Abstract
Concentrations and distribution of octylphenol (OP), nonylphenol (NP), and bisphenol A (BPA) in surface water and suspended particulate matter (SPM) from the north Tai Lake basin, China were studied. Aqueous and particulate (dry weight) concentrations for OP, NP, and BPA varied from 10.5–1,175 ng/L to <1.52–5,365 ng/g, respectively. The spatial distribution of endocrine-disrupting chemicals (EDCs) in dissolved and particulate phases showed that the amount of EDCs in water that were adsorbed to SPM gradually increased from upstream to downstream. There were good correlations between particulate EDCs and particulate organic carbon, with correlation coefficients of 0.46–0.57. Regression analysis of in situ SPM–water partition coefficients (log K’ oc) and log K ow for EDCs indicated that the hydrophobicity of chemicals greatly contributed to their SPM–water partitioning. Strong positive correlations (r = 0.68–0.82) among in situ log K’ oc of OP, NP, and BPA and flow velocity of water were observed, indicating the critical importance of riverine hydrodynamics on the sorption of these compounds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Phenolic endocrine-disrupting chemicals (EDCs), such as octylphenol (OP), nonylphenol (NP), and bisphenol A (BPA), have caused great concern in environmental science and policy due to their ubiquitous and adverse effects on numerous organisms. Most phenolic EDCs are ultimately discharged from sewage treatment plants and urban landfill sites (Soares et al. 2008). Once they enter the aquatic environment, phenolic EDCs partition to sediments, particles, and lipids where organic matter abounds. Studies on sorption and partitioning of phenolic EDCs in aquatic systems have been conducted by employing high sorbate levels and minimizing other processes, such as volatilization and degradation, in the laboratory (Johnson et al. 1998, Düring et al. 2002). However, extrapolation of experimental results under well-controlled boundary conditions to field conditions remains challenging due to variations in environmental parameters (Navarro et al. 2009). Generally, partitioning of organic pollutants depends on some environmental parameters, like organic carbon content and riverine hydrodynamics. Hydrophobic partitioning can be considered the main mechanism for sorption of phenolic EDCs to organic matter, owing to their high octanol–water partition coefficients (log K ow) of 3.12–4.48 (Tan et al. 2007). Field studies have shown that about 40–80 % of the total OP and NP remain in the dissolved phase in the riverine environment (Li et al. 2004). Therefore, hydrodynamics may play an important role in controlling sorption kinetics of phenolic EDCs and thus their in situ solid–water partition coefficients (log K oc). However, information on the relation between flow dynamics and solid–water partitioning coefficients of phenolic EDCs has rarely been reported.
Tai Lake (30°5′–32°8′N, 119°8′–121°5′E) is one of the five largest freshwater lakes in China and its basin area is approximately 36,895 km2. Water resources in the Tai Lake basin are very important for drinking, tourism, fisheries, agriculture, shipping, and irrigation. However, over-exploration and over-utilization of water resources have caused degradation of the lake ecosystem and deterioration of water quality in the basin in the past few decades. This is especially evident in the north Tai Lake basin, where a large amount of urban pollutants are discharged into the surface water, which now faces annual algal blooms and emerging organic contamination. The objectives of this study were to (1) determine the concentrations and spatial distribution of phenolic EDCs in surface water and suspended particulate matter (SPM) from the north Tai Lake basin, and (2) clarify the relationships among hydrodynamics, particulate organic carbon (POC), and in situ solid–water partitions in order to reveal the importance of complex hydrodynamic systems in influencing the environmental behavior and fate of organic contaminants.
Materials and Methods
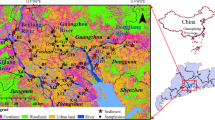
Surface water samples were collected with pre-rinsed 2-L glass bottles in the north Tai Lake basin in October 2011. All sampling sites were located in the Jinghang Canal and its tributaries, except S6 and S7 (Fig. 1). Water velocity was measured in situ with a global Flow Probe FP 211 (Global Water, USA) and the average value of three repeated measurements was adopted. Surface water samples were filtered through a pre-baked and pre-weighed 0.7-μm glass fiber membrane (GF/F) by portable vacuum pump in situ. Each aliquot filtrate was spiked with 100 ng/L di-n-butyl phalate-d4 as the surrogate standard and extracted by solid-phase extraction (SPE) cartridge (LC-18, Suplco, USA). Pretreatment of water samples was the same as that described in our previous study (Zhang et al. 2009). Filters loaded with SPM were freeze-dried and determined gravimetrically. Briefly, the spiked filters were ultrasonically extracted in triplicate with 20 mL of DCM/acetone (1:1) for 20 min. Then, the concentrated extract (about 1 mL) was dissolved again into 500 mL ultrapure water and the solution was loaded with the pre-conditioned SPE cartridge. The target compounds were sequentially eluted with hexane, hexane/DCM (1:1, v/v), and acetone/methanol (1:1, v/v). The second and third fractions were used for analyzing the OP, NP, and BPA. Internal standard was added into the concentrated elution (0.2 mL) prior to gas chromatography–mass spectrometry (GC–MS) analysis. GC–MS (Agilent GC 6890 coupled with Agilent MS 5975) and HP-5MS fused silica capillary column (30 m, 0.25 mm i.d., and 0.25 μm film thickness) were employed. The analysis conditions with GC–MS were the same as that in our previous study (Zhang et al. 2009).
Recoveries of EDCs were checked through triplicate analyses of distilled water and particulate materials spiked with known levels of all target compounds. The average recoveries of water and particulate materials were 82.3–96.5 and 78.2 %–113 %, respectively. Moreover, recovery of surrogate standard ranged from 76.6 to 121 % in all field samples. The limits of quantification (LOQ) of EDCs were calculated based on ten times the signal versus noise (S/N) value. The LOQs were 1.22–26.1 ng/L for surface water and 1.52–32.6 ng/g for SPM. For every batch of 10 samples, a solvent blank and procedural blank were run to check for contamination. There was no blank correction to be performed due to undetected amounts of EDCs.
Another water sample was taken from each sampling site to collect SPM. The SPM was acidified with HCl to remove carbonates. Then, SPM was dried to determine the particulate organic carbon (POC) using the elemental analyzer (Elementar vario macro EI, Germany).
Results and Discussion
Phenolic EDCs were detected in all the water samples at concentrations above their respective LOQs, which suggests that they are ubiquitous in the aquatic environment of the north Tai Lake basin. The concentrations varied from 10.5 to 52.9 ng/L for OP, from 89.3 to 1,189 ng/L for NP, and from 23.7 to 1,175 ng/L for BPA, with average values of 26.5, 388, and 270 ng/L, respectively. For SPM, the concentrations were within the range of <1.52–573 ng/g for OP, 224–5,365 ng/g for NP, and < 8.39–2,682 ng/g for BPA, with average concentrations of 272, 2,381, and 743 ng/g, respectively. Spatial distribution of dissolved EDCs (Fig. 2a) showed that river water at upstream sites (S1–S5) had higher NP concentrations than that in other reaches of the Jinghang Canal. NP was also the dominant component among the three phenolic EDCs in surface water, with the maximum concentration of 1,189 ng/L occurring at S4, followed by S2 and S3. All three sites are situated downstream of the Changzhou urban zone. Concentrations of dissolved NP at these sites were significantly higher than that at the upstream site (S1), indicating the possible input of NP from the Changzhou urban zone, where there is one of the major NP manufacturers with annual production capacity of ≥5,000 tons in China (Huang et al. 2011). The urban zone is generally considered to cause strong impacts on the increase of APs. A statistically greater (p = 0.01) concentration of APs was found downstream rather than upstream of urban centers in the USA (Kolpin et al. 2004). Thus, Changzhou city could be considered as the major discharge source of NP in the north Tai Lake basin.
Unlike dissolved EDCs, the average concentrations of particulate EDCs at midstream and downstream sites (S8–S13) were higher than at upstream sites (Fig. 2b), and their average ratios (Conc.Mid/downstream/Conc.Upstream) were 1.18 for OP, 2.01 for NP, and 3.78 for BPA. The results suggested that more EDCs might partition from the dissolved phase to SPM along the canal in light of their high octanol–water partition coefficients (log K ow) of 3.12–4.48 (Tan et al. 2007). Li et al. (2004) reported that particulate NP increased from upstream to downstream of the Han River in Korea for all seasons and further illustrated that NP was readily absorbed to SPM with a rapid SPM–water partitioning equilibrium. Hence, it is reasonable to expect that the SPM–water partition process would have a strong impact on the behavior and fate of phenolic EDCs in river water of the north Tai Lake basin.
The in situ organic carbon-normalized partition coefficient (K’ oc) between SPM and water is defined in Eq. (1) as:
where POC is the fraction of particulate organic carbon; C s represents the particulate EDC concentration (μg/g); and Caq denotes the dissolved EDC concentration (μg/L). The unit of the partition coefficient (K d) is L/kg. The in situ log K’ oc values of phenolic EDCs in rivers and lake bays of the north Tai Lake basin were in the range of 3.74–6.53 for OP, 4.81–6.56 for NP, and 3.15–6.08 for BPA, with averages of 5.73, 5.74, and 5.10, respectively. Measured in situ log K’ oc of OP in this study was similar with the results found in the Elbe River, Germany (5.52) (Heemken et al. 2001), but they were one order of magnitude greater than the results from the rivers of Japan (4.65) (Isobe et al. 2001). Measured in situ log K’ oc values for NP in surface waters of the study area were close to those from other literature (5.24–5.86) (Heemken et al. 2001; Patrolecco et al. 2006). Similarly, in situ log K’ oc values of BPA in this study were also one order of magnitude greater than those measured in the Tiber River, central Italy (4.05–4.23) (Patrolecco et al. 2006) and the Pearl River, south China (4.34) (Gong et al. 2012). The results suggested that the measured in situ SPM–water log K’ oc values in this study were similar to those reported in other field studies. And, despite variations in environmental parameters like SPM component that exist among different rivers, the in situ log K’ oc of phenolic EDCs reported in different studies were always within a general range.
Adsorption of organic contaminants to SPM is related with particulate organic carbon content. POC (%) in all SPM samples from the rivers of the study area varied from 0.62 to 1.83 % with the mean value of 1.31 %. The significantly positive correlation between POC (%) and concentrations of particulate EDCs was found with correlation coefficients (r) of 0.46 (p > 0.05) for OP and 0.48 (p > 0.05) for NP. For BPA, the correlation coefficient was 0.18 (p ≥ 0.05), but became 0.57 (p ≥ 0.05) when the outliers (S6 and S7) were excluded (Fig. 3).The results indicated that SPM with higher organic carbon was likely to adsorb more EDCs, and particulate organic carbon played an important role in controlling EDCs adsorption to SPM in the rivers of the north Tai Lake basin.
The one-parameter linear free energy relationships (op-LFERs) model, which is based on first-order molecular connectivity indices, has been used to estimate the values of log K oc by using the octanol–water partitioning coefficient (log K ow). The log K ow represents selective affinity of hydrophobic chemicals to organic phases. Chemicals with higher log K ow have a greater possibility to be adsorbed onto organic matter. Relational equations between log K oc and log K ow for EDCs are written as follows (Isobe et al. 2001):
The predicated K oc values were 3.52, 3.81, and 2.94 for OP, NP, and BPA with the respective log K ow values of 4.12, 4.48 (Ahel and Giger 1993), and 3.31 (Staples et al. 2000). Evidently, all measured in situ log K’ oc values of OP, NP, and BPA in the surface water of the north Tai Lake basin were about two orders of magnitude greater than the predicted log K oc, indicating that affinity of phenolic EDCs to SPM was higher than that expected solely due to their hydrophobic nature.
Equilibrium partitioning of phenolic EDCs in SPM–water phases may be significantly influenced by other environmental parameters, such as hydrodynamics, in light of their water solubility of 5.40–120 mg/L (20°C) (Tan et al. 2007). The linear regression relationship between in situ SPM–water partition coefficients (log K’ oc) of phenolic EDCs and the velocity of river waters (μ) were plotted in Fig. 4. Strong positive correlations between in situ log K’ oc values and flow velocities for all three EDCs were found with coefficients (r) of 0.68–0.82 (p < 0.05). Moreover, almost all of the field samples were within the 95 % confidence intervals of the regression equations, showing that higher flow velocity, which ranged from 0.06 to 0.12 m/s, can cause more dissolved EDCs to adsorb onto SPM. It is conceivable that the dilution with freshwater caused not only higher flow velocity, but also lower concentrations of phenolic EDCs in the dissolved phase, which led to greater in situ log K’ oc values. Zhang et al. (2009) reported the contribution of dilution to the declines of OP and NP concentrations in the Jialu River of China were 57.8 % and 38.8 %, respectively, indicating that physical dilution is an important process to determine the concentrations of APs in water. As shown in Fig. 4, the slopes of the regression equations varied in order of BPA (35.5) > OP (26.2) > NP (19.0). Interestingly, changes of solubility in water (20°C) agreed well with the order of BPA (120 mg/L) > OP (12.6 mg/L) > NP (5.40 mg/L) (Tan et al. 2007). This result may indicate that the impact of hydrodynamics on field-measured in situ log K’ oc values were different for different hydrophobic chemicals. Additionally, adsorption kinetics for different compounds was related with respective solubility in water.
Assessment of log K oc becomes important in the prediction of the behavior and fate of organic pollutants in the environment by using a modeling approach. Therefore, correlation analysis between the average in situ log K’ oc and log K ow for three phenolic EDCs in surface waters of the north Tai Lake basin was carried out (Fig. 5):
The correlation equation in this study was similar to that in Eq. (2) derived from batch experiments in the laboratory, except for a lower slope (0.54) and a higher intercept (3.37). The intercept of this correlation equation represents the residual data variations, not modeled by log K ow, thus indicating that binding mechanisms other than hydrophobic effects may occur, such as hydrogen binding and π-π interactions (Gong et al. 2012). The lower slope also suggests that the in situ log K’ oc was less correlative with log K ow and disequilibrium between measured dissolved and particulate EDCs may exist in the surface water bodies of the north Tai Lake basin.
Overall, our results showed that the prediction of the SPM–water partitioning of phenolic EDCs in rivers of the north Tai Lake basin can be achieved based on the correlation equation of in situ log K’ oc with log K ow. However, field-measured in situ log K’ oc values were greatly influenced by hydrodynamics and thereby caution should be exercised in the application of the sorption data obtained by batch experiments and existing empirical equations from other literature in simulation of contaminant behavior and fate in dynamic river ecosystems.
References
Ahel M, Giger W (1993) Aqueous solubility of alkylphenols and alkylphenol polyethoxylates. Chemosphere 26:1461–1470
Düring RA, Krahe S, Gäth S (2002) Sorption behavior of nonylphenol in terrestrial soils. Environ Sci Technol 36:4052–4057
Gong J, Ran Y, Chen D, Yang Y, Zeng EY (2012) Association of endocrine-disrupting chemicals with total organic carbon in riverine water and suspended particulate matter from the Pearl River, China. Environ Toxicol Chem 31:2456–2464
Heemken O, Reincke H, Stachel B, Theobald N (2001) The occurrence of xenoestrogens in the Elbe River and the North Sea. Chemosphere 45:245–259
Huang Y, Wong C, Zheng J, Bouwman H, Barra R, Wahlström B, Neretin L, Wong M (2011) Bisphenol A (BPA) in China: a review of sources, environmental levels, and potential human health impacts. Environ Int 42:91–99
Isobe T, Nishiyama H, Nakashima A, Takada H (2001) Distribution and behavior of nonylphenol, octylphenol, and nonylphenol monoethoxylate in Tokyo metropolitan area: their association with aquatic particles and sedimentary distributions. Environ Sci Technol 35:1041–1049
Johnson A, White C, Besien T, Jürgens M (1998) The sorption potential of octylphenol, a xenobiotic oestrogen, to suspended and bed-sediments collected from industrial and rural reaches of three English rivers. Sci Total Environ 210:271–282
Kolpin DW, Skopec M, Meyer MT, Furlong ET, Zaugg SD (2004) Urban contribution of pharmaceuticals and other organic wastewater contaminants to streams during differing flow conditions. Sci Total Environ 328:119–130
Li D, Kim M, Shim WJ, Yim UH, Oh JR, Kwon YJ (2004) Seasonal flux of nonylphenol in Han River, Korea. Chemosphere 56:1–6
Navarro A, Endo S, Gocht T, Barth JAC, Lacorte S, Barceló D, Grathwohl P (2009) Sorption of alkylphenols on Ebro River sediments: comparing isotherms with field observations in river water and sediments. Environ Pollut 157:698–703
Patrolecco L, Capri S, De Angelis S, Pagnotta R, Polesello S, Valsecchi S (2006) Partition of nonylphenol and related compounds among different aquatic compartments in Tiber River (central Italy). Water Air Soil Pollut 172:151–166
Soares A, Guieysse B, Jefferson B, Cartmell E, Lester J (2008) Nonylphenol in the environment: a critical review on occurrence, fate, toxicity and treatment in wastewaters. Environ Int 34:1033–1049
Staples CA, Dorn PB, Kleĉka GM, O’Block ST, Branson DR, Harris LR (2000) Bisphenol A concentrations in receiving waters near US manufacturing and processing facilities. Chemosphere 40:521–525
Tan BLL, Hawker DW, Müller JF, Leusch FDL, Tremblay LA, Chapman HF (2007) Modelling of the fate of selected endocrine disruptors in a municipal wastewater treatment plant in South East Queensland, Australia. Chemosphere 69:644–654
Zhang YZ, Tang CY, Song XF, Li FD (2009) Behavior and fate of alkylphenols in surface water of the Jialu River, Henan Province, China. Chemosphere 77:559–565
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 41203081, 21277083, 41071164) and Central Level Scientific Research Institutes for Basic R&D Special Fund Business (GYK1291101). The authors also thank Kate Bentsen (Chinese Research Academy of Environmental Science) for editing the manuscript for grammar.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Yz., Meng, W. & Zhang, Y. Occurrence and Partitioning of Phenolic Endocrine-Disrupting Chemicals (EDCs) Between Surface Water and Suspended Particulate Matter in the North Tai Lake Basin, Eastern China. Bull Environ Contam Toxicol 92, 148–153 (2014). https://doi.org/10.1007/s00128-013-1136-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-013-1136-y