Abstract
Introduction
Transcobalamin (TC) transports cobalamin (vitamin B12) from plasma into cells. Its congenital deficiency is a rare autosomal recessive disorder due to mutations in the TCN2 gene. It causes intracellular cobalamin depletion with early onset in the first months of life, failure to thrive with pallor due to megaloblastic anemia. It can be associated with pancytopenia, gastrointestinal symptoms with vomiting, diarrhea, and neurological complications with myelopathy. Aggressive vitamin B12 parenteral therapy must be instituted early and continuously. Retinopathy and maculopathy are rarely associated with this condition.
Subject
We report the electrophysiological results of one TC-deficient patient diagnosed at the age of 4 months immediately and continuosly treated by hydroxocobalamin IM. Her visual function was followed by eight ophthalmological assessments, eight flash-ERG, six EOG, one mf-ERG, and seven P-ERG recordings over a 10-year period, between the age of 2y 9 m and 12y 6 m.
Results
Her ophthalmological assessment including visual acuity, fundi, optical coherent tomography (OCT), and retinal nerve fiber layer (RNFL) remained normal. From the age of 2y 9 m to 5y, dark-adapted and light-adapted flash-ERGs, EOGs and pattern-ERG were normal. From the age of 6y 4 m to 12y 6 m, dark-adapted flash-ERGs and EOGs remained normal. Cone a-wave amplitudes remained normal, whereas cone b-wave and flicker-response amplitudes were decreased. At the age of 12y 6 m, mf-ERG N1P1 amplitudes on the central 30° were decreased. From the age of 7y 4 m to 12y 6 m, P-ERG P50 amplitudes were decreased with no N95.
Comments
While clinical and anatomical assessments remained normal over a 10-year period, patient’s electrophysiological results suggested the progressive onset of a subclinical retinopathy of inner-cone dystrophy type, and a subclinical maculopathy on the central 30° including the ganglion cell layer deficiency on the central 15°, despite continuous intramuscular treatment, RPE and scotopic system remaining normal. The origins of such subclinical retinopathy and maculopathy are unknown and independent of early disease identification and aggressive intramuscular hydroxocobalamin therapy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Transcobalamin (TC) is one of the cobalamin (vitamin B12) transporters. It transports cobalamin from blood into cells, thus ensuring cobalamin uptake for its intracellular metabolism. TC deficiency is a very rare recessively inherited disorder of cobalamin transport due to mutations in the TCN2 gene [1].
It causes intracellular cobalamin depletion with early clinical symptoms in the first months of life. Patients usually present with pallor due to megaloblastic anemia that could be associated with pancytopenia, gastrointestinal symptoms with vomiting, diarrhea, failure to thrive, and if undiagnosed and/or untreated neurological complications [2].
TC deficiency results in depletion of methylcobalamin (MeCbl) and mitochondrial adenosylcobalamin (AdoCbl) causing an increase in plasma homocysteine and plasma and urine methylmalonic acid concentrations and a decrease in methionine concentration [2,3,4].
Prognosis of TC deficiency can be severe if not identified and appropriately treated early on. Long-term intramuscular hydroxocobalamin injections must be initiated early [4,5,6]. A few cases of retinopathy have been described in suboptimally treated patients with TC deficiency, but it remains unclear whether such a complication could also occur in long-term treated patients.
Our aim was to describe the evolution of the visual function in one TC-deficient child diagnosed at the age of 4 months and immediately treated by hydroxocobalamin injections from the time of diagnosis to last visit. Her visual function was assessed by eight flash-ERG, six EOG, one mf-ERG, and seven P-ERG recorded between the ages of 2 years 9 months, and 12 years 6 months (last visit).
This long-term follow-up of visual electrophysiological results shows the progressive development of a subclinical retinopathy of inner-cone dystrophy type and a subclinical maculopathy on the central 30°, including ganglion cells deficiency on the central 15°, despite continuous intramuscular injection of hydroxocobalamin and normal ophthalmological assessments including visual acuity, fundi, optical coherence tomography (OCT) and retinal nerve fiber layer (RNFL).
Subject
Clinical presentation
The patient presented here was already described at the age of 22 months old as patient n°1 in Schiff et al. [4] and as patient n°14 in Trakadis et al. [6]. She was born of consanguineous parents. Her brother died at the age of 3 months within a context of acute anemia five years earlier. Her four years older sister had no medical history. The proband presented with recurrent episodes of diarrhea with stomatitis at the age of one month and was admitted for gastroenteritis and an acute febrile episode with pancytopenia with megaloblastic bone marrow changes and pallor at the age of four months. TC deficiency was then suspected based on the medical history and accumulation of total homocysteine and methylmalonic acid in the setting of normal vitamin B12 blood concentration. TC deficiency was further confirmed by identification of homozygous TCN2 exon skipping splice variant (c.580 + 1G > C homozygous: exon 4 skipping) [4]. She was treated with intramuscular injections of hydroxocobalamin initially 1 mg daily for one week with normalization of hematological and metabolic parameters and then once a month associated with betaine and folinic acid. Hydroxocobalamin injections frequency was increased to twice monthly at the age of 2y 9 m due to mild increased urine methylmalonic acid concentration and once a week at the age of 7y in an attempt to prevent potential visual abnormalities. From the age of 7y 9 m to last visit, hydroxocobalamin injections were rescheduled to twice injections per week due to the occurrence of mild flash-ERG abnormalities recorded at that age. At last visit at the age of 12y, this patient was perfectly healthy with normal hematological and metabolic parameters. She is treated with hydroxocobalamin intramuscular injections 2 mg twice weekly associated with oral betain (1000 mg/day), oral L-methionine (25 mg/d), and oral folinic acid (5 mg/week). She exhibits mild school delay without intellectual disability.
Visual assessment
Fundi and visual acuity (VA: tests: Rossano-Weiss chart and/or Cadet chart before the age of 6, Monoyer chart after the age of 6). At the age of 2y 9 m, her fundi were normal. At the age of 3y 10 m, she had myopia and high astigmatism: right eye VA was: 20/60 with − 0.25(− 4.25)0° and left eye VA: 20/80 with − 0.25(− 5)175°. Appropriate correction was prescribed but not worn regularly. She had no strabismus with normal ocular motility. At the age of 5y, right eye VA was: 20/40 with + 1(− 5)5° and left eye VA: 20/30 with + 0,5(− 6)170°. This optical correction was then worn regularly. Cornea and lens were clear, and her fundi were normal with large optic disks of slightly colobomatous appearance. At the age of 7y 4 m, right eye VA was: 20/50 with + 0.50(− 4.50)0° and left eye VA: 20/30 with 0(− 5.50)170°. She was orthophoric; ocular motility was normal. Fundi were normal. At the age of 9y 2 m, right eye VA was: 20/25 with − 2,5(− 4.5)0° and left eye VA: 20/20, with 0(− 5.5)175°. At the age of 12y 6 m, right eye VA was: 20/30 and left eye VA: 20/25 with the optical correction previously prescribed, right or left eye visual acuity being not improvable.
Totals of eight flash-ERG, six EOG, one mf-ERG and seven P-ERG were recorded between the age of 2y 9 m and 12y 6 m.
Materials and methods
Fundus imaging optical coherence tomography (OCT) and retinal nerve fiber layer (RNFL) were performed with a Zeiss device Cirrus HD-OCT 5000 at the age of 9y 2 m and 12y 6 m.
Electrophysiological device used for all recordings was a MonPackOne (Metrovision, 59 Perenchies, France). All procedures were conducted according to ISCEV standard protocols adapted to children [7]: flash-ERG [8], EOG [9], mf-ERG [10], P-ERG [11]. Procedures, recording protocols, and signal processings were identical over the 10-year follow-up. Artifact rejections are included in the processing program in order to have reliable results. Responses with high noise level are excluded. All results were duplicated (or triplicated if necessary), controlled, and reproductible. Subject’s fixation was stable.
Eight flash-ERG were recorded between the age of 2y 9 and 12y 6 m. The child was never sedated. Her pupils were fully dilated. Child size skin electrodes were used for all recordings (Comepa, Neurocom, 93 Bagnolet, France) [12,13,14]. The two active electrodes were applied on each inferior eyelid. The reference electrode was placed on the forehead. The two earlobes were connected to the ground. Before each flash-ERG recording, the child was dark-adapted for 20 mn. The two dark-adapted flash-ERG responses were then recorded, i.e., rod-response to dim white flash (3.10–3 cd.s/m2) (0.5 Hz–4 averagings) and mixed-response to the standard flash (SF: 3 cd.s/m2) (0,1 Hz–4 averagings). After 10 mn of light adaptation to a white rod saturating background (30 cd/m2), the three light-adapted flash-ERG responses were recorded to the standard flash (SF), i.e., photopic oscillatory potentials (Phot-Ops—0.75 Hz–50 averagings—bandwidth 70–100 Hz), cone-response (2 Hz–4 averagings), and flicker-response (30 Hz – 20 averagings).
Six EOG were recorded at the age of 5y, 6y 4 m, 7y 4 m, 8y 4 m, 9y 2 m, and 12y 6 m with natural pupils, bright background: 500 cd/m2.
One mfERG was recorded with fully dilated pupils, eye by eye, at the age of 12y 6 m (61 hexagons, 75 Hz, hexagon luminance: 200 cd/m2). One active contact lens electrode was placed on the cornea previously anesthetized (ERG-Jet, Fabrinal Switzerland) and one reference skin electrode (Kendall Q-trace gold 5500 electrode Janken Med Inc CA-US) on the ipsilateral temple. Optimal optic correction was adapted to the distance of stimulation (i.e., 30 cm).
Seven P-ERG were recorded with natural pupil sizes and optimal refraction (60 averagings, check size 40’—2 Hz) at the age of 3y 10 m, 5y, 6y 4 m, 7y 4 m, 8y 4 m, 9y 2 m, and 12y 6 m. Each active skin electrode was applied on each inferior eyelid. The reference electrode was placed on the forehead. The two earlobes were connected to the ground.
Result interpretation. Each result was compared to those of age-matched group by the same experienced interpreter over the 10-year follow-up. They were normal or abnormal, i.e., with decreased amplitude beyond 1 SD or significantly decreased amplitude beyond 2 SD.
Results
Visual acuity was increased from the first measurement at the age of 2y 9 m to the fourth one at the age of 6y 4 m and then stabilized associate with consistent wear of her refractive correction. Her visual acuity decreased slightly between the age of 9y 2 m and 12y 6 m, while the optimal optical correction was worn (Table 1).
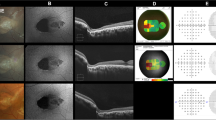
Fundi remained normal over the 10-year follow-up period (represented in Fig. 1 at the age of 9y 2 m and Fig. 2 at the age of 12y 6 m). Optical coherence tomography (OCT) (Fig. 1) and retinal nerve fiber layer (RNFL) were normal at the age of 9y 2 m, and 12y 6 m (Figs. 2 and 3).
Flash-ERG. The eight rod- and mixed-responses remained normal over the 10-year follow-up period (Figs. 4, 5 and 6). Light-adapted responses were normal at the age of 2y 9 m and 3y 10 m (Fig. 4). Form the age of 6y 4 m to 9y 2 m, the cone b-wave, and flicker-response amplitudes were decreased as the cone a-wave amplitudes were normal (Figs. 5 and 6). At the age of 12y 6 m, cone a-wave and flicker-response amplitudes were decreased, and cone b-wave amplitude was more decreased than previously. Phot-OP2 and OP3 amplitudes were nearly normal at the age of 5y and 6y 4 m, decreased at the age of 8y 4 m, and absent at the age of 12y 6 m. Phot-OP recordings were not interpretable at the age of 7y 4 m and 9y 2 m.
Standard flash-ERG. Responses recorded at the age of 5y, 6y4m and 7y4m. Right eye: upper line- Left eye: lower line. Black arrow Nl ampl and IT. Red arrows: decreased ampl < M.V—1SD, Nl IT. Rod- and mixed-responses were normal, cone a-waves were normal, cone b-wave and flicker-response amplitudes were decreased < M.V–1SD
Standard flash-ERG. Responses recorded at the age of 8y4m, 9y2m and 12y6m. Right eye: upper line- Left eye: lower line. Black arrow: Nl ampl and IT. Red arrow: decreased ampl < M.V–2SD. Rod- and mixed-responses remained normal. Cone a-waves remained normal except at the age of 12y6m; cone b-wave and flicker-response amplitudes were decreased < M.V–2SD
All six EOG recorded at the age of 5y, 6y4m, 7y4m, 8y4m, 9y2m, and 12y 6 m were normal (not shown).
Mf-ERG was recorded at the age of 12y 6 m when recording conditions were reliable (Fig. 7). The results were analyzed eye by eye and ring by ring. Central peak was decreased more on the right eye than on the left one. All N1P1 amplitudes were decreased on the central 30°.
Mf- ERG recorded at the age of 12y 6 m. Normal subject’s LE results (Nl s) at the age of 15y: Upper line. A map of local responses colored according to eccentricity. In red: foveal peak on central 5 degrees. In green: annular ring 2°–5°, yellow ring: 5°–10°, blue ring: 10°–15°, gray ring: 15°–20°. B Statistical analysis: normal results highlighted in green. C. 3D map with central peak colored in pink. Our patient’s results. Middle line: Right (RE) and Lower line: Left eye (LE): A- local responses, B- statistical analysis and C- 3D maps. A- Right and left eyes local response amplitudes inside the blue dotted circles were significantly decreased on an macular surface of 30°. The central peak amplitudes shown by a red arrow were also decreased. Noises were very low confirming the good recording quality. Abscissa 100 ms—ordinate: 0,50 µV B- Amplitude analyses – all amplitudes were decreased C- more evident on the 3D maps
Seven P-ERG were recorded between at the age of 3y 10 m and 12y 6 m (Fig. 8). When the first one was recorded (at the age of 3y 10 m), optical correction for astigmatism was prescribed recently. RE and LE pattern-ERG were discernible with an atypical waveform. At the age of 5y, optical correction was worn only few hours a day. However, RE and LE pattern-ERG were discernible, RE P50 amplitude was decreased, and RE and LE N95 amplitudes were normal. Optical correction was then worn permanently. At the age of 6y 4 m, RE and LE pattern-ERG were normal. At the age of 7y 4 m, RE and LE pattern-ERG were not interpretable. At the age of 8y 4 m and 9y 2 m, RE and LE P50 amplitudes were decreased, and RE and LE N95 amplitudes were null. At the age of 12y 6 m, RE P50 and N95 amplitudes were null, LE P50 amplitude was decreased, and N95 amplitude was null.
P-ERG (pediatric protocol: skin electrodes, 60 reversals). Normal subject’s (Nl s) at the age of 5y. Right eye: RE, Left eye: LE. Patient’s results. Normal at the age of 5y and 6y4m. P50 wave amplitudes decreased and N95 wave amplitudes null at the age of 8y4m, 9y2m and 12y 6 m. Abscissa: 50 ms—Ordinate: 2 µV. Vertical black arrow: normal ampl & implicit time (IT); vertical red arrow: –2SD < ampl < –1SD; horizontal red arrow: ampl & IT non-recordable
Comments
To our knowledge, 60 cases of TC deficiency were published between 1990 and 2021 [1, 4, 6, 15,16,17,18,19,20,21,22,23,24,25,26]. Among those 60 cases, only five patients were reported with visual impairment (8%): case 1—one with macular dysfunction at the age of 9 years (Schiff et al. [4]), case 2—one with retinopathy at the age of 13 years [26], case 3—one with retinopathy at the age of 15 years (Trakadis et al. [6]), case 4—one with maculopathy at the age of 16 years (Souied et al. [18]), case 5—one with maculopathy at the age of 29 years having evolved to rod-cone dystrophy at the age of 48 (Dharmasena et al. [20]). In those five cases, TC deficiency was diagnosed very early in life with the classical clinical symptoms (pallor, hematological, digestive, and neurological deterioration). Early intramuscular hydroxocobalamin treatment was initiated in three patients for one year and then switched to oral hydroxocobalamin (case 1) [4], (case 3) [6] and (case 4) [18]. In patient case 5 [20], intramuscular hydroxocobalamin was given for two years, and thereafter, no intramuscular or oral hydroxocobalamin treatment was given until the age of 17 years [18]. At the age of 17 years as epilepsy worsened and TC level was found to be reduced, three intramuscular hydroxocobalamin injections of 5 mg per week were started again. His visual acuity decreased at the age of 29 years with bull’s-eye maculopathy. In patient case 2[26],Footnote 1 oral hydroxocobalamin treatment was given at the age of 17 months with hematological improvement. However, diagnosis of TC deficiency was made at the age of 13 years only when retinopathy and maculopathy were discovered associated with metabolic deficiencies. Intramuscular hydroxocobalamin treatment has been then instituted weekly and continuously. Retinopathy and maculopathy remained stable over a 20-year period.
Our patient’s TC deficiency was diagnosed early in life. She was regularly and continuously treated with hydroxocobalamin intramuscular injections (Table 1). Her ophthalmological assessments were regular over the 10-year period of follow-up. Before the age of 5 years, she did not wear her prescribed optical correction on a regular basis. Her myopic astigmatism could have caused refractive amblyopia with limited visual acuity. She has been wearing her optical correction permanently from the age of 5 years. Visual acuity has been improved because refractive amblyopia was corrected. Visual acuity was normal at the age of 9y 2 m and slightly decreased on RE at the age of 12y 6 m (last visit). Her fundi, neuroretinal layers (OCT), and retinal nerve fiber layers (RNFL), were normal at the age of 9y 2 m and 12y 6 m, i.e., no clinical or visible anatomical signs of retinopathy or maculopathy. OCT is an anatomical result which can be normal when retinal function can be abnormal. These results are complementary.
However, the evolution of her retinal function assessed by electrophysiology differed from those clinical or anatomical results. Throughout the 10-year follow-up period, all electrophysiological recordings were conducted with the same device, in the same conditions, and analyzed by the same experienced physicians.
Flash-ERGs test the global retinal function of all rods and their bipolar cells (i.e., rod-response), of all rods and cones and their respective bipolar cells (i.e., mixed-response), (rod- and mixed-responses corresponding to dark-adapted responses), and of all cones and their bipolar cells (i.e., light-adapted responses). The eight flash-ERGs recorded on our patient showed that rod- and mixed-responses were normal, i.e., that her scotopic system was and remained normal. However, progressive decreases of cone b-wave and flicker-response amplitudes suggested that her photopic system was gradually dysfunctioning in the inner retinal layers. These results qualified a retinopathy of inner-cone dystrophy type.
EOGs reflect the functioning of the retinal pigment epithelium when the a-waves of the flash-ERG mixed-responses are normal. As it was the case and all EOGs normal, the pigment epithelium functioned normally.
Mf-ERG tests the local retinal function of the photopic system on small juxtaposed surfaces located on the posterior pole. Mf-ERG recordings are not possible before the age of 12y as they need a long and stable central fixation with few blinkings to be reliable. A total recording time being at least 4 mn per eye, the child should be old enough to be able to cooperate. It was the case for our patient. The very low noise of the mf-ERGs indicates that her central fixation was stable during the two recording sessions (right and left eyes). The amplitude of the central peak of right and left eyes corresponds to the response of the central 5°. The functioning of these few central retinal degrees generates visual acuity. The amplitude of the central peak was more decreased on the right eye than on the left one. This suggested that this right central area functioned less well than the left one. This was clinically confirmed by the right visual acuity which was a little lower (20/30) than the left one (20/25). The mf-ERG responses on the central macular 30° were decreased corresponding to a dysfunction of the right and left macular areas. These results suggested the presence of a functional subclinical maculopathy over the central 30°.
P-ERG P50 wave is mostly generated by the first two layers of the photopic system on the central macular 15° (cone and bipolar cell layers) with contribution of ganglion cells [27], P-ERG N95 wave is generated by the third retinal layer (ganglion cell layer) where the density of ganglion cells is high [28]. The evolution of the P-ERG wave amplitudes is very interesting. P-ERG amplitudes were normal as soon as astigmatism was corrected by the permanent wearing of optical correction (at the age of 5y and 6y 4 m). At the age of 8y 4 m and 9y 2 m, P50 amplitudes were decreased, and N95 amplitudes were null on both eye and on the left eye at the age of 12y 6 m. P50 and N95 amplitudes were null on the right eye at the age of 12y 6 m. These N95 null amplitudes strongly suggested a dysfunction of ganglion cells located in the third retinal layer (ganglion cell layer) in the macular area tested (over the central 15°).
The maculopathy described here differs from that observed in children with myopic astigmatism. In the latter, the extramacular zones usually function normally (normal dark-adapted and light-adapted flash-ERGs) [29] unless there is a myopic degeneration, which was not in our case.
Mf-ERG results have highlighted a functional maculopathy over the central 30° through the first two retinal layers (cone and bipolar cell layers) and P-ERG a functional maculopathy over the central 15° and through the three macular retinal layers, i.e., including ganglion cell level. Chan et al. [30] reported that cobalamins are intracellular neuroprotective agents particularly in ganglion cells. Cobalamins eliminate oxidative agents that lead to cellular apoptosis. The possible reduction of intracellular cobalamin due to TC deficiency even early compensated by appropriate dose of intramuscular hydroxocobalamin may induce gradual ganglion cell dysfunctions.
The five cases of retinopathies or maculopathies described so far in TC deficiency were evident clinically. No functional tests were used to find out whether or not the ganglion cell layer was affected. In a case of cblG defect, ganglion cell dysfunctions were observed by electrophysiological results and were attributed to the cytotoxic impact of homocysteine [31]. In our case of TC deficiency, the mechanisms of the ganglion cell dysfunctioning are probably multifactorial.
In early onset of severe cblC defects, retinopathy and maculopathy are observed frequently [32, 33] and appear early in life. However, Gaillard et al. [32, 34] suggested that retinal dysfunction are likely to be more frequent than these clinically evident and could be evidenced by electrophysiological recordings. However, ERG recordings are the exception in those cases.
The mechanism of the observed retinopathy of inner-cone dystrophy type which probably originates from the bipolar cell layers is unclear. It could be the result of the feedback of ganglion cell dysfunctions. This remains to be elucidated [2, 33, 35, 36].
Our electrophysiological results and their evolutions over a long period (flash ERG + multifocal ERG + P-ERG) were coherent and complementary. All together they support a subclinical macular dysfunction without anatomical impairment. They may or not precede the onset of clinical retinopathy and/or maculopathy. The current electrophysiological results do not predict the future. Longer-term patient’s retinal evolution is warranted to answer this question.
To our knowledge, no case of TC deficiency without fundus signs has been followed over a long term by visual electrophysiology. Our data support that electrophysiology tests should be included in the long-term follow-up management of TC deficient patients. If systematically done, these recordings may reveal subclinical retinal dysfunction more frequently than the few clinically described.
Conclusion
Retinopathy and maculopathy are ultrarare in TC deficiency. To our knowledge, our case with electrophysiological dysfunctions, i.e., subclinical retinal signs is unique to date. The evolution of electrophysiological results confirmed the progressive retinal and macular dysfunctions over a 10-year period. They highlighted the presence of a subclinical retinopathy of inner-cone dystrophy type and maculopathy on the three retinal layers with no visual clinical or anatomical signs of alert: visual acuity, fundi, OCT, and RNFL remaining normal. Interestingly, aggressive and early initiation of hydroxocobalamin injections were of help in preventing neurological and probably anatomical retinal deteriorations, and hopefully helping that these retinal changes remain clinically silent sustainably. The five retinopathies and/or maculopathies published in TC deficiency appeared as treatment was oral and/or discontinuous. They may have been preceded by abnormal electrophysiological results. We suggest that it might be valuable to monitor by electrophysiology the visual function of all patients with TC deficiencies as this might reveal subclinical retinopathies and/or maculopathies that do not yet have clinical or anatomical manifestation. Early identification of retinal dysfunction might be an argument to establish or reinforce the intramuscular hydroxocobalamin treatment to improve patients’ long-term visual outcomes.
Notes
Currently being submitted for publication after this paper was submitted and not avalaible on line when this article was reviewed. Information given by the corresponding author.
Abbreviations
- Ampl:
-
Amplitude(s)
- EOG:
-
Electro-oculogram
- ERG:
-
Electroretinogram
- IT:
-
Implicit time
- LE:
-
Left eye
- Mean value:
-
M.V
- mf-ERG:
-
Multifocal ERG
- Nl:
-
Normal
- OCT:
-
Optical coherent tomography
- OHCbl IM:
-
Intramuscular hydroxocobalamin injection
- P-ERG:
-
Pattern-electroretinogram
- RE:
-
Right eye
- RNFL:
-
Retinal nerve fiber layer
- RPE:
-
Retinal pigment epithelium
- SD:
-
Standard deviation
- TC deficiency:
-
Transcobalamin deficiency
- VA:
-
Visual acuity
- 9y2m:
-
9 Years 2 months
References
Ratschmann R et al (2009) Transcobalamin II deficiency at birth. Mol Genet Metab 98(3):285–288
Watkins D, Rosenblatt DS, Fowler B (2012) Disorders of cobalamin and folate transport and métabolism. In: Saudubray JM, Van Den Berghe G, Walter JH (eds) Inborn metabolic diseases, 5th edn. Springer-Verlag, Berlin Heidelberg, pp 386–402
Fowler B (1998) Genetic defects of folate and cobalamin metabolism. Eur J Pediatr 157(Suppl 2):S60–S66
Schiff M et al (2010) Should transcobalamin deficiency be treated aggressively? J Inherit Metab Dis 33(3):223–229
De Lonlay P et al (2013) Prise en charge médicale et diététique des maladies héréditaires du métabolisme. Springer-Verlag, Paris, Berlin, Heidelberg, New-York, pp 343–356
Trakadis YJ et al (2014) Update on transcobalamin deficiency: clinical presentation, treatment and outcome. J Inherit Metab Dis 37(3):461–473
Fulton AB et al (2006) Pediatric clinical visual electrophysiology: a survey of actual practice. Doc Ophthalmol 113(3):193–204
McCulloch DL et al (2015) ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol 130(1):1–12
Constable PA et al (2017) ISCEV Standard for clinical electro-oculography (2017 update). Doc Ophthalmol 134(1):1–9
Hood DC et al (2012) ISCEV standard for clinical multifocal electroretinography (mfERG)(2011 edition). Doc Ophthalmol 124(1):1–13
Bach M et al (2013) ISCEV standard for clinical pattern electroretinography (PERG): 2012 update. Doc Ophthalmol 126(1):1–7
Fulton AB, Hartmann EE, Hansen RM (1989) Electrophysiologic testing techniques for children. Doc Ophthalmol 71(4):341–354
Kriss A (1994) Skin ERGs: their effectiveness in paediatric visual assessment, confounding factors, and comparison with ERGs recorded using various types of corneal electrode. Int J Psychophysiol 16(2–3):137–146
Bradshaw K, Hansen R, Fulton A (2004) Comparison of ERGs recorded with skin and corneal-contact electrodes in normal children and adults. Doc Ophthalmol 109(1):43–55
Barshop BA et al (1990) Transcobalamin II deficiency presenting with methylmalonic aciduria and homocystinuria and abnormal absorption of cobalamin. Am J Med Genet 35(2):222–228
Kaikov Y et al (1991) Transcobalamin II deficiency: case report and review of the literature. Eur J Pediatr 150(12):841–843
Monagle PT, Tauro GP (1995) Long-term follow up of patients with transcobalamin II deficiency. Arch Dis Child 72(3):237–238
Souied EH et al (2001) Retinal degeneration associated with congenital transcobalamin II deficiency. Arch Ophthalmol 119(7):1076–1077
Prasad C et al (2008) Transcobalamin (TC) deficiency–potential cause of bone marrow failure in childhood. J Inherit Metab Dis 31(Suppl 2):S287–S292
Dharmasena A, Calcagni A, Kerr AR (2008) Retinopathy in inherited transcobalamin II deficiency. Arch Ophthalmol 126(1):141–142
Nissen PH et al (2010) Transcobalamin deficiency caused by compound heterozygosity for two novel mutations in the TCN2 gene: a study of two affected siblings, their brother, and their parents. J Inherit Metab Dis 33(Suppl 3):S269–S274
Unal S et al (2015) Transcobalamin II deficiency in four cases with novel mutations. Turk J Haematol 32(4):317–322
Nashabat M et al (2017) Long-term outcome of 4 patients with transcobalamin deficiency caused by 2 novel TCN2 mutations. J Pediatr Hematol Oncol 39(8):e430–e436
Unal S et al (2019) Different presentations of patients with transcobalamin II deficiency: a single-center experience from Turkey. Turk J Haematol 36(1):37–42
Khera S, Pramanik SK, Patnaik SK (2019) Transcobalamin deficiency: vitamin B12 deficiency with normal serum B12 levels. BMJ Case Rep 12(10):232319
Chorfi S, Mitchell GA, Qian CX (2021) Retinopathy of transcobalamin II deficiency: long-term stability with treatment. Ophthalmology 128(7):992
Luo X, Frishman LJ (2011) Retinal pathway origins of the pattern electroretinogram (PERG). Invest Ophthalmol Vis Sci 52(12):8571–8584
Curcio CA, Allen KA (1990) Topography of ganglion cells in human retina. J Comp Neurol 300(1):5–25
Hull S et al (2015) Congenital high myopia and central macular atrophy: a report of 3 families. Eye (Lond) 29(7):936–942
Chan W et al (2018) Cobalamin-associated superoxide scavenging in neuronal cells is a potential mechanism for vitamin B12-deprivation optic neuropathy. Am J Pathol 188(1):160–172
Poloschek CM et al (2005) Disturbed visual system function in methionine synthase deficiency. Graefes Arch Clin Exp Ophthalmol 243(5):497–500
Gaillard MC, Matthieu JM, Borruat FX (2008) Retinal dysfunction in combined methylmalonic aciduria and homocystinuria (Cblc) disease: a spectrum of disorders. Klin Monbl Augenheilkd 225(5):491–494
Weisfeld-Adams JD et al (2015) Ocular disease in the cobalamin C defect: a review of the literature and a suggested framework for clinical surveillance. Mol Genet Metab 114(4):537–546
Garcia-Gonzalez JM, Neiweem AE, Grassi MA (2015) Cobalamin C deficiency-associated pigmentary retinopathy. JAMA Ophthalmol 133(12):e152161
Brooks BP et al (2016) Ophthalmic manifestations and long-term visual outcomes in patients with cobalamin C deficiency. Ophthalmology 123(3):571–582
Bonafede L et al (2015) Cobalamin C deficiency shows a rapidly progressing maculopathy with severe photoreceptor and ganglion cell loss. Invest Ophthalmol Vis Sci 56(13):7875–7887
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Informed consent
Parents’patient have consented to the submission of the case report for submission to the journal.
Statement of human rights
Patient’s human rights and her well-being were respected throughout the clinical assessments and electrophysiological recordings over all assessment period.
Statement on the welfare of animals
This article does not contain studies with animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rigaudière, F., Nasser, H., Delouvrier, E. et al. Subclinical maculopathy and retinopathy in transcobalamin deficiency: a 10-year follow-up. Doc Ophthalmol 144, 53–65 (2022). https://doi.org/10.1007/s10633-021-09849-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-021-09849-5