Abstract
Zebrafish (Danio rerio) embryos and larvae were selected to investigate the potential risk and aquatic toxicity of a widely used pharmaceutical, naproxen. The acute toxicity of naproxen to embryos and larvae was measured, respectively. The histopathology was investigated in the liver of zebrafish larvae after 8-day embryo-larvae exposure to naproxen. The values of 96-h LC50 were 115.2 mg/L for embryos and 147.6 mg/L for larvae, indicating that zebrafish embryos were more sensitive than larvae to naproxen exposure. Large suites of symptoms were induced in zebrafish (D. rerio) early life stages by different dosages of naproxen, including hatching inhibition, lower heart rate, and morphological abnormalities. The most sensitive sub-lethal effect caused by naproxen was pericardial edema, the 72-h EC50 values of which for embryos and larvae were 98.3 and 149.0 mg/L, respectively. In addition, naproxen-treated zebrafish larvae exhibited histopathological liver damage, including swollen hepatocytes, vacuolar degeneration, and nuclei pycnosis. The results indicated that naproxen is a potential threat to aquatic organisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, the consumption of pharmaceuticals has increased substantially throughout the world. Pharmaceuticals, which are detected in surface water and occasionally in groundwater (Ternes 1998; Heberer 2002; Zuccato et al. 2006) because of incomplete removal in wastewater treatment plants, have become important pollutants of the aquatic environment (Kümmerer 2009). Many drugs have been presumed to be persistent pollutants because their continual infusion into the aquatic environment serves to sustain perpetual life cycle exposure for aquatic organisms (Daughton and Ternes 1999).
As a non-steroidal anti-inflammatory drug (NSAIDs), naproxen is used worldwide to reduce pain, inflammation, and fever. Approximately, 3000 tons of naproxen was produced during the year 2003 in the world (Zhang 2003). Naproxen is detected at concentrations ranging from nanogram per liter to microgram per liter in the environment (Tixier et al. 2003; Wang et al. 2010). Despite being not intrinsically persistent (half-lives of 27 days), naproxen is considered to be a pseudo-persistent compound in the aquatic environment due to its huge production and consumption (Ternes 1998; Isidori et al. 2005; Grenni et al. 2013). An investigation showed that patients with preexisting medical conditions (e.g., diabetes) appeared to have a significantly higher risk for serious cardiovascular events associated with the long-term treatment of naproxen (Huang et al. 2006). Naproxen remains active after discharged to the environment and may have adverse effects on any aquatic organisms by interfering with their biological systems (Wiegel et al. 2004). Exposure to 0.1 μg/L of naproxen resulted in a decrease in egg fertilization of on Florida flagfish (Jordanella floridae) over one complete life cycle (121 days; Nesbitt 2011). Differential protein expression was observed in gill tissue of zebrafish after a 14-day exposure to naproxen at a concentration of 10 μg/L, indicating that altered gene expression may occur in fish subjected to environmentally realistic levels of naproxen exposure (Adhikari 2012). Some studies focused on the acute toxicity of naproxen on the invertebrate species (Cleuvers 2003; Santos et al. 2010). Relatively low concentration (median effective concentration (EC50) = 0.33 mg/L) of naproxen could inhibit the growth population of crustaceans (Ceriodaphnia dubia) after exposure for 7 days (Isidori et al. 2005). However, studies of naproxen toxicity on aquatic vertebrate species are scarce.
Zebrafish (Danio rerio) is an excellent vertebrate model for assessing the toxicity of novel compounds, pollutants, and pharmaceuticals due to its external fertilization, transparent embryos, rapid embryonic developmental cycle, and large clutch sizes (Ali et al. 2011; Di Paolo et al. 2015; Wernersson et al. 2015). Moreover, the zebrafish genome is completely sequenced, which is 83 % identical to the human gene (with one gap) (Allende et al. 1996). Zebrafish liver resembles the mammalian liver on the morphological and functional level (Hibiya et al. 1982). Compared to zebrafish adults, zebrafish embryos can be recommended as an alternative model system, since it is considered not to perceive pain or other discomfort (Braunbeck et al. 2005; Strähle et al. 2012). From a hepatotoxicity testing perspective, the liver in the zebrafish embryos is fully functioning with active drug metabolism at 72 h post-fertilization (hpf) (Alderton et al. 2010). Some studies showed that zebrafish embryos are suitable to detect human hepatotoxicants (Jones et al. 2009; Amali et al. 2006; Driessen et al. 2013). In the present study, the acute toxicity and histopathological effects of naproxen were assessed using the zebrafish embryos and larvae. The aims of this study were to (1) determine the lethal and sub-lethal effects and (2) explore the liver histopathological changes of naproxen in the early life stages of zebrafish.
Materials and methods
Experimental chemicals
Naproxen ((S)-6-Methoxy-alpha-methyl-2-naphthaleneacetic acid; CAS no. 22204-53-1) was purchased from Sigma-Aldrich (USA). The stock solution of naproxen of 20,000 mg/L was prepared in acetone (CNW, China, HPLC) and stored at 4 °C in darkness. Following initial range-finding experiments, test solutions were prepared by dilution of the stock solution with the final concentration not above 0.3 % acetone (Hallare et al. 2006). Other chemicals used in this study were of analytical grade.
Zebrafish maintenance and embryo collection
Sexually mature zebrafish (D. rerio) were kept in 25-L tanks at a water temperature of 26.0 ± 1.0 °C, a hardness of 250 mg/L, a pH value of 7.5 ± 0.5, and a dissolved oxygen concentration of 10.5 ± 0.5 mg/L. A constant day to night rhythm (14/10 h) was maintained. The fish were fed twice each day with commercially frozen red mosquito larvae sterilized by UV lamps. The day before a test was performed, male and female adult fish (female/male ratio was 1/2) were stocked in a translucent plastic box (12 cm × 20 cm × 12 cm) with a mesh insert (mesh size is 3–4 mm) to prevent the fish from eating their eggs. Spawning and fertilization took place within 30 min after the onset of light in the morning. The eggs were collected in Petri dishes and rinsed several times with reconstituted water (ISO 7346/3: 294 mg/L CaCl2·2H2O, 123 mg/L MgSO4·7H2O, 123 mg/L NaHCO3, and 5.5 mg/L KCl), which had been ventilated to nearly 100 % oxygen saturation. Healthy and normally developing embryos were selected within 2 hpf for exposure tests. For valid experiments, eggs were obtained only from spawns with a fertilization rate higher than 90 %.
Acute toxicity tests on zebrafish
Embryonic acute toxicity test
The embryonic acute toxicity test was conducted following the fish embryo toxicity (FET) test (OECD 2013) with some modification. Test solutions with a naproxen concentration of 0, 10, 20, 50, 75, 100, 125, 150, 175, 200, and 240 mg/L were made up using reconstituted water, designed on the basis of pre-experiment data. For exposure of zebrafish embryos, 2.0 mL of these solutions and two fertilized eggs were transferred to individual wells of a 24-well microtiter plate. Twenty wells in each plate contained four test concentrations evenly and the other four wells filled up with 2.0 mL of 0.3 % acetone were served as the control. Each test concentration was replicated five times, with 10 embryos per replicate. The exposure solution was renewed every 24 h to keep the appropriate concentration of drug and water quality. The embryos were incubated under a temperature of 26.0 ± 1.0 °C with a 14:10 light/dark cycle and monitored at specific time points (8, 24, 48, 72, 96, and 120 h). Lethal and sub-lethal endpoints, including lethality, heartbeat, hatching rate, and abnormality, were observed and recorded with an inverted microscope (Nikon TE2000-U).
Larvae acute toxicity test
Acute exposure of the larvae was conducted following the method performed by Giari et al. (2012), with some modification. Test solutions contained a series of naproxen dosages (0, 10, 20, 50, 75, 100, 125, 150, 175 mg/L) and were made up using reconstituted water. For exposure of zebrafish larvae, 10.0 mL of these solutions and 10 newly hatched larvae (hatching less than 1 h) that had not been exposed to naproxen during the embryonic development were transferred to individual wells of a six-well plate. Four wells in each plate contained four test concentrations evenly, and the other two wells filled up with 10.0 mL of 0.3 % acetone were served as the control. Each test concentration was replicated three times. The exposure solution was renewed every 24 h to keep the appropriate concentration of drug and water quality. The larvae were incubated under a temperature of 26.0 ± 1.0 °C with a 14:10 light/dark cycle. During the experiment, larvae were not fed, and dead individuals were removed immediately. The absence of heartbeat and/or immobility and/or absence of respiration movement and/or lack of reaction mechanism stimulus could be used to determine the death of larvae (OECD 1998). The lethal rate and malformation rate of larvae were examined microscopically at specific time points (24, 48, 72, 96, and 120 h) for all treatment and control groups.
Histopathological examination
Embryo-larvae exposure test
Zebrafish embryos within 2 hpf were collected to be exposed to naproxen for 8 days in accordance with OECD guidelines no. 212 (fish, short-term toxicity test on embryo and sac-fry stages). Based on the acute toxicity results, 10, 50, 75, and 100 mg/L naproxen solutions were prepared using reconstituted water. Of these solutions with 10 fertilized eggs, 10.0 mL was transferred to individual wells of a six-well plate. Four wells in each plate contained four test concentrations evenly, and the other two wells filled up with 10.0 mL of 0.3 % acetone served as the control. Each test concentration was replicated five times. The six-well plates were stored in an incubator at 26.0 ± 1.0 °C under a 14:10 light/dark photoperiod. The exposure solution was renewed every 24 h to keep the appropriate concentration of drug and water quality. During the 8-day exposure test, no feeding was provided, and dead individuals were removed immediately. After exposure for 5 and 8 days, larvae from the embryo-larvae exposure test were collected for histopathological analysis, respectively.
Histopathological measurements
Of each test group, four randomly selected larvae were fixed in Bouin solution, consisting of 15 volumes picric acid (Aldrich, USA), five volumes formaldehyde (Aladdin, China), and one volume pure acetic acid (CNW, China) and stored at 4 °C. Samples were dehydrated and embedded according to the following procedure: rinsing in 50, 75, 85, 95, and 100 % ethanol (CNW, China) for 15 min three times, respectively, infiltration with paraffin. Serial sections of 5-μm thickness were prepared on a microtome (Leica RM2016, China), and mounted on slides. Slides were deparaffinized, rehydrated, stained with hematoxylin and eosin (H&E) staining kit (Beyotime, China), dehydrated and covered with a glass coverslip. For each fish, the histology of liver was qualitatively described after observation with an inverted microscope (Nikon TE2000-U).
Statistical analysis
All statistical analysis was undertaken using SPSS 19.0 software. Data were expressed as the mean ± standard deviation (SD). Differences were determined by one-way analysis of variance (ANOVA) with a Dunnett’s post hoc test, and the significance was set at P < 0.05. For acute toxicity tests, the median lethal concentration (LC50) and EC50 values with 95 % confidence intervals for naproxen were calculated by concentration-response regression using probit analysis.
Results
Lethal effects of naproxen
Naproxen had lethal ability to zebrafish embryos and larvae. The LC50 values of naproxen to zebrafish embryos and larvae at the different time points are shown at Table 1. According to the 96-h LC50 values, embryos were more sensitive to naproxen exposure compared to larvae.
Embryonic developmental effects of naproxen
Hatching rate
For embryos in the solvent control, 10 and 20 mg/L naproxen groups, the hatching rates were above 80 % at 72 hpf and almost all the surviving embryos hatched at 96 hpf (Fig. 1). Compared to the control, the hatching rates of embryos exposed to 50 mg/L naproxen were significantly reduced at 72 hpf (P < 0.05). However, no significant hatching inhibition was observed at 96 and 120 hpf, which indicated that naproxen delayed the hatching of zebrafish embryos. After exposure to 75 and 100 mg/L of naproxen, the hatching rates were 18 and 4 % at 72 hpf, respectively. The hatching of embryos was accomplished at 120 hpf, and the final hatching rates were up to 64 and 36 %, which were significantly lower than that of the control. As the zebrafish embryos without hatching were dead at 120 hpf, the high lethality was inferred to induce the lower hatching rates.
Heart rate
For the control group, average heart rate (48 hpf) of zebrafish embryos was 124 ± 10 beats/min. The heart rates reduced with the increasing drug concentration and were significantly inhibited in embryos of the 100 and 125 mg/L exposure group compared with those of the control group (P < 0.05; Fig. 2).
Teratogenic effects
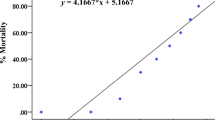
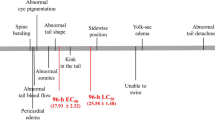
Naproxen induced a suite of morphological abnormalities during the embryonic development. The frequently observed abnormalities were pericardial edema, yolk sac edema, and hemagglutination (Fig. 3a). The most sensitive sub-lethal effect caused by naproxen was pericardial edema, which appeared at a concentration of 20 mg/L, and the rate of occurrence increased with an increasing drug concentration (Fig. 4). The percentage of abnormality was from nearly 10 % at a concentration of 20 mg/L to 62 % at a concentration of 100 mg/L after 72-h exposure. However, the rate of pericardial edema reduced after exposure to 125 mg/L of naproxen. Since the mortality increased at this exposure concentration, there were less zebrafish embryos to observe. Other abnormalities caused by naproxen were weak pigmentation, hemorrhage, yolk condensation, and trunk abnormalities including without somites, tail not detached, axial malformation, and tail twisting (Fig. 3b). As is shown in Table 2, the 72-h EC50 values of zebrafish embryos are pericardial edema (98.3 mg/L) < hemagglutination (120.7 mg/L) < yolk sac edema (122.9 mg/L).
a Embryo with pericardial edema (PE) and yolk sac edema (YSE) after 72-h exposure to naproxen at the concentration of 50 mg/L (a); Embryo with PE and YSE after 72-h exposure to naproxen at the concentration of 75 mg/L (b); Embryo with PE, hemagglutination (HE), and YSE after 72-h exposure to naproxen at the concentration of 100 mg/L (c); Embryo with PE, HE, and YSE after 72-h exposure to naproxen at the concentration of 125 mg/L (d). b Abnormalities of embryos exposed to naproxen at specified time points. a Weak pigmentation at 48 h; b PE without somites (woS) and tail not detached at 48 h; c hemorrhage (H) at 72 h; d yolk condensation (YC) at 72 h; e PE, HE, and YSE at 72 h; f axial malformation (AM) at 96 h; g axial malformation (AM) at 96 h; h tail twisting (TT) at 120 h. c Abnormalities of larvae exposed to naproxen at specified time points. a Normal larva at 48 h; b normal larva at 72 h; c PE and axial malformation (AM) at 48 h; d PE, YSE, and AM at 72 h; e AM at 72 h
For the larvae acute toxicity test, sub-lethal effects were observed when the exposure concentration was not lower than 75 mg/L, including pericardial edema, yolk sac edema, and axial malformation (Fig. 3c). Pericardial edema was the most sensitive sub-lethal effect, the rate of which increased with increasing naproxen concentrations, showing a dose-response relationship (Fig. 5). The percentage of pericardial edema was from nearly 6.7 % at a concentration of 75 mg/L to 66.7 % at a concentration of 175 mg/L after 72-h exposure. The 72-h EC50 values with their 95 % confidence intervals of naproxen for pericardial edema were 149.0 mg/L (131.6–177.3) for larvae (Table 2).
Toxicity of naproxen in embryo-larval stages of zebrafish
Survival rate
As shown in Fig. 6, no mortality was observed for the control during the 8-day exposure of embryo-larval zebrafish to naproxen. After the short-term exposure, the survival rates were 90 and 50 % at the test concentration of 10 and 50 mg/L, respectively. When the test concentrations were up to 100 and 75 mg/L, the survival rate was zero after exposure for 6 and 8 days, respectively.
Hepatic histopathology
Livers of zebrafish exhibited histopathological changes following naproxen treatment compared to the control (Fig. 7a). After 5-day exposure, there were some morphological alterations in the higher concentration (75, 100 mg/L) exposure groups (Fig. 7b, c), in which swelling of hepatic cells and hepatocellular vacuolar degeneration were observed and cell borders became obscure. Particularly, nuclei pycnosis occurred at the concentration of 100 mg/L. Exposure to 10 mg/L naproxen for 8 days elicited a little hepatocellular vacuolar degeneration and nuclei pycnosis (Fig. 7d), while relatively worse phenomenon was observed in the 50 mg/L group (Fig. 7e). Hepatic lesions became much more severe in 75 mg/L group (Fig. 7f), in which nuclei pycnosis was observed along with concomitant hepatocyte necrosis and cytolysis.
Liver histopathology of zebrafish after exposure to different dosage of naproxen. Photomicrographs of liver sections (5 μm) stained with hematoxylin and eosin. a Livers from control group; b livers from zebrafish after 5-day exposure to 75-mg/L naproxen; c livers from zebrafish after 5-day exposure to 100-mg/L naproxen; d livers from zebrafish after 8-day exposure to 10-mg/L naproxen; e livers from zebrafish after 8-day exposure to 50-mg/L naproxen; f livers from zebrafish after 8-day exposure to 75-mg/L naproxen. Pyknotic nuclei (arrow), swelling of hepatic cells (oval), and hepatocellular vacuolar degeneration (asterisks) were observed
Discussion
Although many studies indicate that the widespread occurrence of naproxen was in low-level concentrations (ng/L–μg/L) in the aquatic environment (Peng et al. 2008), chronic exposures and the potential for some pharmaceuticals to bioaccumulate may lead to deleterious effects in aquatic organisms (Cleuvers 2004). In this study, the values of 96-h LC50 were 115.2 mg/L for embryos and 147.6 mg/L for larvae, indicating that zebrafish embryos were more sensitive to naproxen than larvae. The 72-h EC50 values for pericardial edema of embryos and larvae were 98.3 and 149.0 mg/L, respectively, which also demonstrated a higher tolerance of larvae to naproxen. Many previous studies have reported that aquatic embryos showed stronger sensitivity than adults and juveniles when exposed to some organic chemicals, such as formaldehyde and 3,4-dichloroaniline (Ton et al. 2012; Zhu et al. 2013). The factors, which determine the sensitivity of the toxic strength on embryos and larvae, still need to be studied, and one possible factor is the chemicals’ capacity to permeate the embryonic membrane (Embry et al. 2010). The embryonic membrane contains several hydrophilic groups such as carboxyl, amino, sulfate, phosphate, amide, and hydroxyimidazole (Bhainsa and D’Souza 2008; Yan and Viraraghavan 2003). Naproxen contains several hydrophilic groups including carboxylic acid. Therefore, the polar groups of naproxen and those present in the embryonic membrane would interact in solution. Li et al. (2007) indicated that chemicals must have interacted with the cell membrane to penetrate the cell and affected the function of the target biomolecule before inducing toxicity. On the other hand, the presence of solvents, such as DMSO, might decrease the barrier function of the chorion and make an influence via increased availability of chemicals inside the chorion (Braunbeck et al. 2015). However, the effects of solvents acetone on membranes are rarely reported. From the comprehensive lethal and sub-lethal results, the embryo stage is identified as the most sensitive period in the acute toxicity test for naproxen.
The acute lethal effects of naproxen to other aquatic organisms are shown in Fig. 8. The values of 96-h LC50 for zebrafish embryos and larvae were higher than 96-h LC50 of freshwater planarians (Dugesia japonica) (Li 2013) and cnidarians (Hydra attenuata) (Quinn et al. 2008), 48-h LC50 of cladocera (Daphnia longispina), 24-h LC50 of protozoa (Paramecium caudatum) (EI-Bassat et al. 2012), rotifers (Brachionus calyciflorus), and crustaceans (Thamnocephalus platyurus) (Isidori et al. 2005), and lower than the fish Lepomis macrochirus and rainbow trout (Oncorhynchus mykiss) (Rodriguez et al. 1992). Thus, naproxen seems to be more toxic to aquatic invertebrates than aquatic vertebrates, and zebrafish is a relatively sensitive species compared to other fish. It is unclear why there is a large difference in naproxen toxicity levels between vertebrates and invertebrates, but it may be attributed to physiological differences between the taxa or to differences in the respective mechanism of naproxen toxicity. A possible explanation is that the appearance and evolution of liver might have improved the detoxification of naproxen.
The heart is the first functional organ formed in zebrafish, and heart rate is an important toxicology endpoint in the fish embryo test (Glickman and Yelon 2002; OECD 2013). In the present study, the heart rates of embryos significantly reduced after exposure to 125 and 175 mg/L of naproxen. The most sensitive acute embryo and larval stage sub-lethal endpoint appeared to be pericardial edema. Similarly, David and Pancharatna (2009) found that 10–100 μg/L of ibuprofen could cause lower heart rate and pericardial edema in zebrafish embryos. Non-steroidal anti-inflammatory drugs are acting by inhibition of either cyclooxygenase enzymes (COX), cyclooxygenase-1 (COX-1), or cyclooxygenase (COX-2) involved in the synthesis of different prostaglandins from arachidonic acid (Prescott and Yost 2002). Both cDNAs of zCOX-1 and zCOX-2 were widely expressed during development of zebrafish, and zCOX-1 was evident in the embryonic vasculature (Grosser et al. 2002). In zebrafish, COX-1-derived prostaglandins are demonstrated to be necessary for segmentation, and pharmacological inhibition zCOX-1 activity leads to effects in heart formation and shortening of intersomitic vessels (Cha et al. 2005, 2006). Naproxen showed both high COX-1 and COX-2 inhibitory activity (Hecken et al. 2000). Therefore, it can be inferred that the lower heart rate, pericardial edema, and teratogenic effects induced by naproxen might be attributed to the inhibition of COX, which is the rate-limiting enzyme for the synthesis of prostaglandins. In addition, heart abnormalities could also result from the yolk sac edema. During the embryonic development of oviparous fish, embryos use endogenous yolk nutrients previously accumulated in the oocyte (Raldúa et al. 2008). Yolk sac impairment might block nutrient supply during embryonic development, thus, the heart function would be affected by energy limitation (Kodde et al. 2007). This is also a potential for pericardial edema.
The liver is the site of a major portion of metabolism and detoxification of contaminants (Treinen-Moslen 2001; Stancová et al. 2015), and also a critical target for xenobiotic-induced toxicity (Driessen et al. 2013). Histopathology indicated that the larval zebrafish liver is particularly sensitive to naproxen. Obvious hepatic reactions in zebrafish early stages were observed after exposure to naproxen for 8 days at concentrations of 10 and 50 mg/L, including swelling of hepatic cells, hepatocellular vacuolar degeneration, nuclei pycnosis, and obscure cell borders, which can be interpreted as signs of an activated stress status of fish which mobilize their energy storage and modify the structure of their organelles related to the need of intensified detoxification capacities (Triebskorn et al. 2004). In this study, abnormalities were not observed when the exposure concentration was lower than 75 mg/L in the larvae acute toxicity test at 72 hpf, which might be correlated with the metabolism and detoxification of naproxen in the liver. However, hepatocyte necrosis and cytolysis were observed after exposure to 75 mg/L for 8 days. Abnormalities, including pericardial edema, yolk sac edema, and axial malformation that occurred when the exposure concentration was above 75 mg/L, might be due to the hepatocyte necrosis and loss of detoxification function. All zebrafish were dead after 6 days in the 100 mg/L exposure group, indicating that hepatic lesions became more severe with increasing naproxen concentrations, and more abnormalities were induced to cause death when detoxification capability was exceeded.
Conclusion
In summary, the results demonstrated that waterborne exposure to naproxen could induce a large suite of negative influences on zebrafish over embryo and larval stages, including hatching inhibition, lower heart rate, and increased malformation. According to the LC50 and EC50 values in the zebrafish acute toxicity test, embryos showed stronger sensitivity than larvae when exposed to naproxen. The most sensitive acute embryo and larvae stage sub-lethal endpoint seems to be pericardial edema. In addition, short-term exposure to naproxen led to apparent pathological liver damage in zebrafish larvae. However, since the concentrations of naproxen in the aquatic environment are much lower than the concentrations used in this study, it is likely that chronic effects in the long-term exposure would be further studied to improve the environmental risk assessment of naproxen.
References
Adhikari PR (2012) Proteomic responses in the gill of zebrafish following exposure to ibuprofen and naproxen. Dissertation, University of North Texas, Texas
Alderton W, Berghmans S, Butler P, Chassaing H, Fleming A, Golder Z, Richard F, Gardner I (2010) Accumulation and metabolism of drugs and CYP probe substrates in zebrafish larvae. Xenobiotica 40(8):547–557
Ali S, van Mil HG, Richardson MK (2011) Large-scale assessment of the zebrafish embryo as a possible predictive model in toxicity testing. PLoS One 6:e21076
Allende ML, Amsterdam A, Becker T, Kawakami K, Gaiano N, Hopkins N (1996) Insertional mutagenesis in zebrafish identifies two novel genes, pescadillo and dead eye, essential for embryonic development. Gene dev 10(24):3141–3155
Amali AA, Rekha RD, Lin CJ, Wang WL, Gong HY, Her GM, Wu JL (2006) Thioacetamide induced liver damage in zebrafish embryo as a disease model for steatohepatitis. J Biomed Sci 13(2):225–232
Bhainsa KC, D’Souza SF (2008) Removal of copper ions by the filamentous fungus, Rhizopus oryzae from aqueous solution. Bioresource Technol 99:3829–3835
Braunbeck T, Böttcher M, Hollert H, Kosmehl T, Lammer E, Leist E, Rudolf M, Seitz N (2005) Towards an alternative for the acute fish LC50 test in chemical assessment: the fish embryo toxicity test goes multi-species—an update. Altex 22(2):87–102
Braunbeck T, Kais B, Lammer E, Otte J, Schneider K, Stengel D, Strecker R (2015) The fish embryo test (FET): origin, applications, and future. Environ Sci Pollut Res 22(21):16247–16261
Cha YI, Kim SH, Solnica-Krezel L, Dubois RN (2005) Cyclooxygenase-1 signaling is required for vascular tube formation during development. Dev Biol 282:274–283
Cha YI, Solnica-Krezel L, DuBois RN (2006) Fishing for prostanoids: deciphering the developmental functions of cyclooxygenase-derived prostaglandins. Dev Biol 289:263–272
Cleuvers M (2003) Aquatic ecotoxicity of pharmaceuticals including the assessment of combination effects. Toxicol Lett 142(3):185–194
Cleuvers M (2004) Mixture toxicity of the anti-inflammatory drugs diclofenac, ibuprofen, naproxen, and acetylsalicylic acid. Ecotox Environ Safe 59:309–315
Daughton CG, Ternes TA (1999) Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ Health Persp 107(Suppl 6):907–938
David A, Pancharatna K (2009) Developmental anomalies induced by a non-selective COX inhibitor (ibuprofen) in zebrafish (Danio rerio). Environ Toxicol and Phar 27:390–395
Di Paolo C, Seiler TB, Keiter S, Hu M, Muz M, Brack W, Hollert H (2015) The value of zebrafish as an integrative model in effect-directed analysis—a review. Environ Sci Eur 27(1):8
Driessen M, Kienhuis AS, Pennings JLA, Pronk TE, van de Brandhof EJ, Roodbergen M, Spaink HP, van de Water B, van der Ven LTM (2013) Exploring the zebrafish embryo as an alternative model for the evaluation of liver toxicity by histopathology and expression profiling. Arch Toxicol 87:807–823
EI-Bassat RA, Touliabah HE, Harisa GI (2012) Toxicity of four pharmaceuticals from different classes to isolated plankton species. Afr J Aquat Sci 37:71–80
Embry MR, Belanger SE, Braunbeck TA, Galay-Burgos M, Halder M, Hinton DE, Léonard MA, Lillicrap A, Norberg-King T, Whale G (2010) The fish embryo toxicity test as an animal alternative method in hazard and risk assessment and scientific research. Aquat Toxicol 97:79–87
Giari L, Dezfuli BS, Astolfi L, Martini A (2012) Ultrastructural effects of cisplatin on the inner ear and lateral line system of zebrafish (Danio rerio) larvae. J Appl Toxicol 32:293–299
Glickman NS, Yelon D (2002) Cardiac development in zebrafish: coordination of form and function. Seminars in cell & developmental biology. Academic 13:507–513
Grenni P, Patrolecco L, Ademollo N, Tolomei A, Caracciolo AB (2013) Degradation of gemfibrozil and naproxen in a river water ecosystem. Microchem J 107:158–164
Grosser T, Yusuff S, Cheskis E, Pack MA, FitzGerald GA (2002) Developmental expression of functional cyclooxygenases in zebrafish. Proc Natl Acad Sci 99:8418–8423
Hallare A, Nagel K, Köhler HR, Triebskorn R (2006) Comparative embryotoxicity and proteotoxicity of three carrier solvents to zebrafish (Danio rerio) embryos. Ecotox Environ Safe 63:378–388
Heberer T (2002) Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: a review of recent research data. Toxicol Lett 131:5–17
Hecken A, Schwartz JI, Depré M, Lepeleire I, Dallob A, Tanaka W, Wynants K, Buntinx A, Arnout J, Wong PH, Ebel DL, Gertz BJ, Schepper PJ (2000) Comparative inhibitory activity of rofecoxib, meloxicam, diclofenac, ibuprofen, and naproxen on COX-2 versus COX-1 in healthy volunteers. J Clin Pharmacol 40:1109–1120
Hibiya T, Yokote M, Oguri M, Sato H, Takashima F, Aida K (1982) An atlas of fish histology normal and pathological features. Kodansha LTD, Tokyo
Huang WF, Hsiao FY, Wen YW, Tsai YW (2006) Cardiovascular events associated with the use of four nonselective NSAIDs (etodolac, nabumetone, ibuprofen, or naproxen) versus a cyclooxygenase-2 inhibitor (celecoxib): a population-based analysis in Taiwanese adults. Clin Ther 28:1827–36
Isidori M, Lavorgna M, Nardelli A, Parrella A, Previtera L, Rubino M (2005) Ecotoxicity of naproxen and its phototransformation products. Sci Total Environ 348:93–101
Jones M, Ball JS, Dodd A, Hill AJ (2009) Comparison between zebrafish and Hep G2 assays for the predictive identification of hepatotoxins. Toxicology 262(1):13–14
Kodde IF, van der Stok J, Smolenski RT, Jong JW (2007) Metabolic and genetic regulation of cardiac energy substrate preference. Comp Biochem Physiol A Mol Integr Physiol 146:26–39
Kümmerer K (2009) Antibiotics in the environment—a review—part II. Chemosphere 75:435–441
Li MH (2013) Acute toxicity of 30 pharmaceutically active compounds to freshwater planarians, Dugesia japonica. Toxico Enviro Chem 95:1157–1170
Li L, Gao HW, Ren JR, Chen L, Li YC, Zhao JF, Zhao HP, Yuan Y (2007) Binding of Sudan II and IV to lecithin liposomes and E. coli membranes: insights into the toxicity of hydrophobic azo dyes. BMC Struct Biol 7:16
Nesbitt R (2011) Effects of chronic exposure to ibuprofen and naproxen on Florida flagfish (Jordanella floridae) over one complete life-cycle. Dissertation, University of Ontario Institute of Technology, Ontario
OECD (1998) Test No.212: Fish, short-term toxicity test on embryo and sac-fry stages. OECD guidelines for the testing of chemicals, section 2. OECD Publishing, Paris
OECD (2013) Test no. 236: fish embryo acute toxicity (FET) test, OECD guidelines for the testing of chemicals, section 2. OECD Publishing, Paris
Peng XZ, Yu YY, Tang CM, Tan JH, Huang QX, Wang ZD (2008) Occurrence of steroid estrogens, endocrine-disrupting phenols, and acid pharmaceutical residues in urban riverine water of the Pearl River Delta, South China. Sci Total Environ 397(1):158–166
Prescott SM, Yost HJ (2002) The COXes of Danio: from mechanistic model to experimental therapeutics. Proc Natl Acad Sci 99:9084–9086
Quinn B, Gagné F, Blaise C (2008) An investigation into the acute and chronic toxicity of eleven pharmaceuticals (and their solvents) found in wastewater effluent on the cnidarian, Hydra attenuata. Sci Total Environ 389:306–314
Raldúa D, André M, Babin PJ (2008) Clofibrate and gemfibrozil induce an embryonic malabsorption syndrome in zebrafish. Toxicol Appl Pharm 228:301–314
Rodriguez C, Chellman K, Gomez S, Marple L (1992) Environmental assessment report pursuant to 21 CFR 25.31(a) submitted to the US FDA in support of the new drug application (NDA) for naproxen for over-the-counter use. Hamilton Pharmaceuticals Limited, Puerto Rico
Santos LHMLM, Araújo AN, Fachini A, Pena A, Delerue-Matos C, Montenegro MCBSM (2010) Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. J Hazard Mater 175(1):45–95
Stancová V, Ziková A, Svobodová Z, Kloas W (2015) Effects of the non-steroidal anti-inflammatory drug (NSAID) naproxen on gene expression of antioxidant enzymes in zebrafish (Danio rerio). Environ Toxicol Phar 40:343–348
Strähle U, Scholz S, Geisler R, Greiner P, Hollert H, Rastegar S, Schumacher A, Selderslaghs I, Weiss C, Witters H, Braunbeck T (2012) Zebrafish embryos as an alternative to animal experiments—a commentary on the definition of the onset of protected life stages in animal welfare regulations. Reprod Toxicol 33:128–32
Ternes TA (1998) Occurrence of drugs in German sewage treatments plants and rivers. Water Res 32:3245–3260
Tixier C, Singer H, Oellers S, Muller S (2003) Occurrence and fate of carbamazepine, clofibric acid, diclofenac, ibuprofen, ketoprofen and naproxen in surface waters. Environ Sci Technol 37:1061–1068
Ton SS, Chang SH, Hsu LY, Wang MH, Wang KS (2012) Evaluation of acute toxicity and teratogenic effects of disinfectants by Daphnia magna embryo assay. Environ Pollut 168:54–61
Treinen-Moslen M (2001) Toxic responses of the liver. In: Klaasen CD (ed) Casarett and Doull’s toxicology. McGraw-Hill, New York, pp 471–489
Triebskorn R, Casper H, Heyd A, Eikemper R, Köhler HR, Schwaiger J (2004) Toxic effects of the non-steroidal anti-inflammatory drug diclofenac: part II. Cytological effects in liver, kidney, gills and intestine of rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 68:151–166
Wang L, Ying GG, Zhao JL, Yang XB, Chen F, Tao R, Liu S, Zhou LJ (2010) Occurrence and risk assessment of acidic pharmaceuticals in the Yellow River, Hai River and Liao River of north China. Sci Total Environ 408:3139–3147
Wernersson AS, Carere M, Maggi C et al (2015) The European technical report on aquatic effect-based monitoring tools under the water framework directive. Environ Sci Eur 27(1):1–11
Wiegel S, Aulinger A, Brockmeyer R, Harms H, Loffler J, Reincke H, Schmidt R, Stachel B, Von Tumpling W, Wanke A (2004) Pharmaceuticals in the River Elbe and its tributaries. Chemosphere 57:107–126
Yan GY, Viraraghavan T (2003) Heavy-metal removal from aqueous solution by fungus Mucor rouxii. Water Res 37:4486–4496
Zhang L (2003) Marketing analysis of naproxen (in Chinese). China pharmacy 14(6):326–328
Zhu B, Liu TQ, Hu XG, Wang GX (2013) Developmental toxicity of 3,4-dichloroaniline on rare minnow (Gobiocypris rarus) embryos and larvae. Chemosphere 90:1132–1139
Zuccato E, Castiglioni S, Fanelli R, Reitano G, Bagnati R, Chiabrando C, Pomati F, Rossetti C, Calamari D (2006) Pharmaceuticals in the environment in Italy: causes, occurrence, effects and control. Environ Sci Pollu Res 13:15–21
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation of China (No. 41101499) and the national key technology R&D program of the Ministry of Science and Technology of China (2012BAJ24B01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Henner Hollert
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 22 kb)
Rights and permissions
About this article
Cite this article
Li, Q., Wang, P., Chen, L. et al. Acute toxicity and histopathological effects of naproxen in zebrafish (Danio rerio) early life stages. Environ Sci Pollut Res 23, 18832–18841 (2016). https://doi.org/10.1007/s11356-016-7092-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-7092-4