Abstract
Occurrence of five estrogens, including estrone (E1), 17β-estradiol (E2), estriol (E3), 17α-ethynylestradiol (EE2) and bisphenol A (BPA) in water, sediment and biota in Northern Taihu Lake, were investigated and their ecological risk was evaluated. Most of the target estrogens were widely distributed in the eight studied sampling sites, and their levels showed a regional trend of Gong Bay > Meiliang Bay > Zhushan Bay. The average concentrations of E1, E2, E3, EE2 and BPA ranged from 3.86 to 64.4 ng l−1, 44.3 to 64.1 μg kg−1 dry weight and 58.6 to 115 μg kg−1 dry weight in water, sediments and biota, respectively. In most cases, the average concentrations of BPA and E2 were higher than those of other estrogens. E1, E3 and EE2 were found to be accumulated in river snails with bioaccumulation factor values as high as 14,204, 35,327 and 20,127 l kg−1, respectively. E3 was also considered to be accumulated in clams. The evaluation of environmental risk showed that the occurrence of E2 and EE2 in lakes might pose a high risk to aquatic organisms. These findings provide important information for estrogen control and management in the studied area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In past decades, the occurrence of estrogens in aquatic environments has attracted increasing attention because they can exert their biological activity on non-targeted organisms and be harmful to the ecosystems and the health of animals and humans (Gineys et al. 2010). Many estrogens are continuously released into the environment through wastewater treatment plant (WWTP) effluents, surface non-point runoff of manure and sewage sludge used in agriculture, and atmospheric deposition of particulates and aerosols (Björkblom et al. 2008; Kuster et al. 2004). Estrogen residues have been widely detected in surface water, sediment, groundwater and aquatic biota. Although these compounds are usually detected in the environment at trace levels (Arditsoglou and Voutsa 2012; Isobe et al. 2006; Lei et al. 2009; Rocha et al. 2013; Shi et al. 2013), it has been proven that estrogens can induce toxic effects, for example, feminization of fishes, even at low ng g−1 concentrations (Froehner et al. 2012; Hernando et al. 2006). Thus, the characterization and risk assessment for estrogens are very necessary for their environmental control and management.
Taihu Lake is the third largest freshwater lake in China (Qin et al. 2007), located in the southeastern Yangtze River Delta with an average water depth of 1.9 m and a surface water area of 2,338 km2. Over 100 rivers discharge into the lake, and the surroundings comprise one of the most industrialized areas in China with high population density, urbanization and economic development. The lake water is used for agricultural and industrial purposes, and it is the source of drinking water for Shanghai, Suzhou and Wuxi. However, the water supply faces potential ecological and human risks due to the increasing population, industrial and agricultural development, and deteriorated water quality (Qiao et al. 2006). Estrogens, as one of the important pollutants, have been found in the aqueous phage of Taihu Lake. Shen et al. (2001) investigated hormone-disrupting activity in water collected at Meiliang Bay, Taihu Lake, and found that the estrogenic activities (estradiol equivalents: 2.20–12.1 ng l−1) were mainly caused by natural and synthetic hormones. Lu et al. (2011). Yan et al. (2012) detected environmental estrogens in Taihu Lake water and assessed the estrogenic biological effects and feminization risk in fish. The available research has focused on the measurement and assessment of estrogens in the aqueous phage. Given the relatively low polarity of estrogens, with octanol-water partition coefficients (K ow) mostly between 103 and 105 (Kuster et al. 2004), they are easily adsorbed into sediment and accumulated and magnified in the biota (Lai et al. 2002), which might pose a threat to ecological health. However, little literature on the status of estrogens in the sediment and biota in Taihu Lake is available. Thus, it is necessary to determine the levels of estrogens in the sediment and biota in Taihu Lake and to evaluate their potential ecological risk.
In this study, five estrogens, including estrone (E1), 17β-estradiol (E2), estriol (E3), 17α-ethynylestradiol (EE2) and bisphenol A (BPA), were chosen as target estrogens. E1, E2, E3 and EE2 are steroidal estrogens (Kuster et al. 2004); BPA, the monomer used in the manufacture of epoxy resins and polycarbonate plastics, is another example of important environmental estrogens (Sun et al. 2012). We simultaneously investigated their status in water, sediment, and biota in Northern Taihu Lake. Their concentrations were compared with other locations, and their bioaccumulation factors (BAFs) were calculated. Finally, their potential ecological risk to aquatic organisms was evaluated. The results will provide important information for estrogen control and management of this area.
Materials and methods
Study areas and sampling locations
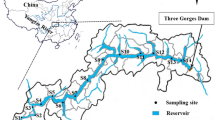
The sampling sites were set in several districts in the northern part of Taihu Lake according to the flow characteristics of Taihu Lake and the water transfer route of the diversion project. A total of three regions (8 sampling sites) were chosen, including Gong Bay (S1, the outlet of Wangyu River; S2, Xiaogongshan; S3, Dagongshan), Meiliang Bay (S4, Tuoshan; S5, Yuantoubao; S6, Zhihu Harbor) and Zhushan Bay (S7, Caoqiao River; S8, the outlet of Zhushan Bay). Their locations are shown in Fig. 1. The sampling was performed in May 2013.
Surface water, sediment and biota samples were collected simultaneously, placed on ice and transported to the laboratory as soon as possible. Eight water samples were collected in glass bottles that had been pre-cleaned and rinsed with methanol, stored at 4 °C and extracted within 48 h. The pH, dissolved oxygen and temperature of water samples are shown in Table S1. Eight sediment samples were collected with a Peterson grab sampler from 10 cm below the sediment surface. The sediments were freeze-dried, ground and sieved at 0.125 mm. For biota samples, fish and two mollusc species (river snail and clam) were collected in each sampling site. However, in S7, we did not find the clam. Thus, a total of 23 samples were found. Fish fillets as well as the visceral mass of crustaceans were homogenized separately. Sediment and biota samples were stored at −20 °C until further analysis.
Chemicals and materials
The standard mixture of five estrogens (each at 1 mg ml−1) in methanol was purchased from Sigma-Aldrich (St. Louis, MO, USA). The deuterated internal standard was 5 mg ml−1 [2, 4, 16, 16-2H4] 17β-estradiol (E2-d4) in methanol, purchased from J&K Chemical, Ltd. (Beijing, China). HPLC grade methanol (MeOH), acetonitrile (ACN), hexane and acetone were purchased from Merck (Darmstadt, Germany). Analytical grade ammonia, ammonium acetate and hydrochloric acid were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Neutral alumina (75–147 μm) was purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Before usage, it was heated in a muffle furnace at 450 °C for 4 h. All standards and working solutions were stored at 4 °C in silanized brown glass bottles with Teflon-lined caps. Deionized water was obtained from a Milli-Q water purification system (Millipore, Bedford, MA).
Analytical procedures
Water sample preparation and analysis
First 50 ng E2-d4 was added to a 0.5-l water sample as the internal standard, and the pH was adjusted to 4 with diluted hydrochloric acid. Then the sample was vacuum-filtered through a 0.45-μm glass fiber filter to remove particles. An Oasis HLB (hydrophilic-lipophilic balance) cartridge (500 mg, 6 ml, Waters, USA) was used to extract analytes. Before extraction, the cartridge was sequentially pre-conditioned with 5 ml MeOH and 10 ml deionized water. The sample was passed through the cartridge at a flow rate of about 5 ml min−1. Then the cartridge was vacuum dried for 30 min to remove excess water. The analytes were eluted with 10 ml ACN containing 5 % ammonia at a flow rate of about 1 ml min−1. The eluate was concentrated to 1 ml by a quantitative concentrator (Buchi R-200, Flawil, Switzerland). The analysis was conducted using a high-performance liquid chromatography/tandem mass spectrometry (HPLC/MS/MS) system. The performance parameters are shown in Table S2.
Sediment sample preparation and analysis
The pretreated sediment (5 g) was placed in a 20-ml amber vial and spiked with 250 ng E2-d4 as internal standard. The mixture was blended and soaked in 10 ml MeOH that was evaporated overnight. The internal standard method was performed following the methods developed by Salvia et al. (2012) and Ding et al.(2011) with minor modification. Then the mixture was placed in a 22-ml stainless steel extraction cell and mixed with diatomaceous earth to increase the contact-surface between the sediment particles and extraction solvent and prevent clogging of the extraction cell.
Extraction was performed by pressurized liquid extraction (PLE) using a Dionex ASE 350 (Dionex, Germany). Optimized extraction conditions were as follows: extraction solvents, acetone/MeOH (1:1, v:v); extraction temperature, 100 °C; static extraction time, 10 min; number of cycles, 2; flush volume, 60 %; nitrogen purge time, 120 s. The obtained 25 ml extract was concentrated to about 1 ml by a quantitative concentrator and then was mixed with 200 ml of deionized water. Subsequent depuration of extract was performed by the methods used in water samples. Finally, the eluate was concentrated to 1 ml. The analysis was carried out using HPLC/MS/MS (Table S2).
Biota sample preparation and analysis
Freeze-dried biota sample (1 g) was spiked with E2-d4 (50 µg kg−1), mixed with diatomaceous earth and placed in a 22-ml stainless steel extraction cell containing 5 g neutral alumina. Different PLE conditions were tested, and the optimized conditions were as follows: extraction solvents, ACN/MeOH (1:1, v:v); extraction temperature, 60 °C; static extraction time, 5 min; number of cycles, 3; flush volume, 60 %; nitrogen purge time, 120 s. After extraction, approximately 40 ml of extract was stored at −20 °C overnight to make fatty deposits. The deposited fat was removed by passing through a 0.22-μm polytetrafluoroethylene filter. Then the extract was concentrated to about 20 ml by a quantitative concentrator and mixed thoroughly with 20 ml of ACN and hexane. The mixture was allowed for the decantation, and the supernatant was abandoned. This step was repeated once. Finally, the extract was concentrated to 1 ml and analyzed by HPLC/MS/MS (Table S2).
The BAF was used to quantitatively describe bioaccumulation in this study. The BAF is defined as the ratio of the target compound concentration in the aquatic organisms to the concentration in water. The weight of the organism is presented on a wet weight basis and the unit of BAF is l kg−1 (Arnot and Gobas 2006). Water contents of the river snail, clam and fish samples are 80.4, 79.7 and 84.9 %, respectively.
Quality control
The concentrations of different compounds and matrices were determined using an internal standard method. Calibration curves of method were verified by analyzing seven concentrations of mixture standard solutions with linearity in the range of 1–1,000 ng l−1, 1–500 and 1–500 μg kg−1 for water, sediment and fish, respectively. The linearity of calibration curve was confirmed. The relative standard deviations (RSDs) were determined by triplicate analyses of samples spiked at 100 ng l−1, 50 and 50 μg kg−1 for water, sediment and fish, respectively. The RSD values were less than 14.7 %, indicating satisfactory precision. The limits of detection (LODs) were calculated at a signal-to-noise ratio (S/N) of 3. The linearity range, correlation coefficient and LODs are listed in Table 1.
Results and discussion
Estrogens in water
Concentrations of the target estrogens in the surface water in Northern Taihu Lake are listed in Table 2. The concentrations of E2 ranged from 40.0 to 117 ng l−1, with the highest mean concentration (65.4 ng l−1), which was slightly higher than that of BPA (64.4 ng l−1). However, BPA had the highest maximum concentration up to 194 ng l−1, far higher than E2 (117 ng l−1). The frequent detection of BPA probably results from the extensive manufacturing, usage and industrial discharge in the Northern Taihu basin. Although the detection frequency of E1 was high, E1 had relatively low concentrations ranging from 1.81 to 28.8 ng l−1. On the contrary, although EE2 concentrations in the aqueous phase were higher than those of E1, EE2 was detected less frequently. The possible reason is that EE2 is easily transformed into phase II metabolites (conjugates of glucuronic acid and sulfate) in the liver of the organism and expelled into the intestines or gut (Blewett et al. 2013). The concentration and detection frequency of E3 were similar to those of EE2. The infrequent detection of E3 is possibly due to the degradation of E3 in the aquatic environment (Gomes et al. 2004). Similar detection frequencies of EE2 and E3 were also observed in Jiulongjiang River (Zhang et al. 2012), in which EE2 was not detected in any samples from Jiulongjiang River, and the detection frequency of E3 was only 16 %. However, the results were not exactly consistent with the previous study in Taihu Lake (Yan et al. 2012), in which E2, E3 and BPA were detected at all sites, while the others were detected at most sites.
The total concentrations of estrogens at different positions were quite different. The spatial differences might be due to the water in the lake. The diverting water from the Yangtze River enters into the lake from Gong Bay (S1, S2 and S3). High estrogen concentrations were detected in the Yangtze Rive, which might be one of the results of high estrogen concentrations in this bay (Hu et al. 2013). S1 was located at the outlet of Wangyu River, which has the highest estrogen levels. Due to dilution, the concentrations of ∑5ES at S2 and S3 were lower. The Meiliang Bay (S4, S5, and S6) and Zhushan Bay (S7 and S8) are near the Wuxi and Yixing, respectively. They receive a large amount of effluent from WWTPs as well as untreated domestic sewage (Shen et al. 2001), which might be the main source of estrogens in both bays (Zhang et al. 2014).
The occurrence of estrogens has been widely reported in surface water around the world (Table S3). In this study, the concentrations of estrogens in Northern Taihu Lake were slightly higher than those in rivers in Portugal (Rocha et al. 2013), the Aegean Sea in Greece (Arditsoglou and Voutsa 2012) and lakes in Korea (Kim et al. 2007), but much lower than in streams in the USA (Kolpin et al. 2002). In comparison to other locations in China, the levels of estrogens in Taihu Lake were much lower than in the Jiulongjiang River (Zhang et al. 2012), higher than in the Yellow River (Wang et al. 2012) and similar to the rivers in Tianjin (Rao et al. 2013). Compared with other available investigations on estrogens in Taihu Lake (Shen et al. 2001; Yan et al. 2012), the results of the present study were slightly higher.
Estrogens in the sediments
Concentrations of estrogens in surface sediments in Northern Taihu Lake are shown in Table 2. All estrogens were detected at all sites. Concentrations of E1, E2, E3, EE2 and BPA ranged from 5.49 to 164 μg kg−1 (mean 44.3 μg kg−1, dw), 9.78 to 151 μg kg−1 (mean 46.0 μg kg−1, dw), 8.66 to 203 μg kg−1 (mean 58.2 μg kg−1, dw), 4.32 to 184 μg kg−1 (mean 45.5 μg kg−1, dw) and 6.31 to 291 μg kg−1 (mean 64.1 μg kg−1, dw), respectively. The ∑5ES levels in sediment samples (as well as water and animals) collected from Gong Bay showed a trend of S1 > S2 > S3. In addition, the concentration of ∑5ES at S1 was also the highest among the eight sediment samples (Table 2), which was the same as their concentrations in water. Since estrogens have low polarity and high octanol-water partition coefficients, they are easily adsorbed into the sediment (Lei et al. 2009; Zhang et al. 2014). Thus, the high estrogen level in the sediment might be from water. However, no linear relationships were found between the estrogen levels in water and sediments in this study. Several studies suggested that the sorption of estrogens onto sediments had a relationship with the properties of estrogens (such as the octanol-water partition coefficient and solubility in water) and sediment [such as the organic carbon content and particle size (Sun et al. 2012)], pH and salinity of the water (Li et al. 2007) and coexisting substances [such as organic compounds (Yu and Huang 2005), heavy metals and surface-active compounds (Li et al. 2007)] in water. The high concentrations in the sediment of North Taihu Lake might be due to these conditions, which need to be further analyzed.
There are many studies in the literature on the status of estrogens in sediments (Table S4). Compared with these investigations, higher concentrations of estrogens were found in this study. The EE2 concentrations were much higher than those in the Aegean Sea (Arditsoglou and Voutsa 2012), Brazilian mangrove sediments (Froehner et al. 2012), Tokyo Bay (Isobe et al. 2006) and rivers in Tianjin, China (Lei et al. 2009). Although the BPA concentrations were lower than those in the Pearl River Delta, China (Gong et al. 2011), the concentrations in this study were still high.
Estrogens in biota samples
Estrogens with high sorption efficiencies are predicted to be bioaccumulated in aquatic and terrestrial species (Nallani et al. 2012). However, limited studies on their occurrence in fish and other aquatic organisms are available (Liu et al. 2011). Concentrations of estrogens in the biota from Taihu Lake are shown in Table 2. As can be seen, the concentrations of estrogens in biota samples collected at S1 ranged from 364 to 562 μg kg−1, which were much higher than those collected at other sites in Northern Taihu Lake. The high concentrations might be due to the high estrogen levels in water and sediment. The average concentration of E2 (117 μg kg−1) was found to be the highest among the five estrogens, while the level of E1 (58.6 μg kg−1) was the lowest. The concentrations of estrogens in different species at each site are shown in Fig. 2. E1, E2 and BPA were detected in all 23 samples, while the detection frequencies of EE2 and E3 were 91.3 and 65.2 %, respectively. River snails had the highest estrogen concentrations in S1. However, in S7 and S8, fish had the highest estrogen concentrations. The S1 had the highest estrogen concentrations in sediment (Table 2). Snails live in sediment; thus, the estrogen concentrations in sediment might have major influences on its accumulation in snails. On the other side, in S7 and S8, the estrogen concentrations in sediment were very low, and the estrogen level in snails in this site was also very low.
The calculated BAFs are shown in Table 3. Because the bioaccumulation is species-specific, significant differences were observed for the BAFs of the three aquatic species, however, it showed the following trend: river snail > clam > fish. BAFs did not increase with the trophic level; this result was consistent with the study of Lai et al. (2002). The bioaccumulation is also dependent on a range of factors such as gender, reproductive status, life stage, size as well as the environmental conditions (Liu et al. 2011). Therefore, it is difficult to compare BAFs from one location to another. In general, if BAFs are greater than 5,000 in aquatic organisms, the chemical is considered to be “bioaccumulative” (Kelly and Gobas 2001). In the present study, E3, EE2 and E1 were found to be bioaccumulated in river snail, because their BAFs were as high as 35,327, 20,127 and 14,204 l kg−1, respectively. The BAF of E3 in clams was 7,539 l kg−1, so the compound was also considered to be bioaccumulated in clams.
Ecological risk assessment
The possible risk from the presence of estrogens in water was also evaluated in the present study on the basis of the calculated risk quotient (RQ). RQs were expressed as the ratio of the measured environmental concentrations (MECs) to the predicted no-effect concentrations (PNECs). Maximum concentrations detected in surface water were used in this study. An assessment factor of 1,000 was used for the determination of PNECs from the lowest values of EC50 or LC50 or NOEC obtained from the literature (Hernando et al. 2006; Kolpin et al. 2002; Martin et al. 2012; Roepke et al. 2005; Rose et al. 2002; Stasinakis et al. 2012; Tompsett et al. 2013), where EC50, LC50 and NOEC are the median effect concentration, median lethal concentration and non-observed effect concentration, respectively.
Different risk assessment levels were established as follows. Compounds with an RQ lower than 0.1 pose a low risk to aquatic organisms, a medium risk for values between 0.1 and 1, and a high risk for values higher than 1 (Hernando et al. 2006; Verlicchi et al. 2012). As shown in Table 4, the RQ values from E2 and EE2 were 9,070 and 4,653, respectively, which are responsible for the highest environmental risk and suggest high risk to aquatic organisms. BPA exhibited medium risk with an RQ value of 0.071, although its MEC was the highest among the five estrogens. E1 exhibited medium risk as well. The RQ value of E3 was 0.015, so low risk was suspected to occur in Taihu Lake. Sun et al. (2013) collected the MEC data from the scientific papers published in the last decade, then evaluated the ecological risk of eight typical estrogenic EDCs in effluents from sewage plants. They found EE2, E2 and E1 had high ecological risk. The RQ of EE2 was highest and ranged from 10 to 103. The RQ of E3 was lowest in the four steroidal estrogens. BPA had smaller RQ values compared with steroidal estrogens. These results and trends were consistent with our present study. It should be noted that the toxicity of estrogens might exhibit a difference due to synergistic or antagonistic effects. In this study, RQs were calculated for individual compounds.
Conclusions
Among five studied estrogens, E1, E2 and BPA were widely distributed in water, while E3 and EE2 were less frequently detected. All the target estrogens were widely found in sediments and biota. The concentrations of estrogens in sediments collected from Gong Bay were relatively higher than those from other locations. E1, E3 and EE2 were considered to be accumulated in river snails with values of BAF higher than 5,000, and E3 was predicted to be accumulated in clams. The risk assessment showed that E2 and EE2 might pose high risk to aquatic organisms. This study provided the first information on the status and potential risk of estrogens in Northern Taihu, which is very useful for the environmental management of estrogens in the studied area.
References
Arditsoglou, A., & Voutsa, D. (2012). Occurrence and partitioning of endocrine-disrupting compounds in the marine environment of Thermaikos Gulf, Northern Aegean Sea, Greece. Marine Pollution Bulletin, 64(11), 2443–2452.
Arnot, J. A., & Gobas, F. A. (2006). A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environmental Reviews, 14(4), 257–297.
Björkblom, C., Salste, L., Katsiadaki, I., Wiklund, T., & Kronberg, L. (2008). Detection of estrogenic activity in municipal wastewater effluent using primary cell cultures from three-spined stickleback and chemical analysis. Chemosphere, 73(7), 1064–1070.
Blewett, T., MacLatchy, D. L., & Wood, C. M. (2013). The effects of temperature and salinity on 17-α-ethynylestradiol uptake and its relationship to oxygen consumption in the model euryhaline teleost (Fundulus heteroclitus). Aquatic Toxicology, 127, 61–71.
Ding, Y., Zhang, W., Gu, C., Xagoraraki, I., & Li, H. (2011). Determination of pharmaceuticals in biosolids using accelerated solvent extraction and liquid chromatography/tandem mass spectrometry. Journal of Chromatography A, 1218(1), 10–16.
Froehner, S., Machado, K. S., Stefan, E., Bleninger, T., da Rosa, E. C., & de Castro Martins, C. (2012). Occurrence of selected estrogens in mangrove sediments. Marine Pollution Bulletin, 64(1), 75–79.
Gineys, N., Giroud, B., & Vulliet, E. (2010). Analytical method for the determination of trace levels of steroid hormones and corticosteroids in soil, based on PLE/SPE/LC-MS/MS. Analytical and Bioanalytical Chemistry, 397(6), 2295–2302.
Gomes, R., Avcioglu, E., Scrimshaw, M., & Lester, J. (2004). Steroid-estrogen determination in sediment and sewage sludge: a critique of sample preparation and chromatographic/mass spectrometry considerations, incorporating a case study in method development. Trac-Trends in Analytical Chemistry, 23(10), 737–744.
Gong, J., Ran, Y., Chen, D.-Y., & Yang, Y. (2011). Occurrence of endocrine-disrupting chemicals in riverine sediments from the Pearl River Delta, China. Marine Pollution Bulletin, 63(5), 556–563.
Hernando, M. D., Mezcua, M., Fernandez-Alba, A. R., & Barcelo, D. (2006). Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta, 69(2), 334–342.
Hu, X., Shi, W., Cao, F., Hu, G., Hao, Y., Wei, S., et al. (2013). Bioanalytical and instrumental analysis of estrogenic activities in drinking water sources from Yangtze River Delta. Chemosphere, 90(7), 2123–2128.
Isobe, T., Serizawa, S., Horiguchi, T., Shibata, Y., Managaki, S., Takada, H., et al. (2006). Horizontal distribution of steroid estrogens in surface sediments in Tokyo Bay. Environmental Pollution, 144(2), 632–638.
Kelly, B. C., & Gobas, F. (2001). Bioaccumulation of persistent organic pollutants in lichen-caribou-wolf food chains of Canada’s Central and Western Arctic. Environmental Science and Technology, 35(2), 325–334.
Kim, S. D., Cho, J., Kim, I. S., Vanderford, B. J., & Snyder, S. A. (2007). Occurrence and removal of pharmaceuticals and endocrine disruptors in South Korean surface, drinking, and waste waters. Water Research, 41(5), 1013–1021.
Kolpin, D. W., Furlong, E. T., Meyer, M. T., Thurman, E. M., Zaugg, S. D., Barber, L. B., et al. (2002). Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: A national reconnaissance. Environmental Science and Technology, 36(6), 1202–1211.
Kuster, M., José López de Alda, M., & Barceló, D. (2004). Analysis and distribution of estrogens and progestogens in sewage sludge, soils and sediments. Trac-Trends in Analytical Chemistry, 23(10), 790–798.
Lai, K. M., Scrimshaw, M. D., & Lester, J. N. (2002). Prediction of the bioaccumulation factors and body burden of natural and synthetic estrogens in aquatic organisms in the river systems. Science of the Total Environment, 289(1–3), 159–168.
Lei, B., Huang, S., Zhou, Y., Wang, D., & Wang, Z. (2009). Levels of six estrogens in water and sediment from three rivers in Tianjin area, China. Chemosphere, 76(1), 36–42.
Li, J., Zhou, B., Shao, J., Yang, Q., Liu, Y., & Cai, W. (2007). Influence of the presence of heavy metals and surface-active compounds on the sorption of bisphenol A to sediment. Chemosphere, 68(7), 1298–1303.
Liu, J., Wang, R., Huang, B., Lin, C., Wang, Y., & Pan, X. (2011). Distribution and bioaccumulation of steroidal and phenolic endocrine disrupting chemicals in wild fish species from Dianchi Lake, China. Environmental Pollution, 159(10), 2815–2822.
Lu, G., Yan, Z., Wang, Y., & Chen, W. (2011). Assessment of estrogenic contamination and biological effects in Lake Taihu. Ecotoxicology, 20(5), 974–981.
Martin, J., Camacho-Munoz, D., Santos, J. L., Aparicio, I., & Alonso, E. (2012). Occurrence of pharmaceutical compounds in wastewater and sludge from wastewater treatment plants: Removal and ecotoxicological impact of wastewater discharges and sludge disposal. Journal of Hazardous Materials, 239–240, 40–47.
Nallani, G. C., Paulos, P. M., Venables, B. J., Edziyie, R. E., Constantine, L. A., & Huggett, D. B. (2012). Tissue-specific uptake and bioconcentration of the oral contraceptive norethindrone in two freshwater fishes. Archives of Environmental Contamination and Toxicology, 62(2), 306–313.
Qiao, M., Wang, C., Huang, S., Wang, D., & Wang, Z. (2006). Composition, sources, and potential toxicological significance of PAHs in the surface sediments of the Meiliang Bay, Taihu Lake, China. Environment International, 32(1), 28–33.
Qin, B., Xu, P., Wu, Q., Luo, L., & Zhang, Y. (2007). Environmental issues of lake Taihu, China. Hydrobiologia, 581(1), 3–14.
Rao, K., Lei, B., Li, N., Ma, M., & Wang, Z. (2013). Determination of estrogens and estrogenic activities in water from three rivers in Tianjin, China. Journal of Environmental Sciences (China), 25(6), 1164–1171.
Rocha, S., Domingues, V. F., Pinho, C., Fernandes, V. C., Delerue-Matos, C., Gameiro, P., et al. (2013). Occurrence of Bisphenol A, Estrone, 17 beta-Estradiol and 17 alpha-Ethinylestradiol in Portuguese Rivers. Bulletin of Environmental Contamination and Toxicology, 90(1), 73–78.
Roepke, T. A., Snyder, M. J., & Cherr, G. N. (2005). Estradiol and endocrine disrupting compounds adversely affect development of sea urchin embryos at environmentally relevant concentrations. Aquatic Toxicology, 71(2), 155–173.
Rose, J., Holbech, H., Lindholst, C., Norum, U., Povlsen, A., Korsgaard, B., et al. (2002). Vitellogenin induction by 17beta-estradiol and 17alpha-ethinylestradiol in male zebrafish (Danio rerio). Comparative Biochemistry and Physiology. Toxicology and Pharmacology, 131(4), 531–539.
Salvia, M.-V., Vulliet, E., Wiest, L., Baudot, R., & Cren-Olivé, C. (2012). Development of a multi-residue method using acetonitrile-based extraction followed by liquid chromatography–tandem mass spectrometry for the analysis of steroids and veterinary and human drugs at trace levels in soil. Journal of Chromatography A, 1245, 122–133.
Shen, J., Gutendorf, B., Vahl, H., Shen, L., & Westendorf, J. (2001). Toxicological profile of pollutants in surface water from an area in Taihu Lake, Yangtze Delta. Toxicology, 166(1), 71–78.
Shi, W., Hu, G., Chen, S., Wei, S., Cai, X., Chen, B., et al. (2013). Occurrence of estrogenic activities in second-grade surface water and ground water in the Yangtze River Delta, China. Environmental Pollution, 181, 31–37.
Stasinakis, A. S., Mermigka, S., Samaras, V. G., Farmaki, E., & Thomaidis, N. S. (2012). Occurrence of endocrine disrupters and selected pharmaceuticals in Aisonas River (Greece) and environmental risk assessment using hazard indexes. Environmental Science and Pollution Research International, 19(5), 1574–1583.
Sun, Y., Huang, H., Sun, Y., Wang, C., Shi, X.-L., Hu, H. Y., et al. (2013). Ecological risk of estrogenic endocrine disrupting chemicals in sewage plant effluent and reclaimed water. Environmental Pollution, 180, 339–344.
Sun, K., Jin, J., Gao, B., Zhang, Z., Wang, Z., Pan, Z., et al. (2012). Sorption of 17α-ethinyl estradiol, bisphenol A and phenanthrene to different size fractions of soil and sediment. Chemosphere, 88(5), 577–583.
Tompsett, A. R., Wiseman, S., Higley, E., Giesy, J. P., & Hecker, M. (2013). Effects of exposure to 17alpha-ethynylestradiol during larval development on growth, sexual differentiation, and abundances of transcripts in the liver of the wood frog (Lithobates sylvaticus). Aquatic Toxicology, 126, 42–51.
Verlicchi, P., Al Aukidy, M., & Zambello, E. (2012). Occurrence of pharmaceutical compounds in urban wastewater: removal, mass load and environmental risk after a secondary treatment—a review. Science of the Total Environment, 429, 123–155.
Wang, L., Ying, G–. G., Chen, F., Zhang, L. J., Zhao, J. L., Lai, H. J., et al. (2012). Monitoring of selected estrogenic compounds and estrogenic activity in surface water and sediment of the Yellow River in China using combined chemical and biological tools. Environmental Pollution, 165, 241–249.
Yan, Z., Lu, G., Liu, J., & Jin, S. (2012). An integrated assessment of estrogenic contamination and feminization risk in fish in Taihu Lake, China. Ecotoxicology and Environmental Safety, 84, 334–340.
Yu, Z., & Huang, W. (2005). Competitive sorption between 17α-ethinyl estradiol and naphthalene/phenanthrene by sediments. Environmental Science and Technology, 39(13), 4878–4885.
Zhang, Z., Ren, N., Kannan, K., Nan, J., Liu, L., Ma, W., et al. (2014). Occurrence of endocrine-disrupting phenols and estrogens in water and sediment of the Songhua river, northeastern China. Archives of Environmental Contamination and Toxicology, 66(3), 361–369.
Zhang, X., Zhang, D., Zhang, H., Luo, Z., & Yan, C. (2012). Occurrence, distribution, and seasonal variation of estrogenic compounds and antibiotic residues in Jiulongjiang River, South China. Environmental Science and Pollution Research, 19(5), 1392–1404.
Acknowledgments
This research work was financially supported by the National Natural Science Foundation of China (no. 51109062), China Postdoctoral Science Foundation (no. 2012M511674), China Postdoctoral Science Special Foundation (No. 2013T60496), Fundamental Research Funds for the Central Universities of Hohai University (no. b11020087) and the Postdoctoral Science Foundation of Hohai University (No. 411102).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Wang, Q., Hu, L. et al. Occurrence of estrogens in water, sediment and biota and their ecological risk in Northern Taihu Lake in China. Environ Geochem Health 37, 147–156 (2015). https://doi.org/10.1007/s10653-014-9637-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-014-9637-0