Abstract
The grafting of cotton fabrics with diallyldimethylammonium chloride was studied. The objective was to improve reactive dye adsorption and antibacterial properties. The cotton fabric was modified with the diallyldimethylammonium chloride at room temperature using the redox initiator system of potassium persulfate as an initiator and N,N,N′,N′-tetramethylethylenediamine as an accelerator. It was found that the nitrogen content of the cationized cotton fabrics increased with an increase in the diallyldimethylammonium chloride concentration and stayed constant at a concentration of 40% (v/v). The adsorption of the reactive dyes on the cotton fabrics was investigated. The contact time of the reactive dye adsorption on the modified cotton fabrics reached equilibrium at 30 min. The dye adsorption properties of the modified cottons depended on the concentration of the diallyldimethylammonium chloride, dyes concentration, chemical structure, and molecular weight of the reactive dyes. By grafting the fabrics with the diallyldimethylammonium chloride, the cotton fabric's antibacterial function was also enhanced.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cotton fabrics have been widely used in the textile industry because of their natural abundance, better conductivity of heat, easy dyeability, and excellent moisture adsorption. However, the dyeing of the cotton fabrics with reactive dyes requires high salt concentration and leads to environmental problems.
The modification of the cotton has been studied. Many papers have been carried out by different methods, such as the coating method (Kwon et al. 2020; Li et al. 2022; Sallehudin 2022), chemical modification e.g., N-halamine and flame retardant chemical (Chen et al. 2023), a copper phthalocyanine derivative and benzophenone (Hu et al. 2023) and grafting techniques with various monomers, e.g., [2-(methacryloxy) ethyl] trimethylammonium chloride and methyacrylamide (Wang et al. 2022), 3-chloro-2-hydroxypropyltrimethyl ammonium chloride (Zhai et al. 2022) and 4-vinylbenzyl chloride (Tsimpouki et al. 2023). From previous reports, the purpose of cationized cotton fabrics was salt free dyeing with the reactive dyes. In addition, the other properties of the modified fabrics were improved, such as antibacterial, flame retardant, and wearing properties.
Niu et al. studied the modification of the cotton fabrics by a bifunctional cationic polymer for salt-free reactive dyeing. The bifunctional cationic polymer was prepared by free radical polymerization between dimethyl diallyl ammonium chloride and allyl glycidyl ether. The dyeability of the modified sample was improved by the cationic polymer (Niu et al. 2020). The two cationic compounds, such as 3-chloro-2-hydroxypropyltrimethyl ammonium chloride and the copolymer of the dimethyl diallyl ammonium chloride and glycidyl ether were modified onto the cotton fabrics. They could be dyed with the reactive dyes using the salt-free dyeing process. (Zhai et al. 2020).
Setthayanond et al. cationized cotton fabric with the 3-chloro-2-hydroxypropyltrimethyl ammonium chloride. The salt-free reactive dyeing was achieved, which led to a reduction in the chemical in reactive dyeing. (Setthayanond et al. 2023). Cationic cotton prepared by using the 3-chloro-2-hydroxypropyltrimethyl ammonium chloride for the salt-free dyeing with the reactive dye. The dye adsorption and dyeing processes were improved. The dye uptake of the modified fabric increased by 92% compared with the traditional dyeing process (Zhai et al. 2023). Prus/ et al. studied the mechanism of bonding between the 3-chloro-2-hydroxypropyltrimethyl ammonium chloride cationized cellulose and the reactive dyes. The modified cellulose formed an electrostatic interaction with the sulphonic acid group of the anionic reactive dye. Also, the dye was exhausted rapidly without the addition of electrolytes (Prus/ et al. 2022).
The copolymer of poly(4-vinylbenzyl chloride) with triethylamine was applied to the cotton fabrics for the salt-free reactive dyeing application. The modified cotton showed high exhaustion under eco-friendly conditions (Tsimpouki et al. 2023). Wu et al. prepared cationic cotton fabrics by using polyhexamethylene guanidine. The salt-free dyeing of the modified fabrics was studied in comparison with the raw cotton. The exhaustion rate of the modified cotton increased by 20–30% (Wu et al. 2023). In addition, the cotton fabric was treated with poly(amidoamine) dendrimer to study the dyeability of dye from seaweed. The percent dye exhaustion of the modified cotton fabric was higher than that of the untreated sample. (Kadir et al. 2023).
Many research papers reported that other properties of the modified cotton fabrics with different chemicals. Chen et al. prepared a multifunctional fabric consisting of flame retardant with antibacterial and hydrophobic properties. The cotton fabric was treated with a modifying agent containing a nitrogen flame retardant chemical and an antibacterial agent based on N-halamine (Chen et al. 2023). The photosensitive textile was prepared by grafting the cotton fabric with C.I. Reactive Blue 21 (a copper phthalocyanine derivative) and benzophenone derivatives. The fabric exhibited photo-induced antibacterial activity (Hu et al. 2023). Wang et al. modified the cotton fabric by grafting with a copolymer of the [2-(methacryloxy) ethyl] trimethylammonium chloride and methyl acrylate. The treated fabric exhibited antibacterial property and comfort properties such as water absorption softness and air permeability (Wang et al. 2022).
According to previous reports, water was frequently used as the reaction medium in the cationization of cellulose. To improve the reaction efficiency of the cationization, Odabas et al. cationized Kraft pulp with 2, 3-epoxypropyltrimethylammonium chloride (EPTMAC) using water-miscible organic solvents in the system, such as isopropanol, tetrahydrofuran, and dimethyl sulfoxide. To the cellulose, the EPTMAC created a covalent bond. When tetrahydrofuran was used in place of 90% of the water, the reaction efficiency increased by a factor of ten when compared to the cationization in the water. Also, the degree of substitution increased with the use of organic solvents. They found that the kind of organic solvent and the cationization reagent concentration determined the degree of substitution. Additionally, the zeta potential of the pulp changed from negative (for the original and blank materials) to positive (for the cationized material) as a result of the cationization process, indicating that the cationization of the cellulose was successful (Odabas et al 2016).
The grafting technique was performed in many systems such as gamma-rays (Hidzir et al. 2020), UV-irradiation (Neubertov/a et al. 2020; Pereira et al. 2022), and benzoyl peroxide (Tariq et al. 2021). These methods were carried out at high temperature or with expensive equipment. Also, the pretreatment of the cotton fabric with the cationic substances occurred at high temperature. Application of the cotton fabric with quaternary ammonium compounds by the redox initiator system of the potassium persulfate and N,N,N′N′-tetramethylethylenediamine to enhance cationicity and dyeability on the fabric are very interesting. The advantage of this method is the reaction occurs at room temperature, and it is easy to control the polymerization reaction.
The present study was carried out in order to grafting the cotton fabrics with diallyldimethylammonium chloride for improving the dye adsorption and antibacterial properties. In this work, the cotton fabric was modified by grafting with the diallyldimethylammonium chloride (a cationic monomer) at room temperature. The reaction was initiated by the potassium persulfate and N,N,N′N′-tetramethylethylenediamine as initiator and accelerator, respectively. The dye adsorption was studied to elucidate the adsorption properties of the modified cotton fabrics. Also, the antibacterial property of the modified cotton fabrics was examined.
Experimental
Materials
The woven cotton fabric used in this research was scoured, bleached, and mercerized cotton and received from Rajamangala University of Technology Krungthep. Diallyldimethylammonium chloride (DADMAC) and N,N,N′N′,-tetramethylethylenediamine (TMEDA) were purchased from Fluka. Potassium persulfate, from.
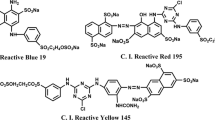
M&B Laboratory Chemical, was used as received. The reactive dyes (from Modern Dyestuffs and Pigment, Co., Ltd.) used for this studied were C.I. Reactive Blue 171 (RB 171), C.I. Reactive Red 195 (RR 195) and C.I. Reactive Yellow 184 (RY 184). The reactive dyes are widely use in dyeing cotton fabric. Thus, they were selected for this study. The differences in molecular weight and length of molecules were taken into consideration while selecting the dyes. The chemical structures and physical properties of the dyes are shown in Fig. 1 and Table 1.
Preparation of grafted cotton fabrics
Cotton fabric was padded with a solution containing the diallyldimethylammonium chloride monomer (DADMAC), K2S2O8 (2% by weight of monomer), and TMEDA (2% by weight of monomer) to 100% wet pickup, followed by drying at room temperature for 2 h. The concentrations of DADMAC solution were 10, 20, 30, 40, 50, and 60% (v/v). The treated sample was then washed with distilled water at a temperature of 80 °C for 2 h (Chantawon and Kusuktham 2023) and rinsed with distilled water for three times. Then, the fabric was dried at temperature of 80 °C for 2 h (Chantawon and Kusuktham 2023).
Characterization
FTIR measurement
FTIR spectra analysis of the cotton and modified cotton were identified in KBr pellets by using an Omnic Nicolet Impact 400 D FTIR spectrophotometer (New York, U.S.A.).
Elemental analysis
The elemental analysis of the unmodified and modified cotton fabrics was measured using a Perkin-Elmer CHNS/O Analyzer 2400 Series II (Shelton, U.S.A.).
Morphology of cotton fabrics
The surface morphologies of the cotton fabrics were examined via Scanning Electron Microscope (SEM: JEOL JSM-5410LV; Japan).
Determination of contact time
The contact time measurements of the reactive dye adsorption on the unmodified and modified cotton fabrics were conducted in a thermostat water bath at temperature of 80 °C. The fabric (0.5 g) and the volume of dye solution (25 ml) at a concentration of 50 mg/l in a vial bottle were mixed for determination of the dye adsorption. The remaining reactive dyes left in the solution were monitored at certain intervals by a Spectronic 21 UV spectrophotometer. The absorbance values of the dye solutions were used to calculate the dye concentration by comparing the calibration curves, as shown in Fig. 2.
Effect of DADMAC concentration on dye adsorption
The cotton fabric (0.5 g) and 150 ml of a 50 mg/l reactive dyes solution were added to the 250 ml Erlenmeyer flask (the liquor ratios = 1: 300). The mixture was carried out in the water bath shaker at 200 rpm and heated at temperature of 80 °C for 30 min to allow equilibrium adsorption. After the determined time, the dye concentration was measured using a Spectronic 21 UV spectrophotometer at the maximum wavelength (λmax) of the absorbance. The amount of adsorption capacity at equilibrium qe (mg/g) was calculated by using the following equation:
where Co and Ce are the initial and final dye concentrations (mg/l) after adsorption on the cotton fabric, respectively. V is the volume of each reactive dye solution (l), and W is the weight of cotton fabric (g).
Dye adsorption isotherms study
The equilibrium adsorption isotherm was investigated by mixing 0.5 g of the cotton fabric with 150 ml of reactive dyes solution at temperature of 80 °C for 30 min. The initial dye concentration ranged from 10 to 130 mg/l. The residue concentration of each dye was measured by a Spectronic 21 UV spectrophotometer and calculated from the calibration curve.
Antimicrobial study
The assay used for measuring antibacterial properties was based on "AATCC Test Method 100-1998, Antibacterial Finishes on Textile Materials: assessment of Antibacterial Finishes on Textiles." The bacterial species used here was Gram-positive Staphylococcus auresus (AATCC No. 6538). The antimicrobial activity of the DADMAC modified cotton fabrics was evaluated. The percent reduction of bacteria was calculated by the following formula.
R = %reduction A = the number of bacteria recovered from the inoculated and treated test specimen swatches in the jar incubated over the desired contact period (24 h) B = the number of bacteria recovered from the inoculated and treated test specimen swatches in the jar immediately after inoculation (at 0 h contact time).
Results
The cotton fabric was grafted with the DADMAC at a concentration of 10–60% (v/v). Figure 3 shows FTIR spectra of the unmodified and modified cotton fabrics with the DADMAC at a concentration of 40% (v/v).
In the FTIR spectrum of the cotton, the adsorption band at 3411 cm−1 corresponded to the absorbance of -OH functional groups (see Fig. 3a). Gigac et al. reported that the FTIR spectrum of the poly(DADMAC) consisted of the –CH3 stretching at 1477 cm−1, the –CH2 group at 2945 cm−1, C–N stretching at 1105 cm−1 and the quaternary ammonium groups at 961 cm−1 (Gigac et al. 2023). In the FTIR spectrum of the modified cotton, it appeared the adsorption spectrum at 1477 and 2945 cm−1 (see Fig. 3b). They were due to C–H bending and stretching of methyl groups. In addition, it appeared the absorption spectrum at 961 cm−1 was attributed to quaternary ammonium groups, confirming the grafting of the poly(DADMAC) onto the cellulose structure. The graft copolymerization between the cotton fabric and the DADMAC may be described by the following sequence reaction, as shown in Scheme 1.
First, the mixing of the oxidizing agent (potassium persulfate) and the reducing agent (TMEDA) in the redox polymerization system produced the free radicals. Huang et al. used redox initiators that included N,N,N,′N′-tetramethylethylenediamine (TMEDA) and potassium persulfate to create hydrogel. The redox initiation system's initial mechanism was propose. They showed how the TMEDA radical forms by cleaving the –CH3 group's C–H bond to produce the radical. As a result, this mechanism in Scheme 1 (reaction 1) mentioned it (Huang et al. 2017).
The free radical then transferred to the cellulose molecule and formed the cellulose macroradical. In order to create the grafted cotton fabric, it finally attacked the DADMAC monomer.
Kameya et al. used electron spin resonance to study the radical formation of cellulose. They found that the C(5) of the glucose unit could be formed the molecular mechanism of the radical formation (Kameya et al. 2013). Therefore, the cotton fabric may be grafted with the DADMAC at the glucose unit's C5 position. In addition, the most active sites that were susceptible to the grafting of numerous monomer units or polymers are the –OH groups found at the C2, C3, and C6 atoms of cellulose chains (Kumar et al. 2018) Since the cellulose radical can be either O-centered or C-centered, both options should be grafted with the poly(DADMAC). Scheme 1 showed the poly(DADMAC) grafting at the O-centered of C6 only.
The nitrogen content analysis of the products is shown in Fig. 4.
The result shows that the %N increased with an increase in the DADMAC concentration and constant at the 40% (v/v) DADMAC concentration. From this result, it supported the interaction between the cotton fabric and the DADMAC. However, the homopolymer developed as the cotton fabric was grafted into the reaction media stopped the %N content from increasing. In a subsequent experiment, the dye adsorption of the cotton fabrics was studied as a function of the DADMAC concentration.
Figure 5 shows scanning electron micrographs of the unmodified and modified cotton fabrics.
The result shows that the unmodified cotton fabric had a smooth surface and ribbon-like cotton strands (see Fig. 5a). The surface of the modified cotton fabric showed a cluster of the poly(DADMAC) connected to the cotton fibers as a result of the grafting reaction of the poly(DADMAC) (see Fig. 5b). Also, the fibers were also enlarged and made rounder by the grafting process.
Contact time determination
The reactive dyes used in this study are C.I. Reactive Blue 171 (RB 171), C.I. Reactive Red 195 (RR 195), and C.I. Reactive Yellow 184 (RY 184). The contact time (ct) of the dyes' adsorption on the cotton fibers was examined using these dyes.
In general, the following steps often take place when the reactive dye solution comes into contact with the cotton fabric. First, the dye molecules diffuse from the solution to the fabric. Then, they are attracted to the fibers and deposited on the cotton fibers surface. After that, the dye deposited on the fiber surface generates a concentration gradient, which is a driving force for the movement of the reactive dye from the surface to the interior of the fiber (Perkins 1996). In this study, the contact time of dyes adsorption on the unmodified and modified cotton fabrics with the DADMAC were examined.
The result is shown in Fig. 6. According to this result, the modified cotton textiles had a greater adsorption rate than the unmodified ones. Also, the results show that the dye adsorption rate was high at the initial period of contact time, which was indicative of a potent attraction between the anionic dye molecules and the cationic groups on the modified cotton fabric. The result indicated that the quaternary ammonium groups on the modified cotton fabrics played an important role in the properties of the fabrics (see Fig. 6a–c).
The attraction between the dyes and the fibers promoted the movement of the dye from the solution to the substrate of the adsorbent. Modification of the cotton fabric with the DADMAC caused an ionic interaction between the dyes and the fibers (see Scheme 2). The dyes were attracted to the dye sites (–N+R4 groups) of the fibers. When the quaternary ammonium groups of the poly(DADMAC) were occupied by a reactive dye molecules, they could not adsorb other dye molecules (see Scheme 2). This caused a limited amount of the dyes to adsorb on the cotton substrate and led to saturation of adsorption. Also, it was due to the slight decrease in the driving force of the dye solutions (Sumari et al 2016). Hence, from this result, the adsorption reached equilibrium at the contact time of 30 min.
Effect of DADMAC concentration on dyes adsorption
The cotton fabric was treated with DADMAC at concentrations ranging from 10 to 60% (v/v). At different DADMAC concentrations, the reactive dyes' adsorption into cotton fabrics was investigated. The results are shown in Table 2 and Fig. 7, respectively.
The result showed that the modified cotton fabric's adsorption capacity at equilibrium (qe) was greater than that of the unmodified cotton fabric (see Fig. 7). The positively charged quaternary ammonium groups in the treated cotton fabric increased the anionic dyes' ability to bind with the reactive dyes. This caused an increase in the dye adsorption. Also, the dye adsorption increased with an increase in the DADMAC concentration (see Fig. 7). This resulted from the high dye sites (–N+R4 groups) on the modified cotton fabrics. Hence, the contact between the modified cotton fabric with the –N+R4 groups and the anionic reactive dyes was an electrostatic interaction (Prus/ et al. 2022). Scheme 2 displayed a schematic of the reactive dye adsorption on the modified cotton fabric.
From Fig. 7, it was seen that the RB 171, RR 195, and RY 184 had equilibrium adsorption (qe, mg/g) values of 7.28 mg/g, 8.91 mg/g, and 9.45 mg/g, respectively. All three dyes, the RB 171 with six sulfonic groups, had the lowest dye adsorption. This resulted from the high molecular weight and complex molecular structure of the RB 171 (RB 171 molecular weight = 1418.9 g/mol; see Table 1 and Fig. 1).
In contrast with the RB 171, the RR 195 and RY 184, which had five or two sulfonic groups and small molecules with low molecular weights were adsorbed higher than the RB 171. (RR 195 molecular weight = 1168.3 g/mol and RY 184 molecular weight = 620.54 g/mol; see Table 1 and Fig. 1). Hence, the RB 171's molecular structure prevented the mobility of the dye to the surface of the cotton fabric. The same findings were published by Filipkowska et al., Sumari et al., and Wang et al. (Filipkowska et al. 2002, Sumari et al. 2016, and Wang et al. 2018). Additionally, it was found that the dye's ability to adsorb to the substrate was influenced by its chemical structure (Wang et al. 2018). In conclusion, the DADMAC concentration, the chemical structure and molecular weight of the dyes, and interactions between the adsorbent (cotton fabric) and the adsorbate (dye solution) were the parameters that influenced the dye adsorption.
Adsorption isotherms
The effect of dye concentration on the adsorption isotherm of the cotton fabric was studied. The reactive dyes' adsorption isotherms on untreated and treated cotton fabrics are depicted in Fig. 8.
The result showed that the cationic samples had better dye adsorption than the unmodified ones. This result may be explained by the fact that the anionic reactive dyes were successfully adsorbed onto the treated substrates by the ionic interaction in the modified cotton with the cationic monomer (DADMAC), which supplied cationic sites (–N+R4 groups) onto the substrate (see Scheme 2). The anionic reactive dye was adsorbed at the active sites, which were the quaternary ammonium groups (cationic groups) (Roy et al. 2023). It can be seen from the results that the modified cotton fabrics showed saturation adsorption at 8.53 mg/g, 10.5 mg/g, and 11.89 mg/g for RB 171, RR 195, and RY 184, respectively (see Fig. 8). Additionally, the dye adsorption increased abruptly at the initial dye concentration (Co) until equilibrium was established.
From Fig. 8, it can be seen that the adsorption capacity of the cellulose substrate increased with an increase in the initial dye concentration. The initial dyes concentration served as the driving force behind this. (Jawada et al. 2019, Rápó and Tonk 2021, and Roy et al. 2023,) As a result, dye molecules began to diffuse and mass transfer from the solution to the active dye sites of the modified cotton fabric. Rápó and Tonk found a similar finding in their investigation into the variables influencing the adsorption of synthetic dyes (Rápó and Tonk 2021). Additionally, Roy et al. claimed that the reactive dyes' adsorption on the cotton fabric treated with chitosan was influenced by the original dye concentration (Roy et al. 2023). Thus the initial dye concentration affected the reactive dyes' ability to adsorb on the cotton fabric.
Antibacterial property
The antibacterial property of the unmodified and modified cotton fabrics are shown in Table 3 and Figs. 9, 10.
The results showed that the DADMAC-modified cotton textiles inhibited bacterial growth. As the DADMAC concentration increased, the number of bacteria decreased further (see Table 3). The modified cotton materials' antibacterial properties were also evident after washing. However, in washed materials, the reduction of germs reduced (see Table 3). This could be because the physical contact between cotton fibers and poly(DADMAC) has been eliminated.
The cell surface of the Gram-positive bacteria had a negative charge (Mizerska et al. 2009). The treatment of the cotton fabrics with the DADMAC led to the appearance of the quaternary ammonium groups on the cotton fabrics. Then, the interaction between the modified cotton fabrics and the bacteria was electrostatic interaction. The positively charged in the cotton fabric may interact with the negatively charged bacterial walls and membranes via electrostatic forces, leading to killing the germs (Yuen and Yung 2013). Therefore, the grafting of the cotton fabric with the poly(DADMAC) had antibacterial property.
Conclusion
The diallyldimethylammonium chloride was grafted onto cotton fabric using potassium persulfate as the initiator and N,N,N′,N′-tetramethylethylenediamine as the accelerator. The nitrogen content of the modified cotton fabrics increased with an increase in the diallyldimethylammonium chloride concentration. The opposing charges between the cationized cotton fabric and an anionic reactive dye molecule caused the modified cotton fabric to absorb dye. Factors affecting the dyes adsorption were the diallyldimethylammonium chloride concentration, dye concentration, molecular weight, and molecular structure of the reactive dyes. Additionally, the antibacterial property of the cotton fabric was improved by the diallyldimethylammonium chloride.
References
Chantawong N, Kusuktham B (2023) Modification of silk fabrics with diallyldimethylammonium chloride. Polym Bull 80:9669–9684. https://doi.org/10.1007/s00289-022-04438-1
Chen X, Ding F, Zhang S, Liu Y, Hou X, Ren X (2023) Flame-retardant, antibacterial and hydrophobic multifunctional coatings on cotton fabrics via layer-by-layer self-assembly. Cellul 30:6679–6694. https://doi.org/10.1007/s10570-023-05287-5
Filipkowska U, Klimiuk E, Grabowski S, Siedlecka E (2002) Adsorption of reactive dyes by modified chitin from aqueous solutions. Pol J Environ Stud 11:315–323
Gigac J, Fmišerová M, Russ A (2023) Effect of precoating on properties of functional coating and electrical conductivity of inkjet-printed electronic. Cellul Chem Technol 57:133–142. https://doi.org/10.35812/CelluloseChemTechnol.2023.57.14
Hidzir NM, Radzali NAM, Rahman IA, Shamsudin SA (2020) Gamma irradiation-induced grafting of 2-hydroxyethyl methacrylate (HEMA) onto ePTFE for implant applications. Nucl Eng Technol 52:2320–2327
Hu L, Song X, Li M, Xie K, Hou A (2023) Scalable fabrication of benzophenone/ phthalocyanine-decorated photodynamic cotton fabrics for enhanced dye degradation and antibacterial performance. Cellul 30:4683–4696. https://doi.org/10.1007/s10570-023-05141-8
Huang C, Li Y, Duan L, Wang L, Ren X, Gaoa G (2017) Enhancing the self-recovery and mechanical property of hydrogels by macromolecular microspheres with thermal and redox initiation systems. RSC Adv 7:16015–16021. https://doi.org/10.1039/c7ra00317j
Jawada AH, Mubaraka NSA, Sabar S (2019) Adsorption and mechanism study for reactive red 120 dye removal by cross-linked chitosan-epichlorohydrin biobeads. Desalin Water Treat 164:378–438. https://doi.org/10.5004/dwt.2019.24438
Kadir MIA, Ahmad MR, Jabbar HA (2023) Dyeability of surface-modified cotton and silk fabrics with PAMAM dendrimer using Sargassum sp. seaweed extract. Trends Sci 20:1–12. https://doi.org/10.48048/tis.2023.5807
Kameya H, Ukai M (2013) Analysis of relaxation behavior of free radicals in irradiated cellulose using pulse and continuous-wave electron spin resonance. InTech, http://intechopen.com/chapters/45617
Kumar R, Sharma RK, Singh AP (2018) Grafted cellulose: a bio-based polymer for durable applications. Polym Bull 75:2213–2242. https://doi.org/10.1007/s00289-017-2136-6
Kwon J, Jung H, Jung H, Lee J (2020) Micro/nanostructured coating for cotton textiles that repel oil, water, and chemical warfare agents. Polym 12:1–14. https://doi.org/10.3390/polym12081826
Li S, Yu L, Xiong J, Xiong Y, Bi S, Quan H (2022) Facile fabrication of superhydrophobic and flame-retardant coatings on cotton fabrics. Polym 14:1–18. https://doi.org/10.3390/polym14235314
Mizerska U, Fortuniak W, Chojnowsk J, Hałasa RT (2009) Polysiloxane cationic biocides with imidasonium salt (ImS) groups, synthesis and antibacterial properties. Eurpean Polym J 45:779–787. https://doi.org/10.1016/j.eurpolymj.2008.11.045
Neubertová V, Slepičková Kasálková N, Vokatá B, Bačáková L, Švorčík V, Kolská Z (2022) Influence of UV irradiation and subsequent chemical grafting on the surface properties of cellulose. Cellul 29:1405–1418. https://doi.org/10.1007/s10570-022-04426-8
Niu T, Wang X, Wu C, Sun D, Zhang X, Chen Z, Fang L (2020) Chemical modification of cotton fabrics by a bifunctional cationic polymer for salt-free reactive dyeing. ACS Omega 5:15409–15416. https://doi.org/10.1021/acsomega.0c01530
Odabas N, Amer H, Bacher M, Henniges U, Potthast A, Rosenau T (2016) Properties of cellulosic material after cationization in different solvents. ACS Sustain Chem Eng 4:2295–2301. https://doi.org/10.1021/acssuschemeng.5b01752
Pereira C, Baumann JS, Humblot V, Falentin-Daudré C (2022) Biological properties of direct grafting by ultraviolet irradiation of vinyl benzyl phosphonic acid onto titanium surfaces. React Funct Polym 173:1–16. https://doi.org/10.1016/j.reactfunctpolym.2022.105215
Perkins WS (1996) Textile coloration and finishing. Carolina Academic Press, North Carolina
Pruś S, Kulpiński P, Matyjas-Zgondek E, Wojciechowski K (2022) Eco-friendly dyeing of cationised cotton with reactive dyes: mechanism of bonding reactive dyes with CHPTAC cationised cellulose. Cellul 29:4167–4182. https://doi.org/10.1007/s10570-022-04521-w
Rápó E, Tonk S (2021) Factors affecting synthetic dye adsorption; desorption studies: a review of results from the last five years (2017–2021). Polym 26:1–31. https://doi.org/10.3390/molecules26175419
Roy MN, Hossain MT, Hasan MZ, Islam K, Rokonuzzaman M, Islam MA, Khandaker S, Bashar MM (2023) Adsorption, kinetics and thermodynamics of reactive dyes on chitosan treated cotton fabric. Text Leather Rev 6:211–232. https://doi.org/10.31881/TLR.2023.030
Sallehudin ME, Affandi NDN, Bonnia NN (2022) Preparation and characterization of TiO2-polysiloxane coated cotton yarn at different coating methods. Mater Today: Proc 51:1303–1308. https://doi.org/10.1016/j.matpr.2021.10.345
Sumari SM, Hamzah Z, Kantasamy N (2016) Adsorption of anionic dyes from aqueous solutions by calcined and uncalcined Mg/Al layered double hydroxide. MJAS 20:777–792. https://doi.org/10.17576/mjas-2016-2004-104
Tsimpouki L, Papapetros K, Anastasopoulos C, Sygellou L, Soto-Beobide A, Andrikopoulos KS, Voyiatzis GA, Bokias G, Kallitsis JK (2023) Water-soluble quaternized copolymers as eco-friendly cationic modifers of cotton fabrics for salt-free reactive dyeing applications. Cellul 30:6031–6050. https://doi.org/10.1007/s10570-023-05220-w
Wang J, Gao Y, Zhu L, Gu X, Dou H, Pei L (2018) Dyeing property and adsorption kinetics of reactive dyes for cotton textiles in salt-free non-aqueous dyeing systems. Polym 10:1–16. https://doi.org/10.3390/polym10091030
Wang P, Zhang MY, Qu J, Wang L, Geng JZ, Fu FY, Liu XD (2022) Antibacterial cotton fabric prepared by a “grafting to” strategy using a QAC copolymer. Cellul 29:3569–3581. https://doi.org/10.1007/s10570-022-04469-x
Wu Y, Niu T, Zhai X, Sun D, Zhang X, Fang L (2023) Chemical modification of cotton fabrics with Polyhexamethylene guanidine for salt-free dyeing with reactive dyes. J Nat Fibers 20:1–13. https://doi.org/10.1080/15440478.2022.2156963
Yuen JWM, Yung JYK (2013) Medical implications of antimicrobial coating polymers-organosilicon quaternary ammonium chloride. Modern Chem Appl 1:1–3. https://doi.org/10.4172/2329-6798.1000107
Zhai X, Ma J, Wu Y, Niu T, Sun D, Fang L, Zhang X (2022) Investigation on dyeing mechanism of modified cotton fiber. RSC Adv 12:31596–31607. https://doi.org/10.1039/d2ra05668b
Acknowledgments
Study was funded by Rajamangala University of Technology Krungthep
Funding
Rajamangala University of Technology Krungthep.
Author information
Authors and Affiliations
Contributions
Miss Boonsri Kusuktham wrote all the manuscript and prepared all figures.
Corresponding author
Ethics declarations
Conflict of interest
Author declare that she has no conflicts of interest.
Ethical approval
The author has compliance with ethical standards.
Consent to participate
Author agrees to participate in the journal.
Consent for publication
Author agrees to publish article in this journal.
Financial interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kusuktham, B. Grafting of cotton fabrics with diallyldimethylammonium chloride. Cellulose 31, 2651–2666 (2024). https://doi.org/10.1007/s10570-024-05739-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-024-05739-6