Abstract
Transcobalamin (TC) deficiency (OMIM# 275350) is a rare, autosomal recessive disorder that presents in early infancy with a broad spectrum of symptoms, including failure to thrive, megaloblastic anemia, immunological deficiency, and neurological symptoms. Here we report a study of a family (parents and three children) with two children suffering from TC deficiency caused by two different mutations in the TCN2 gene. Initially, molecular genetic analysis of genomic DNA revealed a heterozygous mutation in the +1 position of exon 7 (c.1106+1 G > A) in the father and all three children. Bioinformatic analysis indicates that this mutation causes exon skipping, and further experiments supported this hypothesis and suggested that the mutant allele undergoes nonsense-mediated messenger RNA (mRNA) decay. We did not identify further mutations in genomic DNA that could explain TC deficiency in the two children. However, further efforts using complementary DNA (cDNA) derived from RNA from blood leukocytes identified a large deletion removing the entire exon 8, resulting in a frameshift and a premature stop codon (p.E371fsX372) in the mother and the two affected children. Our data indicate that if exon-by-exon DNA sequencing of genomic DNA does not uncover mutations corresponding to the phenotype, a systematic search for other mutations should be initiated by sequencing cDNA or using semiquantitative methods to detect large deletions in TCN2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Impaired synthesis of the cobalamin transporting protein transcobalamin (TC) may lead to cobalamin (Cbl) deficiency and thereby to symptoms such as megaloblastic anemia, a defective immune system, and neurological disease (Rosenblatt and Fenton 2001). Cbl is bound and transported by three proteins: haptocorrin, intrinsic factor, and TC, each important in the different steps of uptake and transport of Cbl (Nexo 1998). Intrinsic factor is needed for gastrointestinal uptake of Cbl, and haptocorrin carries the major part of the circulating Cbl. TC is a plasma protein needed for cellular uptake of Cbl throughout the body. Once in the cell, Cbl acts as coenzymes for methylmalonyl-coenzyme A (CoA) mutase and methionine synthetase. TC deficiency (OMIM# 275350) is a rare autosomal recessive disorder that normally presents in early infancy as failure to thrive, megaloblastic anemia, and in some patients, immunological deficiency and neurological symptoms (reviewed in Rosenblatt and Fenton 2001; Cooper and Rosenblatt 1987). These symptoms are a consequence of the lack of intracellular Cbl, whereas plasma Cbl is usually in the normal range due to the fact that the majority of plasma Cbl is bound by haptocorrin.
The TC gene (TCN2) spans approximately 18 kb of genomic DNA and consists of nine coding exons (Regec et al. 1995). To date, a limited number of studies has reported patients affected with TC deficiency, and as Li and colleagues (Li et al. 1994a) reported on the first identification of genetic mutations in TCN2 in one affected child and his parents, only ten families have been reported in whom the genetic defect is described. Despite the relatively low number of cases, a variety of different molecular causes for the defect, including gross deletions (Li et al. 1994a) (Haberle et al. 2009), small insertions and deletions (Li et al. 1994a, b; Ratschmann et al. 2009), nonsense mutations due to nucleotide substitutions (Li et al. 1994b; Prasad et al. 2008), and splice-site mutations (Namour et al. 2003) have been reported. Furthermore, rare events such as an intronic insertion leading to a cryptic exon (Haberle et al. 2009) and RNA mutations due to an unexplained defect in RNA editing (Qian et al. 2002), have been reported. Here we add two new mutations to the short list of TCN2 defects. The mutations were identified in a family with two compound heterozygous children, one diagnosed with full-blown signs of deficiency at the age of 1 year and one detected at birth. Both parents and the twin brother of the youngest child were heterozygous and displayed a reduced level of circulating TC.
Patients, materials, and methods
Patients and collection of samples
A family—mother, father, and three children—was studied. The case study is presented in the “Results” section. Blood was collected for biochemical examinations in test tubes without anticoagulants. Serum was separated and kept at –20°C until analyzed. Whole blood for RNA analysis was collected in test tubes from Paxgene Blood RNA System, (Preanalytics, Greiner Bio-One, Frickenhausen, Germany) and kept at –80°C until purification of RNA. Whole blood for DNA analysis was collected in test tubes with ethylenediaminetetraacetate (EDTA) and kept frozen at –20°C until isolation of DNA. We obtained control samples from 54 normal individuals through the local blood bank. The study was performed according to the Declaration of Helsinki, and the parents of the three children gave informed consent.
Methods
Biochemical analysis
Hematological parameters were analyzed by routine methods at the local hospital laboratory. Total and holo-TC and total haptocorrin was analyzed as previously described (Nexo et al. 2000, 2002; Morkbak et al. 2005). Methylmalonic acid was determined by gas chromatography mass spectrometry (GC–MS) according to Rasmussen (1989), and total homocysteine in plasma was determined with an enzymatic high-performance liquid chromatography (HPLC) method (Huijgen et al. 2004). Plasma cobalamin was analyzed with a competitive immunometric method on a ADVIA Centaur XP (Siemens, Uppsala, Sweden).
Genetic analysis
Genomic DNA was purified from whole blood using the Puregene genomic DNA purification kit (Qiagen, Copenhagen, Denmark).
RNA was purified with the Paxgene Blood RNA kit (Preanalytix, Greiner Bio-One, Frickenhausen, Germany). Synthesis of complementary DNA (cDNA) was done with 0.1 µg of the purified RNA as template in a reaction mixture of 10 mM Tris-hydrochloric acid (HCl) (pH 8.3), 1 U/µl RNase inhibitor, 1 mmol/l deoxyribonucleoside triphosphate [deoxyadenosine triphosphate (dATP), deoxythymidine triphosphate (dTTP), deoxycytidine triphosphate (dCTP), deoxycytidine triphosphate (dGTP)], 2.5 µmol/l 16-mer d(T)16 primer, 50 mmol/l potassium chloride (KCl), 6.25 mmol/l magnesium chloride (MgCl2), and 2.5 U avian myeloblastosis virus (AMV) reverse transcriptase (all reagents from Applied Biosystems, Nærum, Denmark) in a reaction volume of 20 µl. The reaction mixture was incubated for 90 s at 94°C followed by 30 min at 42°C and finally at 94°C for 1 min.
Using genomic DNA as template, we performed polymerase chain reaction (PCR) of the coding region of TCN2 using ten sets of oligonucleotide primers. The primers were designed to amplify exons 1–9, including a minimum of 13 nucleotides flanking the intron–exon border. Using cDNA as template, we amplified the protein coding region of TCN2 in two overlapping reactions. PCR primers were designed to amplify a fragment covering exons 1–7 (1,270 bp) and a fragment covering exons 7–9 (653 bp). All primer sequences are available on request from the authors. PCR products were electrophoresed in 2% agarose gels using standard procedures. We purified PCR products using either the Illustra GFX PCR Purification Kit (GE Healthcare, Hillerød, Denmark) or MicroSpin S-400 columns (GE Healthcare). We sequenced the amplicons in both directions using BigDye terminator vers. 1.1 (Applied Biosystems) and separated the ethanol-precipitated fragments on an Applied Biosystems 3130 Genetic Analyzer (Applied Biosystems).
Bioinformatic analysis
We aligned sequence traces to the TCN2 reference sequence (NM_000355.2) using SeqScape (version 2.5; Applied Biosystems). Using the Splice Site Prediction Tool (Reese et al. 1997) (http://www.fruitfly.org/seq_tools/splice.html), we analyzed the c.1106+1 G > A variant for potential exon skipping.
Results
We report two new TCN2 mutations for TC in a Swedish family in which two of three children suffered from lack of the vitamin B12-binding protein TC. A pedigree of the family is presented in Fig. 1.
Pedigree of the family investigated. Squares denote male and circle denote female. Half-filled square/circle represents heterozygous individuals, whereas full (black) circle/square represents compound-heterozygous transcobalamin-deficient individuals. The identified mutations are indicated at each individual. The exon 8 deletion (c. exon 8 del) mutation was not analyzed in the boy twin, as sample material for cDNA synthesis was not available. This is indicated by a ?
The case story of the index patient was previously described by Gustafsson et al. (2007). In brief, the patient was born in 2004 as the first child of nonconsanguineous healthy parents after an uneventful pregnancy. He appeared healthy at birth but starting at 2 weeks developed symptoms mimicking cystic fibrosis. Repeat testing of sweat chloride did not confirm the diagnosis. At 4 months of age, he developed megaloblastic anemia, leukopenia, and thrombocytopenia, requiring several blood transfusions and immunoglobulin infusions weekly. A bone marrow biopsy demonstrated myelodysplastic features. Blood parameters responded well when at the age of 6 months he was put on an 8-week trial of orally administered 1 mg vitamin B12 (cyanocobalamin) and 5 mg folic acid daily. After treatment termination, blood values deteriorated again. At the time of diagnosis at the age of 1 year, he displayed high levels of both plasma methylmalonic acid and homocysteine, and no TC could be detected in his plasma (Table 1). Based on the results, injection with 1 mg vitamin B12 every week was started and resulted in normalized blood values and disappearance of lung symptoms. At the age of 5 years, the boy was treated with vitamin B12 injection, 1 mg, every other week. He had delayed speech, slightly delayed psychomotor development, and some clumsiness, but otherwise developed very well.
The twin brother and sister of the index patient were born in 2007 after an uneventful pregnancy, and both appeared healthy at birth. The mother was treated with vitamin B12, 1 mg orally daily during the second and third trimester. Immediately after birth and removal of blood samples for measurement of methylmalonic acid and homocysteine, the twins were injected with 1 mg of vitamin B12. Based on the results (Table 1) only the girl was continued on vitamin B12 injections, 1 mg every other week. From 2 years of age, a daily oral dose of 1 mg vitamin B12 was added to maintain normal levels of methylmalonic acid and homocysteine. At the age of 2.5 years, the girl had a slightly delayed psychomotor development compared with her twin brother but still well within the normal range. As apparent from Table 1 both the index patient and his little sister lack TC, whereas the brother and both parents had low circulating levels of TC but normal levels of holo-TC. Total TC in the brother was low compared with the level observed in the parents. This is in agreement with a previous study showing that total TC at birth is about 50% of the values observed in the mother (Obeid et al. 2006). The level of total TC in the brother increased within the first 2 months to values around 200 pmol/L. Both parents and the children had unremarkable levels of haptocorrin. At birth, the twin lacking TC showed increased levels of both methylmalonic acid and homocysteine, whereas the twin not lacking TC had unremarkable levels of the two markers of vitamin B12 deficiency.
To clarify the genetic cause for the lack of TC, initial efforts focused on direct sequencing of all nine protein coding exons of the TCN2 gene using genomic DNA as template. This resulted in identification of 15 DNA variants, the majority being frequent single nucleotide polymorphisms (SNPs) annotated in the dbSNP (http://www.ncbi.nlm.nih.gov/sites/entrez?db=snp) (data not shown). However, a mutation in the +1 position of exon 7 (c.1106+1 G > A) in the father and all three children is not in the dbSNP and was not identified in 54 control samples. This variant potentially results in skipping of exon 7, as determined by bioinformatic analyses (data not shown).
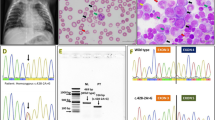
DNA sequencing of genomic DNA did not reveal further mutations. Haplotypes deduced from the SNPs identified indicated that the two children that lacked detectable TC received the same haplotype from the mother, whereas the mothers other haplotype was passed on to the twin not lacking TC (data not shown), suggesting that the mother as well carried a genetic defect giving rise to impaired synthesis of TC. Amplification of a fragment covering exons 7 and 8 of the TCN2 gene from cDNA derived from RNA from blood leukocytes revealed an intriguing pattern (Fig. 2). The mother showed two almost equally strong bands, one corresponding to the wild-type TCN2 allele of 653 bp and the other approximately 100-bp shorter than the wild-type band. The father showed the wild-type band and in addition a very faint band approximately 200-bp shorter. Complementary DNA from the two presumed homozygous children, from whom samples for cDNA synthesis were available, showed a short clear band, representing the mother’s mutated allele; and a shorter, very faint band, representing the father’s mutated allele (Fig. 2).
Analysis of messenger RNA (mRNA) isolated from peripheral leukocytes. Agarose gel electrophoresis of amplified complementary DNA (cDNA) fragments spanning exons 7 and 8 of TCN2 in heterozygous and homozygous individuals with transcobalamin (TC) deficiency. M 100-bp marker. The father has a heterozygous c.1106+1 G > A mutation, resulting in a wild-type band and a faint band corresponding to an allele lacking exon 7. The mother displayed heterozygosity for the wild-type allele and an allele lacking exon 8. Child 1 and child 2: homozygous children displaying compound heterozygosity for each of the parent’s mutant alleles. A control sample is homozygous for the normal allele
Sequence analysis of the amplified cDNA fragments covering exons 7 and 8 from the father revealed wild-type sequence (Fig. 3), in accord with the observation that the wild-type allele was by far the most abundant (Fig. 2).
Sequence data of the identified mutations of TCN2. Representation and sequence data of the identified mutations in TCN2 and the consequence in the corresponding messenger RNA (mRNA). a Part of the sequence of the amplified complementary DNA (cDNA) fragment covering exons 7 and 8 from the father, revealing wild-type sequence, and part of the sequence of exon 7 and intron 7 from genomic DNA illustrating the presumed exon-skipping mutation. b Part of the cDNA sequence covering exons 7–9 of a compound heterozygous child and the cDNA sequence of the heterozygous mother, displaying overlapping sequence of exons 8 and 9. The full and dotted lines under exon 8 indicate that the full range of the exon 8 deletion at the level of genomic DNA is unknown
Sequence analysis of amplified cDNA fragments covering exons 7 and 8 from the mother revealed the mutation from the mother and the two homozygous children to be a deletion of exon 8, resulting in a frameshift and a premature stop codon in the beginning of exon 9 (p.E371fsX372) (Fig. 3). Based on the above, we conclude that the affected children received a TCN2 allele with a mutation resulting in skipping of exon 7 from the father and an allele with an exon 8 deletion from the mother.
Discussion
Here we describe two new mutations giving rise to symptomatic TC deficiency, which confirms that biochemical symptoms of TC deficiency are present at birth, even though the child is born apparently healthy. We identified two mutations: One, detectable in genomic DNA, is a potential splice-site mutation (c.1106+1 G > A) in the invariable +1 position at the 5′ splice site. Mutations in this position have been shown to be disease causing in a range of conditions due to exon skipping (Krawczak et al. 1992), and using the Splice-Site Prediction tool, this mutation was predicted to result in skipping of exon 7. This hypothesis was further supported by amplification of an amplicon covering exon 7 from cDNA. The heterozygous sample from the father resulted in a wild-type band and a very faint shorter band, consistent with a wild-type allele of 653 bp and a mutated allele of 487 bp lacking exon 7. Amplification products of this fragment in both homozygous children (child 1 and child 2) also revealed a faint band of the same size (Fig. 2). Skipping of exon 7 would result in a frameshift leading to a predicted protein with a shift in reading frame at amino acid position 315 followed by a premature termination (p.M315fsX326). DNA sequencing of genomic DNA from the father and mother revealed no further mutations, leaving the TC deficiency in the two children only partially explained. The deletion of exon 8 identified from the cDNA synthesized from leukocyte RNA solved the problem and confirmed the compound heterozygosity for TC deficiency.
Amplification of the cDNA fragment covering exons 7 and 8 in the two affected children exclusively revealed two mutant fragments, in size corresponding to a mutant allele lacking exon 7 and a mutant allele lacking exon 8 and only wild-type sequence when the father’s cDNA was used as template. This strongly supports the idea that the c.1106+1 G > A mutation results in exon skipping and nonsense-mediated mRNA decay (NMD). The mRNA encoded by the allele harboring the exon 8 deletion seems to remain intact, as demonstrated from the two bands of equal intensity in the mother in Fig. 2. This mechanism has been described previously and is due to a process in which only premature stop codons in the last exon or in the penultimate exon 50–55 bp before the exon–exon boundary in general escapes NMD (Isken and Maquat 2007; Khajavi et al. 2006).
The exon 8 deletion was not identified in genomic DNA and would not have been detected if examination of cDNA had been omitted. This is a problem that needs attention in several other diseases in which molecular genetic diagnosis is important, since variation in exon copy numbers, such as large deletions or duplications spanning one or several exons, would be missed with methods for mutation detection using genomic DNA as a template. In several diseases, this problem is solved by complementing a screening method, such as DNA sequencing, with semiquantitative PCR methods, such as, e.g., multiplex ligation-dependent probe amplification, which reliably detects large genomic deletions and duplications (Sellner and Taylor 2004). Following this, the deletion breakpoints could be identified by long-range PCR and sequencing. Another possibility—as employed in this study—is to use RNA as a template for cDNA synthesis and to sequence cDNA. Recently, Haberle and colleagues suggested RNA-based diagnostics of mutations in TC using cDNA obtained from skin fibroblasts as an important part of the genetic analysis to avoid false negative genetic diagnostics (Haberle et al. 2009). These authors used fibroblasts isolated and cultured, a laborious process demanding dedicated cell-culture facilities not available in all diagnostic laboratories. In our study, we show that RNA can be isolated successfully from blood using a blood-drawing system with RNA-preserving additives and that TC mRNA is present in blood leukocytes in amounts sufficient for allowing molecular genetic investigations.
Interestingly, even if the protein had been synthesized based on the mutated TCN2 gene, it is very unlikely that the proteins would have been able to bind Cbl. The crystal structure of TC shows that the Cbl molecule is located between the N-terminal α-domain and the C-terminal β-domain (Wuerges et al. 2006). The two mutations are both predicted to result in prematurely terminated proteins removing, in the case of the c.1106+1 G > A mutation, the entire β-domain containing eight β-sheets and the α13-barrel and, in the case of the exon 8 deletion, the last six β-sheets (Wuerges et al. 2006).
In conclusion, we report two new mutations related to TC deficiency. Our results further stress that using both genomic DNA and cDNA may be useful in a clinical setting. Had we chosen only to use cDNA in the diagnostic setup, we might very well have missed the c.1106+1 G > A mutation, as this was not identified using DNA sequencing of cDNA. Therefore, in the genetic workup of future TC-deficient patients, it is important to follow two lines in parallel, both analysis of genomic DNA and cDNA, or to use other methods to detect large deletions.
Abbreviations
- Cbl:
-
cobalamin
- TC:
-
transcobalamin
- TCN2 :
-
gene encoding transcobalamin
- SNP:
-
single nucleotide polymorphism
- NMD:
-
nonsense-mediated messenger RNA (mRNA) decay
References
Cooper BA, Rosenblatt DS (1987) Inherited defects of vitamin B12 metabolism. Annu Rev Nutr 7:291–320
Gustafsson B, Karpati F, Nordenstrom A, Soderhall S, Sander B, Nordvall M et al (2007) Myelodysplastic features and symptoms mimicking cystic fibrosis in a child with an intracellular vitamin B 12 deficiency. Pediatr Blood Cancer 49:1054–1055
Haberle J, Pauli S, Berning C, Koch HG, Linnebank M (2009) TC II deficiency: avoidance of false-negative molecular genetics by RNA-based investigations. J Hum Genet 54:331–334
Huijgen HJ, Tegelaers FP, Schoenmakers CH, Pronk-Admiraal CJ, Ekema S (2004) Multicenter analytical evaluation of an enzymatic method for the measurement of plasma homocysteine and comparison with HPLC and immunochemistry. Clin Chem 50:937–941
Isken O, Maquat LE (2007) Quality control of eukaryotic mRNA: safeguarding cells from abnormal mRNA function. Genes Dev 21:1833–1856
Khajavi M, Inoue K, Lupski JR (2006) Nonsense-mediated mRNA decay modulates clinical outcome of genetic disease. Eur J Hum Genet 14:1074–1081
Krawczak M, Reiss J, Cooper DN (1992) The mutational spectrum of single base-pair substitutions in mRNA splice junctions of human genes: causes and consequences. Hum Genet 90:41–54
Li N, Rosenblatt DS, Kamen BA, Seetharam S, Seetharam B (1994a) Identification of two mutant alleles of transcobalamin II in an affected family. Hum Mol Genet 3:1835–1840
Li N, Rosenblatt DS, Seetharam B (1994b) Nonsense mutations in human transcobalamin II deficiency. Biochem Biophys Res Commun 204:1111–1118
Morkbak AL, Pedersen JF, Nexo E (2005) Glycosylation independent measurement of the cobalamin binding protein haptocorrin. Clin Chim Acta 356:184–190
Namour F, Helfer AC, Quadros EV, Alberto JM, Bibi HM, Orning L et al (2003) Transcobalamin deficiency due to activation of an intra exonic cryptic splice site. Br J Haematol 123:915–920
Nexo E (1998) Cobalamin binding proteins. In: Kräutler B, Arigoni D, Golding B (eds) Vitamin B12 and B12-proteins. Wiley-VCH, Weinheim, pp 461–475
Nexo E, Christensen AL, Petersen TE, Fedosov SN (2000) Measurement of transcobalamin by ELISA. Clin Chem 46:1643–1649
Nexo E, Christensen AL, Hvas AM, Petersen TE, Fedosov SN (2002) Quantification of holo-transcobalamin, a marker of vitamin B12 deficiency. Clin Chem 48:561–562
Obeid R, Morkbak AL, Munz W, Nexo E, Herrmann W (2006) The cobalamin-binding proteins transcobalamin and haptocorrin in maternal and cord blood sera at birth. Clin Chem 52:263–269
Prasad C, Rosenblatt DS, Corley K, Cairney AE, Rupar CA (2008) Transcobalamin (TC) deficiency-potential cause of bone marrow failure in childhood. J Inherit Metab Dis doi:10.1007/s10545-008-0864-3
Qian L, Quadros EV, Regec A, Zittoun J, Rothenberg SP (2002) Congenital transcobalamin II deficiency due to errors in RNA editing. Blood Cells Mol Dis 28:134–142
Rasmussen K (1989) Solid-phase sample extraction for rapid determination of methylmalonic acid in serum and urine by a stable-isotope-dilution method. Clin Chem 35:260–264
Ratschmann R, Minkov M, Kis A, Hung C, Rupar T, Muhl A et al (2009) Transcobalamin II deficiency at birth. Mol Genet Metab 98:285–288
Reese MG, Eeckman FH, Kulp D, Haussler D (1997) Improved splice site detection in Genie. J Comput Biol 4:311–323
Regec A, Quadros EV, Platica O, Rothenberg SP (1995) The cloning and characterization of the human transcobalamin II gene. Blood 85:2711–2719
Rosenblatt DS, Fenton WA (2001) Inherited disorders of folate and cobalamin transport and metabolism. In: Scriver CR, Berning C, Valle D, Sly WS (eds) The metabolic and molecular bases of inherited disease. McGraw Hill, New York, pp 3897–3933
Sellner LN, Taylor GR (2004) MLPA and MAPH: new techniques for detection of gene deletions. Hum Mutat 23:413–419
Wuerges J, Garau G, Geremia S, Fedosov SN, Petersen TE, Randaccio L (2006) Structural basis for mammalian vitamin B12 transport by transcobalamin. Proc Natl Acad Sci USA 103:4386–4391
Acknowledgement
The technical assistance of Anna Lisa Christensen, Jette Fisker Pedersen, Kirsten Kruse Olsen, Kirsten Hald, and Birgit W Mortensen is warmly appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Brian Fowler
References to electronic databases: OMIM: http://www.ncbi.nlm.nih.gov/omim, GenBank: http://www.ncbi.nlm.nih.gov/Genbank/, Splice Site Prediction Tool: http://www.fruitfly.org/seq_tools/splice.html, dbSNP: http://www.ncbi.nlm.nih.gov/projects/SNP/
Competing interest: None declared
Rights and permissions
About this article
Cite this article
Nissen, P.H., Nordwall, M., Hoffmann-Lücke, E. et al. Transcobalamin deficiency caused by compound heterozygosity for two novel mutations in the TCN2 gene: a study of two affected siblings, their brother, and their parents. J Inherit Metab Dis 33 (Suppl 3), 269–274 (2010). https://doi.org/10.1007/s10545-010-9145-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10545-010-9145-z