was also observed with lower protozoan parasites densities found in adult fish (mean = 8.5 ± 0.98 SE [n = 68]) compared with juvenile fish (mean = 17.2 ± 2.9 SE [n = 19]).
In addition, we observed a marginally significant negative association between protozoan parasite density and THg concentration in adult fish (r 2 = 0.055, p = 0.055, linear regression), a relationship that was not observed in juvenile fish nor with mature trematode density. Therefore, increased tissue THg concentrations may be not only be affecting the fish’s physiological function, but it may also be impacting the survival of the fish’s parasites because adult largemouth bass collected in the delta can have 2–3 times greater Hg concentrations then juvenile fish (Davis et al. 2008).
Conclusion
Biomarkers may provide insight into the health of a population as chemical-induced alteration of physiological processes may indirectly lead to decreases in fitness and survival (Fossi 1998; Huggett et al. 1992; Connon et al. 2011). To our knowledge, we provide one of the few studies that examined biomarkers of effect in contaminated fish collected from the Sacramento-San Joaquin Delta (Spearow et al. 2011; Ostrach et al. 2008) and one of the first to examine the effect of Hg exposure even though the delta supports several sensitive and declining fish species and has a long history of Hg pollution. Our histopathology data suggest that juvenile largemouth bass may be experiencing sublethal effects (immunosuppression) and may be more sensitive than adult fish to increased MeHg exposure. MT gene expression appears to be driven by Cu and Zn exposure, and the relationship with MeHg exposure is not well understood and may be complex if existent. Our results reflect the importance of performing field-based studies because quantifying parasites, a stressor found in the natural environment, was a useful indicator of fish health. Furthermore, our findings support the use of histopathology as a tool for assessing health status changes due to Hg exposure.
References
Adams SM, Bevelhimer MS, Greeley MS, Levine DA, Teh SJ (1999) Ecological risk assessment in a large river-reservoir: 6. Bioindicators of fish population health. Environ Toxicol Chem 18:628–640
Barst BD, Gevertz AK, Chumchal MM, Smith JD, Rainwater TR, Drevnick PE et al (2011) Laser ablation ICP-MS co-localization of mercury and immune response in fish. Environ Sci Technol 45:8982–8988
Berntssen MG, Hylland K, Julshamn K, Lundebye AK, Waagbø R (2004) Maximum limits of organic and inorganic mercury in fish feed. Aquat Nutr 10:83–97
Bloom NS (1992) On the chemical form of mercury in edible fish and marine invertebrate tissue. Can J Fish Aquat Sci 49:1010–1017
Boudou A, Delnomdedieu M, Georgescauld D, Ribeyre F, Saouter E (1991) Fundamental roles of biological barriers in mercury accumulation and transfer in freshwater ecosystems. Water Air Soil Pollut 56:807–821
Chmielnicka J, Brzeznicka EA (1978) The influence of selenium on the level of mercury and metallothionein in rat kidneys in prolonged exposure to different mercury compounds. Bull Environ Contam Toxicol 19:183–190
Compeau GC, Bartha R (1985) Sulfate-reducing bacteria: principal methylators of mercury in anoxic estuarine sediment. Appl Environ Microbiol 50:498–502
Connon RE, Beggel S, D’Abronzo LS, Geist JP, Pfeiff J, Loguinov AV et al (2011) Linking molecular biomarkers with higher level condition indicators to identify effects of copper exposures on the endangered delta smelt (Hypomesus transpacificus). Environ Toxicol Chem 30:290–300
Davis JA, May MD, Greenfield BK, Fairey R, Roberts C, Ichikawa G et al (2002) Contaminant concentration in sport fish from the San Francisco Bay 1997. Mar Pollut Bull 44:1117–1129
Davis JA, Greenfield BK, Ichikawa G, Stephenson M (2008) Mercury in sport fish from the Sacramento-San Joaquin Delta region, California, USA. Sci Total Environ 391:66–75
Finkelstein M, Grasman KA, Croll DA, Tershy BR, Keitt BS, Jarman WM et al (2007) Contaminant-Associated alteration of immune function in black-footed albatross (Phoebastria nigripes) a North Pacific predator. Environ Toxicol Chem 26:1893–1903
Foe C, Davis J, Schwarzbach S, Stephenson M, Slotton D (2003) Conceptual model and working hypotheses of mercury bioaccumulation in the bay-delta ecosystem and its tributaries. Available at: http://loer.tamug.edu/calfed/FinalReports.htm. Accessed 28 March 2012
Fossi MC (1998) Biomarkers as diagnostic and prognostic tools for wildlife risk assessment: integrating endocrine-disrupting chemicals. Toxicol Ind Health 14:291–309
Gehringer D (2007) Molecular, histological, and behavioral differences in largemough bass (Micropterus salmoides) and topsmelt (Atheriniops affinis) exposed to methylmercury. Master’s thesis, CSU State Monterey Bay, Seaside
Gonzalez P, Dominique Y, Massabuau JC, Boudou A, Bourdineaud JP (2005) Comparative effects of dietary methylmercury on gene expression in liver, skeletal muscle, and brain of the zebrafish (Danio rerio). Environ Sci Technol 39:3972–3980
Gotelli NJ, Ellison AM (2004) A primer of ecological statistics. Sinauer Associates, Sunderland
Hamilton S, Mehrle P (1986) Metallothionein in fish: Review of its importance in assessing stress from metal contamination. Trans Am Fish Soc 115:596–609
Hammerschmidt CR, Sandheinrich MB, Wiener JG, Rada RG (2002) Effects of dietary methylmercury on reproduction of fathead minnows. Environ Sci Technol 36:877–883
Hastings RA, Eyheralde I, Dawson SP, Walker G, Reynolds SE, Billett MA et al (1999) A 220-kDa activator complex of the 26 S proteasome in insects and humans. J Biol Chem 274:25691–25700
Hollis L, Hogstrand C, Wood CM (2001) Tissue-specific cadmium accumulation, metallothionein induction, and tissue zinc and copper levels during chronic sublethal cadmium exposure in juvenile rainbow trout. Arch Environ Contam Toxicol 41:468–474
Huggett RJ, Kimerle RA, Mehrle PMJ, Bergman HL (1992) Biomarkers: biochemical, physiological, and histological markers of anthropogenic stress. Lewis, Boca Raton
Hurley JP, Benoit JM, Babiarz CL, Shafer MM, Andren AW, Sullivan JR et al (1995) Influences of watershed characteristics on mercury levels in Wisconsin Rivers. Environ Sci Technol 29:1867–1875
Keating MH, Mahaffey KR, Schoeny R, Rice GE, Bullock OR, Ambrose Jr RB et al. (1997) Mercury study report to congress, vol 1 Executive summary. EPA-452/R-97-003. United States Environmental Protection Agency, Washington DC
Kevin M, Sam C, Susan S (2000) Multivariate statistics for wildlife and ecology research. Springer, New York
Lebel J, Mergler D, Branches F, Lucotte M, Amorim M, Larribe F et al (1998) Neurotoxic effects of low-level methylmercury contamination in the Amazonian Basin. Environ Res 79:20–32
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25:402–408
Mason RP, Reinfelder JR, Morel FMM (1991) Bioaccumulation of mercury and methylmercury. Water Air Soil Pollut 80:915–921
Melwani AR, Bezalel SN, Hunt JA, Grenier JL, Ichikawa G, Heim W et al (2009) Spatial trends and impairment assessment of mercury in sport fish in the Sacramento–San Joaquin Delta watershed. Environ Pollut 157:3137–3149
Moyle PB (2002) Inland fishes of California. University of California Press, Berkeley
National Research Council (2000) Toxicological effects of methylmercury. National Academy Press, Washington, DC
Office of Environmental Health Hazard Assessment (1999) Overview of San Francisco Bay sport fish contamination and response activities. Available at: http://www.oehha.org/fish/pdf/fishup99.pdf. Accessed 28 March 2012
Ostrach DJ, Low-Marchelli JM, Eder KJ, Whiteman SJ, Zinkl JG (2008) Maternal transfer of xenobiotics and effects on larval striped bass in the San Francisco Estuary. Proc Natl Acad Sci U S A 105:19354–19359
Pfaffl MW, Tichopad A, Prgomet C, Neuvians T (2004) Determination of stable housekeeping genes, differentially regulated target genes, and sample integrity: Bestkeeper-Excel based tool using pair-wise correlations. Biotechnol Lett 26:509–515
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Rothman KJ (1990) No adjustments are needed for multiple comparisons. Epidemiology 1:43–46
Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S (eds) Bioinformatics methods and protocols: methods in molecular biology. Humana Press, Totowa, pp 365–386
Schaffter RG (1998) Growth of largemouth bass in the Sacramento-San Joaquin Delta. Interagency Ecological Program Newsletter 11. California Department of Water Resources, Sacramento, pp 27–30
Schlenk D, Zhang YS, Nix J (1995) Expression of hepatic metallothionein messenger RNA in feral and caged fish species correlates with muscle mercury levels. Ecotoxicol Environ Saf 31:282–286
Schmitt CJ, Brumbaugh WG (1990) National contaminant biomonitoring program: Concentrations of arsenic, cadmium, copper, lead, mercury, selenium, and zinc in U.S. freshwater fish, 1976–1984. Arch Environ Contam Toxicol 19:731–747
Schwarzbach S, Adelsbach T (2002) Field assessment of avian mercury exposure in the Bay-Delta ecosystem. Task 3B. CALFED Bay-Delta Program Final Report. United States Geological Survey, Sacramento
Spearow JL, Kota RS, Ostrach DJ (2011) Environmental contaminant effects on juvenile striped bass in the San Francisco estuary, California, USA. Environ Toxicol Chem 30:393–402
Sweet LI, Zelikoff JT (2001) Toxicology and immunotoxicology of mercury: a comparative review in fish and humans. J Toxicol Environ Health B Crit Rev 4:161–205
Tchounwou PB, Ayensu WK, Ninashvili N, Sutton D (2003) Environmental exposure to mercury and its toxicopathologic implications for public health. Environ Toxicol 18:149–175
United States Geological Survey (2005) Mercury contamination from historic gold mining in California. Fact Sheet number FS-061-00
Verreault J, Skaare JU, Jenssen BM, Gabrielsen GW (2004) Effects of organochlorine contaminants on thyroid hormone levels in arctic breeding glaucous gulls, Larus hyperboreus. Environ Health Perspect 112:532–537
Wolfe MF, Schwarzbach S, Sulaiman RA (1998) Effects of mercury on wildlife: a comprehensive review. Environ Toxicol Chem 17(2):146–160
Acknowledgments
The views expressed in this article are those of the author and do not necessarily reflect the position or policy of the Department of Toxic Substances Control, the California Environmental Protection Agency, or the State of California. The Earl and Ethel Myers Marine Biology and Oceanographic Trust provided funding for this project and San Francisco Estuary Institute provided data use from the Fish Mercury Project. CDFG-MPSL staff also provided Hg and trace metal analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gehringer, D.B., Finkelstein, M.E., Coale, K.H. et al. Assessing Mercury Exposure and Biomarkers in Largemouth Bass (Micropterus Salmoides) from a Contaminated River System in California. Arch Environ Contam Toxicol 64, 484–493 (2013). https://doi.org/10.1007/s00244-012-9838-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-012-9838-4