Abstract
Estrogens have been widely detected in water and might pose a potential threat to the aquatic ecosystem. However, little information is available about the occurrence, multi-phase fate and potential risks of estrogens in Hanjiang River (HR). In this work, the concentration, multi-phase distribution and risk assessment of eight estrogens were studied by investigating surface water and sediment samples from HR during two seasons. These samples were analyzed using the solid-phase extraction (SPE) and liquid chromatography-mass spectrometry (LC-MS). The concentrations of eight estrogens were 4.5–111 ng/l in surface water and 1.7–113 ng/g dry weight in sediments. 4-nonylphenol (NP) was the predominant estrogen in both water and sediments. The estrogens showed significantly spatial variability, with the highest average concentration in the lower section of HR (p < 0.01, F > 12.21). Meanwhile, NP, 17α-estradiol (αE2), Bisphenol A (BPA) and 4-tert-octyphenol (OP) in surface water exhibited higher concentrations in summer than in winter (p < 0.05, F > 4.62). The sediment-water partition coefficients of estrogens suggested that these compounds partitioned more to particulate phase. Risk assessment indicated that estriol (E3) was the main contributor to the total estradiol equivalent concentration. Moreover, estrogen mixtures could pose high ecological risks to aquatic organisms in surface water. Overall, estrogens are ubiquitous in HR, and their potential ecological risks should not be neglected.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Estrogens, an important class of endocrine disrupting chemicals (EDCs), have potential endocrine disrupting effects on wildlife and human beings (Chen et al. 2018; Saeed et al. 2017; Zhou et al. 2016). Natural estrogens primarily consist of estrone (E1), 17β-estradiol (βE2), 17α-estradiol (αE2), and estriol (E3). They are excreted by humans and livestock (Huang et al. 2013; Nie et al. 2015). Among the synthetic estrogens, 17α-ethinylestradiol (EE2) is a commonly bioactive medication for humans, livestock and aquaculture activity (Song et al. 2018). Bisphenol A (BPA), 4-tert-octyphenol (OP) and 4-nonylphenol (NP) are widely used in industrial goods and commercial products, such as detergents, plastics and thermal paper (Liu et al. 2017a; Zhang et al. 2014a). Owing to their estrogenic potency, some estrogens (e.g. BPA, NP) have been banned or their use strictly regulated (Liu et al. 2017b). However, high levels of these compounds are still found in the environment due to their continuous release (Pojana et al. 2007; Salgueiro-González et al. 2015; Song et al. 2018).
Generally, estrogens in surface waters are mainly derived from the effluents of wastewater treatment plants (WTPs), surface runoff from manure-fertilized fields and direct discharge from livestock and aquaculture farms (Björkblom et al. 2008; Kuster et al. 2004; Wang et al. 2015). Because of their moderate hydrophobic, with octanol-water partition coefficients (logKow) mostly between 3 and 5, estrogens are easily adsorbed into sediments (Lei et al. 2009; Praveena et al. 2016; Wang et al. 2014). These pollutants in sediments would also be released into the water if river flow conditions change (e.g. river flow, volume, water pH, temperature) (Peck et al. 2009; Pignotti and Dinelli 2018; Rivas-Rivera et al. 2014). Additionally, estrogens in sediments might induce negative effects to benthic organisms and even threaten the aquatic ecosystem through food chain (Ke et al. 2015; Tiwari et al. 2016). However, the potential risks in sediments are often neglected due to the complexity of the interaction of water–sediment system (Liu et al. 2017b). Estradiol equivalent (EEQ) and risk quotient (RQ) approaches are valuable in screening the level of risk assessment (Diao et al. 2017). They have been frequently applied to evaluate environmental risks of estrogens to aquatic ecosystems (Yang et al. 2015; Zhang et al. 2014b). To date, many studies about the risk assessment of estrogens have mainly focused on surface water (Laurenson et al. 2014; Liu et al. 2016; Nie et al. 2015). Consequently, it is important to study the behavior of estrogens in sediment-water system and assess their potential ecological risks to aquatic organisms in sediments.
Hanjiang River (HR), located in the central China, is the largest branch of the Yangtze River. It has a watershed covering approximately 159,000 km2. HR is an important surface water source of the South-to-North Water Transfer Project (SNWTP) (Ai et al. 2015). In addition, it is also an important center of aquaculture and agriculture in China (Wang et al. 2013). Recently, intensive agricultural activities, high population density and the water transfer project have significantly influenced the river (Hu et al. 2018). Many micro-organic pollutants, such as organohalogen compounds, perfluorinated compounds and polycyclic aromatic hydrocarbons have been detected in the HR (Sun et al. 2017; Wang et al. 2013). Nevertheless, there are no studies systematically investigating the estrogens in multiple phases from this river. Thus, eight estrogens, including E1, αE2, βE2, E3, EE2, NP, OP, and BPA were selected according to their ubiquitous distribution, potential ecological effects and persistence (Wang et al. 2016; Zhang et al. 2016). The objectives of this work were: (1) to investigate the occurrence and spatial-temporal variation of eight estrogens in HR; (2) to discuss their partition behavior between water and sediment phase; (3) to estimate their individual and combined effects for both water and sediments based on EEQ and RQ methods.
Materials and methods
Sampling
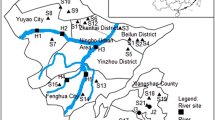
The Danjiangkou Reservoir (DJKR), located in the middle reach of HR, is the key hydraulic project in the HR basin (Fig. 1). It consists of the Dan Reservoir and the Han Reservoir. A total of 13–14 × 1012 m3 of water from the DJKR is transferred to Northern China every year due to the SNWTP. As shown in Fig. 1, DJKR separates the HR into the upper HR and lower HR. Therefore, the studied area was divided into 3 sectors to study spatial distribution of estrogens. About 188 samples were collected from 47 sampling sites in HR and DJKR in the summer (June 2015) and winter (November 2014). Two liters of surface water sample (0–0.5 m) was obtained with amber glass bottle. These bottles were pre-cleaned with methanol and ultrapure water. All water samples were filtered using glass fiber filters (0.45 µm), and then stored at 4 °C. They were analyzed within 24 h after collection. Surface sediment samples (0–10 cm) were collected by a stainless steel grab. Then they were covered with aluminum foil, placed into polytetrafluoroethylene bags and finally kept at −20 °C for further pretreatment. At each site, triplicate water or sediment samples were collected and mixed to obtain one composite sample.
The sampling sites of water and sediment samples collected from HR (a Study area, b Sampling sites in Danjiangkou reservoir, c Sampling sites in HR). Sampling sites in HR include 14 mainstreams (M1–M14) and 14 tributaries (T1–T14). Tributaries are T1 (Ju River), T2 (Bao River), T3 (Lengshui River), T4 (Xushui River), T5 (Xun River), T6 (Yue River), T7 (Du River), T8 (Nan River), T9 (Tangbai River), T10 (Xiao River), T11 (Man River), T12 (Fangjiatai River), T13 (Dongjing River), T14 (Hanbei River). Sites 1–7 belong to the upper section of HR (UHR), while sites 8–14 belong to the lower section of HR (LHR)
Chemical analysis
Eight estrogen compounds (E1, αE2, βE2, E3, EE2, NP, OP, and BPA) and internal standards (βE2-d2 and BPA-d6) were obtained from Sigma-Aldrich (St. Louis, USA). Dichloromethane and methanol used (HPLC grade) were purchased from Thermo Fisher Scientific (Sunnyvale, USA). The physicochemical parameters of target estrogens are described in Table S1.
All water and sediment samples were pretreated following a previously reported method (Wang et al. 2016; Yang et al. 2015). Detail procedures of sample extraction are available in the Supplementary materials (S1).
To detect dissolved organic carbon (DOC), water samples were firstly filtered and acidified to pH 3.0. For sediments, diluted HCl was added to move carbonates. Then DOC and total organic carbon (TOC) determination was performed by a total organic carbon analyzer (Elementar, Germany). The limit of detection of TOC and DOC was 0.01% and 0.02 mg/l, respectively.
Quality assurance and quality control (QA/QC)
All samples were spiked with the surrogates to identify the procedural performance and matrix effects. A matrix spike, a solvent blank and a procedural blank were run for each set of samples (n = 10). No target estrogens were detected in the procedural blanks. The recoveries of the estrogens in all samples were 68–115%. The relative standard deviations (RSD) (n = 3) were 4–12% for water and 6–15% for sediment. The limit of detection (LOD) was calculated at the signal-to-noise (S/N) ratio of 3. It was 0.2–1.7 ng/l for water and 0.3–1.5 ng/g dry weight (dw) for sediment. The estrogen concentrations in all samples were corrected by the surrogate recoveries.
Sediment-water partition coefficient
The distribution of estrogens between sediment and water was assessed qualitatively using the organic carbon normalized partition coefficient (Koc′).
Where Cw is the concentration of the estrogen in water (ng/l); Cs is concentration of the estrogen in sediment (ng/kg); foc is the organic carbon fraction in sediment (%); Kp is the sediment-water partition coefficient (l/kg).
Koc is the partition coefficient at laboratory conditions, and is predicted according to the model reported in previous studies (Gong et al. 2012; Isobe et al. 2001) as follows:
Kow is the octanol-water partition coefficient, which could be obtained from the literature (Ying et al. 2002).
Risk assessment
Estrogenic activity
To assess the mixture effects of estrogens, the estradiol equivalent (EEQ) was used and calculated (Wang et al. 2016) as follows:
Where EEQi is the the estradiol equivalent of compound i; MECi is the measured environmental concentration of compound i; EEFi is the estradiol equivalency factor relative to βE2. In this study, the EEF values of E1, E3, EE2, NP, OP and BPA were shown in Table S1.
The EEQ in sediments was calculated according to the method reported by Jin et al. (2013).
Where Cs, i is the concentration of compound i in the sediment (ng/g); Koc, i calculated on the basis of its Kow according to the Eq. (2); ƒoc is the sediment fraction of organic carbon (%).
Risk quotient (RQ)
According to European Commission Technical Guidance Document (EC 2003), the RQ approach was used to estimate potential ecological risk of estrogens in surface water from HR.
Where PNEC is the predicted no effect concentration; MEC is the estrogen concentration in water. A common risk criteria was used: RQ ≥ 1, high risk; 0.1 ≤ RQ < 1, medium risk; and 0.01 ≤ RQ < 0.1, low risk (Hernando et al. 2006).
In this study, PNEC values of E3 and NP were calculated by assessment factor method using the Eq. (6). Due to sufficient chronic toxicity data, PNEC values of E1, βE2, E3, BPA and OP were obtained by species sensitivity distribution (SSD) method according to the Eq. (7).
Where NOEC is the no observed effect concentration; AF is the assessment factor; 5%SSD is the 5th percentile effect concentration derived from SSD. The NOEC values used in this study were collected from the EPA ECOTOX database. For the same specie, the lowest NOEC value was used when more than one value was available. Therefore, the calculated PNEC value in our study was 4.32, 0.75,15, 0.35, 6, 26.54 and 137.42 ng/l for E1, βE2, E3, EE2, NP, ng/l for OP and BPA, respectively (Table S3 and Fig. S1).
The risk assessment for sediments was performed using the following equation (Zhao et al. 2011).
Based on the concept of concentration addition (CA), the risk quotient for the mixture (MRQ) was calculated as follows:
Where RQi is the risk quotient of compound i.
Statistical procedures
Spatial and seasonal variations were studied using one-way analysis of variance (ANOVA) with Tukey’s post hoc test. The relationships between Kp values of target estrogens and TOC or DOC were conducted by Person’s correlation analysis. All statistical analyses were processed using SPSS 16.0 (SPSS Inc., Illinois). The critical level of significance was set at 0.05. For compound concentration below the LOD, concentrations in samples were reported as not detected (nd). Half of the LOD was substituted as the default value in the statistical analysis.
Results
Concentrations of estrogens in surface water and sediments
The residue levels of eight estrogens in surface water from HR during two seasons are summaried in Table 1. Among the analyzed compounds, NP, OP and E3 were the most frequently measured estrogens, with detection frequencies more than 60% in all seasons. NP was the dominant compound, averagely contributed to 33–35% of the total estrogens. BPA and OP followed, and they were in the similar level range (nd-30 ng/l). However, EE2 was found to be the pollutant with the lowest mean concentration (summer: 0.16 ng/l; winter: 0.14 ng/l). Among four natural estrogens, E3 showed the highest average concentrations (summer: 3.6 ng/l; winter: 2.7 ng/l). αE2 followed, which was measured in more than 50% of sampling sites and the concentration up to 2.8 ng/l.
In sediments, all estrogens were quantified in 54–81% of sampling sites. The concentrations of these compounds were nd-38 ng/g dw in summer and nd-29 ng/g dw in winter (Table 1). Similar to surface water, NP was still the predominant compound in sediments with mean contributions of 25.8–27.2% in two seasons. BPA was the second most abundant estrogen, with the average concentration of 6.1 ng/g dw in summer and 5.3 ng/g dw in summer, followed by OP (summer: 5.8 ng/g dw; winter: 4.4 ng/g dw). Although EE2 has a relatively high Kow (4.15), it was also the lowest detected compound in sediments. Among the natural estrogens, αE2 was found to be dominant, followed by E3, E1 and βE2, with the concentrations of nd-17 ng/g dw, nd-8.6 ng/g dw, nd-3.2 ng/g dw and nd-0.99 ng/g dw, respectively.
Spatial and temporal distribution of estrogens
As present in Fig. 2, there were obvious spatial variations in the total concentrations of eight estrogens for both surface water and sediments (p < 0.01, F > 12.21). They showed an increasing trend in the order: the DJKR < the upper section of HR (UHR) < the lower section of HR (LHR). When estrogens were grouped into two families, significantly highest average concentrations of these estrogens in surface water were still observed in the LHR (p < 0.02, F > 14.10). Likewise, similar spatial trend was also discovered for synthetic estrogens in sediments (p < 0.05, F > 5.80). For natural estrogens, their concentrations among three sectors did not differ significantly (p > 0.05, F < 2.46). However, the highest mean concentration was also found in the LHR. Moreover, relatively higher levels of estrogens were measured at sites T14 and T13 (tributaries) than those at sites M14 and M13 (mainstreams).
Generally, the concentrations of total estrogens in surface water were greater in summer than those in winter (Fig. 2). For individuals, significant seasonal differences were only found for αE2, NP, OP and BPA (p < 0.05, F > 10.37) (Table 1). However, no significant seasonal variations were observed for all estrogens in sediments (p > 0.05, F < 1.53).
K p values of estrogens in HR
The average Kp values of eight estrogens in HR were 610–1951 l/kg in summer, while those were 803–2919 l/kg in winter (Table 2). The logKoc′ values for all estrogens were in the range of 4.62–5.21. The corresponding predicted logKoc values were 2.44–3.81. In the present study, the contents of TOC in sediments were 0.21–4.94% (mean: 1.19%) in summer and 0.69–4.71% (mean: 1.94%) in winter. The concentrations of DOC in surface water were 3.35–18.39 mg/l (mean: 6.43 mg/l) in summer and 0.34–27.87 mg/l (mean: 5.87 mg/l) in winter. Person correlation analysis revealed that in summer TOC was significantly positive correlated with αE2 and NP (p < 0.05), while DOC was significantly positive in correlation with BPA (p < 0.05) (Fig. 3). In winter, E1 and NP showed the significantly positive relationship with TOC. However, E3 was significantly positively related to DOC (p < 0.05).
Risk assessment of target estrogens in HR
The EEQ values of selected estrogens in HR are illustrated in Fig. 4. The EEQ of αE2 was not calculated because of its limited toxicity data. The total EEQ in the surface water was 1.65 ± 0.87 ng/l in summer and 1.32 ± 0.75 ng/l in winter, which was much higher than those in sediments (summer: 0.03 ± 0.02 ng/l; winter: 0.04 ± 0.03 ng/l). Among all compounds, E3 was the most important estrogen and averagely accounted for 44.2–46.8% of the total EEQ in surface water. βE2, EE2 and E1 followed, with the average contributions of 21.8–23.5%, 17.1–18.7% and 12.8–13.7%, respectively. Similarly, E3 was still the main contributor to the total EEQ in sediments, which averagely accounted for 77.5% in summer and 81.7% in winter. E1 followed, accounting for about 9.3–13.3% of the total EEQ. Although NP, OP and BPA had relatively higher concentrations, they only contributed to less than 1% of total EEQ.
From Fig. 5, the individual RQs of target estrogens suggested that E1, βE2, E3, EE2 and OP could pose medium risks in most water samples. BPA showed the lowest RQ values, but it could still cause at least low risks at 76–90% of sampling sites. As for NP, it had the highest RQ, with a maxium RQ value up to 8. In sediments, only E3 posed low risks at 45–70% of the sampling sites in two seasons. The RQ values of the other estrogens were much lower than 0.01 in all seasons. The MRQs of seven estrogens in both surface water and sediments were calculated and listed in Table 3. In surface water, at least 70% of sampling sites had potential ecological risks in all seasons. The proportions of water samples causing high risks were 18.8% in summer and only 8.3% in winter, while those posed medium risks were 29.1% in summer and 18.8% in winter. For sediments, total estrogens showed medium risks in 14.6% of samples in summer. However, only 8.3% of samples were observed in winter.
Discussion
Composition of estrogen in water and sediments
The occurrence of estrogens in our study suggested that NP was the dominant compound in surface water. This result was consistent with those found in surface water from Pearl River Estuary (Diao et al. 2017), Luoma Lake (Liu et al. 2017a), and Taihu Lake (Liu et al. 2016). From Table S1, NP has the lower water solubility value than OP and BPA, but it was measured at the highest concentration in this study. As reported by China Petroleum and Chemical Industry Federation, NP is widely used in household goods in China (Zhang et al. 2014b), with an annual usage of about 50,000 tons. As a commonly used synthetic estrogen, EE2 showed the lowest concentration, probably due to its relatively lower solubility (Table S1). The relativley higher concentration of E3 was related to the fact that E3 is the main metabolite of E2, EE2 and E1 and can reach a maximum during the pregnancy of mammals (Lei et al. 2009; Nie et al. 2015). Very few studies have reported the occurrence of αE2 in surface water (Yang et al. 2015). However, it was measured with high detection frequency in our study. It was reported that αE2 is the common natural estrogen excreted by livestock (e.g. dairy and beef cattle) (Mashtare et al. 2011). Obviously, the levels of E1, NP, OP and BPA in the studied area were lower than those in most rivers or lakes in the world (Table S2). αE2 was in the comparable concentration with that of water samples from East Dongting Lake and Honghu Lake (Yang et al. 2015), Three Gorges Reservoir (Wang et al. 2016). Meanwhile, the concentrations of βE2, E3 and EE2 in our study were slight higher than those reported in Songhua River (Zhang et al. 2014b) and Rivers in Spain and Italy (Esteban et al. 2014; Pignotti and Dinelli 2018), but much lower than those in Three Gorges Reservoir (Wang et al. 2016), Honghu Lake and East Dongting Lake (Yang et al. 2015).
Similar to surface water, NP was still the predominant compound in sediments, suggesting its strong adsorption affinity to sediments. Because of its lowest concentration in water, EE2 was also found to be the lowest detected compound in sediments. Compared with E1 and E3, αE2 has relatively higher Kow value (Table S1), leading to its strong adsorption onto sediments. Moreover, βE2 can be degraded into αE2 in cattle excrement (Furuichi et al. 2006). Therefore, αE2 was dominant among the natural estrogens. In comparison with previous results in the world (Table S2), the residue levels of E1, βE2, E3, EE2 and OP in HR were comparable to those reported in Songhua River (Zhang et al. 2014b), Yellow River (Wang et al. 2012), Rivers in Italy (Pignotti and Dinelli 2018) and waters in England (Hibberd et al. 2009), but lower than those in East Dongting Lake and Honghu Lake (Yang et al. 2015). αE2 was within the ranges reported in Three Gorges Reservoir (Wang et al. 2016), Honghu Lake and East Dongting Lake (Yang et al. 2015). As the main estrogens in the studied area, NP and BPA were measured at relatively lower levels compared with most rivers.
Factors influencing the spatial and temporal distribution of estrogens
The highest concentrations of estrogens in the LHR could be due to the following reasons. According to the field survey, the LHR runs through many large cities, including Zhongxiang, Jinmen, Xiangyang, Xiantao, Qianjiang and Wuhan (Fig. 1), which have more developed industry and higher population density. Chemical industries, pulp industries, car manufacturing industry and food processing industries are clustered in this region. About 1.3 × 109 tons of wastewater enter into HR without any treatment every year (Zeng et al. 2014). Previous studies have shown that industrial effluent and demestic sewage are the main pathways of natural (e.g. E1, αE2, βE2, and E3) and synthetic estrogens (e.g. EE2, BPA, OP and NP) into the environment (Praveena et al. 2016; Zhou et al. 2016). Additionally, the lower section region is one of the most important agricultural areas in HR basin. Fishing ponds and poultry breeding farms are intensively distributed in this area (Li et al. 2013). Thus, agricultural waste and manure might be closely associated with the high concentrations of E1, αE2, βE2, and E3 (Shore and Shemesh 2016). Futhermore, the SNWTP might increase pollutant concentration of the lower section by increased hydraulic retention time and reduced dilution capacity (Yang et al. 2012). Finally, the tributaries (sites T13 and T14) showed higher levels of estrogens than the corresponding mainstreams (sites M13 and M14), indicating that tributaries could discharge estrogens into the main HR.
The seasonal variations of estrogens in surface water from HR are opposite to what is found in Yangtze Estuary (Nie et al. 2015) and Jiulongjiang River (Zhang et al. 2012), but similar to the results in Minho River estuary (Salgueiro-González et al. 2015) and Pearl River (Gong et al. 2012). As described above, αE2 is commonly excreted by livestock, which is mainly as conjugates of sulfuric and glucuronic acids (Baronti et al. 2000). OP and NP are the main metabolisms of octylphenol polyeothoxylates and nonyphenol polyeothoxylates (Liu et al. 2017a). Higher microbial activity in summer could increase the biodegradation rate of these compounds than in winter (Li et al. 2004; Wu et al. 2013). BPA is widely used in food packaging, dental sealants and plastic bottles (Kang et al. 2006). BPA has the highest water solubility value compared to other estrogens (Table S1). Therefore, it could be more easily leached from these materials because of the higher temperature and microbial activity in summer (Tiwari et al. 2016). Besides, more nonpoint source pollutants could enter into river water through strong surface runoff in summer (Liu et al. 2017b). It might result in increasing contributions of these compounds into the river. Finally, these estrogens in sediments could be released and re-enter into water phase due to the heavy rainfall. No significant seasonal variations of all estrogens in sediments suggested that estrogens in sediments were not influenced by the changes of short-term environmental conditions between seasons. The same conclusion has been drawn from previous studies on other EDCs in sediments from the Yellow River Delta (Zhao et al. 2016) and Liao River (Dong et al. 2016).
Partition mechanism of estrogens between water and sediment
The partitioning between water and sediment may not have reached equilibrium in an actual aquatic system, but the Koc′ is still an important parameter to study the transport and fate of organic pollutants (Lei et al. 2009; Nie et al. 2015). It is notable that Koc’ were one to two orders of magnitude higher than their corresponding predicted logKoc. It indicated that estrogens partitioned more to particulate phase than expected from their hydrophobicity. Moreover, hydrophobic interactions were not the dominant sorption mechanism for these compounds. It was further supported by the poor linear correlations between logKow and logKoc for eight estrogens (summer: r = 0.21, p = 0.63; winter: r = 0.10, p = 0.81). This result agreed with the previous studies in the estuary from Jamaica Bay (Ferguson et al. 2001), North Tai Lake (Zhang et al. 2014a), Rivers from Tianjin Area (Lei et al. 2009) and Tokyo metropolitan area (Isobe et al. 2001). There are some possible explanations for this result. Firstly, Koc values were estimated from short-term laboratory experiments, but the interactions between natural colloids and EDCs in the field are of much longer duration (Duering et al. 2002). Secondly, the Koc obtained in the laboratory may be underestimated by minimizing chemical bonding, volatilization, degradation and other processes, which might influence significantly the Koc′ values in natural rivers (Lei et al. 2009). Finally, sorbate concentrations in previous laboratory experiments were commonly conducted at ppm to ppb levels due to analytical limitations. However, those concentrations in the field are generally at low ppb to ppt levels, resulting in higher Koc′ values measured in the field (Gong et al. 2012).
To study the transport mechanism of estrogens in the environment, the relationships between Kp values of these compounds and TOC (DOC) were analyzed. These results demonstrated that TOC in sediments and DOC in water were important factors controlling the dynamic distribution of these estrogens. The positive relationship between TOC and E1, αE2 and NP suggested that they were more likely to be accumulated in sediments, probably associated with their higher logKow values. Owing to their higher water solubility and lower logKow values, BPA and E3 were significantly correlated with DOC rather than TOC. Similar to our results, previous studies also reported the important role of TOC and DOC in the partition of estrogens (Khanal et al. 2006; Zhang et al. 2014b). However, no significant correlations were found between the Kp values of EDCs and TOC in Northern Aegean Sea (Arditsoglou and Voutsa 2012), which might be due to the fact that the paritition of EDCs in surface water is a complex process. It is controlled not only by TOC, but also by other factors such as physical and chemical properties of these compounds, sediment texure, temperature and pH (Wang et al. 2016).
Environmental implication
Estrogens can cause adverse effects on aquatic organisms even at low ng/l levels, such as feminization, hermaphroditism and decreased fertility (Lei et al. 2009; Praveena et al. 2016). Therefore, the EEQ values of estrogens in water and sediments were assessed. The results showed that E3 was the main contributor to the total EEQ in both water and sediments. NP, OP and BPA only contributed to less than 1% of total EEQ due to their low EEFs. Compared with other studies, the total EEQ in surface water from HR was lower than that in Donghu Lake (Jin et al. 2013), Honghu Lake and East Dongting Lake (Yang et al. 2015), Three Gorges Reservoir (Wang et al. 2016), and Luoma Lake (Liu et al. 2017a), where the EEQ was higher than 10 ng/l in water; but comparable or even higher than that in Fenhe River in China (Liu et al. 2017b) and Langat River in Malaysia (Praveena et al. 2016), where the EEQ was nearly 1 ng/l in water.
The individual RQs of target estrogens suggested that NP in surface water posed higher risks to aquatic organisms. In sediments, most estrogens were not likely to pose adverse effects to aquatic organisms with the exception of E3. Generally, the RQs of estrogens in summer were comparable with those in winter, which agreed with the seasonal distribution of estrogens in sediments. Considering the fact that these compounds in the environment normally exist as mixtures, their combined effects should be assessed (Salgueiro-González et al. 2015). The MRQs of seven estrogens in both surface water and sediments suggested that estrogen mixtures posed higher ecological risks in summer than in winter. Overall, the RQs of mixture estrogens in the surface water were higher than those in the sediments. But the result still revealed that estrogens could pose low to medium risks in more than 30% of sediment samples. The accumulated estrogens in sediments might cause secondary pollution and threaten the aquatic ecosystem through food chains (Liu et al. 2016). Therefore, further risk assessment of estrogens needs to be performed in the sediment.
Conclusions
Eight typical estrogens were detected in surface water and sediment samples from HR. The composition of estrogens showed that NP was the dominant compound, with concentrations of nd-48 ng/l in surface water and nd-35 ng/g dw in sediments. The estrogens in surface water and sediments exhibted a similar spatial distribution mainly due to wastewater release, agricultural activities and water transfer project. In addition, obvious seasonal variations of αE2, NP, OP and BPA were only found in surface water. The phase partition of estrogens between water and sediment suggested that TOC and DOC were the important factors controlling their dynamic distribution. Risk assessment demonstrated that estrogens could pose potential ecological risks to aquatic organisms in both water and sediments. HR and DJKR serve as the drinking water source for millions people living in HR Basin and Northern China. Thus, further research is needed to evaluate environmental behaviors and ecological risks of estrogens in HR.
References
Ai L, Shi ZH, Yin W, Huang X (2015) Spatial and seasonal patterns in stream water contamination across mountainous watersheds: Linkage with landscape characteristics. J Hydrol 523:398–408
Arditsoglou A, Voutsa D (2012) Occurrence and partitioning of endocrine-disrupting compounds in the marine environment of Thermaikos Gulf, Northern Aegean Sea, Greece. Mar Pollut Bull 64:2443–2452
Baronti C, Curini R, D’Ascenzo G, Di Corcia A, Gentili A, Samperi R (2000) Monitoring natural and synthetic estrogens at activated sludge sewage treatment plants and in a receiving river water. Environ Sci Technol 34:5059–5066
Björkblom C, Salste L, Katsiadaki I, Wiklund T, Kronberg L (2008) Detection of estrogenic activity in municipal wastewater effluent using primary cell cultures from three-spined stickleback and chemical analysis. Chemosphere 73:1064–1070
Chen W, Pan S, Cheng H, Sweetman AJ, Zhang H, Jones KC (2018) Diffusive gradients in thin-films (DGT) for in situ sampling of selected endocrine disrupting chemicals (EDCs) in waters. Water Res 137:211–219
Diao P, Chen Q, Wang R, Sun D, Cai Z, Wu H, Duan S (2017) Phenolic endocrine-disrupting compounds in the Pearl River Estuary: Occurrence, bioaccumulation and risk assessment. Sci Total Environ 584-585:1100–1107
Dong D, Zhang L, Liu S, Guo Z, Hua X (2016) Antibiotics in water and sediments from Liao River in Jilin Province, China: occurrence, distribution, and risk assessment. Environ Earth Sci 75:1202–1209
Duering RA, Krahe S, Gäth S (2002) Sorption behavior of nonylphenol in terrestrial soils. Environ Sci Technol 36:4052–4057
EC (European Commission) (2003) European Commission Technical Guidance Document in Support of Commission Directive 93//67/EEC on Risk Assessment for New Notified Substances and Commission Regulation (EC) No.1488/94 on Risk Assessment for Existing Substance, Part II, pp. 100–103
Esteban S, Gorga M, Petrovic M, González-Alonso S, Barceló D, Valcárcel Y (2014) Analysis and occurrence of endocrine-disrupting compounds and estrogenic activity in the surface waters of Central Spain. Sci Total Environ 466–467:939–951
Ferguson PL, Iden CR, Brownawell BJ (2001) Distribution and fate of neutral alkylphenol ethoxylate metabolites in a sewage-impacted urban estuary. Environ Sci Technol 35:2428–2435
Furuichi T, Kannan K, Suzuki K, Tanaka S, Giesy JP, Masunaga S (2006) Occurrence of estrogenic compounds in and removal by a swine farm waste treatment plant. Environ Sci Technol 40:7896–7902
Gong J, Ran Y, Chen D, Yang Y, Zeng Eddy Y (2012) Association of endocrine-disrupting chemicals with total organic carbon in riverine water and suspended particulate matter from the Pearl River, China. Environ Toxicol Chem 31:2456–2464
Hernando MD, Mezcua M, Fernández-Alba AR, Barceló D (2006) Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 69:334–342
Hibberd A, Maskaoui K, Zhang Z, Zhou JL (2009) An improved method for the simultaneous analysis of phenolic and steroidal estrogens in water and sediment. Talanta 77:1315–1321
Hu Y, Yan X, Shen Y, Di M, Wang J (2018) Antibiotics in surface water and sediments from Hanjiang River, Central China: Occurrence, behavior and risk assessment. Ecotoxicol Environ Saf 157:150–158
Huang B, Wang B, Ren D, Jin W, Liu J, Peng J, Pan X (2013) Occurrence, removal and bioaccumulation of steroid estrogens in Dianchi Lake catchment, China. Environ Int 59:262–273
Isobe T, Nishiyama H, Nakashima A, Takada H (2001) Distribution and behavior of nonylphenol, octylphenol, and nonylphenol monoethoxylate in Tokyo metropolitan area: Their association with aquatic particles and sedimentary distributions. Environ Sci Technol 35:1041–1049
Jin S, Yang F, Xu Y, Dai H, Liu W (2013) Risk assessment of xenoestrogens in a typical domestic sewage-holding lake in China. Chemosphere 93:892–898
Kang JH, Katayama Y, Kondo F (2006) Biodegradation or metabolism of bisphenol A: From microorganisms to mammals. Toxicology 217:81–90
Ke X, Wang C, Zhang H, Zhang Y, Gui S (2015) Characterization of estrogenic receptor agonists and evaluation of estrogenic activity in the sediments of Liaohe River protected areas. Mar Pollut Bull 100:176–181
Khanal SK, Xie B, Thompson ML, Sung S, Ong SK, van Leeuwen J (2006) Fate, transport, and biodegradation of natural estrogens in the environment and engineered systems. Environ Sci Technol 40:6537–6546
Kuster M, José López de Alda M, Barceló D (2004) Analysis and distribution of estrogens and progestogens in sewage sludge, soils and sediments. TrAC, Trends Anal Chem 23:790–798
Laurenson JP, Bloom RA, Page S, Sadrieh N (2014) Ethinyl estradiol and other human pharmaceutical estrogens in the aquatic environment: A review of recent risk assessment data. AAPS J 16:299–310
Lei B, Huang S, Zhou Y, Wang D, Wang Z (2009) Levels of six estrogens in water and sediment from three rivers in Tianjin area, China. Chemosphere 76:36–42
Li BS, Zhou PJ, Wang XY, Zhu LD (2013) Opportunities and eco-environmental influence of cascade hydropower development and water diversion projects in Hanjiang river basin. J Geol Soc India 82:692–700
Li D, Kim M, Shim WJ, Yim UH, Oh JR, Kwon YJ (2004) Seasonal flux of nonylphenol in Han River, Korea. Chemosphere 56:1–6
Liu D, Liu J, Guo M, Xu H, Zhang S, Shi L, Yao C (2016) Occurrence, distribution, and risk assessment of alkylphenols, bisphenol A, and tetrabromobisphenol A in surface water, suspended particulate matter, and sediment in Taihu Lake and its tributaries. Mar Pollut Bull 112:142–150
Liu D et al. (2017a) Distribution and bioaccumulation of endocrine disrupting chemicals in water, sediment and fishes in a shallow Chinese freshwater lake: Implications for ecological and human health risks. Ecotoxicol Environ Saf 140:222–229
Liu X, Shi J, Bo T, Meng Y, Zhan X, Zhang M, Zhang Y (2017b) Distributions and ecological risk assessment of estrogens and bisphenol A in an arid and semiarid area in northwest China. Environ Sci Pollut Res 24:7216–7225
Mashtare ML, Khan B, Lee LS (2011) Evaluating stereoselective sorption by soils of 17α-estradiol and 17β-estradiol. Chemosphere 82:847–852
Nie M, Yan C, Dong W, Liu M, Zhou J, Yang Y (2015) Occurrence, distribution and risk assessment of estrogens in surface water, suspended particulate matter, and sediments of the Yangtze Estuary. Chemosphere 127:109–116
Peck M, Gibson Richard W, Kortenkamp A, Hill Elizabeth M (2009) Sediments are major sinks of steroidal estrogens in two United Kingdom rivers. Environ Toxicol Chem 23:945–952
Pignotti E, Dinelli E (2018) Distribution and partition of endocrine disrupting compounds in water and sediment: Case study of the Romagna area (North Italy). J Geochem Explor https://doi.org/10.1016/j.gexplo.2018.02.008
Pojana G, Gomiero A, Jonkers N, Marcomini A (2007) Natural and synthetic endocrine disrupting compounds (EDCs) in water, sediment and biota of a coastal lagoon. Environ Int 33:929–936
Praveena SM, Lui TS, Hamin NA, Razak SQNA, Aris AZ (2016) Occurrence of selected estrogenic compounds and estrogenic activity in surface water and sediment of Langat River (Malaysia). Environ Monit Assess 188:442–453
Rivas-Rivera N, Eguren G, Carrasco-Letelier L, Munkittrick KR (2014) Screening of endocrine disruption activity in sediments from the Uruguay River. Ecotoxicology 23:1137–1142
Saeed T, Al-Jandal N, Abusam A, Taqi H, Al-Khabbaz A, Zafar J (2017) Sources and levels of endocrine disrupting compounds (EDCs) in Kuwait’s coastal areas. Mar Pollut Bull 118:407–412
Salgueiro-González N, Turnes-Carou I, Besada V, Muniategui-Lorenzo S, López-Mahía P, Prada-Rodríguez D (2015) Occurrence, distribution and bioaccumulation of endocrine disrupting compounds in water, sediment and biota samples from a European river basin. Sci Total Environ 529:121–130
Shore LS, Shemesh M (2016) Estrogen as an environmental pollutant. Bull Environ Contam Toxicol 97:447–448
Song X, Wen Y, Wang Y, Adeel M, Yang Y (2018) Environmental risk assessment of the emerging EDCs contaminants from rural soil and aqueous sources: Analytical and modelling approaches. Chemosphere 198:546–555
Sun H, Giesy JP, Jin X, Wang J (2017) Tiered probabilistic assessment of organohalogen compounds in the Han River and Danjiangkou Reservoir, central China. Sci Total Environ 586:163–173
Tiwari M, Sahu SK, Pandit GG (2016) Distribution and estrogenic potential of endocrine disrupting chemicals (EDCs) in estuarine sediments from Mumbai, India. Environ Sci Pollut Res 23:18789–18799
Wang B et al. (2013) Distribution of perfluorinated compounds in surface water from Hanjiang River in Wuhan, China. Chemosphere 93:468–473
Wang J, Bovee TFH, Bi Y, Bernhöft S, Schramm KW (2014) Aryl hydrocarbon receptor (AhR) inducers and estrogen receptor (ER) activities in surface sediments of Three Gorges Reservoir, China evaluated with in vitro cell bioassays. Environ Sci Pollut Res 21:3145–3155
Wang L et al. (2012) Monitoring of selected estrogenic compounds and estrogenic activity in surface water and sediment of the Yellow River in China using combined chemical and biological tools. Environ Pollut 165:241–249
Wang W, Ndungu AW, Wang J (2016) Monitoring of endocrine-disrupting compounds in surface water and sediments of the Three Gorges reservoir region, China. Arch Environ Contam Toxicol 71:509–517
Wang Y, Wang Q, Hu L, Lu G, Li Y (2015) Occurrence of estrogens in water, sediment and biota and their ecological risk in Northern Taihu Lake in China. Environ Geochem Health 37:147–156
Wu M et al. (2013) Seasonal and spatial distribution of 4-tert-octylphenol, 4-nonylphenol and bisphenol A in the Huangpu River and its tributaries, Shanghai, China. Environ Monit Assess 185:3149–3161
Yang Q, Xie P, Shen H, Xu J, Wang P, Zhang B (2012) A novel flushing strategy for diatom bloom prevention in the lower-middle Hanjiang River. Water Res 46:2525–2534
Yang Y, Cao X, Zhang M, Wang J (2015) Occurrence and distribution of endocrine-disrupting compounds in the Honghu Lake and East Dongting Lake along the Central Yangtze River, China. Environ Sci Pollut Res 22:17644–17652
Ying GG, Kookana RS, Ru YJ (2002) Occurrence and fate of hormone steroids in the environment. Environ Int 28:545–551
Zeng ZX, Zhang H, Shan BQ, Yang HG (2014) Analysis of industrial pollution sources of the middle and lower reaches of Hanjiang river basin. Resour Environ Yangtze Basin 23:252–259.
Zhang C, Li Y, Wang C, Niu L, Cai W (2016) Occurrence of endocrine disrupting compounds in aqueous environment and their bacterial degradation: A review. Crit Rev Env Sci Technol 46:1–59
Zhang X, Zhang D, Zhang H, Luo Z, Yan C (2012) Occurrence, distribution, and seasonal variation of estrogenic compounds and antibiotic residues in Jiulongjiang River, South China. Environ Sci Pollut Res 19:1392–1404
Zhang YZ, Meng W, Zhang Y (2014a) Occurrence and partitioning of phenolic endocrine-disrupting chemicals (EDCs) between surface water and suspended particulate matter in the North Tai Lake Basin, Eastern China. Bull Environ Contam Toxicol 92:148–153
Zhang Z, Ren N, Kannan K, Nan J, Liu L, Ma W, Qi H, Li Y (2014b) Occurrence of endocrine-disrupting phenols and estrogens in water and sediment of the Songhua River, Northeastern China. Arch Environ Contam Toxicol 66:361–369
Zhao JL, Ying GG, Chen F, Liu YS, Wang L, Yang B, Liu S, Tao R (2011) Estrogenic activity profiles and risks in surface waters and sediments of the Pearl River system in South China assessed by chemical analysis and in vitro bioassay. J Environ Monit 13:813–821
Zhao S, Liu X, Cheng D, Liu G, Liang B, Cui B, Bai J (2016) Temporal–spatial variation and partitioning prediction of antibiotics in surface water and sediments from the intertidal zones of the Yellow River Delta, China. Sci Total Environ 569-570:1350–1358
Zhou LJ, Zhang BB, Zhao YG, Wu QL (2016) Occurrence, spatiotemporal distribution, and ecological risks of steroids in a large shallow Chinese lake, Lake Taihu. Sci Total Environ 557-558:68–79
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (No. 31361140360) and China Postdoctoral Foundation (No. Y71I321K02). The authors are grateful to acknowledge Yang Yuyi and Qi Yueling for their help. The authors also would like to appreciate the reviewers for their suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Supplementary Information
Rights and permissions
About this article
Cite this article
Hu, Y., Yan, X., Shen, Y. et al. Occurrence, behavior and risk assessment of estrogens in surface water and sediments from Hanjiang River, Central China. Ecotoxicology 28, 143–153 (2019). https://doi.org/10.1007/s10646-018-2007-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-018-2007-4