Abstract
In the present paper, we investigated the accumulation of six metals in tilapia (Oreochromis nilocticus) and largemouth bass (Micropterus salmoides) as indicators of the environmental pollution present at three constructed dams in the Yaqui River basin in Sonora, Mexico. The La Angostura (ANG), El Cajon de Onapa (ECO), and El Oviachic (OVI) dams are ecosystems under different degrees of anthropogenic stress. The collected fishes were dissected to obtain liver, gonad, stomach, gill, and muscle samples to determine the metal concentrations of Fe, Mn, Ni, Cu, Zn, and Cr. The results of a PERMANOVA showed that the concentrations of Fe, Cu, and Zn were significantly higher in tilapia liver, stomach, and gill tissues compared with those of the largemouth bass. Also, differences were detected between seasons, with the metal concentrations during the dry season being significantly higher than those of the rainy season (p < 0.001). The results of a principal component analysis showed an association between metals, tissues, and dams with significantly higher (p < 0.001) concentrations in tilapia from the ECO dam compared with those from the ANG and OVI dams. The general distribution of metals in the tissues was as follows: liver > stomach-gills > gonads > muscle. Variations in metal concentrations may be indicative of the different sources of anthropogenic stress in each ecosystem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aquatic ecosystems are subjected to a wide range of pollution sources associated with activities such as tourism, mining, intensive agriculture, and aquaculture. Among contaminants, metalloids are highly persistent that are transformed through biogeochemical cycles into different chemical species within the aquatic ecosystems and may be transferred through food webs. In the aquatic food webs, fishes occupy various trophic levels during their life cycle and thus may reflect variable degrees of contaminant exposure [7].
The biological characteristics of many fish species, such as their longevity, wide-ranging distributions, and feed habits, in addition to the relative facility with which they may be captured and managed, make them suitable to be used as indicators for metal(loids) contamination, which may provide early warnings of their potentially deleterious effects [30, 44]. Tilapia (Oreochromis niloticus) and largemouth bass (Micropterus salmoides) are two fish species that have been widely introduced into various freshwater ecosystems around the world [58], with reported resistant to a wide range of environmental conditions, diseases, and pollution levels [1, 40], and distinctive biological characteristics that make them suitable to assess contamination condition of freshwater ecosystems. Tilapia is benthic omnivorous species that feed mainly on detritus, accumulating contaminants from the fine sediment fraction, while largemouth bass is a predatory fish that is exposed to contaminants via prey items such as insects and other fishes [49].
The Yaqui River is an ecoregion that belongs to the most important basin in northwest Mexico, which harbors several ecosystems, both natural and manmade, with numerous endemic, migratory, and introduced species that depend on the conservation of these ecosystems as well as their functions [34]. The Yaqui River basin provides water for human consumption (e.g., for ~ 375,800 inhabitants around Ciudad Obregon; [37]), and multiple economic activities such as agriculture, cattle raising, pig farming, fish aquaculture, and mining [18, 36]. Previous studies have documented contamination in the basin associated with copper mining in the upper area (around La Angostura dam; [13, 35]) and extensive agricultural production in the lower area around the Yaqui Valley (close to El Oviachic dam; [36]). Metals as Fe, Mn, Ni, Cu, Zn, and Cr are associated with Cu mining and agrochemicals [51]. However, no studies have been performed to assess the contamination by metal(loids) using fishes or other organisms.
Tilapia and largemouth bass were introduced in the Yaqui River basin for economic, alimentary, and tourism purposes [18]. In the present study, three dams of this basin (La Angostura, El Oviachic, and El Cajon de Onapa) were investigated in the dry and rainy season regarding the accumulation of Fe, Mn, Ni, Cu, Zn, and Cr in tissues of these two species with distinctive biological features. To assess the different anthropogenic pressures within each ecosystem, the liver:muscle ratio was also used under the premise that metal(loids) contamination in these species could be associated with the prevailing anthropogenic activities in the ecosystems.
Materials and Methods
Study Area
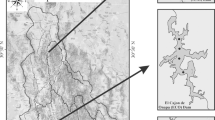
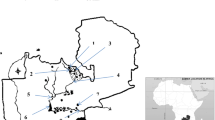
The Yaqui River basin is located in northeastern Mexico (26° 10′– 30° 40′ N and 106° 80′–111° 40′ W; Fig. 1). The watershed begins in the state of Chihuahua, which lies to the east of the state of Sonora, and continues towards the southwest, ending at the Gulf of California [21]. In total, the watershed comprises an area of 71,452 km2 [52].
The dams in the Yaqui River basin are surrounded by different anthropogenic activities. The La Angostura (ANG; 1040 m MSL) dam is situated in the northern portion of the basin in the Nacozari mining district, an important copper mining area [13]. The predominant climate is semiarid with annual average precipitation of 490 mm. The rainy season occurs in July and August, along with the maximum temperatures. The dry season has a minimum of precipitation (average < 50 mm) with high temperatures (from 30 to 40 °C) that produce high rates of evaporation. The El Cajon de Onapa (ECO; 556 m MSL) dam is located on the Sahuaripa River, which is a small tributary of the Yaqui River. The predominant climate around the ECO dam is semiarid-hot (BS1(h)hw) with a monthly average temperature of 32.7 °C and semiarid-mild (BS1hw(e)), with a monthly average temperature of 14.6 °C. The rainy season has an annual average precipitation of 600.8 mm and the dry season has annual average precipitation < 100 mm [23]. The main human activities in this reservoir are sustenance fishing and cattle grazing. In contrast, extensive and technologically intensive agriculture (Yaqui Valley, ~ 230,000 ha; [18]) is the principal activity that surrounds the El Oviachic (OVI; 58 m MSL) dam. This reservoir is located in the lowlands of the Yaqui River basin, near Ciudad Obregon (Fig. 1). The predominant climate around OVI dam is hot arid, predominantly desert (BW(h')w in 52.78% of the area) and steppe (BSo(h')w, in 34.52% of the area), with a mean annual temperature above 18 °C and a dry winter. The precipitation occurs mainly from July to September (Mexican monsoon season) with an average annual precipitation of 366.1 mm [23].
Fish Sampling
Sampling expeditions were carried out in the three dams during the dry (May–June) and rainy (September–October) seasons of 2018. Approximately 21–30 individuals of each fish species were collected per sampling event. Fishes were caught at different sites in each reservoir using commercial 4-cm gill nets (Fig. 1). Fishes were identified and sacrificed by brain spiking according to the Guidelines for the Use of Fishes in Research [5], and their total length and weight were registered. Fish tissue samples (i.e., liver, gonads, stomach, gills, and muscle) were dissected and placed in labeled sterile polyethylene bags, transported to the laboratory, frozen at − 20 °C, and freeze-dried (for 72 h at − 49 °C and 133 × 10−3 mBar) before metal analysis. In the laboratory, tissue samples were weighed to obtain dry weights, homogenized in an agate mortar, and stored at 4 °C. The scalpels, knives, and other dissection tools were stainless and previously conditioned (washed with distilled water, nitric acid 20% v/v, distilled water, and ethylic alcohol) before each specimen and tissue dissection.
The total lengths and weights for each species per dam are summarized in Table 1. In general, largemouth bass total weights and lengths were higher than those of tilapia, which may be explained by species-specific biological characteristics and feeding behaviors. Carnivorous fishes have elongated bodies and small stomachs [56], while omnivorous fishes have short, elliptic bodies and large stomachs [9].
Metal Analysis and Estimation of the Liver:Muscle Ratio
Tissues were digested in a microwave system (MARS-X) in one step. Briefly, 5 mL of concentrated HNO3 was added to 0.250 ± 0.003 g of sample and heated to 100 °C for 5 min, 120 °C for 5 min, and 140 °C for 10 min [25]. The copper (Cu), chromium (Cr), iron (Fe), manganese (Mn), nickel (Ni), and zinc (Zn) concentrations were determined with air-acetylene (Cu, Fe, Mn, Ni, and Zn) and nitrous oxide-acetylene (Cr) flames using the SpectrAA-240 FS absorption atomic spectrophotometer (Varian, Palo Alto, USA). Certified reference material (DORM-4 Fish protein from the National Research Council of Canada) and blanks were used as quality controls. The detection limits were estimated (μg g−1) as Cr = 0.035, Cu = 0.026, Fe = 0.041, Mn = 0.024, Ni = 0.036, and Zn = 0.030. The metal concentrations are reported as dry weights (DWs).
Data Analysis
The metal concentrations in the fish tissues followed a non-normal distribution according to the Shapiro-Wilks and Bartlett tests (p < 0.05). Data were used to calculate the mean and standard deviation of the entire vector. After this, each element was scaled by subtracting the mean and dividing by the standard deviation. Then, a two-way ANOVA was used to compare differences of means among seasons and sampling sites. A principal component analysis (PCA) was used to describe patterns in the levels of contamination among the collected tissues. The PCA was used to interpret the structure of the data via a linear combination of the original variables [32]. To avoid data skewness, all non-normal values were log-transformed ((Log10 X) + 1), following [17]. A distance-based permutational multivariate analysis of variance (PERMANOVA) was used to evaluate the combined effect of sampling sites and seasons on the distribution of all metals [6]. Statistical analyses were performed using NCSS Statistical Software (NCSS, Kaysville, USA), R v. 3.6.1 (R [42]), and PRIMER 7 + PERMANOVA (PRIMER-E Ltd., Devon, UK). The liver:muscle ratio was calculated using the individual concentration of each fish species with Eq. (1) from Kružíková et al. [27]:
Values of the liver:muscle ratio > 1 are indicative of metal contamination in organisms [27]. A one-way ANOVA was also used to evaluate significant differences in the liver:muscle ratio between metals. We assumed that heavily contaminated sites would have higher metal concentrations in liver tissues while slightly contaminated sites would have higher metal concentrations in muscle tissues [2, 27].
Results
The metal concentrations for tilapia and largemouth bass tissues are presented in Fig. 2. The concentrations of the metals varied between tissues, ecosystems, and sampling seasons. Metal concentrations in tilapia tissues followed the order of Fe > Cu > Zn > Mn > Cr > Ni, while in largemouth bass tissues followed the order of Fe > Zn > Cu > Cr > Ni > Mn. Regarding the metal concentrations by tissue, both species presented the same pattern: liver > stomach > gills > gonads > muscle (Fig. 3).
Metal concentrations in tissues of both tilapia and largemouth bass were distinctively grouped under the PCA analysis (Figs. 4 and 5). Tilapia presented high levels of Fe, Mn, and Cu in the stomach, liver, and gills, while largemouth bass presented high levels of Fe, Mn, Cu, and Zn in the liver and gonad. A differentiation among the tissues and ecosystems was also observed, although it was not evident (in most cases) between seasons. The PERMANOVA indicated that significant differences were present among groups of variables and between seasons and ecosystems (p < 0.001). The average similarity present between groups was higher for the three ecosystems when compared with that within groups, indicating that the metal concentrations in both fish species could be compared among dams. A simple analysis showed an average similarity of 85.8%, 84.1%, and 83.4% for OVI-ANG, OVI-ECO, and ANG-ECO, respectively. The metal concentrations were also grouped by this method according to the ecosystem. The ANG and OVI dams presented high similarities (88.0% and 86.6%, respectively) for Fe, Zn, Cu, and Cr, while the ECO dam showed high similarity (83.4%) for Fe, Zn, Cu, and Mn. The Fe, Zn, Cu, and Cr concentrations presented high similarity during the dry (87.0%) and rainy (86.0%) seasons.
Comparison of Metal Concentrations Between Species
The comparison of the magnitude of bioaccumulation indicated clear differences between the two fish species. While similar patterns of metal distributions between species were observed (Tables 2 and 3), the magnitude of bioaccumulation of some of the analyzed metals was significantly higher (p < 0.001) in tilapia tissues compared with that of largemouth bass tissues. In particular, higher values of Fe, Mn, and Cu were observed in tilapia tissues compared with those of largemouth bass tissues. Also, the target organs for metal accumulation were the stomach, liver, and gills, which presented the highest metal concentrations in tilapia for each of the three dam ecosystems. For example, Fe and Mn values in tilapia stomachs presented the highest mean concentrations detected in this study; approximately 80-fold higher compared with those of largemouth bass stomachs collected from the same ecosystems (Fig. 2). A similar scenario was observed with the liver tissues of both species. The mean concentration of Fe in tilapia liver tissues was one- to twofold higher when compared with that of largemouth bass liver tissues. In contrast, the mean Mn concentrations in the liver were (in most cases) seven- to 15-fold higher in tilapia than in the largemouth bass. Moreover, Cu concentrations in tilapia liver tissues were up to 25-fold higher than that of largemouth bass liver tissues (Fig. 2). For this particular element, Cu concentration in tilapia liver tissues was remarkable compared with that of the largemouth bass, and the highest mean Cu concentrations in tilapia and largemouth bass liver tissues were 801.6 ± 297.6 and 30.23 ± 16.85, respectively, which constituted a significant difference concerning the concentration magnitude (p < 0.001).
The highest Fe and Mn concentrations in gill tissues were present in tilapia and were one- to fivefold that of the concentration of largemouth bass gill tissues. However, higher Zn accumulation in gonad tissues was present in the largemouth bass (1096.0 ± 468.5 mg kg-1) compared with that of tilapia, which could be attributed to the size of the tissue (largemouth bass presented larger and bigger gonads). Metal concentrations in the muscle tissues of both species followed similar concentration patterns, where only Cu concentration was significantly higher (p < 0.001) in tilapia compared with that of the largemouth bass. The rest of the analyzed metals in the muscle tissues of both species presented similar orders of magnitude and uniform accumulation patterns regarding the mean concentrations in tilapia muscle tissues. The other metals (i.e., Ni, Zn, and Cr) presented relatively constant concentrations in the aforementioned tissues, with no significant differences (p > 0.001) detected between species (Tables 2 and 3).
Comparison Between Seasons
The comparison between the two sampling seasons showed a pattern among tissues. The concentrations between seasons presented significant differences (p < 0.001) in most of the analyzed tissues, except for tilapia stomachs (Fig. 2). Tilapia stomachs showed variable metal distributions compared with that of the other tissues. The highest Fe and Mn accumulation in tilapia was observed during the rainy season, in contrast to what was observed for the Ni, Cu, Zn, and Cr mean concentrations, which presented higher values during the dry season. On the other hand, the metal concentrations in largemouth bass stomachs were higher during the dry season for Mn, Ni, Cu, Zn, and Cr compared with that of the rainy season. The only exception to this pattern for the largemouth bass stomach tissues was observed with Fe, which presented higher concentrations during the dry season than during the rainy season.
Tilapia and largemouth bass liver and gill tissues showed higher metal concentrations during the dry season than during the rainy season. In addition, tilapia gonad tissues had higher metal concentrations during the rainy season, while largemouth bass gonad tissues had higher metal concentrations during the dry season, excepting Zn in largemouth bass gonads, which was higher during the rainy season, also presenting the highest metal concentration (1096.0 ± 468.5 mg kg−1) for this tissue.
Metal concentrations in muscle tissues were quite variable for both species during the two sampling seasons. In tilapia muscle tissues, the Fe, Mn, and Zn concentrations were higher during the rainy season than during the dry season. The other analyzed metals (i.e., Cu, Ni, and Cr) were higher during the dry season than during the rainy season. Similarly, the metal concentrations in largemouth bass muscle tissues also presented variable distributions. High concentrations of Fe and Mn were recorded during the rainy season, while high concentrations of Ni, Cu, Zn, and Cr were recorded during the dry season. The variation between seasons and among metals was also influenced by the variability among the dams, although the relationship between metal concentrations and seasons among dams was complex.
Comparison Among Dams
The comparison between dams was important to evaluate the connections among the variables analyzed in the present study. Clear associations between dams and metal accumulation patterns in fish tissues were present (Figs. 4 and 5). For example, most of the analyzed tissues from ECO tilapia had the highest Fe and Mn concentrations (the highest mean concentrations in this study) than the concentrations in ANG and OVI tilapia tissues. Finally, the lowest mean concentrations of Fe and Mn were recorded at the OVI dam when compared with that of the ECO and ANG dams. In the case of Cu, this metal tended to present high accumulation in tilapia tissues collected from the ANG and ECO dams when compared with that of the OVI dam. However, the bioaccumulation of Cu in largemouth bass tissues was more variable among dams (Fig. 2). For the particular case of Zn, this metal presented the highest concentration in samples collected from the OVI dam (with few exceptions) compared with that of the ECO and ANG dams for most of the tissues analyzed for both species. For Ni and Cr, their concentrations were more variable among dams, and it was more complicated to establish relationships between these elements and the dams.
The liver:muscle ratios showed differences among the metal accumulation patterns between species, dams, and seasons (Fig. 3). Tilapia samples collected during the dry season from the ANG and ECO dams showed similar metal accumulation patterns (Fig. 2). In those dams, the Fe and Cu liver:muscle ratios were higher than those of the OVI dam, indicating major contamination of those elements. For the OVI dam, only Zn exhibited liver:muscle ratios that tended to be high, although with values < 1. During the rainy season, liver:muscle ratios were similar among tilapia tissues, with Fe and Cu liver:muscle ratios > 1 in the ANG and ECO dams. In contrast, liver:muscle values > 1 were observed for Zn at the OVI dam. During the dry season, OVI largemouth bass showed liver:muscle ratios > 1 for both Fe and Zn. At the ANG dam, the Mn and Zn liver:muscle ratios were the highest (≈ 1), while at the ECO dam Fe liver:muscle ratios were > 1. Nevertheless, for the largemouth bass during the dry season, tissues collected from the OVI and ANG dams presented liver:muscle ratios < 1, although Fe liver:muscle ratios at the ECO dam were > 1. The largemouth bass tissues collected during the rainy season showed similar liver:muscle ratios and all dams presented Fe liver:muscle ratios > 1.
Discussion
The transference of metals to fishes is controlled by abiotic and biotic factors and is frequently driven by alimentary habits, nutritional requirements, and the balance between influx and efflux rates, which result in variable accumulation patterns. The accumulation and distribution of metals in the tissues of the two fish species collected from three ecosystems in this study presented seasonal variability with some defined patterns among ecosystems. For instance, tilapia presented the higher concentrations of the analyzed metals compared with the largemouth bass, likely related to its benthic feed habit. Dwivedi et al. [14] reported similar results for tilapia tissues and considered that the accumulation of metals in tissues was dependent upon the exposure concentration in the sediment and duration as well as other factors, such as salinity, temperature, water hardness, and the metabolism of each species. Metals are linked to the fine component of sediments (mainly fractions < 63 μm; [50]) and are transfer to benthic organisms under specific physicochemical conditions and may be bioaccumulated in tissues depending on biotic factors as their use as micronutrients [28].
The observed significant accumulation of Fe in tilapia is similar to what has been found in previous studies of freshwater ecosystems around the world [24, 38, 55]. This element is one of the most abundant and widely distributed in the earth’s crust (3.50% in the upper continental crust to 7.07% in the bulk continental crust; [48]) and is an essential macro-element that is used in multiple physiological pathways, leading to high requirement rates (30–170 mg Fe kg dry diet−1; [54]). The nutrient requirement rates for both Mn and Cu are also high (1–5 mg and 2–20 mg kg dry diet−1, respectively; [54]), and these metals were found to accumulate at relatively high levels in this study. In contrast, the nutrient requirement rates for Cr and Ni are low (4 mg kg dry diet−1 for Cr and trace requirements for Ni; [11]), and these metals were found to accumulate at relatively low levels in this study. It seems that the principal factor controlling the accumulation of Fe, Mn, and Cu in the fishes in this study was the ample distribution of these elements within the three ecosystems, which is supported by their relatively high content in the crust of the earth (20–600 mg kg−1 in the upper continental crust to 75–1400 mg kg−1 in the bulk continental crust; [48]).
The accumulation of essential metals in the fish tissues in this study was likely linked to their metabolic roles given that the highest levels were found in the gills, gonads, and liver, while the stomach and muscle tissues are considered to be sites for incorporation and storage of these elements, respectively [28]. This may produce a higher tolerance for certain metals in some fish species compared with that of other species in natural ecosystems, culture systems, and in vitro conditions [12].
The stomach accumulates food-associated elements, and the risk of contaminant exposure may be high in fish that feed near the sediment, which would help to explain the high observed accumulation of Fe and Mn in tilapia stomach tissues in this study considering that these elements are associated to oxides and hydroxides present in fine sediments [50, 53, 57]. The accumulation of Fe in the liver is due to the physiological role of the liver in blood cell and hemoglobin synthesis [19], while high levels of Cu in hepatic tissues are usually related to metallothionein (MT) and copper-binding proteins that participate in the storage of metals for use in enzymatic, metabolic, and detoxification processes [12, 46]. The synthesis of MT will be induced by exposure to many metals, including Cr and Ni, that MT may bind for storage and subsequent detoxification, resulting in the accumulation of these elements in various tissues, including the liver [26].
The accumulation of metals in gills is related to the physiological process of metal ion exchange from the water that requires a very large gill surface area to facilitate the rapid diffusion of metal ions [43]. Therefore, this target organ may be used to correlate the metal content in suspended particles and dissolved metals to water levels within aquatic ecosystems [3, 31]. Metal accumulation in gonads can be the result of both dissolved and dietary routes. Metal levels in this tissue are affected by multiple factors, such as gender, reproductive cycles, and/or gonad size [29, 30]. Many authors have reported that females can accumulate five times more metals (e.g., Cu, Fe, Mn, and Zn) compared with males as they are involved in the physiological process of embryonic development [4, 20]. In this study, high levels of Zn were observed in the largemouth bass, which may have been related to the maturity stage of the gonads.
The accumulation of metals in muscle tissues was lower than that of other tissues in this study. Muscles are considered to be depositary tissues because contaminant levels in these tissues represent the quantities that could not be excreted. It is well known that muscle tissues are the least metabolically active tissues in fishes [22, 28]. However, the levels of metal accumulation in fish muscle tissues increase with exposure and are related to the balance between incorporation rates (respiration plus diet), growth rates, and physiological excretion mechanisms [30].
The accumulation of most metals in the tissues of both species in this study was high during the dry season, which had high temperatures with minimum precipitation along the Yaqui River Basin. The accumulation patterns of the different seasons could be associated with high evaporation rates in the dams and anthropogenic activities present. In the case of the OVI dam, dust from the Yaqui Valley that has been reported by Meza-Montenegro et al. [36] may have increased the metal concentrations in the dam due to the metals present in the fine-grained soil fractions. In this ecosystem, metal concentrations appeared to follow trends in both seasons, and differences between the concentrations in tilapia and largemouth bass were present. Nonetheless, some metals showed high liver:muscle ratios for both species during the dry season, suggesting a possible relationship of these metals with agricultural activities. The metals associated with the Yaqui Valley soils are Pb, Hg, Zn, and Cu because they are constituents of some fungicides that are used for agricultural purposes in the region [36]. Also, the use of dithiocarbamate fungicides results in organic Zn and Mn complexes formed by the organic ligands of trace metals and dithiocarbamates [41].
The accumulation of some metals was particularly interesting in the ECO dam. This ecosystem is characterized by agriculture, small-scale cattle farming, and a lack of direct discharges. The Mexican Geology System [45] reported that natural enrichments of Fe (37,000–73,100 mg kg−1), Cu (7–50 mg kg−1), and Zn (57–248 mg kg−1) were present in the superficial sediments of the streams around this ecosystem. Fluvial dragging likely carried these and other metals in the fine sediment fraction to this dam, resulting in the continued exposure and accumulation of metals in fishes, which was most evident in tilapia given their benthic feeding habits. The accumulation of these metals was confirmed by the higher liver:muscle ratios observed in the ECO dam. Moreover, the association to natural sources is additionally possible considering that these metals are also abundant in the crust around the ANG and OVI dams (Fe: 22,100–80,100 mg kg−1 and 24,400–161,000 mg kg−1, respectively; Cu: 35.56–118.50 mg kg−1 and 12.88–43.12 mg kg−1, respectively; and Zn: 50.44–586.9 mg kg−1 and 50.00–129.0 mg kg−1, respectively).
The fishes in the present study exhibited wide inter-specific variations of metal accumulation in all organs, with defined patterns between ecosystems and less defined patterns between seasons. Some metals could be indicative of productive activities in the vicinities of the ecosystems. For instance, mining drainage could be associated with Cu, Mn, and Zn concentrations [35, 39]. A notable accumulation of Cu and Zn was present in both tilapia and largemouth bass, while Mn accumulated to a lesser degree in both species. The accumulation of Cu in both species at the ANG dam was higher than that of the other two dams. The ANG dam is situated near the Nacozari mine district, one of the most important copper ores in northwestern Mexico that produces an average of 132,888 tons of Cu per year. Meza-Figueroa et al. [35] reported that the dispersion of Cu in efflorescence salts formed by evaporation on the unconfined tailings around this mining district was due to heavy wind and rainfall events that frequently occur in this semi-arid area.
The liver:muscle ratio has been utilized in many studies with different fish species around the world to evaluate metal pollution in ecosystems under different conditions ([2, 27, 33]. Abreu et al. [2] found that the sea bass, Dicentrarchus labrax, showed a high liver:muscle ratio in areas contaminated with mercury. The study found that the liver:muscle ratio showed a considerable decrease as the distance from the pollution source increased [2]. In another study, Kružíková et al. [27] found that the non-predatory Chub, Leuciscuscephalus sp., collected from different rivers of the Czech republic, showed significant differences in this ratio among ecosystems, highlighting that the liver:muscle ratio can help to elucidate different metal contamination scenarios [27]. In the present study, tilapia showed higher liver:muscle ratios for metals like Fe, Mn, Cu, and Zn during the two seasons. Also, differences were observed between ecosystems. Tilapia from the ANG ecosystem showed high liver:muscle ratios for most metals, which may have been related to contamination from the Nacozari mining district and from the unconfined tailing of the La Caridad mine that was reported by Meza-Figueroa et al. [35]. That study found that metal concentrations were higher along a southwest to northeast transect, which followed the predominant Weston Super Mare to East North East wind patterns observed at the site, leading towards the ANG dam. Another source of metals in the ANG dam may have been the abandoned mine known as “El Tigre,” which was an important silver and gold ore that is currently adjacent to the ANG dam. Production of the “El Tigre” mine ended in 1938 as a result of the collapse of the price of silver [39], although many streams continue to drain into the ANG ecosystem. Some studies have also documented that gold-silver mine tailings contain elevated levels of many metals (e.g., Ni, Cu, Mn, and Zn; [10, 16]).
Results of the present study suggest that the observed accumulation patterns in both species may have been related to species-specific ecological traits and feeding habits [47]. However, ample differences in interspecific accumulation were observed in both species, and the resulting associations can be difficult to define (e.g., tilapia gonads and largemouth bass stomach). Nevertheless, variations of metal accumulation in largemouth bass stomachs may be attributed to the feeding rates and food availability present in each ecosystem [15]. Some metals may have similar exposure patterns (associated with mineral and chemical species), as well as similar distribution and accumulation mechanisms in the different tissues (e.g., phosphates, calcium granules, and metallothioneins), which have been reported in others studies [12, 46]. In the liver, PCA showed great association within ecosystems in both species, and to a lesser degree between seasons. As previously discussed, and according to Bawuro et al. [8], it is difficult to correlate seasonal variations in metal content among tissues. This could be attributed to the different inputs from river inflows and the intensity of the various anthropogenic activities around the ecosystems.
Conclusions
The metals bioaccumulation was variable among the tissues of both fish species and ecosystems. These variations were likely related to the feeding strategies, ecological habits, and biological characteristics of the species considering the benthic-omnivorous habits of the tilapia and the benthopelagic-carnivorous habits of the largemouth bass. The distribution of metals in the tissues was related to their metabolic roles, with higher levels of accumulation of Fe, Cu, and Zn, which have major rates of requirements for many physiology processes than metals such as Cr, Mn, and Ni. The general distribution of metals among tissues followed the order of liver > stomach > gills > gonads > muscle, which is related to their function in the organism. Seasonal associations were not well defined in this study. The sources of the metals in the three ecosystems may be the result of both natural (e.g., erosion of the crust around the ecosystems) and anthropogenic (e.g., copper mining and intensive agriculture) activities.
References
Abdel-Latif HMR, Abou-Khashaba AM (2017) Subchronic toxicity of Nile tilapia with different exposure routes to Microcystis aeruginosa: Histopathology, liver functions, and oxidative stress biomarkers. Vet World 10:955–963. https://doi.org/10.14202/vetworld.2017.955-963
Abreu SN, Pereira E, Valeá C, Duarte AC (2000) Accumulation of mercury in sea bass from a contaminated lagoon (Ria de Aveiro, Portugal). Mar Pollut Bull 40(4):293–297. https://doi.org/10.1016/S0025-326X(99)00187-3
Alhashmi-Bashir F, Shuhaimi-Othman M, Mazlan AG (2011) Evaluation of trace metal levels in tissues of two commercial fish species in Kapar and Mersing coastal waters, Peninsular Malaysia. J Environ Public Health 2012:352309–352310. https://doi.org/10.1155/2012/352309
Alquezar R, Markich SJ, Booth DJ (2006) Metal accumulation in a common estuarine fish, Tetractenos glaber, in the Sydney region, Australia. Environ Pollut 142:123–131. https://doi.org/10.1016/j.envpol.2005.09.010
American Fisheries Society (2014) Guidelines for the use of fishes in research, vol 2014. American Fisheries Society, Bethesda, p 104
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA for PRIMER: guide to software and statistical methods. PRIMER-E, Ltd., Plymouth
Baptista J, Pato P, Duarte AC, Pardal MA (2013) Organochlorine contaminants in different tissues from Platichthys flesus (Pisces, Pleuronectidea). Chemosphere 93:1632–1638. https://doi.org/10.1016/j.chemosphere.2013.08.028
Bawuro AA, Voegborlo RB, Adimado AA (2018) Bioaccumulation of heavy metals in some tissues of fish in Lake Geriyo, Adamawa State, Nigeria. J Environ Public Health 2018:1854892–1854897. https://doi.org/10.1155/2018/1854892
Beveridge M, Baird DJ (2000) Diet, feeding and digestive physiology. In: Beveridge M (ed) Tilapias: biology and exploitation, 1st edn. University of Stirling, Stirling, pp 59–87
Da Silva EF, Zhang C, Pinto LSS, Patinha C, Reis P (2004) Hazard assessment on arsenic and lead in soils of Castromil gold mining area, Portugal. Appl Geochem 19:887–898. https://doi.org/10.1016/j.apgeochem.2003.10.010
Davis DA, Gatlin DM (1996) Dietary mineral requirements of fish and marine crustaceans. Rev Fish Sci 4:75–99. https://doi.org/10.1080/10641269609388579
De Boeck G, Huong-Ngo TT, Campenhout KV, Blust R (2003) Differential metallothionein induction patterns in three freshwater fish during sublethal copper exposure. Aquat Toxicol 65:413–424. https://doi.org/10.1016/s0166-445x(03)00178-4
De la O-Villanueva M, Meza-Figueroa D, Maier RM, Moreno D, Gómez-Alvarez A, Del Río-Salas R, Mendívil H, Montijo A (2013) Erosive processes in the Presa I mine dam at Nacozari de Garcia, Sonora, and their effect in the dispersion of pollutants. Bol Soc Geol Mex 65:27–38
Dwivedi AC, Tiwari A, Mayank P (2015) Seasonal determination of heavy metals in muscle, gill and liver tissues of Nile tilapia, Oreochromis niloticus (Linnaeus, 1758) from the from the tributary of the Ganga River, India. Zool Ecol 25(2):166–171. https://doi.org/10.1080/21658005.2015.1020012
Ezemonye LI, Princewill O, Adebayo AA, Tongo EI, Ogbomida E (2018) Potential health risk consequences of heavy metal concentrations in surface water, shrimp (Macrobrachium macrobrachion) and fish (Brycinus longipinnis) from Benin River, Nigeria. Toxicol Rep 6:1–9. https://doi.org/10.1016/j.toxrep.2018.11.010
Fashola MO, Ngole-Jeme VM, Babalola OO (2016) Heavy metal pollution from gold mines: environmental effects and bacterial strategies for resistance. Int J Environ Res Public Health 13(11):1047. https://doi.org/10.3390/ijerph13111047
Feng C, Wang H, Lu N, Chen T, He H, Lu Y, Tu XM (2014) Log-transformation and its implications for data analysis. Shanghai Arch Psychiatry 26(2):105–109. https://doi.org/10.3969/j.issn.1002-0829.2014.02.009
Gortáres-Moroyoqui P, Castro-Espinosa L, Naranjo JE, Karpiscak MM, Freitas RJ, Gerba CP (2011) Microbiological water quality in a large irrigation system: El Valle del Yaqui, Sonora México. J Environ Sci Health A Tox Hazard Subst Environ Eng 46:1708–1712. https://doi.org/10.1080/10934529.2011.623968
Gorur FK, Keser R, Akcay N, Dizman S (2012) Radioactivity and heavy metal concentrations of some commercial fish species consumed in the Black Sea Region of Turkey. Chemosphere 87:356–361. https://doi.org/10.1016/j.chemosphere.2011.12.022
Has-Schön E, Bogut I, Kralik G, Bogut S, Horvatić J, Čačić I (2008) Heavy metal concentration in fish tissues inhabiting waters of “Buško Blato” reservoar (Bosnia and Herzegovina). Environ Monit Assess 144:15–22. https://doi.org/10.1007/s10661-007-9627-0
Hendrickson DA, Minckley WL, Miller RR, Siebart DJ, Haddock Minckley P (1980) Fishes of the Rio Yaqui Basin, Mexico and United States. J Ariz Nev Acad Sci 15:65–106
Hosseini M, Nabavi SM, Nabavi SN, Pour NA (2015) Heavy metals (Cd, Co, Cu, Ni, Pb, Fe, and Hg) content in four fish commonly consumed in Iran: risk assessment for the consumers. Environ Monit Assess 187:237. https://doi.org/10.1007/s10661-015-4464-z
INEGI (2018) Anuario estadístico y geográfico de Sonora 2018. Instituto Nacional de Estadística y Geografía, p 639
Jackson BP, Shaw-Allen PL, Hopkins WA, Bertsch PM (2002) Trace element speciation in largemouth bass (Micropterus salmoides) from a fly ash settling basin by liquid chromatography-ICP-MS. Anal Bioanal Chem 374:203–211. https://doi.org/10.1007/s00216-002-1337-4
Jara-Marini ME, Soto-Jimenez MF, Páez-Osuna F (2009) Trophic relationships and transference of cadmium, copper, lead and zinc in a subtropical coastal lagoon food web from SE Gulf of California. Chemosphere 77:1366–1373. https://doi.org/10.1016/j.chemosphere.2009.09.025
Klaassen CD, Lehman-Mckeeman LD (1989) Induction of metallothionein. J Am Coll Toxicol 8:1315–1321
Kružíková K, Kenšová R, Sedláčková L, Jarkovský J, Poleszczuk G, Svobodová Z (2013) The correlation between fish mercury liver/muscle ratio and high and low levels of mercury contamination in Czech localities. Int J Electrochem Sci 8:45–56
Leung HM, Leung AO, Wang HS, Ma KK, Liang Y, Ho KC, Cheung KC, Tohidi F, Yung KKL (2014) Assessment of heavy metals/metalloid (As, Pb, Cd, Ni, Zn, Cr, Cu, Mn) concentrations in edible fish species tissue in the Pearl River Delta (PRD), China. Mar Pollut Bull 78:235–245. https://doi.org/10.1016/j.marpolbul.2013.10.028
Luo Y, Shan D, Zhong H, Zhou Y, Chen W, Cao J, Guo Z, Xiao J, He F, Huang Y, Li J, Huang H, Xu P (2015) Subchronic effects of cadmium on the gonads, expressions of steroid hormones and sex-related genes in tilapia Oreochromis niloticus. Ecotoxicology 24:2213–2223. https://doi.org/10.1007/s10646-015-1542-5
Mackay D, Celsie AKD, Powell DE, Parnis JM (2018) Bioconcentration, bioaccumulation, biomagnification and trophic magnification: a modelling perspective. Environ Sci Process Impacts 20:72–85. https://doi.org/10.1039/c7em00485k
Mahboob-Shazia S, Kauzar S, Jabeen F, Sultana S, Al-Ghanim KA, Hussain B, Al-Misned F, Ahmed Z (2016) Effect of Heavy Metals on Liver, Kidney, Gills and Muscles of Cyprinus carpio and Wallago attu inhabited in the Indus. Braz Arch Biol Technol 59:1–10. https://doi.org/10.1590/1678-4324-2016150275
Mardia KV, Kent JT, Bibby JM (1979) Multivariate analysis. Academic Press, London
Marsalek P, Svobodová Z, Randák T, Vehl J (2005) Mercury and methylmercury contamination of fish from the Skalka Reservoir: A case study. Acta Vet Brno 74:427–434
McCullough EB, Matson PA (2016) Evolution of the knowledge system for agricultural development in the Yaqui Valley, Sonora, Mexico. PNAS 113:4609–4614. https://doi.org/10.1073/pnas.1011602108/-/DCSupplemental
Meza-Figueroa D, Maier RM, de la O-Villanueva M, Gómez-Alvarez A, Moreno-Zazueta A, Rivera J, Campillo A, Grandlic CJ, Anaya R, Palafox-Reyes J (2009) The impact of unconfined mine tailings in residential areas from a mining town in a semi-arid environment: Nacozari, Sonora, Mexico. Chemosphere 77:140–147. https://doi.org/10.1016/j.chemosphere.2009.04.068
Meza-Montenegro MM, Gandolfi AJ, Santana-Alcantar ME, Klimecki WT, Aguilar-Apodaca MG, Del Río-Salas R, De la O-Villanueva M, Gómez-Alvarez A, Mendivil-Quijada H, Valencia M, Meza-Figueroa D (2012) Metals in residential soils and cumulative risk assessment in Yaqui and Mayo agricultural valleys, northern Mexico. Sci Total Environ 433:472–481. https://doi.org/10.1016/j.scitotenv.2012.06.083
Naylor RL, Falcon WP, Puente-González A (2001) Policy reforms and Mexican agriculture: views from the Yaqui Valley. CIMMYT, Mexico
Oberholster PJ, Myburgh JG, Aston PJ, Coetzee JJ, Botha AM (2012) Bioaccumulation of aluminum and iron in the food chain of Lake Loskop, South Africa. Ecotoxicol Environ Saf 75:134–141. https://doi.org/10.1016/j.ecoenv.2011.08.018
Ochoa-Landín L, Pérez-Segura E, Del Río-Salas R, Valencia-Moreno M (2011) Depósitos minerales de Sonora, México. In: Thierry C (ed) Panorama de la geología de Sonora, México. Universidad Nacional Autónoma de México, Mexico, pp 299–331
Pathiratne A, Chandrasekera LW, Pathiratne KA (2009) Use of biomarkers in Nile tilapia (Oreochromis niloticus) to assess the impacts of pollution in Bolgoda Lake, an urban water body in Sri Lanka. Environ Monit Assess 156:361–375. https://doi.org/10.1007/s10661-008-0490-4
Phinney JT, Bruland KW (2009) Trace metal exchange in solution by the fungicides Ziram and Maneb (dithiocarbamates) and subsequent uptake of lipophilic organic zinc, copper and lead complexes into phytoplankton cells. Environ Toxicol Chem 16:2046–2053. https://doi.org/10.1002/etc.5620161009
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna https://www.R-project.org/
Rajkowska M, Protasowicki M (2013) Distribution of metals (Fe, Mn, Zn, Cu) in fish tissues in two lakes of different trophy in Northwestern Poland. Environ Monit Assess 185:3493–3502. https://doi.org/10.1007/s10661-012-2805-8
Segner H, Baumann L (2015) What constitutes a model organism in ecotoxicology? Integr Environ Assess Manag 12:195–205. https://doi.org/10.1002/ieam.1727
SGM (2015) Servicio Geológico Mexicano. Cartas Geológicas del Estado de Sonora, https://www.sgm.gob.mx/CartasDisponibles/. Accessed 23 October 2019
Siscar R, Koenig S, Torreblanca A, Solé M (2014) The role of metallothionein and selenium in metal detoxification in the liver of deep-sea fish from the NW Mediterranean Sea. Sci Total Environ 466–467:898–905. https://doi.org/10.1016/j.scitotenv.2013.07.081
Subotic S, Spasić S, Višnjić-Jeftić Z, Hegediš A, Krpo-Ćetković J, Mićković B, Skorić S, Lenhardt M (2013) Heavy metal and trace element bioaccumulation in target tissues of four edible fish species from the Danube River (Serbia). Ecotoxicol Environ Saf. 98:196–202. https://doi.org/10.1016/j.ecoenv.2013.08.020
Taylor SR, Mclennan SM (1995) The geochemical evolution of the continental crust. Rev Geophys 33:241–265. https://doi.org/10.1029/95RG00262
Taylor GC, Weyl OLF, Cowley PD, Allen MS (2015) Dispersal and mortality of Micropterus salmoides associated with catch and release tournament angling in a South African reservoir. Fish Res 162:37–42. https://doi.org/10.1016/j.fishres.2014.09.014
Tessier A, Rapin F, Carignan R (1985) Trace metals in oxic lake sediments: possible adsorption onto iron oxyhydroxides. Geochim Cosmochim Acta 49:183–194
Vallejo Toro PP, Vásquez Bedoya LF, Correa ID, Bernal GR, Alcántara-Carrió FJ, Palacio Baena JA (2016) Impact of terrestrial mining and intensive agriculture in pollution of estuarine surface sediments: Spatial distribution of trace metals in the Gulf of Urabá, Colombia. Mar Pollut Bull 111:311–320. https://doi.org/10.1016/j.marpolbul.2016.06.093
Vega-Granillo R, Vazquez-Armenta VH, Orozco-Garza A (2011) Structural analysis of the La Colorada Mine, Sonora, Mexico. Rev Mex Cienc Geol 32:239–253
Von Der Heyden BP, Hauser EJ, Mishra B, Martinez GA, Bowie AR, Tyliszczak T, Mtshali TN, Roychoudhury AN, Myneni SCB (2014) Ubiquitous presence of Fe(II) in aquatic colloids and its association with organic carbon. Environ Sci Technol Lett 1:387–392. https://doi.org/10.1021/ez500164v
Watanabe T, Kiron V, Satoh S (1997) Trace minerals in fish nutrition. Aquaculture 151:185–207
Weaver J, Wilson WH, Biegalski SRF, O’Kelly DJ (2009) Evaluation of heavy metal uptake in Micropterus salmoides (Largemouth Bass) of Lake Austin, TX by neutron activation analysis. J Radioanal Nucl Chem 282:443–447. https://doi.org/10.1007/s10967-009-0345-7
Westneat MW, Olsen AM (2015) How fish power suction feeding. PNAS 112:8525–8526. https://doi.org/10.1073/pnas.1510522112
Yi YW, Wang Z, Zhang K, Yu G, Duan X (2008) Sediment pollution and its effect on fish through food chain in the Yangtze River. Int J Sediment Res 23:338–347. https://doi.org/10.1016/s1001-6279(09)60005-6
Zoccal-Garcia DA, Augusto-Cota AD, Alves-Leme GL, Luis-Orsi M (2014) Biology of black bass Micropterus salmoides (Lacepède, 1802) fifty years after the introduction in a small drainage of the Upper Paraná River basin, Brazil. Biodiversitas 15:180–185
Acknowledgments
The authors are grateful to Isabel Ramírez-Hernández, Jorge Cervantes-García, Adolfo Americano, Nicolas Lara-Vázquez, Rodrigo Campa-Molina, and Crisóforo Olaje-Murrieta for their help with fish collection. The authors also thank to Roberto Ochoa-Contreras and Manuel Lastra-Encinas for their technical support with sample processing and preparation. The authors thanks to Dr. Andrea Lievana MacTavish for the English edition of manuscript. This study was supported by the Centro de Investigación en Alimentación y Desarrollo, A.C [project number 10368]. Martinez-Durazo was supported by a scholarship from Consejo Nacional de Ciencia y Tecnología, México.
Availability of Data and Material
As supplementary data, authors provide the tables of metal concentrations in tilapia and largemouth bass tissues.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 39 kb)
Rights and permissions
About this article
Cite this article
Martínez-Durazo, Á., Cruz-Acevedo, E., Betancourt-Lozano, M. et al. Comparative Assessment of Metal Bioaccumulation in Tilapia and Largemouth Bass from Three Dams of the Yaqui River. Biol Trace Elem Res 199, 3112–3125 (2021). https://doi.org/10.1007/s12011-020-02425-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02425-z