Abstract
Sediment constitutes an important sink of endocrine disruptor compounds; however, the potential of sediments to act as a source of endocrine disruptors should be more extensively investigated. The main objective of this study was to determine whether exposure of immature common carp to Uruguay River sediments undergo physiological and endocrine alterations. The lower Uruguay River watershed supports intensive agricultural and forest production, receives municipal sewage discharge and industrial effluent, and a new large pulp mill was constructed in 2006. A 30-day semi-static assay was performed using sediments from four sites along the Uruguay River and compared with an unexposed group in dechlorinated water as a negative control. We focused on two upstream and two downstream sites of a new elemental chlorine free pulp mill. The results showed that plasma vitellogenin levels increased in fish along the river and significant differences were found between the exposed and unexposed groups. Condition factor and gonadosomatic index were not different; however, a significant difference in hepatosomatic index was observed in fish exposed to sediment from an industrial site. A significant reduction in primary spermatocyte accumulation was observed in the exposed group compared with that in the control group, and some individuals exposed to sediments from industrial sites presented with testis–ova. Our results suggest that Uruguay River sediments act as an important source of estrogenic compounds that could be responsible for the alterations observed. Future studies are needed to identify the causal agents and determine exposure routes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Growing scientific evidence indicates that some natural and synthetic chemical compounds interfere with normal endocrine functioning in humans and wildlife. Most of these studies have focused on detecting specific endocrine disruptor compounds (EDCs) in surface water and their potential effects on fish (Sellin et al. 2009, 2011a; Velisek et al. 2011). Recent studies have detected high concentrations of EDCs in sediments, suggesting that the sediments could be responsible for the observed alterations; however, bioavailability of EDCs is complex. Sediments could be acting as a sink and reducing EDC bioavailability or re-releasing the chemical compounds into the water and acting as a source. The possible exposure routes to aquatic organisms include direct uptake of free compounds across the gills or skin and ingestion of sediment particles (Peck et al. 2004). Several laboratories and field studies have reported that fish exposed to sediments experience significant alterations in endocrine functions (Orrego et al. 2005; Kolok et al. 2007; Sellin et al. 2010, 2011b; Kolpin et al. 2013; Jessick et al. 2014). However, the potential of sediments to act as sources for endocrine disruptors should be more extensively investigated.
The lower Uruguay River watershed is characterized by intensive agricultural and forest production. This part of the river receives a variety of municipal sewage discharges and industrial effluents, and a new large pulp mill was constructed in 2006 (Paruelo et al. 2006; Céspedes-Payret et al. 2009; Vega et al. 2009). From 2005 to 2007 several chemicals such as resin acids, phytosterols, polychlorinated dibenzo-p-dioxins, and dibenzofurans have been detected in water and sediments from the lower Uruguay River and bioaccumulation and ecotoxicological effects have been observed in wild fish (Miguez et al. 2010; Saizar et al. 2010).
This study was designed to test the hypothesis that a variety of chemicals found in sediments can display endocrine disrupting activity. The main goal was to determine if immature common carp (Cyprinus carpio) exposed to sediments from the lower Uruguay River exhibit physiological and endocrine alterations as determined by condition factor, hepatosomatic and gonadosomatic indices, plasma vitellogenin levels, and a gonadal histological analysis.
Materials and methods
Sediment samples
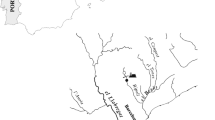
Sediment from four sites along the lower Uruguay River (between S 30°11’, W 57°38’ and S 34°00’, W 58°21’) were collected with an Eckman dredge in December 2006, transported at 4 °C to the laboratory, and stored at −20 °C until bioassay. We focused on two upstream sites from a new elemental chlorine free (ECF) pulp mill: the Paysandú (PY) urban-industrial zone and the Nuevo Berlín (NB) agricultural and forested (Eucalyptus sp.) zone and two downstream sites: the Las Cañas (LC) agricultural and tourist zone and the Juan Lacaze (JL) urban-industrial zone, which are directly influenced by discharge from an elemental chlorine bleached kraft pulp mill (Fig. 1).
Study area and location of the sediment sampling zones. PY Paysandú, NB Nuevo Berlín, LC Las Cañas, JL Juan Lacaze. The grey zones on the Uruguayan territory show land under agricultural activity. The information was obtained from supervised classification of LANDSAT 5 satellite images (Color figure online)
Experimental design
Immature common carp (mean body length, 7.2 ± 0.9 cm; mean weight, 9.0 ± 3.2 g) were obtained from the National Direction of Aquatic Resources Fish Hatchery. The fish were acclimatized for 15 days in an aerated pool (800 L of dechlorinated water, renewed every 2 days), maintained with a constant temperature (22 ± 1 °C), light: dark cycle (12:12 h), and dissolved oxygen (89 ± 1 %). Fish were fed commercial food (Marplatense S.A., Montevideo, Uruguay) ad libitum.
A 30-day semi-static assay (total water renewed every 7 days) was performed in January 2007 using the same environmental conditions as those used for acclimatization. Sixty fish were randomly allocated in 30 L aquaria (four per aquaria) into five groups with 12 fish in each group. One group was placed in dechlorinated water as a negative control, and the others were exposed to sediments from each zone (PY, NB, LC, and JL) a 1:10 w/v rate (Sellin et al. 2011b; Jessick et al. 2014). No mortalities were observed during the assay.
Plasma vitellogenin (VTG) levels
Once the bioassay was completed, blood samples were extracted from the vena caudalis using heparinized syringes for plasma VTG analysis. Plasma was separated by centrifugation (Universal 32R, Hettich Zentrifugen) at 1,500 rpm for 10 min, and plasma VTG was quantified using antibody pre-coated enzyme-linked immunosorbent assay kits (Biosense Laboratory, Bergen, Norway; product no. V01003402) (Nilsen et al. 2004). The microplates were measured at a wavelength of 492 nm in a Biorad 680 microplate reader spectrophotometer (Hercules, CA, USA). The VTG concentration was calculated based on a standard calibration curve and expressed in µg/mL.
Gonadal histology
Gonads were immediately fixed in 10 % phosphate buffered formalin at pH 7.4. They were dehydrated gradually (alcohol 70, 96, and 100 v/v% chloroform) and embedded in paraffin. Sections (5 µm, Reichert–Jung microtome) were rehydrated and stained with hematoxylin and eosin. The fish were sexed and the reproductive maturity of the gonad cells was determined according to Smith and Walker (2004) using an optical microscope (Olympus Vanox; Tokyo, Japan) and photographed using a digital camera.
Physiological indices
The hepatosomatic index (HSI) and gonadosomatic index (GSI) of each fish was determined by dividing the body mass of the fish by the mass of the tissues and multiplying by 100. Condition factor (K) was calculated according to the following equation: (K) = 100 (body mass without organs/standard lengthb), where b is the allometric coefficient, estimated by linear regression after logarithmic transformation of body mass and standard length before starting the assay (n = 60).
Statistical analysis
Normality and homogeneity of variance were verified and a single factor analysis of variance or Kruskal–Wallis test was used to determine differences between the physiological indices and plasma VTG levels. Statistical significance was confirmed by Tukey’s post hoc test A p < 0.05 was considered significant.
Results
Plasma VTG levels and gonadal histology
Plasma VTG levels are presented in Fig. 2. The values increased along a latitudinal gradient from PY to JL. Significant differences were observed among the sediment-exposed groups and the control (Tukey’s HSD, p < 0.05); however, no differences were detected among the exposed groups.
The gonadal histological analysis of females revealed that oocyte stages were not different between exposed and unexposed groups and that all oocytes were in the previtellogenic and perinucleolar stages (Fig. 3C, D). However, sediment-exposed males showed a significant reduction in the number of primary spermatocytes compared with those in the control group (p = 0.01) (Fig. 3A, B), and some individuals exposed to sediments from industrial sites (PY and JL) presented with testis–ova (Table 1).
Photographs of gonad histology in common carp exposed to sediments from different zones along the lower Uruguay River. a Gonads of a male from the control (magnification, 20×); b gonads of a male from the Nuevo Berlín (NB) fish group (magnification, 20×); c gonads of a female from the control (magnification, 20×); d gonads of a female from the NB group (magnification, 10×). PSA primary spermatocyte accumulation, OPP oocytes in previtellogenic and perinucleolar stage, Ct cytoplasm, N nucleus
Physiological indices
Mean (±standard error) values of the physiological indices in the exposed and unexposed groups are given in Table 2. No significant differences were observed among the groups for K or GSI; however, significant differences in HSI were detected. Post-hoc comparisons revealed that fish exposed to sediment from JL had significantly increased HSIs compared with those in the control (p = 0.04) and LC groups (p = 0.02).
Discussion
The main goal of this study was to determine whether immature common carp exposed to Uruguay River sediments exhibit physiological and endocrine alterations. Our results clearly indicate that natural and/or synthetic chemical compounds present in the sediments caused an abnormal and significant induction of the egg-yolk precursor protein VTG in all exposed groups. Elevated levels of VTG in males and immature females were clearly an estrogen-mediated response. The exposure to estrogenic compounds has a transitory effect on VTG production; however, exposure during early life stages could reduce survivability, and the effects on gonadal morphology and reproductive function may be permanent (Hutchinson et al. 2006).
Several known sources of endocrine disruptors are located in the Uruguay River watershed, and previous studies have detected some EDCs in sediments (Saizar et al. 2010) that could be responsible for the observed alterations. The urban-industrial sites (PY and JL) receive untreated municipal sewage effluent containing a complex cocktail of natural (estrone or 17β-estradiol) and synthetic estrogens used in oral contraceptives as well as surfactants used in soaps and detergents (alkylphenols and alkylphenolpolyethoxylates). Furthermore, the plasma VTG concentrations in fish were highest where deposition processes were predominant at JL and where pulp mill effluent was discharged near the sampling site.
The agricultural and forest river sectors (NB and LC) support intense soybean-wheat row crop production, and this agricultural system uses known estrogenic pesticides such as chlorpyrifos, endosulfan, and cypermethrin (Mnif et al. 2011). Additionally, phytoestrogens released by crops as a defense strategy may be reaching the river in overland runoff. In particular, soybeans contain high levels of genistein, daidzein, and glycitein, which can elicit alterations in endocrine function in wildlife and humans (Ng et al. 2006). It is important to note that VTG levels were not affected by the sex ratio, as shown by similar VTG concentrations in the LC and JL groups with opposite sex ratios. This was also true when comparing the PY and NB treatments.
The significant increase in liver mass at JL may have been caused by induction of the hepatic mixed function oxidase system in response to discharge of persistent organic compounds from the pulp mill effluent. Increased protein synthesis generates proliferation of endoplasmic reticulum, which can be reflected in increased hepatocyte size (Munkittrick et al. 1992).
The gonad histology analyses indicated that female fish did not exhibit differences in maturation state; however, sediment-exposed males presented delayed testicular maturation than that in the unexposed group. Jobling et al. (1996) reported that the induction of VTG in males is negatively correlated with testicular maturation, and Devlin and Nagahama (2002) observed retarded gonadal maturation in C. carpio males exposed to estrogenic compounds. Changes in sex ratios and intersex individuals have been reported in common carp exposed to EDCs (Gimeno et al. 1998; Devlin and Nagahama 2002). However, the intersex condition occurs naturally in approximately 5 % of the population in this species (Jobling et al. 1998). Thus, the presence of individuals with testis–ova observed in our study was possibly a natural phenomenon and may not have been caused by exposure to contaminated sediments.
Conclusions
This study is the first report about endocrine disruption in fish exposed to sediment from the lower Uruguay River. The results can be considered a reference condition for monitoring the impacts of the new ECF bleached kraft Eucalyptus pulp mill. Nonpoint (soybean–wheat crops) and point sources (municipal sewage and pulp mill effluent) can explain the VTG induction observed in immature fish, and suggest the presence and bioavailability of EDCs in the sediments. The specific agents responsible for the toxic effects were not identified because it was beyond the scope of this study. Future research is needed to identify the causal agents (natural or synthetic) and to determine exposure routes (e.g., grazing on sediments or bioconcentration from the water column).
References
Céspedes-Payret C, Pineiro G, Achkar M et al (2009) The irruption of new agro-industrial technologies in Uruguay and their environmental impacts on soil, water supply and biodiversity: a review. Int J Environ Health 3:175–197. doi:10.1504/IJENVH.2009.024877
Devlin RH, Nagahama Y (2002) Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture 208:191–364. doi:10.1016/S0044-8486(02)00057-1
Gimeno S, Komen H, Gerritsen AGM, Bowmer T (1998) Feminisation of young males of the common carp, Cyprinus carpio, exposed to 4-tert-pentylphenol during sexual differentiation. Aquat Toxicol 43:77–92. doi:10.1016/S0166-445X(98)00056-3
Hutchinson TH, Ankley GT, Segner H, Tyler CR (2006) Screening and testing for endocrine disruption in fish-biomarkers as “signposts”, not “traffic lights”, in risk assessment. Environ Health Perspect 114:106–114. doi:10.1289/ehp.8062
Jessick AM, Skolness S, Kolok AS (2014) Sandy sediment and the bioavailability of 17β-trenbolone to adult female fathead minnows. Aquat Toxicol 148:48–54. doi:10.1016/j.aquatox.2013.12.025
Jobling S, Sumpter JP, Sheahan D et al (1996) Inhibition of testicular growth in rainbow trout (Oncorhynchus mykiss) exposed to estrogenic alkylphenolic chemicals. Environ Toxicol Chem 15:194–202. doi:10.1002/etc.5620150218
Jobling S, Nolan M, Tyler CR et al (1998) Widespread sexual disruption in wild fish. Environ Sci Technol 32:2498–2506. doi:10.1021/es9710870
Kolok AS, Snowc DD, Kohnod S et al (2007) Occurrence and biological effect of exogenous steroids in the Elkhorn River, Nebraska, USA. Sci Total Environ 388:104–115. doi:10.1016/j.scitotenv.2007.08.001
Kolpin DW, Blazer VS, Gray JL et al (2013) Chemical contaminants in water and sediment near fish nesting sites in the Potomac River basin: determining potential exposures to smallmouth bass (Micropterus dolomieu). Sci Total Environ 443:700–716. doi:10.1016/j.scitotenv.201209063
Miguez D, Carrara MV, Carnikián A et al (2010) Evaluación ecotoxicológica de sedimentos en una zona del Río Uruguay con puntos finales indicadores de toxicidad aguda, sub-letal, crónica, reproductiva y teratogénica. INNOTEC 5:3–10
Mnif W, Hassine AIH, Bouaziz A et al (2011) Effect of endocrine disruptor pesticides: a review. Int J Environ Res Public Health 8:2265–2303. doi:10.3390/ijerph8062265
Munkittrick KR, McMaster ME, Portt CB et al (1992) Changes in maturity, plasma sex steroid levels, hepatic mixed-function oxygenase activity, and the presence of external lesions in lake whitefish (Coregonus clupeaformis) exposed to bleached kraft mill effluent. Can J Fish Aquat Sci 49:1560–1569. doi:10.1139/f92-173
Ng Y, Hanson S, Malison JA et al (2006) Genistein and other isoflavones found in soybeans inhibit estrogen metabolism in salmonid fish. Aquaculture 254:658–665. doi:10.1016/j.aquaculture.2005.10.039
Nilsen BM, Berg K, Eidem JK et al (2004) Development of quantitative vitellogenin-ELISAs for fish test species used in endocrine disruptor screening. Anal Bioanal Chem 378:621–633. doi:10.1007/s00216-003-2241-2
Orrego R, Moraga-Cid G, González M et al (2005) Reproductive, physiological, and biochemical responses in juvenile female rainbow trout (Oncorhynchus mykiss) exposed to sediment from pulp and paper mill industrial discharge areas. Environ Toxicol Chem 24:1935–1943. doi:10.1897/04-251R1.1
Paruelo JM, Guerschman JP, Piñeiro G et al (2006) Cambios en el uso de la tierra en Argentina y Uruguay: marcos conceptuales para su análisis. Agrociencia 10(2):47–61
Peck M, Gibson RW, Kortenkamp A, Hill EM (2004) Sediments are major sinks of steroidal estrogens in two United Kingdom rivers. Environ Toxicol Chem 23:945–952. doi:10.1897/03-41
Saizar C, Boccardi L, Clemente J et al (2010) Línea de base para evaluar el impacto de una planta de celulosa en el Río Uruguay. INNOTEC 5:11–22
Sellin MK, Snow DD, Schwarz M et al (2009) Agrochemicals in Nebraska, USA, watersheds: occurrence and endocrine effects. Environ Toxicol Chem 28:2443–2448. doi:10.1897/09-135.1
Sellin MK, Snow DD, Kolok AS (2010) Reductions in hepatic vitellogenin and estrogen receptor alpha expression by sediments from an agriculturally impacted waterway. Aquat Toxicol 96:103–108. doi:10.1016/j.aquatox.2009.10.004
Sellin MK, Abbott K, Cowman T et al (2011a) Occurrence and endocrine effects of agrichemicals in a small Nebraska, USA, watershed. Environ Toxicol Chem 30(10):2253–2260. doi:10.1002/etc.615
Sellin MK, Conoan NH, Cox MB et al (2011b) The anti-estrogenic activity of sediments from agriculturally intense watersheds: assessment using in vivo and in vitro assays. Aquat Toxicol 105:189–198. doi:10.1016/j.aquatox.2011.04.008
Smith BB, Walker KF (2004) Spawning dynamics of common carp in the River Murray, South Australia, shown by macroscopic and histological staging of gonads. J Fish Biol 64:336–354. doi:10.1111/j.0022-1112.2004.00293.x
Vega E, Baldi G, Jobbágy EG, Paruelo J (2009) Land use change patterns in the Río de la Plata grasslands: the influence of phytogeographic and political boundaries. Agric Ecosyst Environ 134:287–292. doi:10.1016/j.agee.2009.07.011
Velisek J, Stara A, Kolarova J et al (2011) Biochemical, physiological and morphological responses in common carp (Cyprinus carpio L.) after long-term exposure to terbutryn in real environmental concentration. Pestic Biochem Physiol 100:305–313. doi:10.1016/j.pestbp.2011.05.004
Acknowledgments
We express our thanks to the Departments of Virology and Cellular Biology (Science School) for cooperation with the processing of samples and to Mr. Angel Rosano and the Uruguayan Navy for their assistance in the fieldwork. This study was funded by the National Agricultural Research Institute (INIA) Project SA07 and the Environmental Science Master Program.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rivas-Rivera, N., Eguren, G., Carrasco-Letelier, L. et al. Screening of endocrine disruption activity in sediments from the Uruguay River. Ecotoxicology 23, 1137–1142 (2014). https://doi.org/10.1007/s10646-014-1244-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-014-1244-4