Abstract
Increased use of pesticides worldwide has led to damage not only to natural ecosystems but also to nontarget species. This study assessed the effects of different concentrations of the herbicides atrazine, glyphosate, and quinclorac on biochemical parameters, lipid peroxidation, and survival in tadpoles of Lithobates catesbeianus (bullfrog). Two hundred eighty-eight tadpoles were acquired from a frog farm in the south of Brazil. All animals were kept in aquariums under controlled laboratory conditions for 7 days and exposed to commercial formulations of atrazine (5, 10, and 20 μg/L), glyphosate (36, 72, and 144 μg/L), and quinclorac (0.05, 0.10, and 0.20 μg/L) for 7 days thereafter. The concentrations used in this study are similar to the levels of these herbicides found in natural water bodies. After exposure, gill, liver, and muscle samples were removed from each animal for quantitation of glycogen, total lipids, triglycerides, cholesterol, total proteins, and lipid peroxidation. Atrazine, glyphosate, and quinclorac exposure induced a significant decrease in levels of glycogen and total lipids in gill, liver, and muscle. Triglycerides levels in the gill increased after exposure to glyphosate, and decreased after exposure to atrazine and quinclorac; their levels in liver and muscle decreased on exposure to all herbicides. Cholesterol and total protein levels decreased in liver and muscle for all three herbicides. All tissues exhibited increased lipid peroxidation after exposure to all herbicides. In conclusion, exposure to the herbicides tested in this study induced significant changes in biochemical parameters and increased lipid peroxidation levels in tadpoles of L. catesbeianus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
New technologies and the growth of the world population have led to an expansion of global agribusiness. Around the world 1.817 billion tons of grain are produced annually (IGC 2012) leading to the consumption of an average 2.5 million tons of pesticides (Spadotto et al. 2010). This use of agrochemicals has been increasing gradually during recent decades. During the last 10 years alone, worldwide use of agrochemicals increased 93 % (Carneiro et al. 2012) with no attendant increase in arable land, i.e., a greater amount of agricultural chemicals have been applied to the same planting area (Spadotto 2006).
It is widely known that the use of pesticides is related to economic, technical, and social factors (Midio and Martins 1997), but their indiscriminate use has caused severe changes in the balance of ecosystems. Such changes have influenced the flow of energy and the structure and function of natural communities, thus modifying the physical and biotic balance of these environments and causing impacts in exposed individuals at the tissue and molecular levels (Berti et al. 2009; Poleza et al. 2008). According to Blaustein and Johnson (2003), the abusive use of agrochemicals is one of the factors that have contributed to the degradation of habitats and the decrease of biodiversity in aquatic environments, mainly due to the high potential for leaching, persistence, and adsorption to organic matter present in the soil as well as to the water solubility of these chemicals (Moura et al. 2008).
Atrazine, glyphosate, and quinclorac are currently the most widely used herbicides worldwide (Dörfler et al. 1997; Howe et al. 2004; Galon et al. 2009). The mode of action of these agricultural chemicals can include the inhibition of photosynthesis or other enzyme-mediated processes, such as inhibition of essential amino acid synthesis and growth inhibition (Oliveira 2001; Relyea 2005; Tomlin 1994).
Although herbicides target plant species, there is evidence that these agricultural chemicals are also linked to changes in amphibian communities. Exposure of these animals to herbicides can induce direct effects, such as mortality, and indirect effects, such as changes in biochemical and physiological parameters (Ezemonye and Tongo 2009). Excessive use of herbicides is one of the factors described by Blaustein and Johnson (2003) as being responsible for directly affecting the development, reproduction, and survival of amphibian populations around the world.
Changes in biochemical parameters, such as an increase in energy expenditure to detoxify the agrochemicals, can be one response after herbicide exposure. In stressful conditions, such as pesticide exposure, glycogens can provide a reserve of internal energy, and their levels can be quickly depleted (Alkahen 1996; Barton and Iwama 1991; Becker et al. 2009; Chang et al. 2006; Moyes and Schulte 2010; Salbego et al. 2010; Vutukuru 2005).
Lipids also can be a substrate for the production of energy when extra fuel is required or induced by a stressor, thus playing a vital role during biochemical adaptation faced stressful conditions (Champe and Harvey 2006; Gijare et al. 2011; Gurushankara et al. 2007; Honrubia et al 1993; Moyes and Schulte 2010; Zaya et al 2011).
In addition to lipids, triglycerides constitute the main way of storing lipids, which play a vital role in the energy reserves in animals. Exposure to toxic compounds can alter the chemical function in triglycerides due to an increased in energy demand, thus eading to decreased stored triglycerides (El-Banna et al. 2009; Moyes and Schulte 2010; Sawant and Varute 1973).
Cholesterol is an important constituent of cell membranes, and after pesticide exposure, their levels typically decrease, which may be due to an inhibition of cholesterol biosynthesis or the use of fatty deposits as a source of energy after increased energy demand (Aldana-Madrid et al 2012; Champe and Harvey 2006; Ganeshwade 2012; Shakoori et al 1996; Trabalon and Blais 2012).
Proteins may be decreased under stressful conditions and consequent increased energy expenditure after exposure to pesticides. This can occur due to an attempt to detoxify toxic compounds, for increase ATP synthesis, or for the formation of lipoproteins (Ganeshwade 2012; Khan et al. 2003; Rambabu and Rao 1994; Ribeiro et al. 2001; Sak et al. 2006; Salbego et al 2010; Sounderraj et al 2011).
Lipid peroxidation, or oxidation of the lipid layer of the cell membrane, is an important mechanism by which to measure cell injury. Animals exposed to pesticides can increase their levels of lipid peroxidation in tissues, thus leading to a chemical damage and death cell (Al-Othman et al. 2011; Champe and Harvey 2006; El-Banna et al 2009; Patil et al. 2009; Uchendu et al. 2012).
The choice of such biochemical parameters and levels of lipid peroxidation is justified by the fact that the initial effects of the presence of toxic compounds in an organism manifests primarily through changes in biochemical levels before morphological changes become visible. The monitoring of these biochemical responses can provide the earliest warning signs before aspects such as morbidity and mortality become visible in a population. In this way, the effects of pollution in cellular metabolism and their constituents can be considered as reliable indicators in monitoring levels of xenobiotics in the environment (Roy and Hänninen 1993).
Within this context, the present work aimed to evaluate the effects of commercial formulations of the herbicides atrazine, glyphosate, and quinclorac on biochemical parameters, such as total lipids, triglycerides, cholesterol, total proteins, lipid peroxidation levels, and survival in tadpoles of Lithobates catesbeianus exposed at concentrations cited in the literature as commonly found in natural water bodies (Lambropoulou et al. 2002; Marchezan et al. 2007; Paulino et al. 2012; Silva et al. 2003, 2009).
Materials and Methods
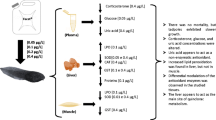
For the purposes of this study, 288 live tadpoles were acquired from a commercial frog farm (Ranasul) in the municipality of Imbé, state of Rio Grande do Sul, Brazil. All tadpoles were 4 months of age and were in the larval stage 26 according to Gosner (1960). The choice of individuals prioritized similar sizes, as recommended by Landis and Yu (2003), as well as the absence of limbs.
The animals were transported in air-filled plastic bags to the Conservation Physiology Laboratory at Pontifícia Universidade Católica do Rio Grande do Sul where they were measured, weighed, and photographed individually. The animals were randomly divided into a control group (n = 24) and killed by the freezing method on arrival at the laboratory for verification of initial conditions, and an intervention group was made consisting of the remaining animals. These animals were placed in 22 aquariums each containing 12 L of water (n = 12), with constant aeration, a water temperature of 22 ± 2 °C, pH 6.2 ± 0.3, and a 12-h light-to-dark cycle.
The tadpoles were fed (5 % of their biomass) once daily with the same fish feed used in the commercial frog farm, thus minimizing any effect of food stress. The acclimation period was 7 days. On day 8 after the start of the experiment, the herbicides were added to the aquarium water. The introduction of liquid-formulation (atrazine and glyphosate) and powder-formulation herbicides (quinclorac) was performed through the dissolution of agrochemical in distilled water and added only once to the water of aquariums in concentration to be used. The exposure period was 7 days.
The experiment was performed in duplicate (two aquariums for each group) with the aquariums subdivided as follows for 7- and 14-day control groups each: atrazine groups = concentrations of 5, 10, and 20 μg/L; glyphosate groups = concentrations of 36, 72, and 144 μg/L; and quinclorac groups = concentrations of 0.05, 0.10, and 0.20 μg/L. In these experiments, we used commercial formulations of atrazine (Primóleo, 400 g/L; Syngenta), glyphosate (Roundup Original, 360 g/L; Monsanto), and quinclorac (Facet, 500 g/kg; Basf).
The chosen concentrations were based on mean values found in natural bodies of water as cited by Lambropoulou et al. (2002), Marchezan et al. (2007), Paulino et al. (2012), and Silva et al. (2003, 2009). At the end of the 14-day study period, all animals were killed by the freezing method. The following structures were removed from each animal by dissection in a glass Petri dish over ice: left and right gills, liver, and muscle. Tissue specimens were then used for biochemical analysis of the levels of glycogen, total protein, lipids, cholesterol, and triglycerides as well as evaluation of lipid peroxidation levels for the different concentrations of pesticides.
The pesticides were applied only once at nominal concentrations. According to Paulino et al. (2012), the concentrations of atrazine found in nature do not follow a pattern but occur in variations, and the contamination of water bodies by atrazine occurs mainly in shallow and water-covered areas (rivers and streams); however, in slow aquatic systems, such as lakes and wetlands, these concentrations can be greater. Atrazine is not strongly adsorbed on sediments, and its low rate of hydrolysis and photolysis can extend its presence in water resulting in half-lives between 34.8, 174, 398, and 742 days at pH of 2.9, 4.5, 6.0, and 7.0, respectively (Solomon et al. 1996). In our work, we used pH of 7.4.
Glyphosate is characterized for being soluble in water, nonpersistent in the environment, without potential for bioaccumulation, and with potential for adsorption in soil (Fernandes and Sarcinelli 2009). According Giesy et al. (2000). The solubility of glyphosate in water is high, and its half-life can range between 7 and 70 days; Quinclorac is shown be persistent in water for ≤31 days after application according Reimche et al. (2008). According to Rodrigues and Almeida (1998), the half-life of quinclorac in water is 21 days. In the field, half-lives of quinclorac were reported to be 18, 36, 44, 50, and 166 days in five different field studies, respectively (McElroy 2006).
All research protocols used in this work were authorized by the Pontifícia Universidade Católica do Rio Grande do Sul Animal Research Ethics Committee with registration number CEUA 11/00250, as set forth in approval letter number 157/11-CEUA, December 2011.
Biochemical Analysis
All experimental determinations in tissue specimens were made by spectrophotometric methods, in quadruplicate, and all results expressed as mg/g of tissue.
Glycogen
Glycogen was extracted by Van Handel’s method (1965) and quantified as glucose after acid hydrolysis (HCl) and neutralization (Na2CO3) using a commercial Glucose Oxidase Kit (Labtest).
Total Proteins
Total protein concentration was determined by the colorimetric biuret method. The intensity of the color formed is proportional to the total protein concentration in the sample. Again, a commercial kit was used (Total Proteins Kit, Labtest).
Lipids, Triglycerides, and Cholesterol
Lipids, triglycerides, and cholesterol were extracted using the chloroform:methanol method (2:1) (Folch et al. 1957). Lipids were determined through the specific sulfo-phospho-vanillin reaction (Frings and Dunn 1970). Triglycerides were determined using the commercial Triglycerides GPO-ANA Kit (Bio-Diagnostic) through the lipoprotein lipase method, whereby triglycerides are hydrolyzed, thus resulting in the release of glycerol, which is then converted, oxidized, and catalyzed by glycerophosphate. The reaction produces a violet color where the intensity is proportional to the concentration of triglycerides in the sample. Cholesterol was determined with the Liquiform Kit (Labtest) using the enzymatic colorimetric method for the determination of total cholesterol in the sample with ready-for-use liquid reagent.
Measurement of Lipid Peroxidation
Lipid peroxidation activity was measured using the thiobarbituric acid-reactive substances (TBA-RS) technique, which consists of heating the sample in the presence of thiobarbituric acid under acidic conditions and measuring the formation of a color product (Buege and Aust 1978). The method used was as follows: 150 μl of 10 % trichloroacetic acid, 50 μl of homogenized tissue, 100 μl of 0.67 % TBA, and 50 μl of distilled water were added to a test tube (total volume = 350 μl). The tube was shaken, incubated at 100 °C for 15 min, and cooled for 10 min. Then 300 μl of n-butyl alcohol was added to the sample for extraction of the colored product from aqueous solution. Tubes were shaken for 45 s and centrifuged for 10 min at 3000 rpm. The supernatant was added to the spectrophotometer cuvette and read at 535 nm. The concentration was expressed in nmol/mg of protein.
Statistical Analysis
The Kolmogorov–Smirnov test for normality, Levene’s test for homogeneity, and one-way analysis of variance followed by Bonferroni correction were used for comparison between the different experimental groups. The significance level was set at 5 %. Statistical analyses were performed using the SPSS Statistics 17.0 for Windows (Zar 1996).
Results
Gills
Glycogen
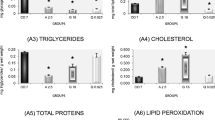
All herbicides, at different concentrations, were associated with significant depletion in levels of glycogen in gill. Animals exposed to atrazine [\({\bar{\text{x}}}\) all concentrations = 28 mg glycogen/g ww (ww)], glyphosate (\({\bar{\text{x}}}\) all concentrations = 27 mg glycogen/g ww), and quinclorac (\({\bar{\text{x}}}\) all concentrations = 24 mg glycogen/g ww) showed a decrease of 97–98 % compared with the 7- (\({\bar{\text{x}}}\) = 1.513 mg glycogen/g ww) and 14-day control groups (\({\bar{\text{x}}}\) = 998 mg glycogen/g ww). The 14-day control group also exhibited a decrease of approximately 34 % in gill glycogen levels in relation to the 7-day control group (Fig. 1a).
(a–f) Levels of glycogen, total lipids, triglycerides, cholesterol, total proteins, and lipid peroxidation in gills of tadpoles of L. catesbeianus exposed to different concentrations of the herbicides atrazine, glyphosate and quinclorac (Cont Controls, A Atrazine, G glyphosate, Q quinclorac). Results are expressed as mean (±) SE. The number 1 under the error bar represents a significant difference compared with the 7-day control group, and the number 2 represents this difference with respect to the 14-day control group (p < 0.05)
Total Lipids
Total lipid levels were decreased after exposure to all concentrations of atrazine (\({\bar{\text{x}}}\) all concentrations = 0.133 mg total lipids/g ww). The decrease was 44 % in relation to the 7-day (\({\bar{\text{x}}}\) = 0.239 mg total lipids/g ww) and 36 % in relation to the 14-day control group (\({\bar{\text{x}}}\) = 0.209 mg total lipids/g ww). Only the highest concentration of glyphosate (\({\bar{\text{x}}}\) = 0.187 mg total lipids/g ww) was associated with a decrease in lipid levels, a 22 and 10 % decrease in relation to the 7-day and 14-day control groups, respectively. Quinclorac (\({\bar{\text{x}}}\) all concentrations = 0.164 mg total lipids/g ww) was associated with a 31 % decrease in lipid levels in gill tissue, at all concentrations of the herbicide, in relation to the 7-day control group. Only the highest concentration (\({\bar{\text{x}}}\) = 0.132 mg total lipids/g ww) was associated with a decrease (of 37 %) in lipid levels compared with the 14-day control group. A 12 % decrease was observed between the 7- and 14-day control groups (Fig. 1b).
Triglycerides
Only the lowest concentrations of atrazine (\({\bar{\text{x}}}\) all concentrations = 0.083 mg triglycerides/g ww) were associated with a decrease in triglyceride levels. Compared with the 7-day control group (\({\bar{\text{x}}}\) = 0.113 mg triglycerides/g ww), this was a 26 % decrease, and it was 34 % in relation to the 14-day control group (\({\bar{\text{x}}}\) = 0.125 mg triglycerides/g ww). Glyphosate (\({\bar{\text{x}}}\) all concentrations = 0.162 mg triglycerides/g ww) was associated with a 43 % increase in triglyceride levels in gill for the lowest concentrations of the herbicide compared with the 7-day control group and a 30 % increase in relation to the 14-day control group. Quinclorac (\({\bar{\text{x}}}\) = 0.090 mg triglycerides/g ww) produced a 28 % decrease in triglyceride levels, but only at the highest concentration of herbicide and compared with the 14-day control group. There was no significant difference between the control groups (Fig. 1c).
Cholesterol
There was no significant change in cholesterol levels in gills of animals exposed to atrazine (\({\bar{\text{x}}}\) all concentrations = 0.088 mg cholesterol/g ww), glyphosate (\({\bar{\text{x}}}\) all concentrations = 0.147 mg cholesterol/g ww), or quinclorac (\({\bar{\text{x}}}\) all concentrations = 0.053 mg cholesterol/g ww) in relation to the 7-day (\({\bar{\text{x}}}\) = 0.217 mg cholesterol/g ww) or 14-day control group (\({\bar{\text{x}}}\) = 0.221 mg cholesterol/g ww) (Fig. 1d).
Total Proteins
There was no significant changes in total protein levels in gills of animals exposed to atrazine (\({\bar{\text{x}}}\) all concentrations = 0.156 mg protein/g ww); for the greater concentrations (72 and 144 μg/L) of the herbicide glyphosate (\({\bar{\text{x}}}\) = 0.054 and 0.061 mg protein/g ww, respectively) and for all concentrations of quinclorac (\({\bar{\text{x}}}\) all concentrations = 0.068 mg protein/g ww), these levels decreased 19, 22, and 24 %,, in relation to the 7-day group (\({\bar{\text{x}}}\) = 0.278 mg protein/g ww) and the 14-day control groups (\({\bar{\text{x}}}\) = 0.281 mg protein/g ww) (Fig. 1e).
Lipid Peroxidation
Exposure to atrazine led to an average increase of 183 % in lipid peroxidation levels (\({\bar{\text{x}}}\) all concentrations = 51 nmol TBARS/mg protein) compared with the 7-day control group (\({\bar{\text{x}}}\) = 18 nmol TBARS/mg protein) and a 219 % increase in relation to the 14-day control group (\({\bar{\text{x}}}\) = 16 nmol TBARS/mg protein). In the glyphosate group, lipid peroxidation (\({\bar{\text{x}}}\) all concentrations = 120 nmol TBARS/mg protein) was 567 % greater in relation to the 7-day control group and 650 % greater in relation to the 14-day control group. The quinclorac group (\({\bar{\text{x}}}\) all concentrations = 190 nmol TBARS/mg protein) appeared to be most impaired in terms of increased lipid peroxidation: Levels were 955 % greater in relation to the 7-day control group and 1,087 % greater compared with the 14-day control group. There was no significant difference in lipid peroxidation levels between the two control groups (Fig. 1f).
Liver
Glycogen
As in gills, exposure to all herbicides at all concentrations was associated with a decrease in glycogen levels in the liver. With atrazine (\({\bar{\text{x}}}\) all concentrations = 113 mg glycogen/g ww), this was a 95 % decrease compared with the 7-day control group (\({\bar{\text{x}}}\) = 2.145 mg glycogen/g ww) and a 91 % decrease in relation to the 14-day control group (\({\bar{\text{x}}}\) = 1.250 mg glycogen/g ww). Glyphosate (\({\bar{\text{x}}}\) all concentrations = 96 mg glycogen/g ww) was associated with a 96 % decrease in relation to the 7-day control group and a 92 % decrease in relation to the 14-day control group. Quinclorac (\({\bar{\text{x}}}\) all concentrations = 56 mg glycogen/g ww) was associated with the most marked decrease in glycogen levels: a 97 % decrease in relation to the 7-day control group and a 95 % decrease compared with the 14-day control group. Furthermore, there was a 41 % decrease in glycogen levels in the 14-day compared with the 7-day control group (Fig. 2g).
(g–l) Levels of glycogen, total lipids, triglycerides, cholesterol, total proteins, and lipid peroxidation in liver of L. catesbeianus tadpoles exposed to different concentrations of herbicides atrazine, glyphosate, and quinclorac (Cont Controls, A Atrazine, G glyphosate, Q quinclorac). Results are expressed as the mean (±) SE. The number 1 under the error bar represents a significant difference compared with the control group at 7 days, and the number 2 represents this difference with respect to the control at 14 days (p < 0.05)
Total Lipids
Atrazine (\({\bar{\text{x}}}\) all concentrations = 0.218 mg total lipids/g ww) produced an 89 % decrease in total lipids in relation to the 7-day control group (\({\bar{\text{x}}}\) = 1.930 mg total lipids/g ww) and a 91 % decrease in relation to the 14-day control group (\({\bar{\text{x}}}\) = 2.387 mg total lipids/g ww). Glyphosate (\({\bar{\text{x}}}\) all concentrations = 0.260 mg total lipids/g ww) and quinclorac (\({\bar{\text{x}}}\) all concentrations = 0.271 mg total lipids/g ww) induced an 86 % decrease in relation to the 7-day control group. Glyphosate exposure induced an 89 % decrease, and quinclorac exposure and 87 % decrease, in total lipid levels in liver compared with levels found in the 14-day control group. There was no significant difference between the 7-dayand 14-day control groups (Fig. 2h).
Triglycerides
All concentrations of atrazine (\({\bar{\text{x}}}\) all concentrations = 0.035 mg triglycerides/g ww) and glyphosate (\({\bar{\text{x}}}\) all concentrations = 0.034 mg triglycerides/g ww) were associated with a 92 % decrease in liver triglyceride levels in relation to the 7-day control group (\({\bar{\text{x}}}\) = 0.417 mg triglycerides/g ww) and a 94 % decrease in relation to the 14-day control group (\({\bar{\text{x}}}\) = 0.618 mg triglycerides/g ww). Quinclorac (\({\bar{\text{x}}}\) = 0.029 mg triglycerides/g ww) was associated with a 93 % decrease compared with the 7-day control group and a 95 % decrease in relation to the 14-day control group. There were no significant differences between the control groups (Fig. 2i).
Cholesterol
Unlike in gills, cholesterol levels in the liver decreased after exposure to herbicides. Atrazine (\({\bar{\text{x}}}\) all concentrations = 0.083 mg cholesterol/g ww) produced an 81 % decrease in liver cholesterol levels compared with levels found in the 7-day control group (\({\bar{\text{x}}}\) = 0.433 mg cholesterol/g ww) and an 88 % decrease compared with levels found in the 14-day control group (\({\bar{\text{x}}}\) = 0.676 mg cholesterol/g ww). Glyphosate (\({\bar{\text{x}}}\) all concentrations = 0.108 mg cholesterol/g ww) produced 75 and 84 % decreases in relation to the 7-day and 14-day control, respectively. Exposure to quinclorac (\({\bar{\text{x}}}\) all concentrations = 0.098 mg cholesterol/g ww) led to a 77 % decrease in liver cholesterol levels in relation to the 7-day control group and an 85 % decrease in relation to the 14-day control group. There was no significant difference between the control groups (Fig. 2j).
Total Proteins
Again, unlike in gills, total protein levels in the liver decreased in response to herbicide exposure. Atrazine exposure (\({\bar{\text{x}}}\) all concentrations = 0.128 mg protein/g ww) produced a decrease of 59 and 52 % in relation to the 7-day (\({\bar{\text{x}}}\) = 0.313 mg protein/g ww) and 14-day control s (\({\bar{\text{x}}}\) = 0.269 mg protein/g ww), respectively. In the glyphosate group (\({\bar{\text{x}}}\) all concentrations = 0.104 mg protein/g ww), the decrease was 67 % in relation to 7-day controls and 61 % in relation to 14-day controls. In the quinclorac group (\({\bar{\text{x}}}\) all concentrations = 0.077 mg protein/g ww), the decrease was 75 % in relation to the 7-day control group and 71 % in relation to the 14-day control group. There was no significant difference in protein levels between the control groups (Fig. 2k).
Lipid Peroxidation
In liver tissue, lipid peroxidation increased after exposure to herbicides. In atrazine-exposed animals (\({\bar{\text{x}}}\) all concentrations = 88 nmol TBARS/mg protein), levels showed an average increase of 418 % in relation to the 7-day control group (\({\bar{\text{x}}}\) = 17 nmol TBARS/mg protein) and 487 % in relation to the 14-day control group (\({\bar{\text{x}}}\) = 15 nmol TBARS/mg protein). In glyphosate-exposed animals (\({\bar{\text{x}}}\) all concentrations = 114 nmol TBARS/mg protein), levels increased 571 % in relation to the 7-day control group and 660 % in relation to the 14-day control group. In the quinclorac group (\({\bar{\text{x}}}\) all concentrations = 248 nmol TBARS/mg protein), levels increased 1,359 % compared with the 7-day control group and 1,553 % in relation to the 14-day control group. The highest concentration of quinclorac was associated with the highest increase in lipid peroxidation levels. There was no significant difference between the control groups (Fig. 2l).
Muscle
Glycogen
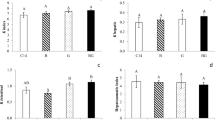
All herbicides were associated with significant glycogen depletion in muscle of exposed animals. In the atrazine group (\({\bar{\text{x}}}\) all concentrations = 10 mg glycogen/g ww), muscle glycogen levels showed a 97 % decrease in relation to the 7-day control group (\({\bar{\text{x}}}\) = 347 mg glycogen/g ww) and a 96 % decrease in relation to the 14-day control group (\({\bar{\text{x}}}\) = 271 mg glycogen/g ww). In glyphosate-exposed animals (\({\bar{\text{x}}}\) all concentrations = 15 mg glycogen/g ww), the decrease was 96 % in relation to the 7-day control group and 94 % in relation to the 14-day control group, and in quinclorac-exposed animals (\({\bar{\text{x}}}\) all concentrations = 08 mg glycogen/g ww), there were 98 and 97 % decreases in relation to the 7- and 14-day control groups, respectively. The 14-day control group also showed a 22 % decrease in glycogen levels in relation to the 7-day control group (Fig. 3m).
(m–r) Levels of glycogen, total lipids, triglycerides, cholesterol, total proteins, and lipid peroxidation in the muscle of Lithobates catesbeianus tadpoles exposed to different concentrations of herbicides atrazine, glyphosate and quinclorac (Cont Controls, A Atrazine, G glyphosate, Q quinclorac). Results are expressed as the mean (±) SE. The number 1 under the error bar represents a significant difference compared with the control group at 7 days, and the number 2 represents this difference with respect to the control at 14 days (p < 0.05)
Total Lipids
Atrazine-exposed animals (\({\bar{\text{x}}}\) all concentrations = 0.052 mg total lipids/g ww) exhibited an 87 % decrease in total lipid levels in relation to those in the 7-day control group (\({\bar{\text{x}}}\) = 0.387 mg total lipids/ g of ww) and an 83 % decrease in relation to the 14-day control group (\({\bar{\text{x}}}\) = 0.310 mg total lipids/g ww). Glyphosate (\({\bar{\text{x}}}\) all concentrations = 0.075 mg total lipids/g ww) was associated with 81 and 76 % decreases in relation to the 7- and 14-day control groups, respectively. In quinclorac-exposed animals (\({\bar{\text{x}}}\) all concentrations = 0.062 mg total lipids/g ww), there was an 84 % decrease in relation to the 7-day control group and an 80 % decrease in relation to the 14-day control group. Furthermore, the 14-day control group showed a 20 % decrease in total lipid levels in relation to the 7-day control group (Fig. 3n).
Triglycerides
Herbicides produced a decrease in triglyceride levels only in relation to the 7-day control group (\({\bar{\text{x}}}\) = 0.140 mg triglycerides/g ww). In atrazine-exposed animals (\({\bar{\text{x}}}\) all concentrations = 0.032 mg triglycerides/g ww), this decrease corresponded to 77 %, in the glyphosate group (\({\bar{\text{x}}}\) all concentrations = 0.048 mg triglycerides/g ww) to 66 %, and in the quinclorac group (\({\bar{\text{x}}}\) = 0.039 mg triglycerides/g ww) to 72 %. There was no significant difference between the 7- and 14-day control groups (Fig. 3o).
Cholesterol
All herbicides produced a decrease in cholesterol levels in muscle tissue. Atrazine (\({\bar{\text{x}}}\) all concentrations = 0.037 mg cholesterol/g ww) induced an 81 % decrease in relation to the 7-day control group (\({\bar{\text{x}}}\) = 0.197 mg cholesterol/g ww) and a 76 % decrease in relation to the 14-day control group (\({\bar{\text{x}}}\) = 0.153 mg cholesterol/g ww). In glyphosate-exposed animals (\({\bar{\text{x}}}\) all concentrations = 0.048 mg cholesterol/g ww), the decrease was 76 % in relation to the 7-day control group and 69 % in relation to the 14-day control group. In the quinclorac group (\({\bar{\text{x}}}\) all concentrations = 0.041 mg cholesterol/g ww), there was a 79 % decrease in relation to the 7-day control group and a 73 % decrease in relation to the 14-day control group. There was no significant difference between the control groups (Fig. 3p).
Total Proteins
The total protein content of muscle tissue in animals exposed to atrazine (\({\bar{\text{x}}}\) all concentrations = 0.191 mg protein/g ww) was 53 % lower in relation to the 7-day control group (\({\bar{\text{x}}}\) = 0.411 mg protein/g ww) and 58 % lower in relation to the 14-day control group (\({\bar{\text{x}}}\) = 0.451 mg protein/g ww). With glyphosate (\({\bar{\text{x}}}\) all concentrations = 0.115 mg protein/g ww), the decrease was 72 % compared with the 7-day control group and 74 % in relation to the 14-day control group. With quinclorac (\({\bar{\text{x}}}\) all concentrations = 0.128 mg protein/g ww), the decrease was 69 and 72 % in relation to the 7- and 14-day control groups, respectively. The 14-day control group showed a 10 % increase in muscle protein levels in relation to the 7-day control group (Fig. 3q).
Lipid Peroxidation
All herbicides were associated with a significant increase in lipid peroxidation in muscle tissue. In atrazine-exposed animals (\({\bar{\text{x}}}\) all concentrations = 66 nmol TBARS/mg protein), levels showed a 214 % increase in relation to the 7-day control group (\({\bar{\text{x}}}\) = 21 nmol TBARS/mg protein) and a 500 % increase in relation to the 14-day control group (\({\bar{\text{x}}}\) = 11 nmol TBARS/mg protein). In the glyphosate group (\({\bar{\text{x}}}\) all concentrations = 129 nmol TBARS/mg protein), levels increased 514 % in relation to the 7-day control group and 1,072 % in relation to the 14-day control group. As in other tissues, quinclorac (\({\bar{\text{x}}}\) all concentrations = 148 nmol TBARS/mg protein) appears to have caused the most marked lipid peroxidation response with exposed animals showing a 604 % increase in levels in relation to 7-day controls and a 1,245 % increase in relation to 14-day controls. There was no significant difference in lipid peroxidation levels between the two control groups (Fig. 3r).
Weight, Size, and Survival
All animals gained weight and increased in size during the experiment period. The weight gain observed in control groups 7 and 14 days was 22 and 18 %, respectively. In the atrazine group, the average weight gain was 14 %, in the glyphosate group was 33 %, and in the quinclorac group was 20 % (Fig. 4s). The increase in size was 2 and 10 % in the 7- and 14-day control groups, respectively. In the atrazine group, the average size increase was 20 %, in the glyphosate group was 17 %, and in the quinclorac group was 13 % (Fig. 4t).
(s–u) Weight gain during the experiment compared with the initial weight of the groups (the value on top of the bar indicates the percentage of weight gain), size at the beginning and end of the experiment (the value on top of the bar indicates the percentage of increase size during the experiment), and survival of tadpoles of L. catesbeianus until the end of the experiment (Cont controls, A atrazine, G glyphosate, Q quinclorac)
Survival in the 7-day and 14-day control groups was 96 and 83 %, respectively. In the atrazine group, average survival was 91 %, in the glyphosate group was 93 %, and in the quinclorac group was 100 %, i.e., there was no mortality in quinclorac-exposed animals (Fig. 4u).
There was no significant variation in pH or in dissolved oxygen in water during the experiment period. Before the introduction of pesticides, aquarium water was approximately 7.2 in the control, atrazine, glyphosate, and quinclorac groups; after exposure, pH was approximately 7.3 in the glyphosate group and 7.4 in the control, atrazine, and quinclorac groups. Before introduction of the herbicides, the level of dissolved oxygen in water was 6.9 mg/L in the control and quinclorac aquariums, 6.8 mg/L in the atrazine aquariums, and 6.6 mg/L in the glyphosate aquariums. After addition of the herbicides to the water, the value of dissolved oxygen was 7.0, 7.3, 6.2 mg/L, and 6.6 mg/L in the control, atrazine, glyphosate, and quinclorac groups, respectively.
Discussion
According to Alkahen (1996), Ganeshwade (2012), and Salbego et al. (2010), nontarget aquatic animals exposed to pesticides can bioaccumulate these toxic compounds in different tissues, which leads to damage, biochemical changes, and increased energy expenditure in an attempt to detoxify the agrochemical. We found similar response patterns in this study despite the use of very low concentrations of all tested herbicides.
The decreases in glycogen levels in gills, liver, and muscle as well as the decrease in total lipid levels in gills and muscle in the 14-day control group compared with the 7-day control group may in fact be due to the availability of food. Unlike the patterns found in a frog farm, where animals are housed in large tanks and fed ad libitum, all food was controlled in this experiment, thus leading to greater competition between individuals due to limited aquarium space and, consequently, lower overall food intake.
In all tissues (gills, liver, and muscle), glycogen levels were severely depleted after exposure to all herbicides and at all concentrations. These decreases accounted to a depletion of >90 % of stores of this polysaccharide. A similar response in glycogen levels in tadpoles has been described by Ezemonye and Ilechie (2007) and Ezemonye and Tongo (2009) after exposure to the pesticides atrazine and basudin.
Glycogen plays an essential role in energy balance because it provides a reserve of internal energy (Moyes and Schulte 2010). Stressful situations during which homeostasis is threatened or disturbed, such as pesticide exposure, lead to induction of physiological responses (Barton and Iwama 1991). Response to this stress consists of a rapid depletion of glycogen levels to meet increased energy demands and assist the metabolic processes involved in the detoxification of pollutants (Alkahen 1996). Furthermore, glycogen can also be used for the of glycoproteins and glycolipids, which are essential constituents of the cell and its membranes (Vutukuru 2005).
The cost of resistance to a toxic compound entails greater energy demand to repair the adverse effects caused by stress. To meet this increased energy demand, glycogen can be rapidly catabolized due to its easy availability to produce energy, thus producing massive depletion of tissue glycogen reserves (Becker et al. 2009; Chang et al. 2006; Salbego et al. 2010; Vutukuru 2005).
In the gills, lipid levels decreased at all concentration of atrazine and quinclorac, but only at the highest concentration of glyphosate. In liver and muscle, total lipid levels decreased after exposure to all herbicides, more markedly in these tissues than in the gills.
Lipids are the body’s main source of energy. Lipids can act as a substrate for the production of energy when extra fuel is required; they are released to meet the increased demand for energy induced by the stressor. Therefore, lipids play a vital role during biochemical adaptations of animals faced with stress conditions. Cells can oxidize fatty acids to increase energy production in response to increased demand, and lipids may be diverted to other metabolic functions essential to the survival of animals faced with a stressful situation (Champe and Harvey 2006; Gijare et al. 2011; Moyes and Schulte 2010).
The decrease in total lipid levels observed in this study, particularly in liver and muscle, in tadpoles exposed to different concentrations of herbicides may also be related to the use of lipids in cell repair and in the organization of these tissues for the formation of lipoproteins, which are an important constituent of cell membranes and organelles.
According to Zaya et al. (2011), exposure to herbicides such as atrazine can increase energy demand and decrease lipid stores, thus altering metabolism and/or lipid mobilization in aquatic animals. This disruption of the lipid energy balance in tadpoles may be due to the massive energy required for detoxification and excretion of the herbicide. A similar decrease in lipid levels was also observed by Gurushankara et al. (2007) and Honrubia et al. (1993) in adult frogs and in tadpoles after exposure to pesticides.
In our experiment, triglyceride levels decreased in the gills of animals exposed to lower concentrations of atrazine and to the highest concentration of quinclorac. In glyphosate-exposed animals, however, these levels increased at lower concentrations. In liver and muscle tissue, triglyceride levels were decreased after exposure to all three herbicides in all concentrations.
The response pattern of lipid and triglyceride levels differed between the tissues studied in the present experiment. Gills exhibited low-intensity depletion of these energy reserves, probably to preserve their osmoregulatory and respiratory capacity.
Triglycerides constitute the main way of storing lipids, which play a vital role as energy reserves in animals (Moyes and Schulte 2010). Exposure to toxic substances, such as pesticides, can cause harmful effects on living organisms and induce oxidation and alterations of the chemical function of triglycerides (El-Banna et al. 2009). In situations of increased energy demand, animals can obtain energy through lipids stored as triglycerides (Landys et al. 2005), and tadpoles seem to use lipids, especially stored triglycerides, as an endogenous energy source (Sawant and Varute 1973).
The decrease in total lipid levels exhibited by Rana perezi tadpoles in response to pesticide exposure might affect triglyceride synthesis and may explain the decrease of these levels in animals exposed to atrazine and quinclorac (Honrubia et al. 1993). A similar strategy was probably verified in this study for the same pesticides, including glyphosate, in liver and muscle tissue of L. catesbeianus tadpoles.
Yet another pathway of response to the stress of pesticide exposure involved increases in triglycerides levels, which may be due to an increase in food intake (Aldana-Madrid et al. 2012; Montgomery et al. 2008; Moyes and Schulte 2010). Animals exposed to glyphosate showed this pattern in gill tissue and by increasing their weight more than other groups, including the control groups (Figs. 1c, 4s).
The greater levels of triglycerides in gills and decreased levels of triglycerides in other tissues in glyphosate-exposed animals may be attributable to decreased oxygen consumption in an attempt to maintain optimal gill function, thus reallocating energy to this vital organ of survival in aquatic animals as a response to herbicide toxicity (Chang et al. 2006). Pãunescu and Ponepal (2011) reported increased triglyceride levels in adult frogs exposed to glyphosate.
In our experiment, only in gills were cholesterol levels unchanged after herbicide exposure. In liver and muscles, cholesterol levels decreased compared with those of control animals. This pattern of change in cholesterol and triglyceride levels appears to reflect an attempt at preservation of gill function, thus ensuring the survival of animals due through a physiological adaptation to agrochemical exposure.
According to Champe and Harvey (2006) and Trabalon and Blais (2012), cholesterol plays an important role as a constituent of cell membranes, and its levels typically decrease after exposure to pesticides (Aldana-Madrid et al. 2012). This decrease in cholesterol levels may be due to an inhibition of cholesterol biosynthesis or to the use of fatty deposits as a source of energy in response to increased demands due to the stress caused by agrochemical exposure (Ganeshwade 2012). A decrease in cholesterol levels also was observed by Shakoori et al. (1996) in fish exposed to pesticides. According to the investigator, this decrease in cholesterol levels may be due to use of fatty acid reserves for energy purposes.
The concentrations of total proteins in the gills were not significantly altered by exposure to atrazine and for the lowest concentrations of glyphosate. Conversely, in gills at the greatest concentrations of glyphosate and for all concentrations of quinclorac, and in liver and muscle at all three concentrations of atrazine, glyphosate, and quinclorac, a decrease was evident after exposure to these herbicides.
According to Salbego et al. (2010), exposure to herbicides can increase energy expenditure in an attempt to detoxify these toxic compounds, thus altering metabolism and leading to low protein levels, which may indicate the mobilization of protein to increase its degradation into amino acids to feed the tricarboxylic acid cycle and increase adenosine triphosphate synthesis, thus satisfying the high energy demands of cells exposed to toxic stress (Ganeshwade 2012).
A pattern of decreased protein levels can indicate physiological acclimatization to high energy demands to compensate for the stress caused by pesticide exposure, or it may reflect a mechanism of formation of lipoproteins, which will be used to repair damaged organelles, cells, and tissues (Rambabu and Rao 1994; Ribeiro et al. 2001; Sak et al. 2006). Khan et al. (2003) and Sounderraj et al. (2011) also reported decreased protein levels in adult frogs after exposure to pesticides.
Although lipid peroxidation levels increased in response to exposure to all herbicides, at all concentrations and in all tissues analyzed, quinclorac appears to be the most damaging of the three herbicides.
Lipid peroxidation, or oxidation of the lipid layer of the cell membrane, is one of the main mechanisms of cell injury due to high concentration of polyunsaturated fatty acids in cells (El-Banna et al. 2009). The oxidative destruction of lipids by means of lipid peroxidation appears to be an inevitable process in tissue injury (Al-Othman et al. 2011).
Animals exposed to pesticides can exhibit increased levels of lipid peroxidation in tissues, which caning turn lead to chemical damage and cell death (Al-Othman et al. 2011; Champe and Harvey 2006; Uchendu et al. 2012). According to Patil et al. (2009), pesticides have been shown to initiate and increase lipid peroxidation in biological membranes in experimental animals exposed to these chemicals, thus leading to changes in the function, structure, and fluidity of cell membranes.
Some investigators have reported increased lipid peroxidation in response to herbicide exposure. Modesto and Martinez (2010) and Lajmanovich et al. (2010) reported that exposure to glyphosate may increase lipid peroxidation in tadpoles; Kadry et al. (2012) reported increased lipid peroxidation in fish exposed to atrazine; and Menezes et al. (2012) noted that quinclorac can increase levels of lipid peroxidation in fish exposed to this pesticide.
In this study, it was found that although the levels of lipid peroxidation had strongly increased due to herbicide exposure, there was not a decrease in survival of these animals. This fact can be explained possibly by increased activity of antioxidant defenses and inhibition to the effects caused by increased lipid peroxidation, thus leading consequently to greater chances of survival of these individuals (Barreiros et al. 2006; Blokhina et al. 2003). However, there have been few studies on increased lipid peroxidation in animals exposed to the herbicides tested herein, particularly quinclorac, which induced the most damage to lipid membranes in relation to the other two herbicides.
Although the present work was performed using concentrations of commercial formulations of atrazine, glyphosate, and quinclorac commonly found in natural water bodies, and these concentrations did not cause high mortality among exposed tadpoles, we found that all three herbicides can alter biochemical parameters and induce lipid peroxidation in these animals. The same pattern of response to exposure, i.e., an intense decrease in levels of all metabolites (glycogen, total lipids, triglycerides, cholesterol, and total proteins) in liver and muscle, was observed with all three herbicides, although only glycogen and total lipid levels decreased in gills, with changes in the other metabolites (triglycerides, cholesterol and total proteins) varying depending on the herbicide.
Such changes in the metabolism of tadpoles exposed to these pesticides may be linked to unsuccessful development, metamorphosis, and reproductive patterns in amphibians. On the basis of these results, we suggest that increased use of agrochemicals may be an important factor in the decrease of amphibian diversity and abundance worldwide. Nevertheless, it bears stressing that this was the pattern of response exhibited by the species L. catesbeianus under laboratory conditions. In the field, or in other species, response patterns may be distinct.
References
Aldana-Madrid ML, Shibayama N, Calderon M, Silva A, Silveira-Gramont MI, Tsutsumi V et al (2012) Hepatic effects from subacute exposure to insecticides in adult male Wistar rats. In: Perven F (ed) Insecticides–advances in integrated pest management. Intech, Rijeka, pp 279–290
Alkahen HF (1996) Effects of lethal and sublethal concentrations of lindane on the behavior and energy reserves of the freshwater fish, Oreochromis niloticus. J King Saud Univ 8:153–164
Al-Othman AM, Khaled SA, Gaber EE, Kareem Y, Zeid AA, Mourad AM et al (2011) Protection of α-tocopherol and selenium against acute effects of malathion on liver and kidney of rats. Afr J Pharm Pharmacol 5:1263–1271
Barreiros ALBS, David JM, David JM, David JP (2006) Estresse oxidativo: Relação entre geração de espécies reativas e defesa do organismo. Quím Nova 29:113–123
Barton BA, Iwama GK (1991) Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu Rev Fish Dis 10:3–26
Becker AG, Moraes BS, Menezes CC, Loro VL, Santos DR, Reichert JM et al (2009) Pesticide contamination of water alters the metabolism of juvenile silvercatfish, Rhamdia quelen. Ecotoxicol Environ Saf 72:1734–1739
Berti AP, Düsman E, Soares LC, Grassi LEA (2009) Efeitos da contaminação do ambiente aquático por óleos e agrotóxicos. Sabios Rev Saúde Biol 4:45–51
Blaustein AR, Johnson PTJ (2003) The complexity of deformed amphibians. Front Ecol Environ 1:87–94
Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–194
Buege JA, Aust SD (1978) Microsomal lipids peroxidation. Methods Enzymol 52:302–310
Carneiro FF, Pignati W, Rigotto RM, Augusto LGS, Rizollo A, Muller NM et al (2012) Dossiê ABRASCO–Um alerta sobre os impactos dos agrotóxicos na saúde. ABRASCO, Rio de Janeiro
Champe PC, Harvey RA (2006) Metabolismo dos lipídeos complexos e colesterol e metabolismo dos esteróides. In: Champe PC, Harvey RA (eds) Bioquímica ilustrada, 2nd edn. Artes Médicas, Porto Alegre, pp 199–242
Chang CC, Lee PP, Hsu JP, Yeh SP, Cheng W (2006) Survival, and biochemical, physiological, and histopathological responses of the giant freshwater prawn, Macrobrachium rosenbergii, to short-term trichlorfon exposure. Aquaculture 253:653–666
Dörfler U, Feicht EA, Scheunert IS (1997) Triazine residues in groundwater. Chemosphere 35:99–106
El-Banna SG, Attia AM, Hafez AA, El-Kazaz SA (2009) Effect of garlic consumption on blood lipid and oxidant/antioxidant parameters in rat males exposed to chlorpyrifos. Slovak J Anim Sci 42:111–117
Ezemonye L, Ilechie I (2007) Acute and chronic effects of organophosphate pesticides (basudin) to amphibian tadpoles (Ptychadena bibroni). Afr J Biotech 6:1554–1568
Ezemonye L, Tongo I (2009) Lethal and sublethal effects of atrazine to amphibian larvae. Jordan J Biol Sci 2:29–36
Fernandes Neto ML, Sarcinelli PN (2009) Agrotóxicos em água para consumo humano: Uma abordagem de avaliação de risco e contribuição ao processo de atualização da legislação brasileira. Eng Sanit Ambient 14(1):69–78
Folch J, Lees M, Sloane-Stanley GHA (1957) Simple method for isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Frings CE, Dunn RA (1970) Colorimetric method for determination of total serum lipids based on the sulfophosphovanillin reaction. Am J Clin Pathol 53:89–91
Galon L, Concenço G, Ferreira EA, Silva AF, Ferreira FA, Noldin JA, Freitas MAM (2009) Competição entre plantas de arroz e biótipos de capim-arroz (Echinochloa spp.) resistente e suscetível ao quinclorac. Planta Daninha 27:701–709
Ganeshwade RM (2012) Biochemical changes induced by dimethoate (Rogor 30 % EC) in the gills of freshwater fish Puntius ticto (Hamilton). J Ecol Nat Environ 4:181–185
Giesy JP, Dobson S, Solomon KR (2000) Ecotoxicological risk assessment for roundup herbicide. Rev Contam Toxicol 167:35–120
Gijare SS, Raja IA, Tanatarpale VT, Kulkarni KM (2011) Lipid changes in the freshwater fish Ophiocephalus punctatus exposed to synthetic pyrethroid cypermethrin. Biosci Biotech Res Commun 4:52–54
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Gurushankara HP, Meenakumari D, Krishnamurthy SV, Vasudev V (2007) Impact of malathion stress on lipid metabolism in Limnonectus limnocharis. Pest Biochem Physiol 88:50–56
Honrubia MP, Herraez MP, Alvarez R (1993) The carbamate insecticide zz-aphox(r) induced structural-changes of gills, liver, gallbladder, heart, and notochord of Rana perezi tadpoles. Arch Environ Contam Toxicol 25:184–191
Howe CM, Berrill M, Pauli BD, Helbring CC, Werry K, Veldhoen N (2004) Toxicity of glyphosate-based pesticides to four North American frog species. Environ Toxicol Chem 23:1928–1938
International Grains Council (2012) Grain market report (no. 427). ICG, London, UK
Kadry SM, Marzouk MS, Amer AF, Hanna MI, Azmy AH, Hamed HS (2012) Vitamin E as antioxidant in female African catfish (Clarias gariepinus) exposed to chronic toxicity of atrazine. Egypt J Aquat Biol Fish 16:83–98
Khan MZ, Tabassum R, Naqvi SNH, Shah EZ, Tabassum F, Ahmad I et al (2003) Effect of cypermethrin and permethrin on cholinesterase activity and protein contents in Rana tigrina (amphibia). Turk J Zool 27:243–246
Lajmanovich RC, Attademo AM, Peltzer PM, Junges CM, Cabana MC (2010) Toxicity of four herbicide formulations with glyphosate on Rhinella arenarum (Anura: Bufonidae) tadpoles: b-esterases and glutathione-s-transferase inhibitors. Arch Environ Contam Toxicol 60:681–689
Lambropoulou DA, Sakkas VA, Hela GD, Albanis TA (2002) Application of solid-phase microextraction in the monitoring of priority pesticides in the Kalamas river (N.W. Greece). J Chromatogr 963:107–116
Landis WG, Yu MH (2003) Introduction to environmental toxicology: impacts of chemicals on ecological systems, 3rd edn. CRC Press, Boca Raton
Landys MM, Piersma T, Guglielmo CG, Jukema J, Ramenofsky M, Wingfield JC (2005) Metabolic profile of long-distance migratory flight and stopover in a shorebird. Proc Royal Soc B 272:295–302
Marchezan E, Zanella R, Avila LA, Camargo ER, Machado SLO, Macedo VRM (2007) Rice herbicide monitoring in two Brazilian rivers during the rice growing season. Sci Agric 64:131–137
McElroy S (2006) Registration of the new active ingredient, Quinclorac (chemical code 128974), contained in the new pesticide product, Ortho Weed B Gon Max Plus Crabgrass Control (EPA Reg. No. 239–2689)
Menezes CC, Leitemperger J, Santi A, Lópes T, Veiverberg CA, Peixoto S et al (2012) The effects of diphenyl diselenide on oxidative stress biomarkers in Cyprinus carpio exposed to herbicide quinclorac (Facet). Ecotoxicol Environ Safe 81:91–97
Midio AF, Martins DI (1997) Herbicidas em alimentos: Aspectos gerais, toxicológicos e analíticos. Livraria Varela, São Paulo
Modesto KA, Martinez C (2010) Effects of roundup transorb on fish: hematology, antioxidant defenses and acetylcholinesterase activity. Chemosphere 81:781–787
Montgomery MP, Kamel F, Saldana TM, Alavanja MCR, Sandler DP (2008) Incident diabetes and pesticide exposure among licensed pesticide applicators: Agricultural Health Study 1993–2003. Am J Epidemiol 167:1235–1246
Moura MAM, Franco DAS, Matallo MB (2008) Impacto de herbicidas sobre os recursos hídricos. Rev Tecnol Inovação Agropecuária 1:142–151
Moyes CD, Schulte PM (2010) Princípios De fisiologia animal. Artmed, Porto Alegre, pp 526–571
Oliveira Jr RS (2001) Mecanismos de ação de herbicidas. In: Oliveira Jr RS (ed) Plantas daninhas e seu manejo. Maringá, Paraná: Napd–Um
Patil JA, Patil AJ, Sontakke AV, Govindwar SP (2009) Oxidative stress and antioxidants status of occupational pesticides exposed sprayers of grape gardens of western Maharashtra (India). J Environ Health Res 9:81–89
Paulino MG, Sakuragui MM, Fernandes MN (2012) Effects of atrazine on the gill cells and ionic balance in a neotropical fish, Prochilodus lineatus. Chemosphere 86:1–7
Păunescu A, Ponepal CM (2011) Effect of Roundup herbicide on physiological indices in marsh frog Pelophylax ridibundus. Scientific Papers, UASVM Bucharest, Bucharest, pp 269–274
Poleza F, Souza RC, Stramosk CA, Rorig LR, Resgalla C Jr (2008) Avaliação da toxicidade aguda para o organismo-teste Vibrio fischeri dos principais herbicidas e inseticidas aplicados na lavoura de arroz irrigado dos estados de Santa Catarina e Rio Grande Do Sul Pesticidas. Rev Ecotoxicol Meio Ambiente 18:107–114
Rambabu JP, Rao MB (1994) Effect of organochlorine and three organophosphate pesticides on glucose, glycogen, lipid and protein contents in tissues of the freshwater snail Bellamya dissimilis (muk ller). Bull Environ Contam Toxicol 53:142–148
Reimche GB, Machado SLO, Golombieski JI, Baumart JS, Braun N, Marchesan E et al (2008) Persistência na água e influência de herbicidas utilizados na lavoura arrozeira sobre a comunidade zooplanctônica de cladocera, copepoda e rotifera. Ciência Rural Santa Maria 38(1):7–13
Relyea RA (2005) The impact of insecticides and herbicides on the biodiversity and productivity of aquatic communities. Ecol Appl 15:618–627
Ribeiro S, Sousa JP, Nogueira AJA, Soares AMVM (2001) Effect of endosulfan and parathion on energy reserves and physiological parameters of the terrestrial isopod Porcellio dilatatus. Ecotoxicol Environ Saf 49:131–138
Rodrigues NR, Almeida FS (1998) Guia de herbicidas (4th ed). Londrina pp 137–142
Roy S, Hänninen O (1993) Biochemical monitoring of the aquatic environment: Possibilities and limitations. In: Richardson M (ed) Ecotoxicology monitoring. VCH-Verlag, Weinheim, pp 119–135
Sak O, Uçkan F, Ergin E (2006) Effects of cypermethrin on total body weight, glycogen, protein, and lipid contents of Pimpla turionellae (Hymenoptera: Ichneumonidae). Belg J Zool 136:53–58
Salbego J, Pretto A, Gioda CR, Menezes CC, Lazzari R, Neto JR et al (2010) Herbicide formulation with glyphosate affects growth, acetylcholinesterase activity, and metabolic and hematological parameters in piava (Leporinus obtusidens). Arch Environ Contam Toxicol 58:740–745
Sawant VA, Varute AT (1973) Lipid changes in the tadpoles of Rana tigrina during growth and metamorphosis. Comp Biochem Physiol 44:729–750
Shakoori AR, Mughal AL, Iqbal MJ (1996) Effects of sublethal doses of fenvalerate (a synthetic pyrethroid) administered continuously for four weeks on the blood, liver and muscles of a freshwater fish, Ctenopharyngodon idella. Bull Environ Contam Toxicol 57:487–494
Silva MD, Peralba MCR, Mattos MLT (2003) Pesticidas. Rev Ecotoxicol Meio Ambiente 13:19–28
Silva DRO, Avila LA, Agostinetto D, Dal Magro T, Oliveira E, Zanella R et al (2009) Monitoramento de agrotóxicos em águas superficiais de regiões orizícolas no sul do Brasil. Ci Rural 39:2383–2389
Solomon KR, Baker DB, Richards RP, Kenneth RD, Klaine SJ, Lapoint TW et al (1996) Ecological risk assessment of atrazine in North American surface waters. Environ Toxicol Chem 15:31–76
Sounderraj SFL, Sekhar P, Kumar PS, Lesley N (2011) Effect of systemic pesticide phosphamidon on haematological aspects of common frog Rana tigrina. Int J Pharm Biol Arch 2:1776–1780
Spadotto CA (2006) Avaliação de riscos ambientais de agrotóxicos em condições brasileiras. Documentos, Jaguariúna, Embrapa Meio Ambiente, 58
Spadotto CA, Scorza Jr RP, Dores EFGC, Gebler L, Moraes DAC (2010) Fundamentos e aplicações da modelagem ambiental de agrotóxicos. Documentos, Campinas, Embrapa Meio Ambiente, 78
Tomlin C (1994) The pesticide manual–Incorporating the agrochemicals handbook, 10th edn. British Council, Cambridge
Trabalon M, Blais C (2012) Juvenile development, ecdysteroids and hemolymph level of metabolites in the spider Brachypelma albopilosum (Theraphosidae). J Exp Zool 317:236–247
Uchendu C, Ambali SF, Ayo JO (2012) The organophosphate, chlorpyrifos, oxidative stress and the role of some antioxidants: a review. Afr J Agric Res 7:2720–2728
Van Handel E (1965) Estimation of glycogen in small amount soft tissue. Anal Biochem 11:256–265
Vutukuru SS (2005) Acute effects of hexavalent chromium on survival, oxygen consumption, hematological parameters and some biochemical profiles of the Indian major carp, Labeo rohita. Int J Environ Res Public Health 2:456–462
Zar JH (1996) Biostatistical analysis, 3rd edn. Prentice-Hall, London
Zaya RM, Amini Z, Whitaker AS, Kohler SL, Ide CF (2011) Atrazine exposure affects growth, body condition and liver health in Xenopus laevis tadpoles. Aquat Toxicol 104:243–253
Acknowledgments
We thank Dr. Taran Grant, Dr. Nelson Ferreira Fontoura, the team at the PUCRS Laboratory of Conservation Physiology, and CAPES for supporting this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dornelles, M.F., Oliveira, G.T. Effect of Atrazine, Glyphosate and Quinclorac on Biochemical Parameters, Lipid Peroxidation and Survival in Bullfrog Tadpoles (Lithobates catesbeianus). Arch Environ Contam Toxicol 66, 415–429 (2014). https://doi.org/10.1007/s00244-013-9967-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-013-9967-4