Abstract
Silver nanoparticles (AgNPs) are among the most produced nanomaterials in the world and are incorporated into several products due to their biocide and physicochemical properties. Since freshwater bodies are AgNPs main final sink, several consequences for biota are expected to occur. With the hypothesis that AgNPs can interact with environmental factors, we analyzed their ecotoxicity in combination with humic acids and algae. In addition to the specific AgNPs behavior in the media, we analyzed the mortality, growth, and phototactic behavior of Chydorus eurynotus (Cladocera) as response variables. While algae promoted Ag+ release, humic acids reduced it by adsorption, and their combination resulted in an intermediated Ag+ release. AgNPs affected C. eurynotus survival and growth, but algae and humic acids reduced AgNPs lethality, especially when combined. The humic acids mitigated AgNP effects in C. eurynotus growth, and both factors improved its phototactic behavior. It is essential to deepen the study of the isolated and combined influences of environmental factors on the ecotoxicity of nanoparticles to achieve accurate predictions under realistic exposure scenarios.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanoparticle (NP) production has increased and diversified exponentially in recent years due to the variety of advantages that these novel materials possess, such as their reduced size (<100 nm), and therefore, high area/volume ratio, which enhances their reactivity and other physicochemical properties (Salem et al. 2022). Silver nanoparticles (AgNPs) are one of the most produced and applied nanomaterials due to their unique biocide and physicochemical properties (Zahoor et al. 2021; Corsi et al. 2022). AgNP production has diversified in a wide range of applications such as medicine, including sanitizing products, medical clothing, and masks used during the COVID-19 pandemic (Al-Radadi and Abu-Dief 2022; Jamunkar et al. 2022; Ahmed et al. 2022), catalysis, electrical and optical devices, food industry, paints, textiles, cosmetics, water treatment, and agriculture (Antezana et al. 2021; Islam et al. 2021; Municoy et al. 2021). Global AgNP production is continuously growing and is expected to reach 800 tons per year by 2025 (Pulit-Prociak and Banach 2016). The main discharge sources of AgNPs are industrial, domestic, and hospital effluents, while surface water bodies are their final sink (Islam et al. 2021). In this context, concern about their environmental fate and toxicity has increased in recent years.
Given the absence of accessible methods to measure AgNPs in natural matrices, the available information regarding environmental concentrations is based on predictions. Therefore, this information is highly variable, as predicted AgNP concentrations in surface water bodies range between 9 × 10−5 and 1.51 μg L−1 (NanoFATE; Batley et al. 2013; Sun et al. 2014, 2016), with some studies reporting up to 10 μg L−1 (Gottschalk et al. 2013). However, predicted environmental concentrations should be interpreted carefully as they are based on static models that do not consider the fast development of AgNP production, or their complete life cycle (Sun et al. 2016). Several reports showed that AgNPs can exert toxic effects on aquatic organisms at levels below the predicted environmental concentrations (Ale et al. 2018a, 2018b; Cazenave et al. 2019; Gutierrez et al. 2021; Andrade et al. 2023). Zooplankton is ubiquitous in almost every freshwater ecosystem, and contributes to nutrients and energy cycling, acting as an intermediate link between primary producers and predators such as fishes, insect larvae, crabs, and shrimps, among others (Mano and Tanaka 2016). Organisms from this community, mainly cladocerans, have been largely used in ecotoxicological studies since they have high sensitivity to environmental changes and xenobiotics (Resh 2008; Ferdous and Muktadir 2009). Previous studies showed that AgNPs are considerably toxic for cladocerans, reporting several effects such as mortality (0.18–1.5 μg L−1), decreased reproduction and growth (0.07–1.25 μg L−1), and gene expression changes (0.43–1.5 μg L−1) (Park and Choi 2010; Poynton et al. 2012; Ribeiro et al. 2014; Hartmann et al. 2019; da Silva et al. 2021; Andrade et al. 2023). Nevertheless, most of the toxicological information refers to Daphnia magna (Gutierrez et al. 2021; Wang and Liu 2022). Although this species is useful for comparative purposes (Liu et al. 2022), it represents neither the variety of functional traits of zooplankton nor holotropical region (Barnett et al. 2007; Gutierrez et al. 2021). Within Cladocera, Chydoridae is the most diverse taxonomic group, including 40 genera and 269 currently known species (43% of cladoceran species) (Sacherová and Hebert 2003; Forró et al. 2008). These organisms differ from daphniids in morphology, physiology, and habitat. They inhabit littoral-benthic zones of freshwater systems which receive the highest load of anthropogenic pollutants (Wang et al. 2017; Zhao et al. 2021; Mergia et al. 2022). Chydorus eurynotus (Sars, 1901) is a representative of this family and has a wide distribution and ubiquity in freshwater systems. This species, together with C. sphaericus, was employed in several toxicological studies, demonstrating its high potentiality as test organism (Monkiédjé et al. 2000; Wang et al. 2012, 2015; Le and Peijnenburg 2013; Song et al. 2015; Yu et al. 2021).
When AgNPs reach aquatic ecosystems, their behavior may vary depending on both the nanoparticles intrinsic properties and environmental factors (Levard et al. 2012; Furtado et al. 2016; Islam et al. 2021). It has been documented that dissolved organic matter (DOM), mainly humic acids, can adsorb and stabilize AgNPs, and reduce their Ag+ release (Ale et al. 2021; Liu et al. 2021; Wang and Liu 2022). DOM can promote AgNPs persistence in water and reduce their bioavailability to freshwater organisms such as Daphniidae (Gao et al. 2009; McLaughlin and Bonzongo 2012; Poda et al. 2013). Wang et al. (2015) reported the mitigation by humic substances of the acute AgNP toxicity on C. sphaericus. However, the effect of these organic substances on the chronic toxicity of NPs on cladocerans has not been reported yet.
Besides humic acids, AgNPs can interact with algae populations in freshwater bodies, which promote Ag+ release mainly due to the oxidation capacity of their exudates (Navarro et al. 2015; Chen et al. 2019; Ponton et al. 2019). Nevertheless, algae have also been reported to reduce AgNP toxicity in Daphniidae, possibly due to both energy supply and interactions with AgNPs (Ribeiro et al. 2014; Harmon et al. 2017; Stevenson et al. 2022; Andrade et al. 2023).
As the effects of combined environmental factors on AgNP behavior and toxicity have been poorly studied, the aims of the present study were to (i) analyze the behavior of AgNPs in presence of humic acids, algae, and their combination; and (ii) analyze the effects of AgNPs on the mortality, growth, and phototactic behavior of C. eurynotus in the presence of humic acids, algae, and their combination.
Materials and methods
Materials and reagents
AgNPs were provided by Nanotek S.A. (nanArgen®, CAS no. 7440-22-4), purity ≥ 99.0%. The capping agent was made of glucose oligomers (mainly nanocrystalline cellulose) and the stabilizing agent was made of polyvinyl pyrrolidone (PVP). Regarding humic acids, a sodic humic salt was purchased from Sigma-Aldrich (Argentina).
Test organisms
Chydorus eurynotus were collected from a shallow lake of the Paraná River alluvial plain. In the laboratory, it was isolated and gradually adapted to experimental conditions for one month. The neonates for the experiments were developed from one parthenogenetic female isolated from the initial stock culture. Laboratory conditions consisted of 12/12 day/night photoperiod, 21 ± 1 °C, and dechlorinated and aerated tap water (pH: 7.1, conductivity: 1020 µs cm−1, total hardness: 180 mg L−1 CaCO3, alkalinity 120 mg L−1 CaCO3, 39 mg L−1 Ca++, 20 mg L−1 Mg++, 146 mg L−1 HCO3−). Media was completely changed twice a week and organisms were fed with Tetradesmus obliquus algae three times a week (APHA 2017).
T. obliquus was isolated from a shallow lake of the Parana River basin. A monospecific culture was grown in sterile conditions in modified Detmer medium for green algae (Watanabe 1960) (KCl: 50, KH2PO4: 50, Ca (NO3)2-4H2O: 360, MgSO4-7H2O: 360, Cl3Fe+: 5, C4H6O6: 5, H3BO3, 2.86, MnCl2-4H2O: 1.81, ZnSO4-7H2O: 0.23, Cl2Cu: 0.05 mg L−1), at 25 °C, with warm-white LED light (50 μmol m2 s−1), and constant aeration. Algae were cropped in the exponential growth phase, resuspended in sterile distilled water, and stocked in the dark at −4 °C. Cell concentration was estimated using an optical microscope (Nikon E100) with a Neubauer chamber.
NPs behavior
UV–Vis spectroscopy was performed using a Jasco V-730 Spectrophotometer (Jasco Analitica Spain, Madrid, Spain) for AgNPs in ultrapure water, in culture water, and with the addition of humic acids. Under the same media conditions, AgNPs were characterized through transmission electron microscopy (TEM) and Fourier transform infrared spectroscopy (FT-IR), obtained over the range of 4000–500 cm−1 using an FTIR-Raman Nicolet iS 50 (Thermo Scientific).
The Ag+ released from the particles was analyzed during 48 h in ultrapure water and each experimental condition: culture water (W), algae added (WA), humic acids added (WH), and algae and humic acids added (WAH). Briefly, 1 mL of the solution was placed in the upper chamber of Vivaspin® 20 centrifugal concentrator (30 kDa molecular weight cutoff ≈4 nm, Sartorius Stedim Biotech GmbH, Göttingen, Germany) and centrifuged at 5000 rpm for 15 s at 25 °C (Antezana et al. 2021; Municoy et al. 2021). In this way, the nanoparticles remained in the upper chamber, while the aqueous filtrate contained the released Ag+. The concentration of Ag in the filtrate was measured consecutively in time during 48 h by atomic absorption spectroscopy. Cumulative doses were calculated using a standard curve and expressed as a function of time. In all cases, results were expressed as mean ± SD from triplicate experiments.
Experimental design
To analyze the AgNP toxicity on C. eurynotus and the effects of algae and humic acids, a set of four experimental conditions was defined as follows: culture water (W), algae addition (WA), humic acids addition (WH), and algae and humic acids addition (WAH). The algae concentration of 10 × 104 cel mL−1 was selected as being the optimum for cladoceran growth, based on previous works and bibliographic information (Savaş and Erdoğan 2006; Rodgher and Espíndola 2008; Andrade et al. 2023). The humic acids concentration of 10 mg L−1 was chosen as it is an average value within the range of environmental concentrations (Wang et al. 2015; Zhang et al. 2019). Both factors were then combined with the AgNP concentrations used in the toxicological tests (Table 1).
Mortality bioassay
AgNP acute toxicity was tested following APHA (2017) guidelines with modifications. The LC50 of AgNP was estimated for each experimental condition mentioned above (W, WA, WH, and WAH). Neonates of C. eurynotus (<24 h) were exposed to a control and five AgNP concentrations (dilution factor 2) (Table 1) for each experimental condition with four replicates containing five organisms each in 20 mL glass containers. Before each bioassay fresh stock solutions (400 µg L−1) were prepared in ultrapure water and stored in the dark to prevent any prior transformation of the nanoparticles (e.g., agglomeration, aggregation, or dissolution). The nominal detected Ag concentration correlated with the product description (purity ≥ 99.0%). The assays were performed in darkness to avoid algae growth and AgNP photodegradation (Li et al. 2013; Andrade et al. 2023). Physicochemical variables (conductivity -µs cm−1, dissolved oxygen -DO, mg L−1, and pH) were measured at the beginning and the end of the bioassays.
Mortality was checked at 48 h under a stereoscopic microscope (Nikon E100). The size of the surviving organisms was registered by measuring from the upper end of the cephalic carapace to the lower end of the valve carapace through a microscale attached to the microscope eyepiece.
Growth bioassay
Neonates of C. eurynotus (<24 h) were individually exposed to AgNPs in 20 mL glass containers with five replicates under the experimental conditions described before (WA and WAH), although treatments without algae were not included as they would limit cladocerans development. A control and two sublethal AgNP concentration (Table 1) were set up based on the WA LC50 (25 and 50%) and considering the predicted environmental concentrations (8.8 × 10−5–10 μg L−1) (Nowack and Mueller 2008; Gottschalk et al. 2013; Maurer-Jones et al. 2013). The media were completely changed every two days and the experiment lasted 10 days accordingly to the life cycle of the test species (Santos-Wisniewski et al. 2006). The molts were counted and removed before every media change, and adult size was registered at the end of the experiment.
Phototactic behavior bioassay
C. eurynotus has negative phototaxis at natural conditions (Cabrera et al. 1997; Sacherová and Hebert 2003; dos Santos Silva et al. 2018). The effect of AgNPs on this behavior was analyzed in C. eurynotus adults at the same four exposure conditions described for mortality (W, WA, WH, and WAH), and at the same AgNP concentrations selected for the growth bioassay (Table 1). The experimental design to assess phototactic behavior was based on previous studies with modifications (Rivetti et al. 2016; De Felice et al. 2019). Specific devices were built consisting of glass rectangular chambers (7.5 × 1 × 1 cm) divided into two zones: light and dark. The light zone was provided with a white LED light placed in one extreme (12 w, 10 cm distance), and the dark one was provided with a black coverage. The experiments consisted of placing adult C. eurynotus individually on the light section of the chamber. Entering into the dark zone was considered a positive expected response. Any failure in this response was considered a behavior alteration. Also, the time spent by organisms to evade the light was recorded and considered for the analysis. Based on preliminary observations, organisms that did not cross the line in a period of 3 min were considered as non-evaders. For each experimental condition and AgNP concentration (Table 1) 15 replicates were performed with one organism each. The evasion behavior was measured after 2 and 24 h of exposure in 50 mL beakers under the same conditions as described before.
Data analysis
The mean Ag+ release from AgNPs was compared among experimental conditions (W, WA, WH, and WAH) through analysis of variance (ANOVA, Tukey post-test) with R package “rstatix” (Kassambara 2020). The means of physicochemical variables (conductivity, DO, and pH) were compared between treatments and through time by ANOVA (Tukey post-test) or Kruskal–Wallis test (KW), as appropriate.
To obtain the 48 h LC50 we performed the Probit analysis (Finney 1971) with the “drc” R package (Ritz et al. 2015). The mean size of surviving organisms of treatments in mortality assays were compared to control through ANOVA (Dunnett post-test).
The phototactic behavior was analyzed through generalized linear mixed effect models with a binomial error distribution and logit link function. The fixed predictors were AgNPs, algae, and humic acids; while time was considered as random effect. The best model was selected based on the lowest corrected Akaike Information Criterion (AICc) through the “dredge” function (“MuMIn” R package) (Barton and Barton 2015), followed by “sw” function to obtain the parameter weights. Finally, the model assumptions were checked.
All data analysis was performed with R Studio software (version 2022.07.1).
Results
NPs behavior
TEM analysis displayed a different size distribution between spherical and non-spherical AgNPs. The first ones were 25 ± 10 nm, while the second ones were 75 ± 11 nm in both ultrapure and culture water (Fig. 1a, b). Moreover, the humic acids tended to coat the NPs (shown as a thin layer around the particle surface) (Fig. 1c).
The UV–visible absorption spectrum presented the typical surface plasmon of AgNPs, with a maximum peak close to 410 nm (Fig. 2a). As previously described, the asymmetry in the plasmon is due to the presence of nanoparticles with triangular or elongated shape (Tak et al. 2015; Andrade et al. 2023). The characteristic peak is conserved in both culture water and with the addition of humic acids (Fig. 2a).
a Surface plasmon resonance of AgNPs in ultrapure water, culture water (W), and with humic acids addition (WH). b Fourier transform infrared spectroscopy (FT-IR) in culture water of AgNPs (W), humic acids, and their combination (WH). c Means and standard deviation of Ag+ ions release (%) by AgNPs over time (h) in ultrapure water and each experimental condition: culture water (W), algae addition (WA), humic acids addition (WH), and algae and humic acids addition (WAH)
FT-IR spectra analyses in culture water for AgNPs and humic acids alone and in combination are demonstrated in Fig. 2b. Humic acids showed a wide band at 3246 cm−1 (H-bonded OH), a strong peak at 1580 cm−1 (COO- of carbonyl), and a peak at 1370 cm−1 (C=O of quinone) and at 1000 cm−1 (C-O stretch or OH deformation of COOH) (Sharan et al. 2018). On the other hand, AgNPs also presented a wide band at 3246 cm−1 (H-bonded OH), a peak at 1288 cm−1 (N-OH complex), and a peak at 1060 cm−1 (C-N of pyrrolidone) (Andrade et al. 2023). Finally, the FT-IR spectra of the combination of AgNPs and humic acids gather the main vibration bands of the two components, leading to an overlap of peaks of 1370 cm−1 (C=O of quinone from humic acids) and appearances of 1580 cm−1 peak (COO- of carbonyl from humic acids) which indicates that the AgNPs and the humic acids may have interacted.
The Ag+ release at 48 h differed between the different experimental conditions (ANOVA F = 9.728, p = 0.0018) (Fig. 2c). In ultrapure water it was 22.87% (±1.13), in culture water (W) it was 36.75% (±2.18), the algae presence (WA) increased it: 47.74% (±6.45), the humic acids presence (WH) decreased it: 22.21% (±4.09), and an intermediate situation was observed when both antagonistic factors were present (WAH): 36% (±3.8).
Mortality bioassay
The physicochemical variables did not change either between treatments or though time (48 h) (ANOVA test, p > 0.05); conductivity: 1225–1535 µs cm−1, DO: 8.53–8.71 mg L−1, and pH: 6.5–7.2.
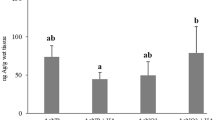
The LC50 of each experimental condition and their dose-response curves are shown in Fig. 3a. The presence of algae decreased the AgNP LC50 by 24% (WA), and the presence of humic acids, by 151% (WH). When both factors were present (WAH), the LC50 decreased 211%; which means 36% more than the additive sum of the observed individual effects (i.e., 175%).
Mortality bioassay of C. eurynotus exposed to AgNPs at four experimental conditions: culture water (W), algae addition (WA), humic acids addition (WH), and algae and humic acids addition (WAH). a Dose response curves and the estimated LC50 and 95% confidence intervals (CI) (µg L−1). b Mean size (µm) and standard deviations of surviving C. eurynotus. Letters indicate significative differences between controls, and * indicates significative differences between treatments and controls
The algae promoted the short-term growth of organisms in absence of AgNPs (ANOVA test F = 13.43, p < 0.001). A significative reduction in surviving C. eurynotus size was registered at concentrations ≥2.5 µg L−1 in the treatments containing only algae (WA) (ANOVA test F = 7.96, p < 0.001). No differences were observed in organism size in treatments containing humic acids (ANOVA test p < 0.05) (Fig. 3b).
Growth bioassay
C. eurynotus adults’ size was significantly lower at 1.5 µg L−1 AgNPs than control in absence of humic acids (WA) (ANOVA test p = 0.001). The number of molts decreased marginally significantly at 1.5 µg L−1 AgNPs than control in absence of humic acids (WA) (ANOVA test p = 0.06). With humic acids addition (WAH), no effects of AgNPs on organisms’ size and molts were observed under the selected concentrations (ANOVA test p > 0.05) (Fig. 4).
Mean and standard deviations of (a) size (µm) and (b) total molts of adult C. eurynotus at the end of the growth bioassay (10 d) of AgNPs at two experimental conditions: culture water with algae addition (WA) and algae and humic acids addition (WAH). * indicates significative differences between treatments and controls
Phototactic behavior bioassay
The selected model for the analysis of the negative phototactic behavior based on the AICc criterion (Table 2) is shown in Table 3. Algae and humic acids were the main predictors that influenced the light evasion response (weights: 1 and 0.92 respectively, Table 2). Only these factors improved significantly the negative phototactic behavior of C. eurynotus by increasing the percentage of organisms that evaded the light (Fig. 5, Table 3). AgNPs did not significantly affect the behavioral response in the tested concentrations (weight: 0.53, Table 2).
The same pattern was observed for the time spent to avoid the light (Cox model, data not shown), as algae and humic acids improved C. eurynotus performance, but AgNPs did not affect it significantly.
Discussion
NPs behavior
The TEM analysis has shown that humic acids stabilized AgNPs by adsorption forming a thin layer around the particles, which was also observed in other studies (Cáceres-Vélez et al. 2019; Ale et al. 2021).
The increase of Ag+ release in culture water compared to ultrapure water, might be due to the presence of CaCO3 in the first, as described in the material and method section. In this context it is probably that the Ag+ interact with the CO32− to form Ag2CO3. This reaction between these ions could produce an increase in the Ag+ release from the nanoparticle since it is consumed by the CO32−. As a result, when the Ag+ release is measure we could find an increase in these values. Moreover, the culture water also has Mg2+ and Ca2+ which can interact with the union sites of the nanoparticle for the Ag+, affecting the liberation of those ions. In this sense, it has been previously shown by other authors that the Ag+ release could be affected by the composition of the culture medium (Liu et al. 2011; Vazquez-Muñoz et al. 2020).
As expected, the presence of Tetradesmus obliquus promoted Ag+ release from AgNPs. Previous studies reported a similar effect with the same (Andrade et al. 2023) and other algae species on AgNP dissolution (Navarro et al. 2015; Chen et al. 2019; Ponton et al. 2019). This result was attributed to the oxidation capacity of some algae exudates such as hydrogen peroxide (Sigg and Lindauer 2015). In the case of T. obliquus, AgNP dissolution could have taken place mainly in the area surrounding the cell wall, as Chlorococcales have a characteristic strong trilaminar cell wall (Burczyk 1973; Allard and Templier 2001), and also, algae wall pores would not allow the entry of AgNPs bigger than 20 nm (Chen et al. 2019).
Conversely, the presence of humic acids reduced the Ag+ released from AgNPs, which agrees with recent studies (Xiao et al. 2020; Ale et al. 2021). Humic acids may have stabilized AgNPs by adsorption as observed in the TEM analysis. Other reports have suggested that humic acids are able to reduce Ag+ ions and form new NPs, thus decreasing Ag+ concentration in the media (Liu et al. 2021).
When both algae and humic acids were present, an intermediate ion release percentage was observed. In this case, a reduction in AgNP dissolution was observed when compared with culture water, indicating that humic acids may have exerted a greater effect on Ag+ release than algae. It has been reported that the presence of humic substances can modify the effects of pollutants in algae in different ways. Humic acids can form a protective coating on algae, decrease Ag+ released by AgNPs, and reduce Ag+ ions, thereby decreasing the uptake of Ag+ ions by algae (Ale et al. 2021; Liu et al. 2021; Popa et al. 2022). On the other hand, the humic substances redox buffering activity may have countered the oxidative effects of algae exudates on AgNPs. In this sense, humic acids can counteract the ability of algae to increase ions release as these substances can reduce reactive oxygen species (ROS) such as hydrogen peroxide present in algae exudates (Fabrega et al. 2009; Sigg and Lindauer 2015; Popa et al. 2022). Nevertheless, the behavior of AgNPs under the combined effect of these environmental factors has not been studied before, therefore, the underlying mechanism needs to be further addressed.
Mortality bioassay
Chydorus eurynotus was sensitive to AgNPs, as acute effects (LC50 = 2.37 µg L−1) were observed in concentrations close to those predicted for surface water bodies (9 × 10−5 and 1.51 μg L−1) (NanoFATE; Batley et al. 2013; Sun et al. 2014, 2016). Although the toxicity of AgNPs was not previously evaluated in this test species, Wang et al. (2012) reported a higher LC50 for C. sphaericus (34 µg L−1). The authors concluded that although silver ions (AgNO3) may play a role in AgNP toxicity, this influence was lower for cladocerans compared to other organisms since they can ingest the nanoparticles. However, it is important to consider the high variability of the acute toxicity of AgNPs, which can be related to test conditions, test species, and the nanoparticles properties; for example, reported LC50 for Daphnia spp. range from 0.26 to 30 µg L−1 (Silva et al. 2014; Carvalho-Pereira et al. 2015; Assis da Silva et al. 2022) and for Ceriodaphnia dubia, from 0.15 to 67 µg L−1 (Angel et al. 2013; Kennedy et al. 2015; Harmon et al. 2017).
The presence of algae decreased the lethality of AgNPs on C. eurynotus by 24%. This agrees with observations in a previous study where the same algae concentration decreased Ceriodaphnia reticulata mortality by AgNPs by 64% (Andrade et al. 2023). In congruence, Stevenson et al. (2017, 2022) reported that a scarce algae provision increased the toxicity of AgNPs in D. pulicaria in terms of survival and reproduction, with consequences at the population level in the long term, the authors discussed that this may be due to effects of nanoparticles on Daphnia sp. feeding. Some authors have attributed this mitigation effect mainly to better nutritional conditions of cladocerans under the presence of food and the possible interactions between algae and AgNPs (Allen et al. 2010; Ribeiro et al. 2014; Harmon et al. 2017). On the one hand, the greater energy available when food was present might allow a better performance of cladocerans in terms of energy allocation for detoxification, growth, and reproduction (Harmon et al. 2017). Furthermore, the interaction between algae and AgNPs could have played an important role as Ag+ release increased in presence of algae, triggering a faster AgNP dissolution. In consequence, this could have reduced of the well-known “Trojan horse” mechanism of toxicity, which implies that silver ions are released once the particles are ingested by the organisms inducing oxidative stress (de Souza et al. 2019; Galhano et al. 2022).
The presence of humic acids had a greater effect on AgNP toxicity, as it reduced their lethality (i.e., increased LC50) by 151%. Humic acids also inhibited the observed reduction in the size of the surviving organisms at the end of the acute assay. This may be due to the decrease of dissolved silver ions in presence of humic acids, which can be due to both adsorption on AgNPs and reduction of Ag+ into new NPs as described before. Wang et al. (2015) reported that humic substances alleviated AgNP lethality on C. sphaericus in a concentration-dependent manner and attributed this to the inhibition of AgNP dissolution by humic acids. Moreover, the authors discussed that humic substances can also act as antioxidants, as they are able to react with ROS caused by AgNPs (Fabrega et al. 2009).
Under the combination of both, algae and humic acids, AgNP toxicity on C. eurynotus decreased in a greater percentage compared with the additive sum of the individual mitigation effects (211%), indicating a possible interaction between both factors. In this sense, Zheng et al. (2022) reported that humic acids promoted the growth, chlorophyll content, and polysaccharide concentration of Scenedesmus capricornus, which belongs to the same family than T. obliquus (Scenedesmaceae). The authors suggested that humic acids may act as a carbon source for energy uptake and growth of algae. In a recent review, Popa et al. (2022) reported that humic substances act as microalgal biostimulants since they can increase the ionic nutrient availability, improve the protection against abiotic stressors (like AgNPs and Ag+), and enhance the accumulation of several compounds such as carotenoids, fatty acids, lipids, and carbohydrates. In this scenario, humic acids could have indirectly improved the nutritional conditions of C. eurynotus, and therefore, increase the magnitude of the toxicity mitigation. There are some reports in the literature on the combined effects of environmental factors such as humic acids and light irradiation regarding AgNP toxicity (Akhil and Sudheer Khan 2017; Zhang et al. 2017), however the underlying mechanism of these interactions needs to be further explored. It should be noted that even under these conditions of reduced toxicity, AgNPs could still represent a risk to aquatic biota, as lethal effects (LC50 ≤ 7.38 µg L−1) were observed at concentrations close to those predicted for the environment. However, it should be considered that these predictions are highly variable, so they must be interpreted carefully.
Growth bioassay
C. eurynotus growth was affected by AgNPs in terms of reduction in size and number of total molts. Although the effects of AgNPs on growth of Chydoridae were not reported before, reductions in growth by these nanoparticles were reported in different Daphniidae cladocerans (Zhao and Wang 2011; Andrade et al. 2023). These negative impacts of AgNPs have been attributed to direct and indirect effects. On the one hand, AgNPs can directly affect cladocerans, as oxidative stress is one of their main reported mechanisms of toxicity (de Souza et al. 2019; Galhano et al. 2022). Furthermore, AgNPs were shown to exert mechanical effects on cladocerans by adhesion to carapace and antennas, and obstruction of filter setae and gut, which ultimately affect their locomotion and feeding (Zhao and Wang 2010; Asghari et al. 2012; Yan and Wang 2021). On the other hand, AgNPs may exert indirect effects on cladocerans by interacting with the food provided in chronic exposures. Although no effects on T. obliquus flocculation were previously observed under similar AgNP concentrations (Andrade et al. 2023), it has been reported that algae can adsorb NPs or uptake Ag+ ions, and therefore, constitute another Ag route of entry into cladocerans’ bodies (Wang et al. 2019; Dang et al. 2021). Moreover, Lekamge et al. (2019) reported that algae previously exposed to AgNPs induced a decrease in the feeding rates of D. carinata, and demonstrated the trophic transference of the particles.
Under the presence of humic acids, the effects of AgNPs on C. eurynotus growth were inhibited. This inhibition by organic matter on the effects of AgNPs to cladoceran growth was not reported before. However, it has been suggested that humic substances can decrease the effects of silver nitrate and other metallic NPs such as zinc and copper oxide on D. magna growth (Glover and Wood 2004; Dai et al. 2020; Ahmed et al. 2021).
Phototactic behavior bioassay
No significant effects of AgNPs were observed on C. eurynotus phototactism at the tested concentrations, despite previous studies registering behavioral alterations in D. magna exposed to these particles. Indeed, Park et al. (2022) reported that AgNPs induced variable alterations in swimming speed at concentrations between 0.1 and 10 µg L−1. Galhano et al. (2020) found that AgNPs altered the cladoceran allocation time in the water column, and Kolkmeier and Brooks (2013) concluded that their phototactic behavior decreased when exposed to silver nitrate. As the behavioral impairments of AgNPs on cladocerans are still incipient and variable, more studies are needed to elucidate the possible mechanisms.
The presence of algae and/or humic substances may directly or indirectly provide cladoceran more energy to react to the stress situation even in presence of AgNPs (Harmon et al. 2017; Popa et al. 2022). These results highlight the importance of considering environmental factors when analyzing behavior endpoints, in order to assess more realistic exposure scenarios, which may have a great effect on such sensitive responses.
Conclusions
The behavior of AgNPs was strongly affected by environmental factors such as algae and humic acids alone and in combination. Algae promoted Ag+ release, and humic substances reduced it, and an intermediate situation was observed when both factors were combined.
Chydorus eurynotus was sensitive to AgNPs, as its survival and growth were affected. The presence of algae and humic acids reduced these effects through different mechanisms. Both environmental factors may have interacted as their combined effect on AgNP toxicity was greater than the sum of the individual effects.
In view of the obtained results, it is essential to analyze the isolated and combined effects of environmental factors on NPs behavior and toxicity, in order to understand and predict these processes under realistic exposure conditions.
References
Ahmed I, Zhang B, Muneeb-Ur-Rehman M, Fan W (2021) Effect of different shapes of Nano-Cu2O and humic acid on two-generations of Daphnia Magna. Ecotoxicol Environ Saf 207:111274. https://doi.org/10.1016/j.ecoenv.2020.111274
Ahmed T, Ogulata RT, Sezgin Bozok S (2022) Silver nanoparticles against SARS-CoV-2 and its potential application in medical protective clothing – a review. J Text Inst 113:2825–2838. https://doi.org/10.1080/00405000.2021.1996730
Akhil K, Sudheer Khan S (2017) Effect of humic acid on the toxicity of bare and capped ZnO nanoparticles on bacteria, algal and crustacean systems. J Photochem Photobiol B Biol 167:136–149. https://doi.org/10.1016/j.jphotobiol.2016.12.010
Al-Radadi NS, Abu-Dief AM (2022) Silver nanoparticles (AgNPs) as a metal nano-therapy: possible mechanisms of antiviral action against COVID-19. Inorg Nano-Metal Chem 1–19. https://doi.org/10.1080/24701556.2022.2068585
Ale A, Bacchetta C, Rossi AS et al. (2018a) Nanosilver toxicity in gills of a neotropical fish: metal accumulation, oxidative stress, histopathology and other physiological effects. Ecotoxicol Environ Saf 148:976–984. https://doi.org/10.1016/j.ecoenv.2017.11.072
Ale A, Galdopórpora JM, Mora MC et al. (2021) Mitigation of silver nanoparticle toxicity by humic acids in gills of Piaractus mesopotamicus fish. Environ Sci Pollut Res 1. https://doi.org/10.1007/s11356-021-12590-w
Ale A, Rossi AS, Bacchetta C et al. (2018b) Integrative assessment of silver nanoparticles toxicity in Prochilodus lineatus fish. Ecol Indic 93:1190–1198. https://doi.org/10.1016/j.ecolind.2018.06.023
Allard B, Templier J (2001) High molecular weight lipids from the trilaminar outer wall (TLS) -containing microalgae Chlorella emersonii, Scenedesmus communis and Tetraedron minimum. Phytochemistry 57:459–467. https://doi.org/10.1016/S0031-9422(01)00071-1
Allen HJ, Impellitteri CA, Macke DA et al. (2010) Effects from filtration, capping agents, and presence/absence of food on the toxicity of silver nanoparticles to Daphnia magna. Environ Toxicol Chem 29:2742–2750. https://doi.org/10.1002/etc.329
Andrade VS, Ale A, Antezana PE et al. (2023) Ecotoxicity of nanosilver on cladocerans and the role of algae provision. Environ Sci Pollut Res 30:27137–27149. https://doi.org/10.1007/s11356-022-24154-7
Angel BM, Batley GE, Jarolimek CV, Rogers NJ (2013) The impact of size on the fate and toxicity of nanoparticulate silver in aquatic systems. Chemosphere 93:359–365. https://doi.org/10.1016/j.chemosphere.2013.04.096
Antezana PE, Municoy S, Pérez CJ, Desimone MF (2021) Collagen hydrogels loaded with silver nanoparticles and cannabis sativa oil. Antibiotics 10. https://doi.org/10.3390/antibiotics10111420
APHA (2017) Standard methods for the examination of water and wastewater. 23rd edn. American Public Health Association, American Water Works Federation, Water Environment Association, Washington, D.C.
Asghari S, Johari SA, Lee JH et al. (2012) Toxicity of various silver nanoparticles compared to silver ions in Daphnia magna. J Nanobiotechnol 10:1–11. https://doi.org/10.1186/1477-3155-10-14
Assis da Silva C, Ribeiro BM, Trotta C do V et al. (2022) Effects of mycogenic silver nanoparticles on organisms of different trophic levels. Chemosphere 308:136540. https://doi.org/10.1016/j.chemosphere.2022.136540
Barnett AJ, Finlay K, Beisner BE (2007) Functional diversity of crustacean zooplankton communities: towards a trait-based classification. Freshw Biol 52:796–813. https://doi.org/10.1111/j.1365-2427.2007.01733.x
Barton K, Barton MK (2015) Package ‘mumin’. R Foundation for Statistical Computing: Vienna, Austria. Version, 1. 18:439. https://cran.hafro.is/web/packages/MuMIn/MuMIn.pdf
Batley GE, Kirby JK, McLaughlin MJ (2013) Fate and risks of nanomaterials in aquatic and terrestrial environments. Acc Chem Res 46:854–862. https://doi.org/10.1021/ar2003368
Burczyk J (1973) The chemical composition of the cell wall of Scenedesmus obliquus. I. General chemical characteristics. Folia Histochem Cytochem 11:119–133
Cabrera S, López M, Tartarotti B (1997) Phytoplankton and zooplankton response to ultraviolet radiation in a high-altitude Andean lake: Short- versus long-term effects. J Plankton Res 19:1565–1582. https://doi.org/10.1093/plankt/19.11.1565
Cáceres-Vélez PR, Fascineli ML, Rojas E et al. (2019) Impact of humic acid on the persistence, biological fate and toxicity of silver nanoparticles: a study in adult zebrafish. Environ Nanotechnol Monit Manag 12:100234. https://doi.org/10.1016/j.enmm.2019.100234
de A de Carvalho-Pereira TS, de S Santos T, Pestana EMS et al. (2015) Natural humic substances effects on the life history traits of Latonopsis australis SARS (1888) (Cladocera - Crustacea). Chemosphere 120:165–170. https://doi.org/10.1016/j.chemosphere.2014.06.025
Cazenave J, Ale A, Bacchetta C, Rossi AS (2019) Nanoparticles toxicity in fish models. Curr Pharm Des 25:3927–3942. https://doi.org/10.2174/1381612825666190912165413
Chen F, Xiao Z, Yue L et al. (2019) Algae response to engineered nanoparticles: current understanding, mechanisms and implications. Environ Sci Nano 6:1026–1042. https://doi.org/10.1039/c8en01368c
Corsi I, Desimone MF, Cazenave J (2022) Building the bridge from aquatic nanotoxicology to safety by design silver nanoparticles. Front Bioeng Biotechnol 10:1–28. https://doi.org/10.3389/fbioe.2022.836742
da Silva MLN, Nogueira DJ, Köerich JS et al. (2021) Multigenerational toxic effects on daphnia magna induced by silver nanoparticles and glyphosate mixture. Environ Toxicol Chem 40:1123–1131. https://doi.org/10.1002/etc.4952
Dai H, Sun T, Han T et al. (2020) Aggregation behavior of zinc oxide nanoparticles and their biotoxicity to Daphnia magna: Influence of humic acid and sodium alginate. Environ Res 191:110086. https://doi.org/10.1016/j.envres.2020.110086
Dang F, Huang Y, Wang Y et al. (2021) Transfer and toxicity of silver nanoparticles in the food chain. Environ Sci Nano 8:1519–1535. https://doi.org/10.1039/d0en01190h
De Felice B, Sabatini V, Antenucci S et al. (2019) Polystyrene microplastics ingestion induced behavioral effects to the cladoceran Daphnia magna. Chemosphere 231:423–431. https://doi.org/10.1016/j.chemosphere.2019.05.115
de Souza TAJ, Souza LRR, Franchi LP (2019) Ecotoxicology and environmental safety silver nanoparticles: an integrated view of green synthesis methods, transformation in the environment, and toxicity. Ecotoxicol Environ Saf 171:691–700. https://doi.org/10.1016/j.ecoenv.2018.12.095
dos Santos Silva E, Rocha O, dos Santos-Wisniewski MJ (2018) Diel vertical migration of Cladocera in a compartment of a tropical reservoir. Acta Limnol Bras 30. https://doi.org/10.1590/S2179-975X13517
Fabrega J, Fawcett SR, Renshaw JC, Lead JR (2009) Silver nanoparticle impact on bacterial growth: effect of pH, concentration, and organic matter. Environ Sci Technol 43:7285–7290. https://doi.org/10.1021/es803259g
Ferdous Z, Muktadir A (2009) A review: potentiality of zooplankton as bioindicator. Am J Appl Sci 6:1815–1819
Finney D (1971) Probit analysis. Cambridge University Press, London, U.K.
Forró L, Korovchinsky NM, Kotov AA, Petrusek A (2008) Global diversity of cladocerans (Cladocera; Crustacea) in freshwater. Hydrobiologia 595:177–184. https://doi.org/10.1007/s10750-007-9013-5
Furtado LM, Bundschuh M, Metcalfe CD (2016) Monitoring the fate and transformation of silver nanoparticles in natural waters. Bull Environ Contam Toxicol 97:449–455. https://doi.org/10.1007/s00128-016-1888-2
Galhano V, Hartmann S, Monteiro MS, et al. (2020) Impact of wastewater-borne nanoparticles of silver and titanium dioxide on the swimming behaviour and biochemical markers of Daphnia magna: an integrated approach. Aquat Toxicol 220. https://doi.org/10.1016/j.aquatox.2020.105404
Galhano V, Zeumer R, Monteiro MS et al. (2022) Effects of wastewater-spiked nanoparticles of silver and titanium dioxide on survival, growth, reproduction and biochemical markers of Daphnia magna. Sci Total Environ 839:156079. https://doi.org/10.1016/j.scitotenv.2022.156079
Gao JIE, Youn S, Hovsepyan A et al. (2009) Dispersion and toxicity of selected manufactured nanomaterials in natural river water samples: effects of water chemical composition. Environ Sci Technol 43:3322–3328. https://doi.org/10.1021/es803315v
Glover CN, Wood CM (2004) Physiological interactions of silver and humic substances in Daphnia magna: effects on reproduction and silver accumulation following an acute silver challenge. Comp Biochem Physiol - C Toxicol Pharmacol 139:273–280. https://doi.org/10.1016/j.cca.2004.12.005
Gottschalk F, Sun T, Nowack B (2013) Environmental concentrations of engineered nanomaterials: review of modeling and analytical studies. Environ Pollut 181:287–300. https://doi.org/10.1016/j.envpol.2013.06.003
Gutierrez MF, Ale A, Andrade V et al. (2021) Metallic, metal oxide, and metalloid nanoparticles toxic effects on freshwater microcrustaceans: an update and basis for the use of new test species. Water Environ Res 93:2505–2526. https://doi.org/10.1002/wer.1637
Harmon AR, Kennedy AJ, Laird JG et al. (2017) Comparison of acute to chronic ratios between silver and gold nanoparticles, using Ceriodaphnia dubia. Nanotoxicology 11:1127–1139. https://doi.org/10.1080/17435390.2017.1399219
Hartmann S, Louch R, Zeumer R et al. (2019) Comparative multi-generation study on long-term effects of pristine and wastewater-borne silver and titanium dioxide nanoparticles on key lifecycle parameters in Daphnia magna. NanoImpact 14:100163. https://doi.org/10.1016/j.impact.2019.100163
Islam MA, Jacob MV, Antunes E (2021) A critical review on silver nanoparticles: from synthesis and applications to its mitigation through low-cost adsorption by biochar. J Environ Manag 281:111918. https://doi.org/10.1016/j.jenvman.2020.111918
Jamunkar R, Shrivas K, Sinha D et al. (2022) Application of silver nanoparticles as a new alternative antiviral agent for SARS-CoV-2: a review. Curr Nanosci 18:465–477. https://doi.org/10.2174/1573413717666211118105415
Kassambara A (2020) rstatix: Pipe-friendly framework for basic statistical tests. R Packag version 0.5. https://cran.r-project.org/web/packages/rstatix/rstatix.pdf
Kennedy AJ, Hull MS, Diamond S et al. (2015) Gaining a critical mass: a dose metric conversion case study using silver nanoparticles. Environ Sci Technol 49:12490–12499. https://doi.org/10.1021/acs.est.5b03291
Kolkmeier MA, Brooks BW (2013) Sublethal silver and NaCl toxicity in Daphnia magna: a comparative study of standardized chronic endpoints and progeny phototaxis. Ecotoxicology 22:693–706. https://doi.org/10.1007/s10646-013-1061-1
Le TTY, Peijnenburg WJGM (2013) Modeling toxicity of mixtures of perfluorooctanoic acid and triazoles (triadimefon and paclobutrazol) to the benthic cladoceran Chydorus sphaericus. Environ Sci Technol 47:6621–6629. https://doi.org/10.1021/es4001104
Lekamge S, Miranda AF, Abraham A et al. (2019) The toxicity of coated silver nanoparticles to Daphnia carinata and trophic transfer from alga Raphidocelis subcapitata. PLoS One 14:1–20. https://doi.org/10.1007/s42452-020-2430-z
Levard C, Hotze EM, Lowry GV, Brown GE (2012) Environmental transformations of silver nanoparticles: Impact on stability and toxicity. Environ Sci Technol 46:6900–6914. https://doi.org/10.1021/es2037405
Li Y, Zhang W, Niu J, Chen Y (2013) Surface-coating-dependent dissolution, aggregation, and reactive oxygen species (ROS) generation of silver nanoparticles under different irradiation conditions. Environ Sci Technol 47:10293–10301. https://doi.org/10.1021/es400945v
Liu W, Zhou QF, Liu JY et al. (2011) Environmental and biological influences on the stability of silver nanoparticles. Chin Sci Bull 56:2009–2015. https://doi.org/10.1007/s11434-010-4332-8
Liu Y, Li C, Luo S et al. (2021) Inter-transformation between silver nanoparticles and Ag+ induced by humic acid under light or dark conditions. Ecotoxicology 30:1376–1385. https://doi.org/10.1007/s10646-020-02284-3
Liu Z, Malinowski CR, Sepúlveda MS (2022) Emerging trends in nanoparticle toxicity and the significance of using Daphnia as a model organism. Chemosphere 291:132941. https://doi.org/10.1016/j.chemosphere.2021.132941
Mano H, Tanaka Y (2016) Mechanisms of compensatory dynamics in zooplankton and maintenance of food chain efficiency under toxicant stress. Ecotoxicology 25:399–411. https://doi.org/10.1007/s10646-015-1598-2
Maurer-Jones MA, Gunsolus IL, Murphy CJ, Haynes CL (2013) Toxicity of engineered nanoparticles in the environment. Anal Chem 85:3036–3049. https://doi.org/10.1021/ac303636s
McLaughlin J, Bonzongo JCJ (2012) Effects of natural water chemistry on nanosilver behavior and toxicity to Ceriodaphnia dubia and Pseudokirchneriella subcapitata. Environ Toxicol Chem 31:168–175. https://doi.org/10.1002/etc.720
Mergia MT, Weldemariam ED, Eklo OM, Yimer GT (2022) Pesticide residue levels in surface water, using a passive sampler and in the sediment along the littoral zone of Lake Ziway at selected sites. SN Appl Sci 4. https://doi.org/10.1007/s42452-022-04966-5
Monkiédjé A, Njiné T, Tamatcho B, Démanou J (2000) Assessment of the acute toxic effects of the fungicide Ridomil plus 72 on aquatic organisms and soil micro-organisms. Environ Toxicol 15:65–70. 10.1002/(SICI)1522-7278(2000)15:1<65::AID-TOX8>3.0.CO;2-K
Municoy S, Antezana PE, Pérez CJ et al. (2021) Tuning the antimicrobial activity of collagen biomaterials through a liposomal approach. J Appl Polym Sci 138:1–13. https://doi.org/10.1002/app.50330
NanoFATE Report on perceived current and future use of silver, ceriumoxide and zincoxide. Nanoparticles, including a compilation of available data on production volumes and predicted environmental concentrations. Gothenburg University Research
Navarro E, Wagner B, Odzak N et al. (2015) Effects of differently coated silver nanoparticles on the photosynthesis of Chlamydomonas reinhardtii. Environ Sci Technol 49:8041–8047. https://doi.org/10.1021/acs.est.5b01089
Nowack B, Mueller NC (2008) Exposure modeling of engineered nanoparticles in the environment. Environ Sci Technol 42:4447–4453. https://doi.org/10.1021/es7029637
Park J, Park C, Lee Y et al. (2022) Acute adverse effects of metallic nanomaterials on cardiac and behavioral changes in Daphnia magna. Environments 9:1–12. https://doi.org/10.3390/environments9020026
Park S-Y, Choi J-H (2010) Geno- and ecotoxicity evaluation of silver nanoparticles in freshwater crustacean Daphnia magna. Environ Eng Res 15:23–27. https://doi.org/10.4491/eer.2010.15.1.428
Poda AR, Kennedy AJ, Cuddy MF, Bednar AJ (2013) Investigations of UV photolysis of PVP-capped silver nanoparticles in the presence and absence of dissolved organic carbon. J Nanoparticle Res 15. https://doi.org/10.1007/s11051-013-1673-7
Ponton DE, Croteau MN, Luoma SN et al. (2019) Three-layered silver nanoparticles to trace dissolution and association to a green alga. Nanotoxicology 13:1149–1160. https://doi.org/10.1080/17435390.2019.1640912
Popa DG, Lupu C, Constantinescu-Aruxandei D, Oancea F (2022) Humic substances as microalgal biostimulants—implications for microalgal biotechnology. Mar Drugs 20:1–27. https://doi.org/10.3390/md20050327
Poynton HC, Lazorchak JM, Impellitteri CA et al. (2012) Toxicogenomic responses of nanotoxicity in Daphnia magna exposed to silver nitrate and coated silver nanoparticles. Environ Sci Technol 46:6288–6296. https://doi.org/10.1021/es3001618
Pulit-Prociak J, Banach M (2016) Silver nanoparticles - A material of the future…?. Open Chem 14:76–91. https://doi.org/10.1515/chem-2016-0005
Resh VH (2008) Which group is best? Attributes of different biological assemblages used in freshwater biomonitoring programs. Environ Monit Assess 138:131–138. https://doi.org/10.1007/s10661-007-9749-4
Ribeiro F, Gallego-Urrea JA, Jurkschat K et al. (2014) Silver nanoparticles and silver nitrate induce high toxicity to Pseudokirchneriella subcapitata, Daphnia magna and Danio rerio. Sci Total Environ 466–467:232–241. https://doi.org/10.1016/j.scitotenv.2013.06.101
Ritz C, Baty F, Streibig JC, Gerhard D (2015) Dose-response analysis using R. PLoS ONE 10:1–13. https://doi.org/10.1371/journal.pone.0146021
Rivetti C, Campos B, Barata C (2016) Low environmental levels of neuro-active pharmaceuticals alter phototactic behaviour and reproduction in Daphnia magna. Aquat Toxicol 170:289–296. https://doi.org/10.1016/j.aquatox.2015.07.019
Rodgher S, Espíndola ELG (2008) The influence of algal densities on the toxicity of chromium for Ceriodaphnia dubia Richard (Cladocera, Crustacea). Braz J Biol 68:341–348. https://doi.org/10.1590/S1519-69842008000200015
Sacherová V, Hebert PDN (2003) The evolutionary history of the Chydoridae (Crustacea: Cladocera). Biol J Linn Soc 79:629–643. https://doi.org/10.1046/j.1095-8312.2003.00216.x
Salem SS, Hammad EN, Mohamed AA, El-Dougdoug W (2022) A comprehensive review of nanomaterials: types, synthesis, characterization, and applications. Biointerface Res Appl Chem 13. https://doi.org/10.33263/BRIAC131.041
Santos-Wisniewski MJ, Rocha O, Guntzel AM, Matsumura-Tundisi T (2006) Aspects of the life cycle of Chydorus pubescens Sars, 1901 (Cladocera, Chydoridae). Acta Limnol Bras 18:305–310
Sars GO (1901) Contributions to the knowledge of the fresh-water Entomostraca of South America, as shown by artificial hatching from dried material. Archiv for Mathematik og Naturvidenskab 24:1–52
Savaş S, Erdoğan Ö (2006) The effect of food (Scenedesmus acuminatus (von Lagerheim) R. H. Chodat) densities and temperature on the population growth of the Cladoceran Ceriodaphnia quadrangula (O. F. Muller, 1785). J Fish Aquat Sci 23:113–116
Sharan BR, Nagaraja MS, Kadalli GG, Champa BV (2018) Fourier Transform Infrared (FTIR) spectroscopy of soil humic and fulvic acids extracted from paddy land use system. Int J Curr Microbiol Appl Sci 7:834–837. https://doi.org/10.20546/ijcmas.2018.705.102
Sigg L, Lindauer U (2015) Silver nanoparticle dissolution in the presence of ligands and of hydrogen peroxide. Environ Pollut 206:582–587. https://doi.org/10.1016/j.envpol.2015.08.017
Silva T, Pokhrel LR, Dubey B et al. (2014) Particle size, surface charge and concentration dependent ecotoxicity of three organo-coated silver nanoparticles: comparison between general linear model-predicted and observed toxicity. Sci Total Environ 468–469:968–976. https://doi.org/10.1016/j.scitotenv.2013.09.006
Song L, Vijver MG, de Snoo GR, Peijnenburg WJGM (2015) Assessing toxicity of copper nanoparticles across five cladoceran species. Environ Toxicol Chem 34:1863–1869. https://doi.org/10.1002/etc.3000
Stevenson LM, Krattenmaker KE, Johnson E et al. (2017) Standardized toxicity testing may underestimate ecotoxicity: Environmentally relevant food rations increase the toxicity of silver nanoparticles to Daphnia. Environ Toxicol Chem 36:3008–3018. https://doi.org/10.1002/etc.3869
Stevenson LM, Krattenmaker KE, McCauley E, Nisbet RM (2022) Extrapolating contaminant effects from individuals to populations: a case study on nanoparticle toxicity to daphnia fed environmentally relevant food levels. Arch Environ Contam Toxicol 83:361–375. https://doi.org/10.1007/s00244-022-00950-7
Sun TY, Bornhöft NA, Hungerbühler K, Nowack B (2016) Dynamic probabilistic modeling of environmental emissions of engineered nanomaterials. Environ Sci Technol 50:4701–4711. https://doi.org/10.1021/acs.est.5b05828
Sun TY, Gottschalk F, Hungerbühler K, Nowack B (2014) Comprehensive probabilistic modelling of environmental emissions of engineered nanomaterials. Environ Pollut 185:69–76. https://doi.org/10.1016/j.envpol.2013.10.004
Tak YK, Pal S, Naoghare PK et al. (2015) Shape-dependent skin penetration of silver nanoparticles: does it really matter? Sci Rep 5:1–11. https://doi.org/10.1038/srep16908
Vazquez-Muñoz R, Bogdanchikova N, Huerta-Saquero A (2020) Beyond the nanomaterials approach: influence of culture conditions on the stability and antimicrobial activity of silver nanoparticles. ACS Omega 5:28441–28451. https://doi.org/10.1021/acsomega.0c02007
Wang F, Guan W, Xu L et al. (2019) Effects of nanoparticles on algae: adsorption, distribution, ecotoxicity and fate. Appl Sci 9:1–15. https://doi.org/10.3390/app9081534
Wang J, Peng J, Tan Z et al. (2017) Microplastics in the surface sediments from the Beijiang River littoral zone: composition, abundance, surface textures and interaction with heavy metals. Chemosphere 171:248–258. https://doi.org/10.1016/j.chemosphere.2016.12.074
Wang T, Liu W (2022) Emerging investigator series: metal nanoparticles in freshwater: transformation, bioavailability and effects on invertebrates. Environ Sci Nano 9:2237–2263. https://doi.org/10.1039/d2en00052k
Wang Z, Chen J, Li X et al. (2012) Aquatic toxicity of nanosilver colloids to different trophic organisms: contributions of particles and free silver ion. Environ Toxicol Chem 31:2408–2413. https://doi.org/10.1002/etc.1964
Wang Z, Quik JTK, Song L et al. (2015) Humic substances alleviate the aquatic toxicity of polyvinylpyrrolidone-coated silver nanoparticles to organisms of different trophic levels. Environ Toxicol Chem 34:1239–1245. https://doi.org/10.1002/etc.2936
Watanabe A (1960) List of algal strains in collection at the Institute of Applied Microbiology, University of Tokyo. J Gen Appl Microbiol 6:283–292. https://doi.org/10.2323/jgam.6.283
Xiao B, Wang X, Yang J et al. (2020) Bioaccumulation kinetics and tissue distribution of silver nanoparticles in zebrafish: the mechanisms and influence of natural organic matter. Ecotoxicol Environ Saf 194:110454. https://doi.org/10.1016/j.ecoenv.2020.110454
Yan N, Wang WX (2021) Novel imaging of silver nanoparticle uptake by a unicellular alga and trophic transfer to Daphnia magna. Environ Sci Technol 55:5143–5151. https://doi.org/10.1021/acs.est.0c08588
Yu Q, Wang Z, Zhai Y, et al (2021) Effects of humic substances on the aqueous stability of cerium dioxide nanoparticles and their toxicity to aquatic organisms. Sci Total Environ 781. https://doi.org/10.1016/j.scitotenv.2021.146583
Zahoor M, Nazir N, Iftikhar M et al. (2021) A review on silver nanoparticles: classification, various methods of synthesis, and their potential roles in biomedical applications and water treatment. Water 13:2216. https://doi.org/10.3390/w13162216
Zhang T, Lu D, Zeng L et al. (2017) Role of secondary particle formation in the persistence of silver nanoparticles in humic acid containing water under light irradiation. Environ Sci Technol 51:14164–14172. https://doi.org/10.1021/acs.est.7b04115
Zhang W, Ke S, Sun C et al. (2019) Fate and toxicity of silver nanoparticles in freshwater from laboratory to realistic environments: a review. Environ Sci Pollut Res 26:7390–7404. https://doi.org/10.1007/s11356-019-04150-0
Zhao CM, Wang WX (2011) Comparison of acute and chronic toxicity of silver nanoparticles and silver nitrate to Daphnia magna. Environ Toxicol Chem 30:885–892. https://doi.org/10.1002/etc.451
Zhao CM, Wang WX (2010) Biokinetic uptake and efflux of silver nanoparticles in Daphnia magna. Environ Sci Technol 44:7699–7704. https://doi.org/10.1021/es101484s
Zhao J, Wang X, Hoang SA et al. (2021) Silver nanoparticles in aquatic sediments: occurrence, chemical transformations, toxicity, and analytical methods. J Hazard Mater 418:126368. https://doi.org/10.1016/j.jhazmat.2021.126368
Zheng X, Xu Z, Zhao D et al. (2022) Double-dose responses of Scenedesmus capricornus microalgae exposed to humic acid. Sci Total Environ 806:150547. https://doi.org/10.1016/j.scitotenv.2021.150547
Funding
This work was supported by the Agencia Nacional de Promoción Científica y Técnica [grant number PICT 2018-01271, PI: JC; and grant number PICT-2020-01206, PI: AA] and the Universidad de Buenos Aires [UBACYT 20020150100056BA and PIDAE 2022, PI: MD].
Author information
Authors and Affiliations
Contributions
Victoria S. Andrade: Conceptualization, Formal analysis, Investigation, Methodology, Visualization, Writing - original draft. Analía Ale: Investigation, Funding acquisition, Methodology, Writing - review & editing. Pablo E. Antezana: Formal analysis, Investigation, Writing - review & editing. Martín F. Desimone: Funding acquisition, Methodology, Resources, Writing - review & editing. Jimena Cazenave: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Writing - review & editing. María F. Gutierrez: Conceptualization, Methodology, Project administration, Resources, Supervision, Writing - review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Andrade, V.S., Ale, A., Antezana, P.E. et al. Environmental factors modify silver nanoparticles ecotoxicity in Chydorus eurynotus (Cladocera). Ecotoxicology 33, 683–696 (2024). https://doi.org/10.1007/s10646-024-02766-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-024-02766-8