Abstract
The cleaning symbiosis in coral reef fish is one of the most remarkable mutualist marine interactions; the main actors are cleaner and client fishes, that communicate via tactile and visual stimulation, and the specific sites where this interaction happens are called cleaning stations. The removal of ectoparasites is a contribution to the health of clients, which may have an important role as herbivores or carnivores, and therefore also a contribution for a healthy ecosystem. The aim of this work was to identify the cleaning interaction as an indicator of reef health in the center-south of the Mexican Caribbean. Hence, we located and described the cleaning stations and the attributes of cleaner and client fishes for three climatic seasons in four locations with different degrees of conservation (i.e., time since declaration as protected areas), in the biosphere reserves of Sian Ka’an and Mexican Caribbean. Bluehead Thalassoma bifasciatum was the dominant cleaner fish in the dry and north-wind seasons, and it interacted with 27 species of client fishes year-round. The frequency of client fishes changes with the seasons; parrotfishes are the favorite clients in the dry season, and surgeonfishes for the north-wind season. We recorded for the first time high-hat Pareques acuminatus acting as a cleaner. Cleaning stations are more numerous in Mahahual, the location with the highest human impact; however, the higher structural complexity and area of this reef can explain the observed diversity of the client and cleaner species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cleaning symbiosis in coral reef fishes happens when microcarnivorous cleaner fishes, colloquially called “doctors of the sea,” eat ectoparasites, mucus, dead or diseased tissue from a bigger fish, or client (Losey 1972), the latter often being of commercial interest (Serranidae and Lutjanidae) or functionally important (e.g. herbivores such as Acanthuridae and Scarinae) for the coral reef (Waldie et al. 2011). The specific places where this happens are called cleaning stations (Arnal et al. 2000), and, in the Atlantic Ocean and the Caribbean, they can be observed on healthy corals or sponges, the latter mainly with cleaner gobies (Côté and Soares 2011). Ecologically, cleaning stations are feeding zones for the cleaner fishes (Hixon and Randall 2018) and high diversity points or local “hotspots” for ectoparasites (Caves 2021). The mutually beneficial behavior promotes the well-being and good health of the client fish as well as influencing its growth rate (Waldie et al. 2011) and stress reduction (Bshary et al. 2007), while the cleaner fishes obtain food (Limbaugh 1961); finally, this symbiotic interaction influences the coral reef integrity because it decreases the number of ectoparasites (Vaughan et al. 2017).
Cleaner fish are divided into two functional categories: obligate and facultative. Obligate cleaners are fishes that will clean throughout their lives, whereas facultative cleaners only clean in one period of their ontogenetic cycle, namely, as juveniles (Whiteman and Côté 2002). Currently, 19 cleaning fish families have been reported to exist, Labridae and Gobiidae being the most representative for coral reef ecosystems (Whiteman and Côté 2002; Hixon and Randall 2018). Additionally, Quimbayo et al. (2021) mentioned nine traits of cleaners to describe the level of specialization in such aspects as mobility, activity period, distance to bottom, schooling size, diet, body size, depth range, pelagic larval duration, and geographical range.
During the last decades, the center and south of Quintana Roo, Mexican Caribbean, have suffered changes due to anthropic pressure (Calderón-Aguilera et al. 2012; Santander-Monsalvo et al. 2018), observed in the increase of overfishing (Figueroa-Zavala et al. 2015), invasive species (Cobián-Rojas et al. 2018; García-Rivas et al. 2018), loss of biodiversity, coral habitat fragmentation, diseases in reef-building corals (Estrada-Saldívar et al. 2020), and the massive arrival of pelagic Sargassum (van Tussenbroek et al. 2017). Due to this, protected marine areas like Sian Ka’an Biosphere Reserve (SKBR) and Mexican Caribbean Biosphere Reserve (MCBR) were created, with the purpose of conservation and preservation of the natural resources, sustainable resource usage, and moderation and/or restriction of excessive tourism; however, the proper functioning of these protected areas is not always evaluated. Furthermore, these anthropogenic and natural disturbances are drivers that affect the abundance, distribution, and diversity of cleaner and client fishes and the cleaning symbiosis interaction (Titus et al. 2015; Tuttle 2017). Therefore, we hypothesized that the cleaning symbiosis in the southern Mexican Caribbean between client and cleaner fishes will be more frequent in healthy and protected coral reefs, where we will expect to find high abundances, diversity and length varieties of client and cleaner fishes, due to the null human presence. The aim of this work was to evaluate the aforementioned marine protected areas’ efficiency using data on cleaner and client fishes and cleaning stations. This information was taken from a variety of coral reefs situations, trying to represent different degrees of conservation (e.g., different ages as protected areas), and to provide additional tools for the reinforcement and management of coral reef ecosystems, as well as new information on this symbiosis for the Mexican Caribbean.
Materials and methods
Study area
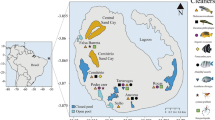
Sian Ka’an Biosphere Reserve (SKBR) was established in 1986; it is a Priority Marine Region that falls into the category “extreme importance” due to high development and surface area of coral reef, seagrass meadows, and good conservation of mangroves (CONANP 2014; Guimarais et al. 2021). Two of our localities, Tampalam and Pulticub (Fig. 1 and Table 1), are within the reserve; however, Pulticub is a transition zone between Sian Ka’an Biosphere Reserve and Mexican Caribbean Biosphere Reserve. In general, the SKBR presents reef-building coral like Acropora palmata and Orbicella annularis; some corals with laminar growth, like Agaricia agaricites and A. tenuifolia; and other corals with massive growth, like Montastraea cavernosa, Pseudodiploria clivosa, and Porites astreoides (Jordán-Dahlgren 1989). The management plan of the reserve (CONANP 2014) considers Tampalam and Pulticub sites for “sustainable use of natural resources.” Tampalam is considered in good conservation condition with presence of A. palmata and O. annularis (Argüelles-Jiménez et al. 2020); it is an important site for reproduction of commercial fish species such as Goliath Grouper Epinephelus itajara (García-Téllez et al. 2022), and the tourism is limited or null. At Pulticub, there is a fishing concession and spiny lobster fishery, as well as low-impact tourism and some housing areas (CONANP 2014).
The Mexican Caribbean Biosphere Reserve (MCBR) was established in 2016 and falls under the category “very important” as a Priority Marine Region (CONANP 2016). The sites studied in the MCBR were Río Indio and Mahahual (Fig. 1 and Table 1), both included in the buffer zone of the reserve. Río Indio is a preservation subzone; it has a small human settlement and low-impact tourism; there are records of red mangrove and seagrass meadows, and in the reef lagoon, there are patches of O. annularis, P. astreoides, and P. porites and small coral recruits of Siderastrea radians. Mahahual belongs in a public-use subzone; it presents high reef complexity compared with reefs further north along the Caribbean coast of the Yucatan peninsula (Argüelles-Jiménez et al. 2020). The dominant corals are Orbicella and Diploria (CONANP 2016). This is a fringing reef with low nutrients (Hernández-Ballesteros et al. 2013), high coastal development, and an increase in massive tourism (Arriaga-Cabrera et al. 1998).
Field work was conducted in 2022 over three climatic seasons: dry (April 04 to 08th), rainy (August 16 to 18th), and north-wind season (November 15 to 17th).
Description and quantification of cleaner and client fishes
The samplings were done in each locality by the same team of three observers, once every season; observers were previously trained to identify fish and shrimp species; unit effort was standardized as cleaning interactions per hour-person. We considered two zones of the reef: the reef lagoon (2-m depth) with snorkel diving and the shallow forereef (~ 8–10 m depth) with scuba diving. After locating cleaning stations, the following data were collected: cleaner and client fish species, abundance, length, time of interaction (Arnal and Côté 1998), and presence of lionfish in the cleaning stations (Tuttle 2017; Tuttle et al. 2021). Fish identification was done with the guide of Humann and DeLoach (2014), and the cleaner shrimp was corroborated with DeLoach et al. (2019).
The cleaning interactions were documented with Sony a6000 and GoPro Hero 9 cameras, providing evidence for such behaviors as client position, “dance,” and body sites where the cleaner fish bit (see Supplementary Material). Size and ontogenetical stage of cleaner and client fish were also noted, as well as identity and abundance of fishes surrounding the interaction (at ~ 2m, during 5 min).
Structural complexity of the substratum and description of cleaning stations
The structural complexity of the substratum was evaluated using the methodology of Polunin and Roberts (1993), i.e., a 6-point scale: 0, no vertical relief; 1, low and sparse relief; 2, low but widespread relief; 3, moderately complex; 4 very complex and numerous caves and fissures; and 5, exceptionally complex with high coral cover and numerous caves and overhangs. As a complement, we estimated the percent coverage of live coral, dead coral, seagrass, sand, rock, and algae, in ~ 1 m2 (Lang et al. 2012). Depth, time of day, and general weather conditions (rain and wind) were also noted.
Just before or after each dive, temperature, salinity, and pH were measured at ~ 1-m depth using a Hanna multiparameter probe model HI98194.
Statistical analysis of data
To compare diversity and other community descriptors, we standardized the sampling effort with rarefaction curves obtained with EstimateS version 9.10 (Colwell 2013). These curves were built by site and by season.
One-way univariate PERMANOVA (α < 0.05) was used to compare the richness of cleaner and client fishes by each locality and season. Two-way multivariate PERMANOVA (α < 0.05) with similarity index of Bray–Curtis was used to detect differences between cleaner and of client fishes by locality, season, and the interaction (locality * season). The program used was PAST version 4.04 (Hammer et al. 2001).
Networks of cleaning interactions were constructed, considering the frequency of cleaning events, relative abundance of species, and richness of cleaner and client fishes. These were done with the function bipartite “plotweb” within the package “bipartite” in R version 4.2.1 (R Core Team 2020).
Results
Behavioral observations
We observed three types of poses in client fish: a) classic vertical static pose (head up or head down) (Fig. 2a), mainly observed in parrotfishes, juvenile wrasses, and damselfishes; b) horizontal static pose (Fig. 2b), surgeonfishes (Acanthurus chirurgus, A. coeruleus, and A. tractus), butterflyfishes (Chaetodon capistratus, Ch. ocellatus, and Ch. striatus), and Yellowtail Damselfish Microspathodon chrysurus; c) horizontal moving pose (Fig. 2c), observed in carnivorous fishes such as French Grunt Haemulon flavolineatum, Bluestriped Grunt H. sciurus, Schoolmaster Lutjanus apodus, Yellowtail Snapper Ocyurus chrysurus, and Guaguanche Sphyraena guachancho. Furthermore, Sparisoma viride was observed with the vertical static pose between the branches of a gorgonian, the behavior known as “pseudo-cleaning.”
The dance observed in the cleaner fish, Bluehead Thalassoma bifasciatum, consisted in the inspection to client fish, some signals with dance around the cleaning station and finally the cleaning act. Cleaners such as sergeant major Abudefduf saxatilis, slippery dick Halichoeres bivittatus, broadstripe goby Elacatinus prochilos, sharknose goby E. evelynae, juvenile French angelfish Pomacanthus paru, juvenile high-hat Pareques acuminatus, and banded coral shrimp Stenopus hispidus did not present dance or specific behaviors before or after the cleaning.
Cleaning stations, cleaner, and client fishes
We observed a total of 63 cleaning stations for 42 person-hours. In the dry season, we located 23 cleaning stations, 12 of them in Mahahual, eight in the reef lagoon reef, and four in the shallow forereef; nine cleaning stations were found in Río Indio, two in Pulticub, and none in Tampalam. The mean time of cleaning interactions for all localities was 10.16 s (± 5.48 s). The preferred biting site for the cleaner fish was along the sides, with 84%. The total abundance of cleaner fishes was 71 individuals divided in four species: juvenile A. saxatilis (~ 3 cm, n = 16), E. prochilos (n = 1), H. bivittatus initial phase (~ 5 cm, n = 2), and T. bifasciatum (3–5 cm, n = 52). The latter was dominant in the reef lagoon of Mahahual, Río Indio, and Pulticub in its initial phase; in the shallow forereef in Mahahual, this same species was dominant, but in the intermediate phase (6 cm).
We sighted 56 individuals of client fish in the dry season, belonging in 7 families and 19 species. The subfamily Scarinae was most abundant (39.3%) and frequent (31%) of all client fish (Fig. 3). The mean length of client fish was 16.8 cm (± 4.7 cm); 57% were adults. Mahahual was the location with most cleaning stations the highest richness of client fish, with redband parrotfish Sparisoma aurofrenatum and striped parrotfish Scarus iseri the major clients (Fig. 4), likewise, Río Indio, the dominant client, was yellowtail parrotfish Sparisoma rubripinne, followed by L. apodus and H. flavolineatum (Fig. 5). Pulticub had only three client fish: A. saxatilis, S. rubripinne, and S. viride (Fig. 6). Finally, in Tampalam, we did not observe any cleaning interaction, although potential cleaner species were present.
In the rainy season, nine cleaning stations were found: six in Mahahual, three in Pulticub, and none in Río Indio (Tampalam could not be visited), all only in the reef lagoon zone (Fig. 7). The mean duration of cleaning interactions was 4.0 s (± 2.0 s). The preferred bite site was along the sides, with an incidence of 91%. Only two cleaner species were recorded in this season: juvenile A. saxatilis (3 cm, n = 4) and T. bifasciatum in the initial phase (~ 3 cm, n = 23); T. bifasciatum was dominant in Mahahual and Pulticub. Both cleaner fishes were seen in Río Indio, but there was no cleaning interaction. The abundance of client fish was 15 individuals, in 6 families and 8 species; S. aurofrenatum and Ch. capistratus had the highest cleaning frequency with a 45%. H. flavolineatum and S. rubripinne were the dominant client fishes at Mahahual (Fig. 8), whereas for Pulticub three client fishes were observed, with only one individual each: Canthigaster rostrata, Ch. capistratus, and S. aurofrenatum (Fig. 9). The mean body size of client fishes was 14.9 cm (± 9.9 cm).
Finally, for the north-wind season, 31 cleaning stations were found: 10 in the Mahahual reef lagoon, 10 in the Mahahual shallow forereef, and 11 in the shallow forereef of Río Indio; none was found in Pulticub and Río Indio reef lagoon, and again Tampalam was not accessed. The mean duration of each cleaning event was 9.6 s (± 6.9 s). The bites of the cleaners were 81% along the sides of the client pectoral fins 15%, and there were some bites in the head, gills, and tail fin, with 1% each. In this season, six cleaner species were recorded (Fig. 5): E. evelynae (1 cm, n = 2), juvenile H. bivittatus (~ 10 cm, n = 3), juvenile P. acuminatus (4 cm, n = 1), juvenile Pomacanthus paru (10 cm, n = 1), the banded coral shrimp S. hispidus (n = 1), and juvenile T. bifasciatum (1–15 cm, n = 135). The latter was the only cleaner in Mahahual shallow forereef and dominant in Río Indio shallow forereef and Mahahual reef lagoon, although in the latter the diversity of cleaner was higher. We observed 102 individuals of client fishes, belonging in 9 families and 21 species. In this season, the client fishes with greater cleaning frequency were A. coeruleus and bicolor damselfish Stegastes partitus (Fig. 10); there was a reduction in the parrotfish clients and an increase in the diversity of client fishes in general. The client fishes frequent (38%) and dominant in Mahahual reef lagoon were adults of White Grunt Haemulon plumierii and H. flavolineatum; in Mahahual shallow forereef the dominant client fish was Blue Chromis Azurina cyanea (Fig. 11); and in Río Indio shallow forereef, Acanthurus tractus and S. iseri (Fig. 12).
Specifically, the presence of lionfish interacting in cleaning stations was not seen. However, lionfish was part of the visual census of coral reef fish community in Mahahual and Río Indio in all climatic seasons.
Statistical analysis
There were clear differences in richness of client fishes by season and locality (Table 2). Specifically, Mahahual and Río Indio were different to Pulticub (Table 3).
The same occurred with the richness of cleaner species, by seasons and by locality, as well as the interaction between season and locality (Table 2). Again, Pulticub was different to Mahahual and Río Indio (Table 3).
Cleaning stations attributes and environmental variables
In general, the structural complexity and types of bottoms were different by season, locality, and reef zone.
In the dry season, the structural complexity of 43% of the cleaning stations was category 2, 39% category 3, and 17% in level 4; massive corals were dominant, with 20% of the total substrate, followed by dead coral and sand, with 18% each, algae with 17%, and other substrates with 27% (Table 4).
In the rainy season, 56% of the cleaning stations were in level 2, 22% in category 4, 11% in level 1 and 11% in level 3, while the benthos composition was 31% sand, 21% algae, 16% sponges, 13% dead coral, and 18% other substrates (Table 5).
In the north-wind season, 52% of cleaning stations were in category 3, 29% in level 2, and 6% in level 4. The bottom was covered by live coral (37%), soft corals (20%), algae (16%), and sand 11% (Table 6).
The environmental variables did not show wide variation by season and locality. The mean temperature for the dry season was 29.0 °C ± 1.3 °C, rainy season 29.7 °C ± 1.0 °C, and north-wind season 30.4 °C ± 1.6 °C. The salinity was constant for the three seasons, with 32.8 to 34.4 psu. The lower pH values were in the rainy season, coinciding with the massive arrival of Sargassum blooms.
Discussion
We did not design our sampling to compare the methods of snorkel vs. scuba diving. However, by any method, the cleaner and client fishes continued their interaction even if the observer came as close to them as 30–40 cm. This result coincides with findings by Giglio et al. (2020).
The cleaner and client fishes in the southern Mexican Caribbean are affected by spatial and temporal factors such as season and degree of conservation (time since establishment as MPA). In contrast to the original hypothesis, we did not observe any cleaning stations at the site with the longest time since establishment as MPA, with the highest degree of conservation and good coral health, whereas the sites with more recent protection and stronger effects of tourism and coastal development had a greater diversity of cleaner and client fishes and cleaning stations. In general, we concur with Arnal et al. (1999), who infer a tendency towards higher activity of cleaning and major ectoparasites availability in sites with tourism impact, some degree of degradation and wastewater discharge, such as Mahahual and Río Indio (Arias-González et al. 2017; Schmitter-Soto et al. 2018; Camacho-Cruz et al. 2020). The reason is that these conditions increase the probability that the client fishes have a high ectoparasite loading (Sasal et al. 2005) and the propagation of bacterial diseases (Narvaez et al. 2021) through the contact with cleaner fishes, for instance the black-spot syndrome (BSS) seen in Mahahual. Moreover, Cheney and Côté (2005) emphasized that the natural variability of cleaning symbioses drives temporal and geographical variation in ectoparasite abundance.
The cleaner fishes of Mesoamerican Reef System are voracious predators of ectoparasites (Grutter 1999), especially in Mahahual and Río Indio, the localities with more recent protection, with higher tourism in the former, where the largest diversity of cleaner and client fish species was reported; furthermore, fishes with ectoparasites on the cheek or the operculum were observed only in Mahahual. The presence of ectoparasite on the client fishes is a signal of local pollution (Sasal et al. 2007); high variation of temperature and salinity are important for the parasite specificity to their host, and environmental stressors can also increase the parasite community (Sikkel et al. 2000; Sasal et al. 2007).
The lack of cleaning interactions at Tampalam, however, could be due to sampling effort. Several authors (Arnal and Côté 1998; Sazima et al. 1999; Whiteman and Côté 2002; Dunkley et al. 2019) have found that cleaner gobies are frequent on live and healthy coral heads of Siderastrea siderea, Montastraea cavernosa, Colpophyllia natans, Orbicella annularis, and Agaricia agaricites; the cleaning stations observed in our study were found on massive corals, sand, algae, sponges, and dead coral. The exception was observed in the Mahahual and Río Indio shallow forereefs, where the cleaning stations occurred on heads of Orbicella faveolata and soft corals.
Without cleaner fishes, coral reefs face a decrease in the richness, abundance, and length of client fishes (Limbaugh 1961; Waldie et al. 2011) or even the disappearance of some species (Limbaugh 1961). This was corroborated in the Río Indio reef lagoon, where there was a considerable decrease of cleaner and client fishes during the rainy season. These changes on the fish community could be explained by stochastic and deterministic processes that affect fish at local scale (Grutter et al. 2003), for instance the coincidence with the Sargassum massive arrival on the coastline. Currently, there is no information about the effect of this macroalga on the cleaning interactions in situ; however, the effects of brown tide, such as darkened color of the water, higher organic matter content in the bottom, stress on coral and seagrass, increased epiphyte cover, and mortality of fauna (van Tussenbroek et al. 2017) are all consequences of Sargassum that possibly affected the cleaner and client fishes. Three months after our first sampling in the Río Indio reef lagoon, the cleaner T. bifasciatum disappeared, and the diversity and abundance of potential client fishes decreased notably, besides a structural change in benthos (pers. obs.); perhaps cleaner and client fishes moved toward colder waters and with less turbidity.
Multiple studies have demonstrated than gobies are quintessential cleaners for the Caribbean (Arnal and Côté 1998; Arnal et al. 2000; Soares et al. 2007; Dunkley et al. 2019); however, in the southern Mexican Caribbean reefs the dominant and most abundant cleaner is T. bifasciatum. The bluehead wrasse has advantages as a cleaner, for instance its vivid colors, which make it a striking species to client fishes (Bellwood et al. 2020), as a “cleaning service signal” (Cheney et al. 2009); high visual resolution (McFarland 1991); high mobility; and a variety of behavioral and morphological adaptations (Baliga and Law 2016).
Halichoeres bivittatus overlaps in ecological functional niche with T. bifasciatum, which is beneficial for H. bivitattus, because of the shelter that it finds among bluehead juveniles. Both species are morphologically similar, in fact the coloration patterns are identical in the initial growth phase. Côté and Brandl (2021) concluded than the differences among wrasse cleaners lie in the cleaning intensity and the habitat preferences, although H. bivittatus was more frequent in bottoms with low and sparse relief, such as seagrass meadows or sand.
The low cleaning activity by gobies Elacatinus prochilos and E. evelynae compared to other studies (Darcy et al. 1974; Whiteman and Côté 2002; Soares et al. 2007; Dunkley et al. 2019) could be due to their reported client fishes being mainly piscivores: in the last decade, this trophic guild has decreased in the region (Schmitter-Soto et al. 2018). Furthermore, the ecotypes of Elacatinus associated with coral-dwelling with cleaning function were uncommon compared with those reported in the Antilles (Côté and Soares 2011; Dunkley et al. 2019; Xavier et al. 2019), depth variation being an important factor to consider (Johnson and Ruben 1988).
Invasive lionfish Pterois volitans is a voracious predator of native cleaner species such as T. bifasciatum, H. bivittatus, Abudefduf saxatilis, and juveniles of such client fish as parrotfishes (Valdez-Moreno et al. 2012; Anton et al. 2016); therefore, the presence of P. volitans in the cleaning stations directly alters the dynamics between native cleaner and client fishes (Tuttle 2017).
Reef size and availability of shelter are essential for the density of cleaner fishes (Youngbluth 1968; Johnson and Ruben 1988; Arnal et al. 1999). Variation in the structural complexity around our cleaning stations and the different bottom types explain the differences among localities and reveal distinct habitat-associated species patterns (Núñez-Lara and Arias-González 1998). For instance, Stegastes partitus and T. bifasciatum were observed interacting in the same habitat in Mahahual shallow forereef; S. partitus is not a cleaner, but it has been reported to regulate access to client fishes or predators (Arnal and Côté 1998; Dunkley et al. 2023), a mutualist cooperation with the wrasse.
Depth has an important role in coral reef fish assemblages (Johnson and Ruben 1988; Arnal et al. 1999). We observed the planktivorous guild only in the shallow forereef at Mahahual, with higher abundance of species such as Azurina cyanea, Clepticus parrae, and Melichthys niger. There is as well a temporal variation of cleaner and client fishes due to their reproductive cycles, where the availability of resources, nictemeral cycles, environmental variables, and local geomorphology (Toro-Ramírez et al. 2017) explain the assemblage changes of coral reef fishes. During the north-wind season, reproductive harems of T. bifasciatum and parrotfishes were observed in Mahahual.
Secondary or incidental juvenile cleaners such as Pomacanthus paru and Pareques acuminatus were observed, a first record of them acting as cleaners to adult high-hat P. acuminatus in the Mexican Caribbean. These cleaner species are considered incidental or secondary because only 20% of their diet are ectoparasites removed from other fish (DeLoach et al. 2019). Specifically, French angelfish P. paru has a wide range of fish clients in the Western Atlantic, such as cryptobenthic fish (Sampaio et al. 2017), groupers, jacks, morays, triggerfish (Sazima et al. 1999), or squirrelfish (Morais et al. 2017). In this study, P. paru was observed for the first time as a cleaner of Guachancho Barracuda and White Grunt. Other facultative cleaner fishes reported for the Caribbean were observed (Côté 2000; DeLoach et al. 2019): such as Spanish hogfish Bodianus rufus, Royal Gramma Gramma loreto, queen angelfish Holacanthus ciliaris, and spotted drum Equetus punctatus, furthermore, cleaner crustaceans as Stenorhynchus seticornis (Medeiros et al. 2011) and Stenopus hispidus (Limbaugh et al. 1961).
In general, the presence and density of cleaning stations, cleaner, and client fishes of the southern Mexican Caribbean vary according to season and the degree of conservation (time since establishment as MPA) of each locality. Sites with tourism and anthropogenic impacts were a hotspot of ectoparasites and, therefore, a greater diversity of cleaner and client fishes with unique behaviors. Local monitoring of water quality and environmental variables that affect these crustaceans is important; however, regional effects are perhaps more important to consider, especially the ever greater seasonal arrival of Sargassum.
Data availability
The dataset analyzed during this work is available with the corresponding author on reasonable request.
References
Anton A, Cure K, Layman CA, Puntila R, Simpson MS, Bruno JF (2016) Prey naïveté to invasive lionfish Pterois volitans on Caribbean coral reefs. Mar Ecol Progress Ser 544:257–269. https://doi.org/10.3354/meps11553
Argüelles-Jiménez J, Alva-Basurto JC, Pérez-España H, Zetina-Rejón MJ, Arias-González JE (2020) The measurement of ecosystem development in Caribbean coral reefs through topological indices. Ecol Indic 110:105866. https://doi.org/10.1016/j.ecolind.2019.105866
Arias-González JE, Fung T, Seymour RM, Garza-Pérez JR, Acosta-González G, Bozec Y-M, Johnson CR (2017) A coral-algal phase shift in Mesoamerican not driven by changes in herbivorous fish abundance. PLOS ONE 12(4):e0174855. https://doi.org/10.1371/journal.pone.0174855
Arnal C, Côté IM (1998) Interactions between cleaning gobies and territorial damselfish on coral reefs. Anim Behav 55:429–1442. https://doi.org/10.1006/anbe.1998.0727
Arnal C, Côté IM, Sasal P, Morand S (2000) Cleaner-client interactions on a Caribbean reef: influence of correlates of parasitism. Behav Ecol Sociobiol 47:353–358. https://doi.org/10.1007/s002650050676
Arnal C, Morand S, Kulbicki M (1999) Patterns of cleaner wrasse density among three regions of the Pacific. Mar Ecol Progress Ser 177:213–220. https://doi.org/10.3354/meps177213
Arriaga-Cabrera L, Vázquez-Domínguez E, González-Cano J, Jiménez-Rosenberg R, Muñoz-López E, Aguilar Sierra V (1998) Regiones Prioritarias Marinas de México. México: Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, Mexico City
Baliga VB, Law CJ (2016) Cleaners among wrasses: phylogenetics and evolutionary patterns of cleaning behavior within Labridae. Mol Phylogenet Evol 94:424–435. https://doi.org/10.1016/j.ympev.2015.09.006
Bellwood DR, Hemingson CR, Tebbett SB (2020) Subconscious biases in coral reef fish studies. BioScience 70(7):621–627. https://doi.org/10.1093/biosci/biaa062
Bshary R, Oliveira RF, Oliveira T, Canário A (2007) Do cleaning organisms reduce the stress response of client reef fish? Front Zool 4(1):21. https://doi.org/10.1186/1742-9994-4-21
Calderón-Aguilera LE, Rivera-Monroy VH, Porter-Boland L et al (2012) An assessment of natural and human disturbance effects on Mexican ecosystems: current trends and research gaps. Biodivers Conserv 21:589–617. https://doi.org/10.1007/s10531-011-0218-6
Camacho-Cruz KA, Ortiz-Hernández MC, Sánchez A et al (2020) Water quality in the eastern karst region of the Yucatan Peninsula: nutrients and stable nitrogen isotopes in turtle grass, Thalassia testudinum. Environ Sci Pollut Res 27:15967–15983. https://doi.org/10.1007/s11356-019-04757-3
Caves EM (2021) The behavioural ecology of marine cleaning mutualisms. Biol Rev 96:2584–2601. https://doi.org/10.1111/brv.12770
Cheney KL, Côté IM (2005) Mutualism or parasitism? The variable outcome of cleaning symbioses. Biol Lett 1:162–165. https://doi.org/10.1098/rsbl.2004.0288
Cheney KL, Grutter AS, Blomberg S, Marshall JN (2009) Blue and yellow signal cleaning behaviour in coral reef fishes. Curr Biol 19:1283–1287. https://doi.org/10.1016/j.cub.2009.06.028
Cobián-Rojas D, Schmitter-Soto JJ, Aguilar-Perera A, Aguilar-Betancourt C et al (2018) Diversidad de las comunidades de peces en dos áreas marinas protegidas del Caribe y su relación con el pez león. Rev Biol Trop 66(1):189–203. https://doi.org/10.15517/rbt.v66i1.28197
Colwell RK (2013) EstimateS: statistical estimation of species richness and shared species from samples. Version 9. – user’s Guide and application at http://purl.oclc.org/estimates.
CONANP, Comisión Natural de Áreas Naturales Protegidas (2014) Programa de Manejo Complejo Sian Ka´an: Reserva de la Biosfera Sian Ka’an, área de protección de flora y fauna Uaymil y Reserva de la Biosfera Arrecifes de Sian Ka’an. Secretaría de Medio Ambiente y Recursos Naturales, Mexico City
CONANP, Comisión Natural de Áreas Naturales Protegidas (2016) Programa de Manejo Reserva de la Biosfera Caribe Mexicano. Secretaría de Medio Ambiente y Recursos Naturales, Mexico City
Côté IM (2000) Evolution and ecology of cleaning symbioses in the sea. Oceanogr Mar Biol Annu Rev 38:311–355
Côté IM, Brandl SJ (2021) Functional niches of cleanerfish species are mediated by habitat use, cleaning intensity and client selectivity. J Anim Ecol 90:2834–2847. https://doi.org/10.1111/1365-2656.13585
Côté IM, Soares MC (2011) Gobies as cleaners. In: Patzner R, Van Tassell JL, Kovacic M, Kapoor BG (eds) The biology of gobies. Science Publishers, St. Helier, UK, pp 525–551
Darcy GH, Maisel E, Ogden JC (1974) Cleaning preferences of the gobies Gobiosoma evelynae and G. prochilos and the juvenile wrasse Thalassoma bifasciatum. Copeia 1974(2):375–379. https://doi.org/10.2307/1442531
DeLoach N, DeLoach A, Humann P (2019) Reef fish behavior. New World, Jacksonville
Dunkley K, Ellison AR, Mohammed RS, Oosterhout C, Whittey KE, Perkins SE, Cable J (2019) Long-term cleaning patterns of the sharknose goby (Elacatinus evelynae). Coral Reefs 38:321–330. https://doi.org/10.1007/s00338-019-01778-9
Dunkley K, Whittey KE, Ellison A, Perkins SE, Cable J, Herbert-Read J (2023) The presence of territorial damselfish predicts choosy client species richness at cleaning stations. Behav Ecol 34(2):269–277. https://doi.org/10.1093/beheco/arac122
Estrada-Saldívar N, Molina-Hernández A, Pérez-Cervantes E, Medellín-Maldonado F, González-Barrios FJ, Álvarez-Filip L (2020) Reef-scale impacts of the stony coral tissue loss disease outbreak. Coral Reefs 39:861–866. https://doi.org/10.1007/s00338-020-01949-z
Figueroa-Zavala B, Correa-Sandoval J, Ruiz-Zárate MA, Weissenberger H, González-Solís D (2015) Environmental and socioeconomic assessment of a poorly known coastal section in the southern Mexican Caribbean. Ocean Coast Manag 110:25–37. https://doi.org/10.1016/j.ocecoaman.2015.02.010
Fulton S, Caamal-Madrigal J, Aguilar-Perera A, Bourillón L, Heyman WD (2018) Marine conservation outcomes are more likely when fishers participate as citizen scientists: case studies from the Mexican Mesoamerican Reef. Citizen Science: Theory and Practice 3(1):1–12. https://doi.org/10.5334/cstp.118
García-Rivas M, Machkour-M’Rabet S, Pérez-Lachaud G et al (2018) Age-dependent strategies related to lionfish activities in the Mexican Caribbean. Environ Biol Fish 101:563–578. https://doi.org/10.1007/s10641-018-0718-2
García-Téllez N, Schmitter-Soto JJ, Barrientos-Medina RC, Herrera-Pavón RL (2022) Goliath grouper Epinephelus itajara (Teleostei: Serranidae) in the Mexican Caribbean: local ecological knowledge and habitat use. Environ Biol Fish 105:669–684. https://doi.org/10.1007/s10641-022-01275-z
Giglio VJ, Nunes JA, Ferreira CE, Blumstein DT (2020) Client reef fish tolerate closer human approaches while being cleaned. J Zool 312(3):205–210. https://doi.org/10.1111/jzo.12814
Grutter AS (1999) Cleaner fish really do clean. Nature 398:672–673. https://doi.org/10.1038/19443
Grutter AS, Murphy J, Howard J (2003) Cleaner fish drives local fish diversity on coral reef. Curr Biol 13:64–67. https://doi.org/10.1016/S0960-9822(02)01393-3
Guimarais M, Zúñiga-Ríos A, Cruz-Ramírez CJ, Chávez V, Odériz I, van Tussenbroek BI, Silva R (2021) The conservational state of coastal ecosystems on the Mexican Caribbean Coast: Environmental Guidelines for their management. Sustainability 13(5):2738. https://doi.org/10.3390/su13052738
Hammer Ø, Harper D, Ryan PD (2001) PAST:Paleontological statistics software package for education and data analysis. Palaeontol Electron 4(1):1–9
Hernández-Ballesteros LM, Elizalde-Rendón EM, Carballo JL, Carricart-Ganivet JP (2013) Sponge bioerosion on reef building corals: dependent on the environment or on skeletal density? J Exp Mar Biol Ecol 441:23–27. https://doi.org/10.1016/j.jembe.2013.01.016
Hixon MA, Randall JE (2018) Coral reef fishes. In: Cochran J, Bokuniewicz J, Yager LP (eds) Encyclopedia of ocean sciences. Academic Press, San Diego, pp 142–150
Humann P, DeLoach N (2014) Reef fish identification: Florida. Caribbean and Bahamas, New World, Jacksonville
Johnson WS, Ruben P (1988) Cleaning behavior of Bodianus rufus, Thalassoma bifasciatum, Gobiosoma evelynae and Periclimenes pedersoni along a depth gradient at Salt River submarine Canyon. St. Croix. Environ Biol Fishes 23(3):225–232. https://doi.org/10.1007/BF00004913
Jordán-Dahlgren E (1989) Efecto de la morfología del sustrato en el desarrollo de la comunidad coralina. An Inst Cienc Mar Limnol Univ Nac Autón México 16:105–118
Lang JC, Kramer PR, Kramer PA, Ginsburg RN (2012) Appendix one: the Atlantic and Gulf Rapid Reef Assessment (AGRRA) protocols: version 5.5. Atoll Research Bull 496:611–624
Limbaugh C (1961) Cleaning symbiosis. Sci Am 205(2):42–49
Limbaugh C, Pederson H, Chance FA Jr (1961) Shrimps that clean fishes. Bull Mar Sci Gulf & Carib 11:237–257
Losey GS (1972) The ecological importance of cleaning symbiosis. Copeia 4:820–833. https://doi.org/10.2307/1442741
McFarland WN (1991) The visual world of coral reef fishes. In: Sale P (ed) The ecology of fishes on coral reefs. Academic Press, San Diego, pp 16–38
Medeiros DV, de Anchieta CC, Nunes J et al (2011) Yellowline arrow crab Stenorhynchus seticornis (Brachyura: Majidae) acting as a cleaner of reef fish, eastern Brazil. Mar Biodivers Rec 10(1):1–4. https://doi.org/10.1017/S1755267211000637
Morais RA, Brown J, Bedard S, Ferreira CEL, Floeter SR, Quimbayo JP, Rocha LA et al (2017) Mob rulers and part-time cleaners: two reef fish associations at the isolated Ascension Island. J Mar Biolog Assoc U.K. 97(4):799–811. https://doi.org/10.1017/S0025315416001041
Narvaez P, Vaughan DB, Grutter AS et al (2021) New perspectives on the role of cleaning symbiosis in the possible transmission of fish diseases. Rev Fish Biol Fisheries 31:233–251. https://doi.org/10.1007/s11160-021-09642-2
Núñez-Lara E, Arias-González J (1998) The relationship between reef fish community structure and environmental variables in the southern Mexican Caribbean. J Fish Biol 53(sA):209–22. https://doi.org/10.1111/j.1095-8649.1998.tb01028.x
Polunin N, Roberts C (1993) Greater biomass and value of target coral-reef fishes in two small Caribbean marine reserves. Mar Ecol Progr Ser 100:167–176. https://doi.org/10.3354/meps100167
Quimbayo JP, Mendes TC, Barneche DR et al (2021) Patterns of taxonomic and functional diversity in the global cleaner reef fish fauna. J Biogeogr 48(10):2469–2485. https://doi.org/10.1111/jbi.14214
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Sampaio CLS, Loiola M, Colman LP et al (2017) Cryptobenthic fish as clients of french angelfish Pomacanthus paru (Pomacanthidae) during cleaning behaviour. Mar Biodivers Rec 10(8). https://doi.org/10.1186/s41200-017-0109-y
Santander-Monsalvo J, Espejel I, Ortiz-Lozano L (2018) Distribution, uses, and anthropic pressures on reef ecosystems of Mexico. Ocean Coast Manag 165:39–51. https://doi.org/10.1016/j.ocecoaman.2018.08.014
Sasal P, Mouillot D, Fichez R, Chifflet S, Kulbicki M (2007) The use of fish parasites as biological indicator of anthropogenic influences in coral-reef lagoons: a case study of Apogonidae parasites in New-Caledonia. Mar Poll Bull 54:1697–1706. https://doi.org/10.1016/j.marpolbul.2007.06.014
Sasal P, Thomas F, Grutter AS (2005) Behavioural aspects of parasitism. In: Rohde K (ed) Marine parasitology. CABI Publishing, Wallingford, pp 259–278
Sazima I, Moura RL, Sazima C (1999) Cleaning activity of juvenile angelfish, Pomacanthus paru, on the reefs of the Abrolhos Archipielago, western South Atlantic. Environ Biol Fishes 56:399–407. https://doi.org/10.1023/A:1007531925845
Schmitter-Soto JJ, Aguilar-Perera A, Cruz-Martínez A et al (2018) Interdecadal trends in composition, density, size, and mean trophic level of fish species and guilds before and after coastal development in the Mexican Caribbean. Biodivers Conserv 27:459–474. https://doi.org/10.1007/s10531-017-1446-1
Sikkel PC, Fuller CA, Hunte W (2000) Habitat/sex differences in time at cleaning stations and ectoparasite loads in a Caribbean reef fish. Mar Ecol Progr Ser 193:191–199. https://doi.org/10.3354/meps193191
Soares MC, Cardoso SC, Côté IM (2007) Client preferences by Caribbean cleaning gobies: food, safety or something else? Behav Ecol Sociobiol 61:1015–1022. https://doi.org/10.1007/s00265-006-0334-6
Titus BM, Daly M, Exton DA (2015) Do reef fish habituate to diver presence? Evidence from two reef sites with contrasting historical levels of scuba intensity in the Bay Islands. Honduras. PLoS ONE 10(3):e0119645. https://doi.org/10.1371/journal.pone.0119645
Toro-Ramírez A, Sosa-López A, Ayala-Pérez LA et al (2017) Abundancia y diversidad de la ictiofauna en la Reserva de la Biósfera Los Petenes, Campeche, México: asociaciones con los ciclos nictemerales y las épocas climáticas. Lat Am J Aquat Res 45(2):311–321. https://doi.org/10.3856/vol45-issue2-fulltext-7
Tuttle LJ (2017) Direct and indirect effects of invasive lionfish on coral-reef cleaning mutualists. Mar Ecol Progr Ser 569:163–172. https://doi.org/10.3354/meps12092
Tuttle LJ, Lamb RW, Stringer AL (2021) Differential learning by native versus invasive predators to avoid distasteful cleaning mutualists. Func Ecol 35:1481–1490. https://doi.org/10.1111/1365-2435.13806
Valdez-Moreno M, Quintal-Lizama C, Gómez-Lozano R, García-Rivas MC (2012) Monitoring and alien invasión: DNA barcoding and the identification of lionfish and their prey on coral reefs of the Mexican Caribbean. PLoS ONE 7(6):e36636. https://doi.org/10.1371/journal.pone.0036636
van Tussenbroek BI, Hernández Arana HA, Rodríguez-Martínez RE et al (2017) Severe impacts of brown tides caused by Sargassum sp. on near-shore Caribbean seagrass communities. Mar Poll Bull 122(1-2):272–281. https://doi.org/10.1016/j.marpolbul.2017.06.057
Vaughan D, Grutter AS, Costello M, Hutson K (2017) Cleaner fishes and shrimp diversity and a re-evaluation of cleaning symbioses. Fish Fish 18(4):698–716. https://doi.org/10.1111/faf.12198
Waldie PA, Blomberg SP, Cheney KL et al (2011) Long-term effects of the cleaner fish Labroides dimidiatus on coral reef fish communities. PLoS ONE 6(6):e21201. https://doi.org/10.1371/journal.pone.0021201
Whiteman EA, Côté IM (2002) Cleaning activity of two Caribbean cleaning gobies: intra and interspecific comparisons. J Fish Biol 60:1443–1458. https://doi.org/10.1111/j.1095-8649.2002.tb02439.x
Xavier R, Mazzei R, Pérez-Losada M, Rosado D et al (2019) A risky business? Habitat and social behavior impact skin and gut microbiomes in Caribbean cleaning gobies. Front Microbiol 10:716. https://doi.org/10.3389/fmicb.2019.00716
Youngbluth MJ (1968) Aspects of the ecology and ethology of the cleaning fish, Labroides phthirophagus Randall. Z Tierpsychol 25:915–932. https://doi.org/10.1111/j.1439-0310.1968.tb00052.x
Acknowledgements
We thank the staff of the marine protected areas (CONANP) for the authorization of fieldwork, María del Carmen García-Rivas and Miguel Ángel Ruiz-Zárate for the revision and suggestions, and Roberto L. Herrera-Pavón for his help in fieldwork.
Funding
This work was financed by Consejo Nacional de Ciencias, Humanidades, Tecnología e Innovación (CONAHCYT) with grant number 1095948.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study was observational, and the ethical approval was not required.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Videos of the types of cleaning interactions. (MP4 41422 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramírez-Ruiz, C.I., Schmitter-Soto, J.J. & Díaz-Osorio, A.C. Interactions of coral reef cleaner species in the Mexican Caribbean. Environ Biol Fish 106, 1831–1850 (2023). https://doi.org/10.1007/s10641-023-01459-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-023-01459-1