Abstract
Glyphosate herbicide is widely used in worldwide crop production. Consequently, its active ingredient, surfactants, and adjuvants commonly reach the aquatic ecosystem, thereby harming the biota. An investigation into how this herbicide affects aquatic species is important, especially in fish, as they have the ability to absorb and concentrate toxins. We aimed to evaluate the effects of glyphosate on the embryonic, larval and adult stages of zebrafish (Danio rerio), an appreciable organismal model. In this sense, we performed a meta-analysis using published articles from online databases (PubMed and ScienceDirect), which covered studies published until 2022. From a massive compilation of studies evaluating the effects of active substance glyphosate and Glyphosate-Based Herbicides (GBH) on zebrafish, we selected 36 studies used in downstream analyses. Overall, we report that glyphosate affects developmental stages and demonstrates toxicity and damage in zebrafish. We observed that embryos exposed to glyphosate exhibit increased mortality. There was also an increase in the number of morphological abnormalities related to yolk sac oedema, pericardial oedema, spinal curvature and body malformations, and a decrease in body size was observed. Furthermore, there was a decrease in the number of beats. The biochemical results demonstrated an increase in reactive oxygen species and antioxidant capacity against peroxyl radicals in the gills. The literature shows that glyphosate decreased the distance covered and the mean speed of the animals and increased the number of rotations. We concluded that glyphosate causes damage in the embryonic, larval and adult stages of this species. These results are valid for zebrafish and can be applied to other freshwater fish species.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The demand and production of pesticides to control unwanted organisms has increased annually worldwide (Zhang and Liu 2017), mainly motivated by the advances in agricultural technology and the demand for greater productivity. Although pesticides may benefit agricultural production, a plethora of ecosystem damage is routinely recorded in empirical studies (Lanzarin et al. 2020; Rumschlag et al. 2020; Ruuskanen et al. 2020; Sulukan et al. 2017; Zhang et al. 2017).
One of the most used pesticides to remove unwanted plants (that is, herbicide) is glyphosate [Glyphosate-Based Herbicides (GBH)]. Since its first commercialisation in the seventies (Baird 1971), GBH has been the most widely used herbicide in the world (Benbrook 2016). For example, until 2014, around 800 million kilograms of GBH were used worldwide, with estimates expected to reach 740 to 920 thousand tons by 2025 (Benbrook 2016; Maggi et al. 2020). In the context of agriculture, glyphosate is mixed with chemical ingredients, such as adjuvants and surfactants (for example, polyoxyethylene amine – POEA and alkyl polyphosphate amine) (FAO 2016). In turn, when disposed in the natural environment, glyphosate can be decomposed into two metabolite types, namely aminomethylphosphonic acid (AMPA) or sarcosine (Borggaard and Gimsing 2008).
The intensified use of glyphosate in agricultural activities, including surfactants, adjuvants and its main metabolite, increases the presence of these compounds in the ecosystem (Benbrook 2016). This herbicide can be found in the aquatic environment through releasing, inadequate packaging disposal, wind, spray diversions and runoff (Silva and FAY 2004). In fact, glyphosate and AMPA have been frequently found in the seawater, urban streams, wetlands, surface water, groundwater, freshwater and water bodies (Aparicio et al. 2013; Coupe et al. 2012; Mercurio et al. 2014; Okada et al. 2018; Ruiz-Toledo et al. 2014). According to the literature, concentrations of 0.70 mg L−1 can be observed on the water surface (Annett et al. 2014; Byer et al. 2008; Peruzzo et al. 2008). This is concerning, as when glyphosate comes into contact with the aquatic environment, it can affect the quality of water, aquatic plants and animals (Dornelles and Oliveira 2014; Hong et al. 2018; Liu et al. 2019; Moreno et al. 2014; Vera et al. 2010).
More specifically, in case of fishes, the glyphosate can be absorbed by gills and through a dietary route during all stages of life (Hued et al. 2012). Glyphosate toxicity can cause oxidative stress, affect the activity of antioxidant enzymes and inhibit acetylcholinesterase (AChE) activity (Glusczak et al. 2011, 2007; Lushchak et al. 2009). In this sense, the use of zebrafish represents an adequate alternative to study the effects of pesticides on water. Zebrafish is an ideal bioindicator of water quality, as it exhibits a rapid development, has a size that is suitable for maintenance (3 to 4 centimetres) and high fertility and meets the 3 Rs criterion (Grunwald and Eisen 2002). Another feature intrinsically associated with zebrafish is that different stages of its development may be evaluated in toxicological studies (embryo: 0–48 h; larva: 2–30 days; adult: 2–3 months). In the embryonic and larval phases, some of the advantages are easy handling and observation of structures (transparent animals) along with rapid development (OECD 2013). Therefore, several characteristics of this animal are highly favourable for use in ecotoxicological studies.
Although specific studies have revealed the effects of glyphosate on zebrafish (Fiorino 2018; Lanzarin et al. 2019; Moraes et al. 2020; Sulukan et al. 2017), a broad and systematic view remains absent. Therefore, a framework that integrates sophisticated analytical approaches to systematically assessing the effects of glyphosate can provide an overview of zebrafish’s sensitivity to the herbicide, with implications relevant to freshwater ecosystems as a whole. Here we performed a systematic review followed by a meta-analysis of glyphosate toxicity during the embryonic, larval and adult stages of zebrafish to identify the effect of glyphosate in a broader spectrum. Specifically, we aimed to investigate 1) the extent to which glyphosate effects differ between stages and 2) which routes are primarily involved in glyphosate toxicity.
Methods
Research sources, identification, and criteria for inclusion of studies
This meta-analytic review was conducted following the PRISMA guidelines for systematic reviews (Liberati et al. 2009). We carried out a PubMed and ScienceDirect full text search, to identify studies that tested the effects of glyphosate on the embryo larval and adult stages of zebrafish.
We used the following search terms: “Glyphosate” AND “zebrafish” AND (“egg” OR “embryo” OR “larvae” OR “adult”), “Glyphosate” AND “danio rerio” AND (“egg” OR “embryo” OR “larvae” OR “adult”), “Roundup” AND “zebrafish” AND (“egg” OR “embryo” OR “larvae” OR “adult”) and “Roundup” AND “danio rerio” AND (“egg” OR “embryo” OR “larvae” OR “adult”). The search was carried out with no limited start date until May 2022, and the keywords were searched in the English language. This yielded a total of 3394 results (see Results section for more details). We initially screened studies based on titles and abstracts. Thereafter, we read the full text to extract all the necessary information.
Some studies were not included in the meta-analysis, only in the review, as they presented insufficient data for comparison. In addition, those that did not provide adequate information regarding the type of pesticide used and/or studies with restricted access to the full text were excluded. Reviews, PhD theses, scientific notes and book chapters were not included. We also disregarded articles in which the active ingredients of different pesticides were combined. In this sense, the articles included met the following criteria: (1) original research, performed with zebrafish exposed to glyphosate; (2) glyphosate reported as one of the sources of exposure; (3) in vivo studies on zebrafish; and (4) results presented in mean and standard deviation (MD ± SD) or mean and standard error (MD ± SE).
To summarise, we only considered articles that included the previously mentioned criteria and directly addressed the effects of glyphosate on embryo-larval and adult stages. For the sake of simplicity and statistical criteria (see Data analysis section), we grouped the obtained data into effects caused by glyphosate on 1) mortality (for example, in hours post fertilisation – hpf), 2) hatched (for example, in hpf), 3) malformation (for example, yolk sac oedema), 4) morphology (for example, body length), 5) heart rate (for example, in hpf), 6) biochemistry (for example, ACAP) and 7) behaviour (for example, rotations).
Data extraction
All search results considered were tabulated in a digital spreadsheet (using Microsoft Excel). The following information was included in this table: authors, year of publication, country, stage of life, active substance glyphosate or GBH, concentration, replacement, dilution, the effect caused, specification of effect and temperature. All extracted information was standardised to the same unit of measure (for example, heart rate per minute). Studies, which tested different concentrations of glyphosate for the same control, were considered as independent sampling units. Although different glyphosate formulations are known, we consider the combined effect of different GBHs and active substance glyphosate, as the data are insufficient to perform separate meta-analyses. However, the active substance glyphosate and its adjuvants can be found together in the aquatic environment. Therefore, we treat the results keeping the combination in mind throughout the manuscript – an approach that has been followed in previous studies (see Battisti et al. 2021 for similar approaches).
We also extracted the sample size (n), mean and standard deviation for the control and experimental groups for each article. When the results were presented in terms of standard error of the mean (SEM, standard error of the mean), we converted this into standard deviation (SD) using an equation that has been previously described in literature (Vesterinen et al. 2014). We extracted mean and standard deviation from a procedure performed using the FIJI software ImageJ (Schindelin et al. 2012).
Data analysis
We performed a meta-analysis on the sample size, mean and standard deviation in order to calculate a standardised measure for each study (that is, effect size). Subsequently, we used the R package meta (Balduzzi et al. 2019) to calculate the standardised mean difference (SMD) as an estimate of effect size on two types of outcomes – continuous and binary data. We regard those instances in which studies have evaluated the effect of glyphosate on quantitatively measured structures as continuous outcomes. In contrast, we consider the instances that have been categorically measured as binary outcomes.
For continuous outcomes (adult: biochemistry and behaviour; embryo and larva: heart rate and morphology), the SMD was estimated using Hedges’ g statistic (Hedges and Olkin 1985), and the between-study variance (τ2) was calculated using the DerSimonian–Laird (DL) method (Dersimonian and Laird 1986). The SMD (effect size) was considered significant when the 95 per cent confidence interval (CI) did not include zero. We also quantified and tested for statistical heterogeneity using Higgin’s I² (Higgins et al. 2003). Moreover, we considered a sub-group analysis to determine the effects of glyphosate based on the hours post fertilisation (hpf) or some specification parameter dependent (see forest plot legends for more details on the sub-group used). Calculated effect sizes and 95 per cent CI were used to generate forest plots. The results were considered significant at the level of p < 0.05. All these procedures involving continuous outcome data were implemented in the “metacont” R function.
For binary outcomes data (embryo and larva: mortality, hatching rate and occurrence of malformations), we calculated the fixed and random effect estimates for meta-analysis using the “metabin” R function. Specifically, we used the Mantel–Haenszel (MH) method with the Hedges estimator of between-study variance (τ2) to calculate the Odds Ratio (OR) with a 95 per cent CI (Mantel and Haenszel 1959; Egger et al. 2001). The results were considered significant at the level of p < 0.05. We also evaluated the degree of residual heterogeneity in our data using Higgin’s I² statistics (Higgins et al., 2003). Similarly, for continuous outcomes data, a sub-group analysis was performed.
To examine the magnitude and direction of the effects of glyphosate concentration level on embryonic, larval and adult stages, we created meta-regression models using the “metareg” function. We also checked the publication bias in our dataset from three approaches. First, we analysed the 10-item checklist of the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE), which is mainly associated with the experimental procedures used in each study (Hooijmans et al. 2014). Second, we graphically inspected asymmetry using funnel plots (R funnel function). Third, we performed the Egger regression test (R “metabias” function), which entails using a quantitative method to test for asymmetry in the funnel plot (Egger et al. 1997). All aforementioned functions were derived from the ‘meta’ package (Balduzzi et al. 2019) in R version 4.0.3.
Results
Systematic search
Database searches resulted in an initial total of 3394 documents (n = 247 from PubMed, and n = 3147 from ScienceDirect) through systematic search (Fig. S1). Duplicates (n = 2024) and other research subtypes such as reviews and book chapters (n = 885) were removed. Furthermore, studies that analysed the effects on other living beings (n = 334) and studies with other contaminants with the exception of glyphosate (n = 68) were rejected. After these exclusions, we obtained a total of 83 articles, which were subjected to a full text reading. In addition, in vitro studies (n = 35) evaluated the effects of exposure of zebrafish cells to glyphosate, effects of other variables with glyphosate, such as pH and sediment (n = 11) and without statistical analysis (n = 1). We could not gain access to the full text of one of the articles, despite searching in all access routes. After selection by title, abstract and full text, 36 original research articles with zebrafish exposed to glyphosate commercial and/or pure were obtained. All these studies were included in the systematic review, and the main characteristics and results are presented in Table S1.
Study characteristics
Taken together, the studies included in this review were published between 2014 and 2022. These studies were carried out in Brazil (n = 14), China (n = 4), India (n = 1), Portugal (n = 3), Turkey (n = 1), United Kingdom (n = 1), Czech Republic (n = 1), United States (n = 5), Mexico (n = 2), France (n = 1), Ukraine (n = 1), Canada (n = 1) and Spain (n = 1). The articles are detailed in Table S1. Of these, 21 articles used zebrafish in the embryonic and larval stages, and 15 studies used zebrafish in the adult stage, two of which evaluated the embryonic, larval and adult stages. The dosages of GBH and active substance used ranged from 0.00005 to 400 mg/L. The active substance used was approximately 99 per cent pure and the GBHs were quite diverse. All selected studies show that exposure of zebrafish, regardless of its stage of life, to some GBHs or active substance induces damage to this fish species.
The findings showed that glyphosate affects development in the embryonic and larval stages. The studies evaluated the mortality and hatching rate of embryos and larvae through exposure to GBHs and active substance at concentrations ranging from 0.00005 to 400 mg/L (de Brito Rodrigues et al. 2019, 2017; Fiorino 2018; Lanzarin et al. 2020, 2019; Sulukan et al. 2017; Uren Webster et al. 2014; Zhang et al. 2017; Forner-Piquer et al. 2021; Díaz-Martín et al. 2021; Díaz-Martín et al. 2021; Pompermaier et al. 2022). The heart rate was also verified to check cardiovascular damage (Gaur and Bhargava 2019; Lanzarin et al. 2019; Roy et al. 2016b; Zhang et al. 2021; Liu et al. 2022; Pompermaier et al. 2022; Díaz-Martín et al. 2021; Díaz-Martín et al. 2021). Changes in morphology and malformations were evaluated through exposure to GBHs and active substance ranging from 0.00005 to 35 mg/L (de Brito Rodrigues et al. 2019; Roy et al. 2016a, 2016b; Sulukan et al. 2017; Zhang et al. 2017; Forner-Piquer et al. 2021; Liu et al. 2022). Biochemical damage was discovered through the analysis of antioxidant enzymes, proteins and other biomarkers (Lanzarin et al. 2019; Panetto et al. 2019; Sulukan et al. 2017; Zhang et al. 2017; Lanzarin et al. 2021; Liu et al. 2022; Pompermaier et al. 2022). Several researchers also verified the effects on behaviour through changes in locomotor activity, aversive behaviour, rotation and other analyses (Bridi et al. 2017; Zhang et al. 2017; Forner-Piquer et al. 2021; Ivantsova et al. 2022). In addition, they found an increase in the gene expression of pax2a, kim1, sod2, cox4i1 and so on (Babich et al. 2020; Ivantsova et al. 2022; Zhang et al. 2021).
Regarding the effects of glyphosate on adult zebrafish, mortality, survival, biochemical and behavioural changes were verified. Two studies evaluated the mortality and survival of animals throughout the experiment (Davico et al. 2021; Falfushynska et al. 2022). During the biochemical analysis, the authors found damages in the gills, liver, brain and muscle in the form of biomarkers such as SOD, CAT, GPx, ROS, ACAP and AChE and changes in the expression of some genes (Jaramillo et al. 2018; Lopes et al. 2018, 2014; Moraes et al. 2020; Santo et al. 2018; Velasques et al. 2016; Faria et al. 2021; Ding et al. 2021; Falfushynska et al. 2022; Giommi et al. 2022). Furthermore, the authors noted effects on behaviour, such as distance travelled, latency to enter the upper zone, average speed, rotations, distance covered, avoidance and so on (Bridi et al. 2017; da Costa Chaulet et al. 2019; da Rosa et al. 2016; Pompermaier et al. 2020; Faria et al. 2021; Mena et al. 2022).
Study quality and risk of bias
Supplementary Fig. S2 presents the ‘high’ or ‘low’ risk of bias in terms of percentages for each domain assessed for risk of bias within each individual study and across studies, respectively. In 100 per cent of the studies (n = 36), there was an adequate distribution of the animals between the groups, and they were similar at the beginning of the treatment. There is a low risk of bias in these two factors, thereby demonstrating a homogeneity of animals between groups. As for allocation secrecy, 100 per cent of the studies (n = 36) did not report whether the group allocation was adequately concealed, and 80,5 per cent of the studies (n = 29) did not mention the random housing of the animals. The random allocation of animals is not yet a standard practice in animal experiments and it may reflect a possible distortion of the global interpretation of the data. In all studies (100 per cent, n = 36), there were no reports about the lack of knowledge regarding the interventions utilised by caregivers. In addition, there were no reports regarding the results being collected randomly and by a blind observer. Usually, in experimental designs with animal models, these procedures are rarely described. All studies (100 per cent, n = 36) did not present other potential sources of bias and incomplete results. In this sense, the objects of comparison (variables) were potentially approximated and analysed with greater certainty. Finally, 91,6 per cent of the studies (n = 33) had a conclusion free of selectivity. Thus, we started with the statistical procedures described, to build a body of evidence on the problem in question.
Meta-analysis
Glyphosate affects mortality and hatching in zebrafish embryos
We observed that embryos exposed to glyphosate exhibit an increase in mortality in 3 hpf (I2 = 63 per cent; p < 0.01), 24 hpf (I2 = 91 per cent; p < 0.01), 48 hpf (I2 = 93 per cent; p < 0.01), 72 hpf (I2 = 95 per cent; p < 0.01) and 96 hpf (I2 = 75 per cent; p < 0.01) (Fig. 1). Thus, the number of dead animals during the entire embryonic and larval stages is higher in groups exposed to this pesticide. The visual inspection of the funnel plot in Supplementary Fig. 3A and the Egger test (t = 2.139, p = 0.0374) indicates the possible presence of publication bias in our results. We performed a meta-regression to check whether there was a relationship between mortality and the dosage of glyphosate or GBH used. The target regression demonstrated a significant positive relationship between embryo mortality and the dosage used (se = 0.0031, p < 0.0001, Supplementary Fig. 4A). The higher the concentration of glyphosate used, the greater is the probability of observing an effect on mortality.
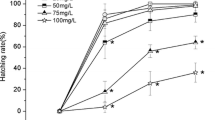
We found mixed results regarding the effects of glyphosate on hatching rates, whereby there was an increase in 48 hpf (I2 = 72 per cent; p < 0.01) and a decrease in 72 hpf (I2 = 94 per cent; p < 0.01), and there was no difference in 96 hpf (I2 = 0 per cent; p = 1.00) (Fig. 2). We observed that glyphosate accelerates the hatching of embryos at 48 hpf. A visual inspection of the funnel plot (Supplementary Fig. 3B) and the Egger test (t = 1.907, p = 0.027) indicates no evidence of publication bias in our results. We observed that the meta-regression showed a significant positive relationship between the hatch rate and the dosage of GBH or active substance used (se = 0.8630, p < 0.0001, Supplementary Fig. 4B).
Glyphosate causes malformations and structural abnormalities in zebrafish embryos
Our results on malformations revealed an increase in the number of morphological abnormalities related to the yolk sac oedema (I2 = 80 per cent; p < 0.01), pericardial oedema (I2 = 75 per cent; p < 0.01), spinal curvature (I2 = 77 per cent; p < 0.01) and body malformations (I2 = 88 per cent; p < 0.01) (Fig. 3). A visual inspection of the funnel plot (Supplementary Fig. 3C) and the Egger test (t = 1.34; p = 0.1919) indicates no evidence of publication bias in our results. It was not possible to analyse this parameter by meta-regression, owing to insufficient observations.
In relation to morphology, we regarded changes mainly associated with a decrease in body size in animals exposed to glyphosate (I2 = 98 per cent; p < 0.01, Fig. 4). A visual inspection of the funnel plot (Supplementary Fig. 3D) and the Egger test (t = 7.051, p = 0.0000014) indicates the possible presence of publication bias in our results. Meta-regression demonstrated a significant positive relationship in glyphosate-exposed embryos on morphology (se = 0.4946, p < 0.0001, Supplementary Fig. 4C).
Glyphosate alters heart rate in zebrafish embryos
Our findings on the heart rate of embryos exposed to glyphosate indicate a decrease in the number of beats in both 48 hpf (I2 = 99 per cent; p = 0) and 72 hpf (I2 = 100 per cent; p = 0) (Fig. 5). A visual inspection of the funnel plot (Supplementary Fig. 3E) and the Egger test (t = 2.89, p = 0.008) indicates the possible presence of publication bias in our results. Meta-regression demonstrated a significant negative effect on heart rate in the case of glyphosate-exposed embryos (se = 4.4679, p < 0.001, Supplementary Fig. 4D). We found that, at lower concentrations, there is a greater likelihood that we will perceive an effect on the heart rate.
Glyphosate changes the reactive oxygen species (ROS) and antioxidant capacity against peroxyl radicals (ACAP) in the gills of zebrafish adults
The biochemical results demonstrated an increase in reactive oxygen species (ROS) in the gills within 24 hours of exposure to glyphosate (I2 = 88 per cent; p < 0.01; Fig. 6). In addition, it increased the antioxidant capacity against peroxyl radicals (ACAP) in the gills within 96 h of exposure (I2 = 61 per cent; p = 0.05). A visual inspection of the funnel plot (Supplementary Fig. 3F) and the Egger test (t = −2.359, p = 0.0333) indicates the possible presence of publication bias in our results. It was not possible to analyse this parameter by meta-regression, owing to insufficient observations.
Glyphosate alters the behaviour of adult zebrafish
We found that glyphosate decreased the distance travelled (I2 = 99 per cent; p < 0.01) and the average speed of the animals (I2 = 88 per cent; p < 0.01). In addition, exposure to glyphosate increased the number of rotations (I2 = 98 per cent; p < 0.01) (Fig. 7). A visual inspection of the funnel plot (Supplementary Fig. 3G) and the Egger test (t = 2.368, p = 0.0280) indicates the possible presence of publication bias in our results. The meta-regression demonstrated a significant positive relationship between adult behaviour and the concentration used (se = 0.6598, p < 0.0001, Supplementary Fig. 4E). The higher the concentration of glyphosate used, the greater is the probability of observing an effect on behaviour.
Discussion
The damage caused by the toxic effects of glyphosate and its additives has been widely reported in the corpus of scientific literature. The use of zebrafish (Danio rerio) as a model for the mechanistic description of its action as well as the ecological implications of using this pesticide have considerably increased in the recent years. Thus, we chose to use the results of works published until 2022 in reference to this species in vivo, thereby compiling the stages of its life cycle (embryonic, larval and adult). Studies show that glyphosate-based herbicides affect the life cycle of species Cantareus aspersus, Lithobates sylvaticus, and Chrysoperla externa (Druart et al. 2017; Lanctôt et al. 2014; Schneider et al. 2009), thereby demonstrating the need for a broader look at these effects. However, there was no record of experimental trials that considered the damage caused by glyphosate during all stages of the lifecycle of the zebrafish fish species. In the natural environment, the fish species comes into contact with the pesticide during all stages of its life.
Due to the chronological variation of glyphosate applications in crops and its permanence of about 60 days in surface waters, studies have shown its prevalent presence in aquatic ecosystems (Annett et al. 2014). In the United States, maximum concentrations of 73 μg/L were found in rivers and streams, <0.02 in lakes and 301 μg/L in swamps (Battaglin et al. 2014). Measured concentrations of glyphosate in surface freshwater ranged from 2.7 to 10.3 mg acid equivalent/L (Córdova López et al. 2019; Ronco et al. 2016). These data raise important concerns about the constant presence and high solubility of glyphosate, as well as its potential threat, caused by exposure to non-target organisms present in the environment. It should be mentioned that the dosages of GBH and active substance used in the studies included in this review ranged from 0.00005 to 400 mg/L. In addition, most studies used concentrations that are not environmentally relevant. Therefore, in the present study, we included all publications, which pertained to the exposure of zebrafish to GBH and/or active substance, in a single document. It is important to mention that there are a lot of GBH formulations around the world. Furthermore, it is very difficult to attribute the toxic effects to the active substance per se due to variations in the commercial formulations available. These GBH formulations include ammonium salt, glycine salt and other chemical substances used to improve plant absorption of the herbicide (Benbrook 2016).
Exposure of fish embryos to GBH presents an increased danger, as the chorionic membrane of the embryo is the first to come into contact with the toxic agent. This chorion is an acellular envelope, which is known to be about 0.5–0.7 μm thick with three layers perforated by pore channels in fertilised eggs (Bonsignorio et al. 1996; Rawson et al. 2000). It acts as a barrier to protect embryos from external stimuli (Tran et al. 2021) and allows molecules to pass into the embryo by passive diffusion (Berghmans et al. 2008). However, the membrane has no protective effect on the development of embryos exposed to organophosphates, which penetrate the chorion and cause lethal effects (Ansari and Ahmad 2010). Objectively, glyphosate induces changes in the chorion structure (Zhang et al. 2017). This occurs because glyphosate accesses the embryo through the chorion pores, mainly due to its low molecular weight, high polarity, low solubility in organic solvents and high solubility in water (Sanchís et al. 2012). This entry into the chorion causes active substance and/or additives present in GBHs to be absorbed by the developing embryo.
In the early period of life, the embryo is in the cleavage and blastula stage, during which cell division occurs (Kimmel et al. 1995). This is the reason behind why this period becomes the most critical for the survival of the embryo itself. The high sensitivity of the organism to glyphosate toxicity during this period was evident. In our meta-analysis, we observed that up to 3 hpf there is an increase in mortality in animals exposed to 0.01 to 15 mg/L of glyphosate. In another study, there was an increase in mortality in embryos (3 hpf) exposed to 15 mg/L of GBH (Lanzarin et al. 2019). It is important to highlight that there are more environmental interferences, in addition to glyphosate, in the natural environment. However, the increased challenges to embryonic survival can lead to population suppression.
During gastrulation (24 hpf), we found an increase in mortality in animals exposed to 0.01–400 mg/L of glyphosate. Furthermore, there was a pronounced increase in mortality in embryos with 22 hpf, which were exposed to 8.5 and 15 mg/L of GBH (Lanzarin et al. 2019). This high mortality is related to developmental delay and embryo malformations. In our work, it was evident that embryos exposed to glyphosate have a smaller body size. This evidence has been reinforced in a study that evaluated gene expression ntl (no tail), which is responsible for the formation of the notochord, which demonstrated that glyphosate could reduce the structure of the notochord related to the smaller size of the body (Odenthal et al. 1996; Zhang et al. 2017; Zou et al. 2009). We observed a dose-effect relationship, such that the higher the concentration of glyphosate used, the greater is the likelihood of anatomical changes, such as body length.
With reference to malformations, the results of our meta-analysis demonstrate an increase in the number of animals with embryonic alterations when exposed to glyphosate. Furthermore, our evidence suggests that the higher the concentration of glyphosate used, the greater is the probability of observing these anatomical damages. Interestingly, these malformations, induced by glyphosate, include oedema in the yolk and pericardial sac, spinal curvature and malformations in the body (head, eye and tail). We demonstrate that, based on the experimental studies analysed, morphological abnormalities are frequently recorded in a wide spectrum of active substance concentrations, including 1–100 mg/L (Sulukan et al. 2017), 100 mg/L (Gaur and Bhargava 2019) and 8.5 mg/L (Lanzarin et al. 2019).
The reason behind the formation of oedema in the yolk and pericardial sac in exposed embryos could be a deficiency of the metabolic system associated with the accumulation of the pesticide (Wu et al. 2017). Alternately, these oedemas can occur due to the inhibition of genes slc2a10/glut10 (Solute carrier family 2 member 10/Glucose transporter 10) or Lrrc10 (Leucine-rich Repeat Containing protein 10) (Kim et al. 2007; Willaert et al. 2012). We can relate oedema to interference in the synthesis and metabolism of lipids present in the yolk sac. Owing to this difficulty in absorbing lipids, which are important for the survival of animals, there was a basic dietary deficiency for the development of organs. Consequently, malformations in the body were observed. With reference to the damage to the formation of the spine, we can list the following: i) an over-expression of growth hormone; ii) decreased collagen in the spine (Çelik et al. 2012); iii) inhibition of gene expression col27a1a (collagen, type XXVII, alpha 1a) and col27a1b (collagen, type XXVII, alpha 1b) (Christiansen et al. 2009); iv) lysyl oxidase inhibition (Snawder and Chambers 1993); v) suppression or down regulation of the gene ptk7 (protein-tyrosine kinase-7) (Hayes et al. 2014).
The effects of morphological changes can affect locomotion. These effects may cause changes in locomotor activity and aversive behaviour after exposure to active substance or Roundup® (GBH), at concentrations of 0.01, 0.065 and 0.5 mg/L (Bridi et al. 2017). By affecting the animal’s morphology in the embryonic and larval stages, it can cause several damages, such as locomotor difficulties. Thus, it increases the susceptibility to predation, difficulty faced while searching for food and finding partners for reproduction. These factors promote impaired development, which, in turn, can lead to death.
In our review, we found that glyphosate increases mortality, accelerates hatching and decreases the heart rate of glyphosate-exposed embryos by 48 hpf. In addition, we discovered that mortality was increased in embryos exposed to concentrations between 2 and 400 mg/L. Another study also demonstrated an increase in mortality in 48 hpf embryos exposed to similar concentrations of 8.5 mg/L and 15 mg/L of GBH (Lanzarin et al. 2019).
With respect to the hatching rate, we present strong evidence that there is a pronounced increase in the number of hatched animals at 48 hpf when exposed to concentrations between 0.01 mg/L and 400 mg/L. Our findings allowed us to observe that the higher the concentration of glyphosate used, the greater is the likelihood of hatch implications. An interesting study showed that at 48 hpf there was an increase in the number of animals hatched in two GBHs, at concentrations of 1.8, 3.6, 8.3 and 18 mg/L (de Brito Rodrigues et al. 2017). This hatch corresponds to the release of individuals from the chorion envelope and marks the end of embryogenesis and the beginning of the larval stage. The reported period for the normal hatching of developing embryos is between 48 and 72 hpf (Kimmel et al. 1995). Therefore, even if the hatch occurred in a time interval considered normal, we found that there was an increase in the number of hatches in the exposed animals, thereby highlighting the interference of the contaminant. While hatching occurs due to the activity of proteolytic enzymes in specialised cells, hormonal metabolic patterns, and the movements of muscle contractions performed by the embryo (Samaee et al. 2015), the increase in hatching rates during this period may probably be attributed to alterations in proteolytic enzymes. Among the candidates, the incubation 1 enzyme (HE1) can be pointed out as the enzyme responsible for breaking the chorionic barrier (Sano et al. 2008).
With reference to the decrease in heart rate of 48 hpf, we showed that it occurred in animals exposed to concentrations between 2 mg/L and 100 mg/L of glyphosate. Different studies found a decrease in the heart rate of embryos exposed to glyphosate in a concentration-dependent manner (Gaur and Bhargava 2019; Lanzarin et al. 2019). It is known that the heart rate of the zebrafish ranges from 120 to 180 beats per minute (bpm) during the early stages of development (Bournele and Beis 2016). It became clear that at lower concentrations, there is a greater likelihood of seeing an effect on heart rate. This decrease may be associated with genes involved in heart contraction and excitation Cacna1C (Calcium Voltage-Gated Channel Subunit Alpha1 C) and ryr2a (Ryanodine receptor 2a), thereby altering calcium homoeostasis through the expression of Heat Shock Protein Family B (Small) Member 11 (hspb11) involved in the signalling pathway of nitric oxide (NO) (Gaur and Bhargava 2019). In addition, another reason behind this phenomenon may be the presence of acetylcholinesterase (AChE) inhibitors in the GBHs, which increase the concentration of acetylcholine in the synaptic cleft, cause continuous acetylcholine receptor stimulation and decrease the heart rate (Lin et al. 2007).
Zebrafish’s cardiac pumping system goes through several essential processes of functional maturation and, once damages occur during development at this stage, they result in congenital cardiac abnormalities (Beis et al. 2005). In association with these results, we found an increase in mortality in animals exposed to glyphosate at 72 hpf. There is evidence that shows an increase in mortality in embryos with 72 hpf at concentrations of 8.5 mg/L and 15 mg/L of commercial glyphosate (Lanzarin et al. 2019). In our review, we found that the decrease in heart rate persisted in larvae at 72 hpf. These results are justified by the prevalence of structural abnormalities caused by glyphosate. This occurred both in the atrium and the ventricle with the rupture of the cardiac wall, thereby leading to the functional impairment of pumping (Lanzarin et al. 2019; Roy et al. 2016b; Yusof et al. 2014).
Adequate cardiac function depends on high energy conversion efficiency. Thus, due to the oedema found in the yolk sac, the normal functioning of the heart is strongly affected, thereby leading to a decrease in heart rate through the reduction of the local energy supply (Raldúa et al. 2008). These damages to the heart lead to systemic pathophysiological implications, affect the availability of oxygen for the basal cell metabolism and impair the blood transport, thereby affecting energy production, which is fundamental to the development of the embryo. We also have to consider that oedema was not always observed in all the studies included in this review. Therefore, we must be a little careful when making the blanket statement that this damage has occurred in all embryos.
There is an explicit relationship between glyphosate accelerating the hatching of embryos by 72 hpf and the metabolic damage caused by cardiac system failure. We observed in our work, that the higher the concentration of glyphosate used, the greater the chances of these effects leading to an outcome of increased hatching and mortality. Hatch rate is a sensitive parameter that is used to assess the interference of chemicals in embryo development (OCDE 2013). Therefore, embryos can become more vulnerable to predatory attacks, mechanical and osmotic stress and chemical compounds present in the external environment, which, in turn, render changes at this stage potentially lethal (Kimmel et al. 1995; Samaee et al. 2015).
At 96 hpf, we only verified that glyphosate increased mortality in animals in contact with the contaminant. However, different studies found that, during this same period, the larvae had lower body length than expected and the head and eyes reduced in size (Zhang et al. 2017). The carbonic anhydrase activity (EC 4.2.1.1) and hexokinase (EC 2.7.1.1) decreased, and an increase in apoptotic response was observed (Panetto et al. 2019; Sulukan et al. 2017).
Unlike larvae, in adult specimens, it was possible to analyse the specific effects of the organ from the perspective of the methodological peculiarity of the assessment of biochemical responses. Animals exposed to glyphosate showed an increase in ROS in the gills (exposed for 24 h), and an increase in antioxidant capacity against peroxyl radicals in the gills (exposed for 96 h) was observed. Adult fish show changes in brain thiol levels as well as increased lipid peroxidation markers in the brain, liver and muscle (Lopes et al. 2018; Santo et al. 2018). The activities of enzymes catalase (EC 1.11.1.6) and glutathione peroxidase (EC 1.11.1.9) showed affected activities in the liver, increase in Antioxidant Capacity Against Peroxyl radicals (ACAP) and Reactive Oxygen Species (ROS) in the gills and the liver (Santo et al. 2018; Velasques et al. 2016). Thus, these enzymatic alterations reduce cellular defences, thereby increasing the susceptibility to toxic xenobiotic substances that would be metabolised under normal conditions as well as impairing gas exchange and osmoregulation.
With respect to the effects related to the behaviour of the species, we observed a decrease in distance travelled and mean speed and an increase in the number of rotations. We verified that there was an explicit relationship between the increase in glyphosate concentration and the presence of disorders associated with behaviour. Adult animals exposed to glyphosate spend more time in the upper zone and less time in the lower part of the test field. Rotational behaviour was increased, and, in turn, they spent less time in the treated range, had impaired memory and showcased reduced aggressive behaviour (Bridi et al. 2017; da Costa Chaulet et al. 2019; da Rosa et al. 2016).
The significant changes found in this review, which have been supported by several studies, demonstrate the potential toxicity of this herbicide for fish populations that live in a contaminated environment. Our data raise important concerns about potential harm to wild fish in the embryonic, larval and adult stages. That being said, the data show that, owing to the impact on the embryonic stage, the chances of mortality increase, or else, complications will continue to persist throughout the life span of the fish species. The resulting impacts can compromise the search for food, the local reproduction and perpetuation of the species and the socialisation.
Although limitations are inherent in systematic review and/or meta-analysis studies, some caveats underlying our analyses need to be considered. First, our compiled dataset covered a small number of studies, which may have made some comparisons difficult. Notably, we recognise that the effects of glyphosate on zebrafish may differ between GBH and active substance. Another caveat in some cases is uncertainty regarding whether the reported effects can only be attributed to the specific impact of the active substance or the adjuvants present in GBH. Furthermore, care should be taken when generalising effects observed at the intracellular level by making extrapolations at the population level. However, owing to the low number of comparable studies in the final sample, it was not possible to separate the effects of glyphosate in the GBH or active substance. Keeping these caveats in mind, we conducted these analyses using methods that seek to minimise the risk of bias in the results and conclusions.
Conclusion
We concluded that GBH and active substance glyphosate can be considered toxic for the development of Danio rerio. In the embryonic and larval stages, they induce an increase in mortality, cause malformations in the body and affect hatching and heart rate. At high concentrations, the revised studies show a greater likelihood of observing an effect on morphology, hatching, malformations and mortality. In addition, the revised studies show this biochemical and behavioural damage, which is caused in the adult stage of this fish’s lifecycle.
We believe that the revised studies indicate damage mainly caused in the embryonic and larval stages of this fish species. Due to lethal effects on animals, or sublethal effects that cause difficulties in predation, feeding, locomotion, reproduction and survival, this and/or other fish species may undergo decline in populations. Therefore, precautionary and damage mitigation measures must be taken in a timely manner to reduce the potential risks of glyphosate to fish and other aquatic species. Hence, our study reinforces the necessary and urgent attention to the risks of glyphosate and its commercial formulations for the health of fish and the entire aquatic system.
References
Annett R, Habibi HR, Hontela A (2014) Impact of glyphosate and glyphosate-based herbicides on the freshwater environment: Impact of glyphosate-based herbicides. J. Appl. Toxicol 34:458–479. https://doi.org/10.1002/jat.2997
Ansari BA, Ahmad MK (2010) Toxicity of pyrethroid Lambda-cyhalothrin and Neemgold to the embryo of zebrafish Danio rerio (Cyprinidae). J Appl Biosci 36:97–100
Aparicio VC, De Gerónimo E, Marino D, Primost J, Carriquiriborde P, Costa JL (2013) Environmental fate of glyphosate and aminomethylphosphonic acid in surface waters and soil of agricultural basins. Chemosphere 93:1866–1873. https://doi.org/10.1016/j.chemosphere.2013.06.041
Babich R, Ulrich JC, Ekanayake EDV, Massarsky A, De Silva PMC, Manage PM, Jackson BP, Ferguson PL, Giulio RTD, Drummond IA, Jayasundara N (2020) Kidney developmental effects of metal-herbicide mixtures: Implications for chronic kidney disease of unknown etiology. Environ Int 144:106019. https://doi.org/10.1016/j.envint.2020.106019
Baird DD (1971) Introduction of a new broad spectrum post emergence herbicide class with utility for herbaceous perennial weed control. In: Proceedings of the 26th North Central Weed Conference. North Central Weed Science Society, Kansas City
Balduzzi S, Rücker G, Schwarzer G (2019) How to perform a meta-analysis with R: a practical tutorial. Evid Based Mental Health 22:153–160. https://doi.org/10.1136/ebmental-2019-300117
Battaglin WA, Meyer MT, Kuivila KM, Dietze JE (2014) Glyphosate and its degradation product AMPA occur frequently and widely in U.S. Soils, surface water, groundwater, and precipitation. JAWRA J Am Water Resour Assoc 50:275–290. https://doi.org/10.1111/jawr.12159
Battisti L, Potrich M, Sampaio AR, de Castilhos Ghisi N, Costa-Maia FM, Abati R, dos Reis Martinez CB, Sofia SH (2021) Is glyphosate toxic to bees? A meta-analytical review. Sci Total Environ 767:145397. https://doi.org/10.1016/j.scitotenv.2021.145397
Beis D, Bartman T, Jin S-W, Scott IC, D’Amico LA, Ober EA, Verkade H, Frantsve J, Field HA, Wehman A, Baier H, Tallafuss A, Bally-Cuif L, Chen J-N, Stainier DYR, Jungblut B (2005) Genetic and cellular analyses of zebrafish atrioventricular cushion and valve development. Development 132:4193–4204. https://doi.org/10.1242/dev.01970
Benbrook CM (2016) Trends in glyphosate herbicide use in the United States and globally. Environ Sci Eur 28:3. https://doi.org/10.1186/s12302-016-0070-0
Berghmans S, Butler P, Goldsmith P, Waldron G, Gardner I, Golder Z, Richards FM, Kimber G, Roach A, Alderton W, Fleming A (2008) Zebrafish based assays for the assessment of cardiac, visual and gut function — potential safety screens for early drug discovery. J Pharmacol Toxicol Methods 58:59–68. https://doi.org/10.1016/j.vascn.2008.05.130
Bonsignorio D, Perego L, Giacco LD, Cotelli F (1996) Structure and macromolecular composition of the zebrafish egg chorion. Zygote 4:101–108. https://doi.org/10.1017/S0967199400002975
Borggaard OK, Gimsing AL (2008) Fate of glyphosate in soil and the possibility of leaching to ground and surface waters: a review. Pest Manag Sci 64:441–456. https://doi.org/10.1002/ps.1512
Bournele D, Beis D (2016) Zebrafish models of cardiovascular disease. Heart Fail Rev 21:803–813. https://doi.org/10.1007/s10741-016-9579-y
Bridi D, Altenhofen S, Gonzalez JB, Reolon GK, Bonan CD (2017) Glyphosate and Roundup® alter morphology and behavior in zebrafish. Toxicology 392:32–39. https://doi.org/10.1016/j.tox.2017.10.007
Byer JD, Struger J, Klawunn P, Todd A, Sverko E (2008) Low cost monitoring of glyphosate in surface waters using the ELISA method: an evaluation. Environ Sci Technol 42:6052–6057. https://doi.org/10.1021/es8005207
Çelik ES, Kaya H, Yılmaz S (2012) Effects of phosalone on mineral contents and spinal deformities in common carp (Cyprinus carpio, L.1758). TrJFAS 12:259–264
Christiansen HE, Lang MR, Pace JM, Parichy DM (2009) Critical early roles for col27a1a and col27a1b in zebrafish notochord morphogenesis, vertebral mineralization and post-embryonic axial growth. PLOS One 4:e8481. https://doi.org/10.1371/journal.pone.0008481
Córdova López AM, Sarmento RA, de Souza Saraiva A, Pereira RR, Soares AMVM, Pestana JLT (2019) Exposure to Roundup® affects behaviour, head regeneration and reproduction of the freshwater planarian Girardia tigrina. Sci Total Environ 675:453–461. https://doi.org/10.1016/j.scitotenv.2019.04.234
Coupe RH, Kalkhoff SJ, Capel PD, Gregoire C (2012) Fate and transport of glyphosate and aminomethylphosphonic acid in surface waters of agricultural basins. Pest Manag Sci 68:16–30. https://doi.org/10.1002/ps.2212
da Costa Chaulet F, de Alcantara Barcellos HH, Fior D, Pompermaier A, Koakoski G, da Rosa JGS, Fagundes M, Barcellos LJG (2019) Glyphosate- and fipronil-based agrochemicals and their mixtures change zebrafish behavior. Arch Environ Contam Toxicol 77:443–451. https://doi.org/10.1007/s00244-019-00644-7
da Rosa JGS, de Abreu MS, Giacomini ACV, Koakoski G, Kalichak F, Oliveira TA, de Alcântara Barcellos HH, Barreto RE, Barcellos LJG (2016) Fish aversion and attraction to selected agrichemicals. Arch Environ Contam Toxicol 71:415–422. https://doi.org/10.1007/s00244-016-0300-x
Davico CE, Pereira AG, Nezzi L, Jaramillo ML, de Melo MS, Müller YMR, Nazari EM (2021) Reproductive toxicity of Roundup WG® herbicide: impairments in ovarian follicles of model organism Danio rerio. Environ Sci Pollut Res 28(12):15147–15159. https://doi.org/10.1007/s11356-020-11527-z
de Brito Rodrigues L, de Oliveira R, Abe FR, Brito LB, Moura DS, Valadares MC, Grisolia CK, de Oliveira DP, de Oliveira GAR (2017) Ecotoxicological assessment of glyphosate-based herbicides: effects on different organisms: ecotoxicity of glyphosate-based herbicides. Environ Toxicol Chem 36:1755–1763. https://doi.org/10.1002/etc.3580
de Brito Rodrigues L, Gonçalves Costa G, Lundgren Thá E, da Silva LR, de Oliveira R, Morais Leme D, Cestari MM, Koppe Grisolia C, Campos Valadares M, de Oliveira GAR (2019) Impact of the glyphosate-based commercial herbicide, its components and its metabolite AMPA on non-target aquatic organisms. Mutation Res Genet Toxicol Environ Mutagen 842:94–101. https://doi.org/10.1016/j.mrgentox.2019.05.002
Dersimonian R, Laird N (1986) Metaanalysis in clinical-trials. Controlled Clin Trials 7:177–188
Díaz-Martín RD, Carvajal-Peraza A, Yáñez-Rivera B, Betancourt-Lozano M (2021) Short exposure to glyphosate induces locomotor, craniofacial, and bone disorders in zebrafish (Danio rerio) embryos. Environ Toxicol Pharmacol 87:103700. https://doi.org/10.1016/j.etap.2021.103700
Díaz-Martín RD, Valencia-Hernández JD, Betancourt-Lozano M, Yáñez-Rivera B (2021) Changes in microtubule stability in zebrafish (Danio rerio) embryos after glyphosate exposure. Heliyon 7(1):e06027. https://doi.org/10.1016/j.heliyon.2021.e06027
Ding W, Shangguan Y, Zhu Y, Sultan Y, Feng Y, Zhang B, Liu Y, Ma J, Li X (2021) Negative impacts of microcystin-LR and glyphosate on zebrafish intestine: Linked with gut microbiota and microRNAs? Environ Pollut 286:117685. https://doi.org/10.1016/j.envpol.2021.117685
Dornelles MF, Oliveira GT (2014) Effect of atrazine, glyphosate and quinclorac on biochemical parameters, lipid peroxidation and survival in bullfrog tadpoles (Lithobates catesbeianus). Arch Environ Contam Toxicol 66:415–429. https://doi.org/10.1007/s00244-013-9967-4
Druart C, Gimbert F, Scheifler R, de Vaufleury A (2017) A full life-cycle bioassay with Cantareus aspersus shows reproductive effects of a glyphosate-based herbicide suggesting potential endocrine disruption. Environ Pollut 226:240–249. https://doi.org/10.1016/j.envpol.2017.03.061
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Egger M, Smith GD, Altman DG (2001) Systematic reviews in health care: meta-analysis incontext. BMJ Publishing Group, London, UK
Falfushynska H, Khatib I, Kasianchuk N, Lushchak O, Horyn O, Sokolova IM (2022) Toxic effects and mechanisms of common pesticides (Roundup and chlorpyrifos) and their mixtures in a zebrafish model (Danio rerio). Sci Total Environ 833:155236. https://doi.org/10.1016/j.scitotenv.2022.155236
Faria M, Bedrossiantz J, Ramírez JRR, Mayol M, García GH, Bellot M, Prats E, Garcia-Reyero N, Gómez-Canela C, Gómez-Oliván LM, Raldúa D (2021) Glyphosate targets fish monoaminergic systems leading to oxidative stress and anxiety. Environ Int 146:106253. https://doi.org/10.1016/j.envint.2020.106253
FAO - Food and Agriculture Organization of the United Nations. 2016. Specifications and evaluations for glyphosate. Disponível em: http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/Specs/Glyphosate_2016_02_10.pdf
Fiorino E (2018) Effects of glyphosate on early life stages: comparison between Cyprinus carpio and Danio rerio. Environ Sci Pollut Res 8:8542–8549
Forner-Piquer I, Faucherre A, Byram J, Blaquière M, de Bock F, Gamet-Payrastre L, Ellero-Simatos S, Audinat E, Jopling C, Marchi N (2021) Differential impact of dose-range glyphosate on locomotor behavior, neuronal activity, glio-cerebrovascular structures, and transcript regulations in zebrafish larvae. Chemosphere 267:128986. https://doi.org/10.1016/j.chemosphere.2020.128986
Gaur H, Bhargava A (2019) Glyphosate induces toxicity and modulates calcium and NO signaling in zebrafish embryos. Biochem Biophys Res Commun 513:1070–1075. https://doi.org/10.1016/j.bbrc.2019.04.074
Giommi C, Ladisa C, Carnevali O, Maradonna F, Habibi HR (2022) Metabolomic and transcript analysis revealed a sex-specific effect of glyphosate in zebrafish Liver. Int J Mol Sci 23(5):2724. https://doi.org/10.3390/ijms23052724
Glusczak L, Loro VL, Pretto A, Moraes BS, Raabe A, Duarte MF, da Fonseca MB, de Menezes CC, de Sousa Valladão DM (2011) Acute exposure to glyphosate herbicide affects oxidative parameters in piava (Leporinus obtusidens). Arch Environ Contam Toxicol 61:624–630. https://doi.org/10.1007/s00244-011-9652-4
Glusczak L, Miron D, dos S, Moraes BS, Simões RR, Schetinger MRC, Morsch VM, Loro VL (2007) Acute effects of glyphosate herbicide on metabolic and enzymatic parameters of silver catfish (Rhamdia quelen). Comp Biochem Physiol Part C Toxicol Pharmacol 146:519–524. https://doi.org/10.1016/j.cbpc.2007.06.004
Grunwald DJ, Eisen JS (2002) Headwaters of the zebrafish — emergence of a new model vertebrate. Nat Rev Genet 3:717–724. https://doi.org/10.1038/nrg892
Hayes M, Gao X, Yu LX, Paria N, Henkelman RM, Wise CA, Ciruna B (2014) ptk7 mutant zebrafish models of congenital and idiopathic scoliosis implicate dysregulated Wnt signalling in disease. Nat Commun 5:4777. https://doi.org/10.1038/ncomms5777
Hedges LV, Olkin I (1985) Statistical methods for meta-analysis. Academic Press, Orlando
Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. Brit Med J 327:557–560
Hong Y, Yang X, Huang Y, Yan G, Cheng Y (2018) Assessment of the oxidative and genotoxic effects of the glyphosate-based herbicide roundup on the freshwater shrimp, Macrobrachium nipponensis. Chemosphere 210:896–906. https://doi.org/10.1016/j.chemosphere.2018.07.069
Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW (2014) SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol 14:43. https://doi.org/10.1186/1471-2288-14-43
Hued AC, Oberhofer S, de los Ángeles Bistoni M (2012) Exposure to a commercial glyphosate formulation (Roundup®) alters normal gill and liver histology and affects male sexual activity of Jenynsia multidentata (Anablepidae, Cyprinodontiformes). Arch Environ Contam Toxicol 62:107–117. https://doi.org/10.1007/s00244-011-9686-7
Ivantsova E, Wengrovitz AS, Souders II CL, Martyniuk CJ (2022) Developmental and behavioral toxicity assessment of glyphosate and its main metabolite aminomethylphosphonic acid (AMPA) in zebrafish embryos/larvae. Environ Toxicol Pharmacol 103873. https://doi.org/10.1016/j.etap.2022.103873
Jaramillo ML, Pereira AG, Davico CE, Nezzi L, Ammar D, Müller YMR, Nazari EM (2018) Evaluation of reference genes for reverse transcription-quantitative PCR assays in organs of zebrafish exposed to glyphosate-based herbicide, Roundup. Animal 12:1424–1434. https://doi.org/10.1017/S1751731117003111
Kim K-H, Antkiewicz DS, Yan L, Eliceiri KW, Heideman W, Peterson RE, Lee Y (2007) Lrrc10 is required for early heart development and function in zebrafish. Dev Biol 308:494–506. https://doi.org/10.1016/j.ydbio.2007.06.005
Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF (1995) Stages of embryonic development of the zebrafish. Dev Dyn 203:253–310. https://doi.org/10.1002/aja.1002030302
Lanctôt C, Navarro-Martín L, Robertson C, Park B, Jackman P, Pauli BD, Trudeau VL (2014) Effects of glyphosate-based herbicides on survival, development, growth and sex ratios of wood frog (Lithobates sylvaticus) tadpoles. II: agriculturally relevant exposures to Roundup WeatherMax® and Vision® under laboratory conditions. Aquatic Toxicol 154:291–303. https://doi.org/10.1016/j.aquatox.2014.05.025
Lanzarin GAB, Félix LM, Santos D, Venâncio CAS, Monteiro SM (2019) Dose-dependent effects of a glyphosate commercial formulation – Roundup® UltraMax - on the early zebrafish embryogenesis. Chemosphere 223:514–522. https://doi.org/10.1016/j.chemosphere.2019.02.071
Lanzarin GAB, Venâncio CAS, Monteiro SM, Félix LM (2020) Behavioural toxicity of environmental relevant concentrations of a glyphosate commercial formulation - RoundUp® UltraMax - During zebrafish embryogenesis. Chemosphere 253:126636. https://doi.org/10.1016/j.chemosphere.2020.126636
Lanzarin G, Venâncio C, Félix LM, Monteiro S (2021) Inflammatory, oxidative stress, and apoptosis effects in zebrafish larvae after rapid exposure to a commercial glyphosate formulation. Biomedicines 9(12):1784. https://doi.org/10.3390/biomedicines9121784
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62:e1–e34. https://doi.org/10.1016/j.jclinepi.2009.06.006
Lin CC, Hui MNY, Cheng SH (2007) Toxicity and cardiac effects of carbaryl in early developing zebrafish (Danio rerio) embryos. Toxicol Appl Pharmacol 222:159–168. https://doi.org/10.1016/j.taap.2007.04.013
Liu N, Zhong G, Zhou J, Liu Y, Pang Y, Cai H, Wu Z (2019) Separate and combined effects of glyphosate and copper on growth and antioxidative enzymes in Salvinia natans (L.) All. Sci Total Environ 655:1448–1456. https://doi.org/10.1016/j.scitotenv.2018.11.213
Liu Z, Shangguan Y, Zhu P, Sultan Y, Feng Y, Li X, Ma J (2022) Developmental toxicity of glyphosate on embryo-larval zebrafish (Danio rerio). Ecotoxicol Environ Saf 236:113493. https://doi.org/10.1016/j.ecoenv.2022.113493
Lopes FM, Sandrini JZ, Souza MM (2018) Toxicity induced by glyphosate and glyphosate-based herbicides in the zebrafish hepatocyte cell line (ZF-L). Ecotoxicol Environ Saf 162:201–207. https://doi.org/10.1016/j.ecoenv.2018.07.005
Lopes FM, Varela Junior AS, Corcini CD, da Silva AC, Guazzelli VG, Tavares G, da Rosa CE (2014) Effect of glyphosate on the sperm quality of zebrafish Danio rerio. Aquat Toxicol 155:322–326. https://doi.org/10.1016/j.aquatox.2014.07.006
Lushchak OV, Kubrak OI, Storey JM, Storey KB, Lushchak VI (2009) Low toxic herbicide Roundup induces mild oxidative stress in goldfish tissues. Chemosphere 76:932–937. https://doi.org/10.1016/j.chemosphere.2009.04.045
Maggi F, la Cecilia D, Tang FHM, McBratney A (2020) The global environmental hazard of glyphosate use. Sci Total Environ 717:137167. https://doi.org/10.1016/j.scitotenv.2020.137167
Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. JNCI J Natl Cancer Inst 22:719–748. https://doi.org/10.1093/jnci/22.4.719
Mena F, Romero A, Blasco J, Araújo CV (2022) Can a mixture of agrochemicals (glyphosate, chlorpyrifos and chlorothalonil) mask the perception of an individual chemical? A hidden trap underlying ecological risk. Ecotoxicol Environ Saf 230:113172. https://doi.org/10.1016/j.ecoenv.2022.113172
Mercurio P, Flores F, Mueller JF, Carter S, Negri AP (2014) Glyphosate persistence in seawater. Marine Pollut Bull 85:385–390. https://doi.org/10.1016/j.marpolbul.2014.01.021
Moraes JS, da Silva Nornberg BF, Castro MR, de, Vaz B, dos S, Mizuschima CW, Marins LFF, Martins C, de MG (2020) Zebrafish (Danio rerio) ability to activate ABCC transporters after exposure to glyphosate and its formulation Roundup Transorb®. Chemosphere 248:125959. https://doi.org/10.1016/j.chemosphere.2020.125959
Moreno NC, Sofia SH, Martinez CBR (2014) Genotoxic effects of the herbicide Roundup Transorb® and its active ingredient glyphosate on the fish Prochilodus lineatus. Environ Toxicol Pharmacol 37:448–454. https://doi.org/10.1016/j.etap.2013.12.012
OECD (2013) Test no. 236: fish embryo acute toxicity (FET) test. https://doi.org/10.1787/9789264203709-en
Odenthal J, Haffter P, Vogelsang E, Brand M, van Eeden FJ, Furutani-Seiki M, Granato M, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Warga RM, Allende ML, Weinberg ES, Nüsslein-Volhard C (1996) Mutations affecting the formation of the notochord in the zebrafish, Danio rerio. Development 123:103–115
Okada E, Pérez D, De Gerónimo E, Aparicio V, Massone H, Costa JL (2018) Non-point source pollution of glyphosate and AMPA in a rural basin from the southeast Pampas, Argentina. Environ Sci Pollut Res 25:15120–15132. https://doi.org/10.1007/s11356-018-1734-7
Panetto OS, Gomes HF, Fraga Gomes DS, Campos E, Romeiro NC, Costa EP, do Carmo PRL, Feitosa NM, Moraes J (2019) The effects of Roundup® in embryo development and energy metabolism of the zebrafish (Danio rerio). Comp Biochem Physiol Part C Toxicol Pharmacol 222:74–81. https://doi.org/10.1016/j.cbpc.2019.04.007
Peruzzo PJ, Porta AA, Ronco AE (2008) Levels of glyphosate in surface waters, sediments and soils associated with direct sowing soybean cultivation in north pampasic region of Argentina. Environ Poll 156:61–66. https://doi.org/10.1016/j.envpol.2008.01.015
Pompermaier A, Kirsten K, Soares SM, Fortuna M, Kalichak F, Idalencio R, Koakoski G, Barreto RE, Barcellos LJG (2020) Waterborne agrichemicals compromise the anti-predatory behavior of zebrafish. Environ Sci Pollut Res 27(31):38559–38567. https://doi.org/10.1007/s11356-020-09862-2
Pompermaier A, Varela ACC, Mozzato MT, Soares SM, Fortuna M, Alves C, Tamagno WA, Barcellos LJG (2022) Impaired initial development and behavior in zebrafish exposed to environmentally relevant concentrations of widely used pesticides. Comp Biochem Physiol Part C Toxicol Pharmacol 257:109328. https://doi.org/10.1016/j.cbpc.2022.109328
Raldúa D, André M, Babin PJ (2008) Clofibrate and gemfibrozil induce an embryonic malabsorption syndrome in zebrafish. Toxicol Appl Pharmacol 228:301–314. https://doi.org/10.1016/j.taap.2007.11.016
Rawson DM, Zhang T, Kalicharan D, Jongebloed WL (2000) Field emission scanning electron microscopy and transmission electron microscopy studies of the chorion, plasma membrane and syncytial layers of the gastrula-stage embryo of the zebrafish Brachydanio rerio: a consideration of the structural and functional relationships with respect to cryoprotectant penetration. Aquac Res 31:325–336. https://doi.org/10.1046/j.1365-2109.2000.00401.x
Ronco AE, Marino DJG, Abelando M, Almada P, Apartin CD (2016) Water quality of the main tributaries of the Paraná Basin: glyphosate and AMPA in surface water and bottom sediments. Environ Monit Assess 188:458. https://doi.org/10.1007/s10661-016-5467-0
Roy NM, Carneiro B, Ochs J (2016a) Glyphosate induces neurotoxicity in zebrafish. Environ Toxicol Pharmacol 42:45–54. https://doi.org/10.1016/j.etap.2016.01.003
Roy NM, Ochs J, Zambrzycka E, Anderson A (2016b) Glyphosate induces cardiovascular toxicity in Danio rerio. Environ Toxicol Pharmacol 46:292–300. https://doi.org/10.1016/j.etap.2016.08.010
Ruiz-Toledo J, Castro R, Rivero-Pérez N, Bello-Mendoza R, Sánchez D (2014) Occurrence of glyphosate in water bodies derived from intensive agriculture in a tropical region of Southern Mexico. Bull Environ Contam Toxicol 93:289–293. https://doi.org/10.1007/s00128-014-1328-0
Rumschlag SL, Mahon MB, Hoverman JT, Raffel TR, Carrick HJ, Hudson PJ, Rohr JR (2020) Consistent effects of pesticides on community structure and ecosystem function in freshwater systems. Ecology https://doi.org/10.1101/2020.02.03.932442
Ruuskanen S, Rainio MJ, Gómez-Gallego C, Selenius O, Salminen S, Collado MC, Saikkonen K, Saloniemi I, Helander M (2020) Glyphosate-based herbicides influence antioxidants, reproductive hormones and gut microbiome but not reproduction: a long-term experiment in an avian model. Environ Pollut 266:115108. https://doi.org/10.1016/j.envpol.2020.115108
Samaee S-M, Rabbani S, Jovanović B, Mohajeri-Tehrani MR, Haghpanah V (2015) Efficacy of the hatching event in assessing the embryo toxicity of the nano-sized TiO2 particles in zebrafish: A comparison between two different classes of hatching-derived variables. Ecotoxicol Environ Saf 116:121–128. https://doi.org/10.1016/j.ecoenv.2015.03.012
Sanchís J, Kantiani L, Llorca M, Rubio F, Ginebreda A, Fraile J, Garrido T, Farré M (2012) Determination of glyphosate in groundwater samples using an ultrasensitive immunoassay and confirmation by on-line solid-phase extraction followed by liquid chromatography coupled to tandem mass spectrometry. Anal Bioanal Chem 402:2335–2345. https://doi.org/10.1007/s00216-011-5541-y
Sano K, Inohaya K, Kawaguchi M, Yoshizaki N, Iuchi I, Yasumasu S (2008) Purification and characterization of zebrafish hatching enzyme – an evolutionary aspect of the mechanism of egg envelope digestion. FEBS J 275:5934–5946. https://doi.org/10.1111/j.1742-4658.2008.06722.x
Santo GD, Grotto A, Boligon AA, Da Costa B, Rambo CL, Fantini EA, Sauer E, Lazzarotto LMV, Bertoncello KT, Júnior OT, Garcia SC, Siebel AM, Rosemberg DB, Magro JD, Conterato GMM, Zanatta L (2018) Protective effect of Uncaria tomentosa extract against oxidative stress and genotoxicity induced by glyphosate-Roundup® using zebrafish (Danio rerio) as a model. Environ Sci Pollut Res 25:11703–11715. https://doi.org/10.1007/s11356-018-1350-6
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. https://doi.org/10.1038/nmeth.2019
Schneider MI, Sanchez N, Pineda S, Chi H, Ronco A (2009) Impact of glyphosate on the development, fertility and demography of Chrysoperla externa (Neuroptera: Chrysopidae): ecological approach. Chemosphere 76:1451–1455. https://doi.org/10.1016/j.chemosphere.2009.05.029
Silva CDS, FAY EF (2004) Agrotóxicos e ambiente, vol. 1. Embrapa Informática Tecnológica, Jaguariúna, Embrapa Meio Ambiente
Snawder JE, Chambers JE (1993) Osteolathyrogenic effects of malathion in xenopus embryos. Toxicol Appl Pharmacol 121:210–216. https://doi.org/10.1006/taap.1993.1147
Sulukan E, Köktürk M, Ceylan H, Beydemir Ş, Işik M, Atamanalp M, Ceyhun SB (2017) An approach to clarify the effect mechanism of glyphosate on body malformations during embryonic development of zebrafish (Daino rerio). Chemosphere 180:77–85. https://doi.org/10.1016/j.chemosphere.2017.04.018
Tran CM, Lee H, Lee B, Ra J-S, Kim K-T (2021) Effects of the chorion on the developmental toxicity of organophosphate esters in zebrafish embryos. J Hazard Mater 401:123389. https://doi.org/10.1016/j.jhazmat.2020.123389
Uren Webster TM, Laing LV, Florance H, Santos EM (2014) Effects of glyphosate and its formulation, roundup, on reproduction in zebrafish (Danio rerio). Environ. Sci. Technol. 48:1271–1279. https://doi.org/10.1021/es404258h
Velasques RR, Sandrini JZ, da Rosa CE (2016) Roundup ® in zebrafish: effects on oxidative status and gene expression. Zebrafish 13:432–441. https://doi.org/10.1089/zeb.2016.1259
Vera MS, Lagomarsino L, Sylvester M, Pérez GL, Rodríguez P, Mugni H, Sinistro R, Ferraro M, Bonetto C, Zagarese H, Pizarro H (2010) New evidences of Roundup® (glyphosate formulation) impact on the periphyton community and the water quality of freshwater ecosystems. Ecotoxicology 19:710–721. https://doi.org/10.1007/s10646-009-0446-7
Vesterinen HM, Sena ES, Egan KJ, Hirst TC, Churolov L, Currie GL, Antonic A, Howells DW, Macleod MR (2014) Meta-analysis of data from animal studies: a practical guide. J Neurosci Methods 221:92–102. https://doi.org/10.1016/j.jneumeth.2013.09.010
Willaert A, Khatri S, Callewaert BL, Coucke PJ, Crosby SD, Lee JGH, Davis EC, Shiva S, Tsang M, De Paepe A, Urban Z (2012) GLUT10 is required for the development of the cardiovascular system and the notochord and connects mitochondrial function to TGFβ signaling. Human Mol Genet 21:1248–1259. https://doi.org/10.1093/hmg/ddr555
Wu M, Pan C, Chen Z, Jiang L, Lei P, Yang M (2017) Bioconcentration pattern and induced apoptosis of bisphenol A in zebrafish embryos at environmentally relevant concentrations. Environ Sci Pollut Res 24:6611–6621. https://doi.org/10.1007/s11356-016-8351-0
Yusof S, Ismail A, Alias MS (2014) Effect of glyphosate-based herbicide on early life stages of Java medaka (Oryzias javanicus): a potential tropical test fish. Mar Pollut Bull 85:494–498. https://doi.org/10.1016/j.marpolbul.2014.03.022
Zhang S, Xu J, Kuang X, Li S, Li X, Chen D, Zhao X, Feng X (2017) Biological impacts of glyphosate on morphology, embryo biomechanics and larval behavior in zebrafish (Danio rerio). Chemosphere 181:270–280. https://doi.org/10.1016/j.chemosphere.2017.04.094
Zhang W, Liu G (2017) Situation and development of worldwide agri-environment: Agricultural land uses, fertilizers consumption and carbon dioxide equivalent emissions. Environ Skeptics and Critics 6(1):1–8
Zhang W, Wang J, Song J, Feng Y, Zhang S, Wang N, Liu S, Song Z, Lian K, Kang W (2021) Effects of low-concentration glyphosate and aminomethyl phosphonic acid on zebrafish embryo development. Ecotoxicol Environ Saf 226:112854. https://doi.org/10.1016/j.ecoenv.2021.112854
Zou S, Kamei H, Modi Z, Duan C (2009) Zebrafish IGF genes: gene duplication, conservation and divergence, and novel roles in midline and notochord development. PLoS One 4:e7026. https://doi.org/10.1371/journal.pone.0007026
Funding
This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível 470 Superior - Brasil (CAPES – PROEX, process number 23038.005450/2020-19).
Author information
Authors and Affiliations
Contributions
JA: Conceptualisation, Methodology, Investigation, Formal analysis, Investigation, Writing - Original Draft. Jaíne is the author of master thesis derived of this manuscript. AAM: Conceptualisation, Methodology, Writing - Review & Editing. MFC: Methodology, Formal analysis, Writing - Review & Editing. FOC: Methodology, Formal analysis, Writing - Review & Editing. VLL: Resources, Writing - Review & Editing, Supervision, Project administration. VLL is the adviser of JA.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ames, J., Miragem, A.A., Cordeiro, M.F. et al. Effects of glyphosate on zebrafish: a systematic review and meta-analysis. Ecotoxicology 31, 1189–1204 (2022). https://doi.org/10.1007/s10646-022-02581-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-022-02581-z