Abstract
This work is devoted to the study of surface properties of cellulose before and after a surface modification. Surface modification of polymeric materials was carried out in two steps: (1) activation by UV irradiation at 254 or 365 nm, followed by (2) chemical grafting with alanine, leucine or curcumin. Two types of cellulose materials, regenerated cellulose and cotton, were studied. The structure of cellulose at different stages of modification was examined by available physical and physico-chemical techniques and antibacterial activity of prepared composites was studied too. Antibacterial assays were performed on selected substrates. The results show that the changes in surface properties depend on the wavelength of UV irradiation as well as on the irradiation time. Smaller molecules of grafted substances (alanine and leucine) are bound not only onto the cellulose surface but also into the cellulose pores in contrast with curcumin. Cellulose substrates modified with alanine, leucine or curcumin show antibacterial activity, especially for S. epidermidis, also slightly against E. coli. The obtained results indicated the strongest antibacterial effect for cellulose grafted with curcumin, where CFU reduced by almost 58% for E. coli and 55% for S. epidermidis in comparison with pristine, alanine and leucine have only smaller effect.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, various methods of surface treatment of different polymer materials have been studied, such as chemical modification (Benkocká et al. 2015; Kolská et al. 2014; Lupínková et al. 2014; Slepička et al. 2011), plasma treatment (Borcia et al. 2011; Chen et al. 2006; Guruvenket et al. 2004; Hegemann et al. 2003; Kolářová et al. 2013; Kolská et al. 2014; Kotál et al. 2007; Kraus et al. 2016; Lupínková et al. 2014; Sarra-Bournet et al. 2006; Slepička et al. 2011; Švorčík et al. 2006), exposure to UV radiation (Alonso et al. 2009; Borcia et al. 2011; Fischbach et al. 2001; Kolská et al. 2018; Shin et al. 2016) or combinations thereof. The aim of all described methods is to change the surface properties of the polymers. They exhibit specific advantages and/or disadvantages. For example, chemical modification by Piranha solutions is fast and easy to use, but it requires work with hazardous corrosive chemicals. Plasma activation, on the other hand, is possible only with proper special equipment. In contrast, effects of UV radiation appear to be less demanding on technical equipment and environmental impact. In order to be effective and cause chemical change, UV light must be absorbed by the polymer substrate. The material which is fully transparent in the UV region will not exhibit a photoinitiation reaction. The UV light absorbing polymer parts may be either backbone monomer repeating units which are able to absorb photochemically significant wavelengths initiating photodegradation. Also, there can be some chromophoric groups located at or attached to the chain ends either present naturally or randomly introduced into polymers (Nechifor 2016). In addition, usage of UV radiation of different wavelengths makes possible to vary depth of the surface modification, reactivity of the modified material and its absorption coefficient. As has been confirmed previously (Silovska et al. 2020) surface activation by UV radiation leads to breaking the original bonds of the macromolecular chains and to the formation of reactive sites on polymer surface and the similar effects could be expected for the cellulose. The UV irradiation can break the original bonds in cellulose structure and create reactive sites on the cellulose surface, which can be chemically grafted with suitable chemical compounds. The exposure of polymer surface to UV light can also lead to an oxidation and creation of polar groups. In several studies, UV radiation was used to introduce the oxygen groups (–COOH, –OH, –COO–) to the polymer surface (Lupínková et al. 2021; Neubertová et al. 2018; Shin et al. 2016; Wang and Brown 2004).
To improve the surface properties for variable applications, polymeric materials are subsequently grafted with different chemical compounds. Recently, different bioactive substances have been used for binding to pre-functionalized polymer surfaces. Grafting of chemical compounds onto polymer surfaces is one of the ways to create new materials with superior properties and potential applications in biomedicine and tissue engineering (Nelson and Cox 2005; Novotna et al. 2013). Bioactive compounds catalyze or elicit a certain reaction in a given biological system and can be both of natural or synthetic. For attachment of bioactive compounds, it is necessary to modify the surface since most polymers are inert in nature. It is important to optimize the surface to incorporate the desired type as well as the number of functional groups. The last phase is to attach the bioactive substance covalently to the modified substrate surface (Goddard and Hotchkiss 2007).
Cellulose is the most promising biopolymer (Kolářová et al. 2013). Biodegradable polymers as antimicrobials are great for prevention of the growth of pathogenic bacteria (Rahmani et al 2016). The application of UV radiation is the most common in synthetic polymers used in biology and medicine, but this type of modification seems to be also a promising alternative method for natural polymers like cellulose (Alonso et al. 2009; Fan et al. 2011). Surface chemistry and morphology of polymers, also including cellulose, have been changed by the photocatalytic process carried out under various working gases (Bačáková and Švorčík 2009; Vosmanská et al. 2016).
Natural products have always played important role in drug discovery, even in infectious diseases (Newman and Cragg 2016). The development of antimicrobial compounds, that can selectively act e.g. as food preservatives, without increasing antimicrobial resistance should be of the utmost importance. The discovery of compounds that can have both antimicrobial and antioxidant activities with no toxic effects on health is therefore highly awaited (Martelli and Giacomini 2018). Recently, interest in plant-derived antimicrobial agents has increased (Rossiter et al. 2017; Silva et al. 2016). Overall, curcumin and its analogues are known to be antimicrobial agents against gram-positive and gram-negative bacteria and are also used as preservatives (Czernicka et al. 2019; Izui et al. 2016; Mun et al. 2013; Teow et al. 2016; Vaughn et al. 2017). Methoxy- and hydroxyl groups are directly involved in antimicrobial activity and the antimicrobial mechanism of curcumin involves interaction with the protein filamenting temperature-sensitive mutant Z (FtsZ) necessary as a cell-inducing agent in bacteria (Kaur et al. 2010; Martelli and Giacomini 2018). The other interesting substances with antibacterial properties are amino acids, which are vital compounds and have many functions in the body. Their antibacterial effect is usually preserved even after grafting on substrates (Benkocká et al. 2015, 2019; Kolská et al. 2014). Activation and subsequent grafting of polymers with amino compounds has great potential to create new materials in the field of biomedicine (Andrade 1985; Benkocká et al. 2015, 2019; Chen et al. 2012; Kolská et al. 2014; Kovac et al. 2008). For example, alanine or leucine were previously tested for their antimicrobial effect but not as individual agent but always in combination with other agents. For example a low molecular weight chitosan (LMWC) and nisin, recognized as cationic antibacterial agents, inhibit bacterial growth by interacting with the anionically charged cell wall. In one study, alanine uptake significantly reduced the anionic cell surface charge of Staphylococcus aureus, resulting from the incorporation of D-alanine into the cell wall (Chen et al. 2012). Also, enzyme D-amino acid oxidase (DAAO) from the yeast Rhodotorula gracilis in the presence of D-alanine excels in antimicrobial activity. These two agents show antibacterial activity against both gram-positive and gram-negative bacteria when tested on plates and reduced their growth by half when tested on liquid cultures (Marcone et al. 2019). Another example is modification of polytetrafluoroethylene (PTFE) by UV excimer lamp with wavelength 172 nm. Heitz et al. 2003 exposed samples to aqueous solutions of alanine, Švorčík et al. 2004 samples firstly irradiated in reactive ammonia atmosphere and then exposed to aqueous solution of alanine, leucine or glycine.. Adhesion and proliferation of rat aortic smooth muscle cells (SMC) and mouse fibroblasts (3T3 cells) were studied. It resulted in a significant increase in the number of adhering cells and in the size of their spreading area and with subsequent amino acids grafted, the effect enhanced.
By modifying surface properties of polymers it is possible to expand the areas of their biological applications. The surface modification can be performed either non-covalently by deposition or spraying of coatings from solution or by covalent attachment od polymer chains using e.g. polymer brushes. Surface properties of PEEK were suitably adapted using a simple strategy based on the covalent surface bonding of polymer chains using free radicals created on the surface of benzophenone groups by UV irradiation for 3 h (Yousaf et al. 2014).
UV radiation was generated by a 230 W lamp radiating at 315–400 nm wavelengths for 3 h. Not only samples in form of foils were irradiated, but also in the form of nanofibers. Polyamide (PA) fibers were treated with UV-A radiation emitted from a tubular low-pressure mercury steam actinic lamp had wavelengths between 300 and 460 nm (Kolská et al. 2018). Changes of the surface properties were significant especially after a short radiation time, a longer irradiation time did not affect the surface properties dramatically. UV radiation has led to changes in surface chemistry, wettability, charge, polarity, roughness and morphology of polymers, which may contribute to improved subsequent adhesion to other materials, chemicals or microorganisms. Effect of UV treatment onto cellulose was tested sporadic in the past, e.g. one study dealt with the exposure of VUV-excimer lamp. The nitrogen atmosphere proved to be the most suitable for cleaning the cellulose surface. The surface was smooth, free of burrs and increased water absorption in the cellulose. The ammonia atmosphere caused the incorporation of large amounts of nitrogen analyzed as amine and amide groups. It had a positive effect on water absorption up to an irradiation time of 20 min, after which the water absorption decreased with increasing irradiation time. Unfortunately, cellulose exposure to VUV is not suitable for creating an antibacterial cellulose surface (Vosmanská et al. 2016). To the best of our knowledge, no study has yet been published on the cellulose modified with compounds tested in this our work (alanine, leucine or curcumin) for development of new materials with antibacterial activity.
In this paper we studied the possibilities of surface modification for cellulose and cotton to prepare new material with antibacterial behavior for usage in medicine. Surface modifications of these potential biomaterials were carried out in two steps: (1) activation by UV irradiation, followed by (2) chemical grafting with alanine, leucine or curcumin. Important surface properties of cellulose and cotton before and after surface activation and chemical modification were studied by the following methods: goniometry for surface polarity and wettability; electrokinetic analyses for surface charge and chemistry; scanning electron microscopy (SEM) for surface morphology; adsorption/desorption isotherms with Brunauer–Emmett–Teller equation (BET) for surface area determination and Barrett–Joiner–Halend model (BJH) for study of materials porosity; X-ray photoelectron spectroscopy for surface chemistry determination. Antibacterial activity was tested on selected substrates.
Experimental
Materials
Regenerated cellulose, thickness 90 µm was provided by GoodFellow Ltd., UK. Cellulose in the form of cotton, perforated, nonwoven, 60 g m−2 was provided by Holzbecher (Czech Republic). DL-alanine (Al) and DL-leucine (Leu) were purchased from Alfa Aesar (Haverhill, USA) and curcumin (Cur) was purchased from Helago (Czech Republic).
Activation by UV irradiation
Surfaces of celluloses under study were firstly activated by UV irradiation at room temperature using one of two UV lamps with wavelength of 254 or 365 nm and a power of 40 W. The samples were irradiated for 10, 30, 60 or 120 min. Due to less significant changes in surface wettability and surface charge after 365 nm UV irradiation, for most of study the samples irradiated only by 254 nm UV irradiation have been used.
Chemical grafting
Substrates were immersed immediately after UV activation in an aqueous solution of concentration of 2 wt% (1) alanine (Al) or (2) leucine (Leu) or (3) methanol solution of curcumin (1 wt%, Cur). Substrates were soaked for 30 min at room temperature (RT). Subsequently, samples were removed and rinsed with distilled water and dried for 30 min at RT in Petri dishes.
Characterization methods
Goniometry
The surface wettability of the cellulose surfaces before and after activation and chemical modification steps was determined by measuring the static contact angle. Contact angle measurement was performed on the DSA30 (KRÜSS GmbH, Germany). Using a needle, 5 drops of 2 µl distilled water were placed on each sample at different positions. In the ADVANCE program, the contact angle was automatically evaluated with the error of 5%.
Electrokinetic analysis
The zeta potential of planar samples was determined by using a SurPASS instrument (Anton Paar, Austria) (Kolská et al. 2018, 2014; Lupínková et al. 2016, 2014). Samples of 2 × 1 cm2 were attached to two holders inside the adjustable gap cell with a slit about 100 µm and measured 4 × with the experimental error of 5%. The measurements were carried out at atmospheric pressure, RT, in a 1 mmol L−1 KCl electrolyte and constant pH of 6.9. The streaming current method and Helmholtz-Smoluchowski equation were taken for zeta potential determination.
Scanning electron microscopy
The morphology of the samples was examined on a scanning electron microscope VEGA (TESCAN, Czech Republic). Individual substrates were placed on standard metal targets using double-sided carbon adhesive tape. The measurement was performed under high vacuum and a secondary electron (SE) detector was used. The conditions of measurements are always indicated in the picture caption.
Surface size and porosity analysis
Specific surface area and total pore volume were determined from adsorption and desorption isotherms using a NOVA3200 (Quantachrome Instruments) and the results evaluated in NovaWin software. The samples were degassed at RT for 8 h and then their adsorption and desorption isotherms were measured with nitrogen (Linde, purity 99,999%) at liquid nitrogen temperature. The 5-point Brunauer–Emmet–Teller (BET) analysis was used to determine the total specific surface area and the 40-point Barret–Joyner–Halenda (BJH) model was used to determine the pore volume. Each sample was measured 5 × with experimental error of 5%, pore size histograms were determined by the density functional theory (DFT) model.
X-ray photoelectron spectroscopy
The concentration of atoms C(1s), O(1s) and N(1s) in the surface layer of cellulose substrates were determined by XPS method using Omicron Nanotechnology ESCAProbe P Spectrometer (Omicron Nanotechnology GmbH, DE). The analyzed areas were 2 × 3 mm2. Spectra were measured (1% assay error), at 2 × 10–8 Pa with monochromatic radiation source (1486.7 eV) in 0.05 eV steps. The elemental composition was determined from the individual peak areas, using CasaXPS software. The error of evaluated element concentration values was below 3%.
Antibacterial tests
Antibacterial effect was studied on cellulose using the drip test (Vosmanská et al. 2015). Escherichia coli (E. coli) and Staphylococcus epidermidis (S. epidermidis) colonies were observed, where E. coli belongs to the gram-negative bacteria group and S. epidermidis to the gram-positive one. To determine the antibacterial activity of the prepared materials both bacterial cultures were grown overnight in lysogeny broth (LB) medium at 37 °C in an orbital shaker. Samples, 3 pieces 1 × 1 cm2 in size, were separately placed in physiological saline under sterile conditions. Aliquots of diluted cultures were seeded in saline with the final concentration for E. Coli and for S. epidermidis of 1 × 104 CFU ml−1 (CFU is the number of colony forming units). The inoculated solutions were incubated for 3 h under static conditions at 24 °C. Both bacterial strains—E. coli and S. epidermidis incubated in pure physiological solutions were used as control ones. LB agar plates were placed in plastic Petri dishes and an aliquot of 25 µl of all samples was dropped. Plates were incubated at 24 °C for E. coli and 37 °C for S. epidermidis for 20 h. Finally, the cultures were counted and the values statistically processed (Vosmanská et al. 2015).
Results and discussion
Determination surface wettability
Static contact angle measurements were used to determine the surface wettability of pristine and modified celluloses. As it is clear from Fig. 1, the wavelength of UV irradiation plays important role for the surface properties of cellulose. Contact angle values for pristine cellulose and that treated by UV irradiation at 365 nm are presented in Fig. 1a. It is clear that the surface wettability has increased only slightly. So that UV treatment at 365 nm wavelength has “little or no” effect on cellulose films regardless of exposure time since this cellulose does not absorb this light (Nechifor 2016).
Contact angle values of the regenerated cellulose polymer a irradiated with UV light at 365 nm—unmodified (pristine), irradiated for 10, 30, 60 and 120 min; b irradiated with UV light at 254 nm—unmodified (pristine); irradiated for 10, 30, 60 and 120 min; after UV activation and subsequent grafting with alanine (Al), leucie (Leu) or curcumin (Cur)
More interesting results obtained at 254 nm wavelength are shown in Fig. 1b. In this case the irradiation has much stronger effect not only on the contact angle, but also on next modification steps. In comparison with irradiation at 365 nm wavelength, the contact angle after 254 nm UV irradiation increases more markedly with the exposure time. Therefore, the irradiation at 254 nm wavelength (UV-C) was chosen for activation of the polymer surfaces under study for other testing. As has been mentioned in Introduction UV irradiation of polymers leads to breaking the original bonds of the macromolecular chains and to the creation of reactive sites and oxygen groups (–COOH, –OH, –COO–) which (1) can be important for subsequent grafting of other chemical compounds and (2) lead to the creation of polar groups on the surface which change the surface chemistry, charge, polarity and wettability. This affect is visible on Fig. 1.
It is clear, that the changes in surface wettability depend on the exposure time. The longer exposure time, the more significant increase of contact angle and corresponding decrease of surface wettability. For this reason, only the samples activated by UV irradiation at 254 nm wavelength for 10, 30, 60 and 120 min exposure times were subsequently grafted selected chemical compounds and used for other study.
The UV irradiation affects not only surface chemistry but also surface morphology and roughness, which may play important role in surface wettability. This will be documented on SEM figures presented below.
Grafting of all tested compounds resulted in other wettability changes, the degree of which depends on: (1) exposure time and (2) tested grafted compound (see Fig. 1b). Alanine (Al) grafting leads to small decrease of contact angle and wettability increase. More significant changes in surface wettability are observed after leucine (Leu) or curcumin (Cur) grafting. While Leu grafting leads to decrease of the contact angle and surface wettability increase, the curcumin (Cur) grafting results in dramatical, increase of contact angle and of surface wettability decrease. The different compound behavior of Al, Leu and Cur molecules is caused by their different chemistry. Small alanine and leucine molecules with polar groups affect significantly the polarity–hydrophility of grafted surfaces. On the other hand, curcumin with its large molecule and hydrocarbons skeleton, gives rise to hydrophobic character of grafted surface.
It is evident that the wettability of modified cellulose depends on the UV light wavelength, irradiation time and grafted compound chemical structure. Cotton a nonwoven fabric was not subjected to contact angle measurement because of its extreme wettability.
It is also clear from Fig. 1b, that grafting of all three tested compounds onto surface irradiated for 60 and 120 min gives similar values of contact angle and surface wettability. Due to this, in next experiments only samples irradiated for 10, 30 and 60 min were used.
Determination of zeta potential
The zeta potential values of both cellulosic materials were determined before and after each modification step. All materials were exposed to UV irradiation at the 254 nm wavelength for 10, 30 and 60 min. Substrates were subsequently grafted with solutions of alanine or leucine or curcumin. Obtained zeta potential values for studied samples are shown in Fig. 2 (for cellulose) and 3 (for cotton). The resulting potential values provide evidence that different surface chemistry affects the surface charge and the zeta potential value as well. After UV irradiation, new reactive spots penetrate the surface, and change the polarity and chemical composition of tested material. These effects have already been discussed earlier (Kolská et al. 2018; Neubertová et al. 2018; Shin et al. 2016; Vosmanská et al. 2016; Wang and Brown 2004). The UV irradiation causes a shift of zeta potential to more negative values. After UV irradiation, the polar groups, mostly with a negative charge (mainly OH– and COO–) (Neubertová et al. 2018), are formed on the polymer surface, which are attractive for subsequent grafting of other chemicals, e.g. tested compounds in our case. The compounds grafting is expected to affect the surface charge and chemistry significantly. As we can see from Figs. 2 and 3 the amount of grafted chemicals under study (and changes in surface chemistry and charge) strongly depends on previous exposure time. It can be explained by the fact the polymer surface is destroyed differently after variable exposure time and when the pores are higher the more compounds are bonded not only to the surface but into the pores (which will be discussed below) and therefore does not affect the surface chemistry so significantly as in the case when compounds are grafted on the surface. After alanine and leucine grafting, the zeta potential is shifted to the much negative values and thus the surface gains more negative charge due to the presence of functional groups on the surface and especially due to a decrease of surface roughness, which will be discussed below (Kolská et al. 2014; Neubertová et al. 2018). Curcumin-grafted substrates also show zeta potential negative shift, that is caused by the negatively charged CH3O– and OH– functional groups as has also been described by (Martelli and Giacomini 2018). The effect is especially pronounced at cotton (see Fig. 3). The changes in zeta potential after grafting of individual tested compounds are dependent on UV exposure time. The longer UV exposure time, the more significant changes in surface charge and chemistry are observed.
Determination of surface morphology
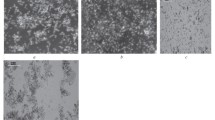
Surface roughness and morphology was investigated on both types of celluloses. Samples were measured before and after 30 min irradiation by the wavelength 254 nm and subsequently grafted with alanine, leucine or curcumin. Surface area and morphology of both unmodified celluloses are shown in Fig. 4, the same but for cellulose irradiated by UV and grafted with tested compounds are shown in Fig. 5 and for cotton in Fig. 6. It is clear that the surface morphology both of celluloses differ significantly. The large pores visible at cotton (Fig. 4, right) make contact angle measurement and the wettability determination on this surface impossible. Comparison of the morphologies at regenerated cellulose in Figs. 4 (left) and 6 shows slight morphology changes after each modification step. Especially after longer irradiation time, the fibers are damaged, merged and connected (Fig. 5c). Sometimes the fibers are more densified (Fig. 5, e, f). The fibers are contracted and the pores become clogged, as can also be evidenced by the subsequent analysis of the porosity of the material.
Determination of surface area and porosity
Regenerated cellulose, unmodified (pristine), irradiated with UV light at 254 nm wavelength for 10, 30 and 60 min and subsequently grafted with alanine, leucine or curcumin were tested for specific surface area and porosity. Values for surface area (determined by BET method) and pore volume (by BJH model) are given in Table 1.
The example of pore size histograms for cellulose before and after irradiation and subsequent grafting with alanine are shown in Fig. 7. From Table 1, it can be concluded that the surface area and the pore volume vary considerably after treatment by UV irradiation. The bonds and the fibers are broken by irradiation and the surface is enlarged. Also, the porosity of the cellulose increases significantly. On the other hand, after binding of alanine and especially of leucine, the surface area becomes smaller and the pore volume are decreasing significantly. This is because smaller molecules bind not only onto the surface, but also into the pores, thus clogging them. While curcumin with larger molecule, does not fit into the pores and remains grafted predominantly on the surface and lead to increase the surface are and porosity. Such grafting of smaller substances into pores has been studied before for various amino- compounds grafted on carbon nanoparticles (CNPs) or PE surface grafted with CNPs on other materials (Žáková et al. 2017). In that previous work we studied CNPs, their grafting by variable amino- compounds (totally different from compounds used in this manuscript) and subsequent grafting of modified CNPs onto polymer substrate. The tested amino- compounds also varied in their structure and geometry. Determination of surface area and porosity had shown that the smaller molecules are able to clog the pores (Slepičková Kasálková et al. 2019; Žáková et al. 2017).
Figure 7 shows the pore size histograms and comparison for samples unmodified, irradiated with UV 254 nm and irradiated with UV 254 nm + subsequently grafted with alanine. One can see how the pore size and distribution vary after the individual activation and subsequent grafting steps. The pore volume increases after UV irradiation due to the cellulose fiber damage. This is visible also at SEM image in Fig. 5. Subsequent grafting of alanine results in clogging larger pores (as was discussed above) and reducing the frequency of these pores in the histogram. This is good confirmation of the fact that small molecules bind not only to the surface but also to larger pores in agreement with the results presented earlier for other compounds of small molecules (Slepičková Kasálková et al. 2019; Žáková et al. 2017).
Analysis of element surface composition
The elemental surface composition was studied for cellulose samples (1) unmodified; (2) irradiated for 30 min (by 254 nm) and (3) subsequently grafted with alanine, leucine or curcumin. XPS measurements were made at 0 or 81° incident angles. Individual representation of elements for individual measurements is presented in Table 2.
Table 2 shows that cellulose consists only of carbon and oxygen, in good agreement with other authors (Vosmanská et al. 2016). When exposed to UV irradiation, the oxygen concentration decreases, so the surface becomes more hydrophobic (with regard to the cleavage of the original bonds and other steps which are described above), as confirmed also by results the contact angle measurement. This is also in agreement with previous study of other authors where cellulose was treated by VUV-excimer lamp (Vosmanská et al. 2016). After grafting of alanine and leucine, with molecules containing amino- groups, a small amount of nitrogen (N (1s)) is detected on the modified cellulose surface. Unexpected low nitrogen amount can be explained by: (1) the fact that the leucine and alanine molecules are very “small” and contain only one –NH2 group, so that the nitrogen concentration on the large cellulose molecules is low and that (2) during the small alanine and leucine molecules are preferably bound into the cellulose pores which was confirmed by BET and BJH measurements discussed above. Curcumin is also largely composed of carbon and oxygen. Observed changes in the amount of carbon and oxygen after curcumin grafting (in comparison with only UV treated cellulose) demonstrates the successful binding of curcumin to the cellulose surface. This behavior was also confirmed by the determination of porosity.
Antibacterial tests
The modified cellulose with significantly changed surface chemistry, wettability and morphology was used for antibacterial testing according to the procedure presented earlier (Vosmanská et al. 2014). The samples were subjected to irradiation at 254 nm wavelength for 10, 30 and 60 min, and subsequently grafted from alanine, leucine or curcumin solutions. Mean values of colony forming units (CFU), together with CFU for control samples, are presented in Fig. 8 for E. coli (a) and S. epidermidis (b). It can be seen from Fig. 8a that the cellulose sample irradiated for only 10 min and grafted with curcumin shows the greatest antibacterial effect for E. coli colonies. Even a short irradiation time has a great influence on the polarity and chemistry of the sample, but longer irradiation times did not result in such a large change. This effect probably combines three important factors: (1) curcumin has the stronger antimicrobial effect in comparison with alanine a leucine; (2) alanine or leucine immobilized on the surface can reduce their original antimicrobial activity (Taniguchi et al. 2016) in comparison with bigger molecules which is immobilized on the surface (Benkocká et al. 2019) and (3) 10 min UV irradiation leads to only small changes in the surface properties (wettability, chemistry and charge) and in turn to only small amount of grafted curcumin on the activated surface. Some earlier studies discussed the possibility that even small amount of grafted compounds preserves their antimicrobial activity after grafting on some substrates (Benkocká et al. 2019; Knapova et al. 2020). No significant effect on E. coli colony was observed on other samples, which is consistent with the known fact that E. coli colonies are highly resistant to many agents (Vosmanská et al. 2016, 2015). Figure 8b clearly shows that the effect on S. epidermidis colonies for selected treatment combination is more significant in comparison with E. coli. The most effective antibacterial effect on S. epidermidis colonies has a curcumin-bound cellulose sample especially after only 10 min UV irradiation. Also, curcumin-grafted samples with exposure time 30 min and 60 min have visible effect in comparison with other tested samples. Samples grafted with alanine and leucine also have mild but still visible antibacterial activity. These results are better in comparison with those from Control samples for both of tested colonies.
Conclusion
Two types of cellulose (regenerated one and cotton) were studied in this work. Firstly, they were activated by UV irradiation at two different wavelengths (365 and 254 nm) for 4 different times (10, 30, 60 and 120 min). The UV activated samples were then chemically grafted with alanine, leucine or curcumin. All of these steps led to the changes in surface properties, surface wettability, surface charge and chemistry, surface area, porosity morphology and subsequently to the antibacterial activity. The most important results are the following: (1) UV activation of cellulose surface depends on: (1) tested wavelength, the 254 nm UV treatment had most significant impact on the surface properties in comparison with 365 nm; (2) the exposure time of UV irradiation, the most significant changes were obtained after 10 min, longer time did not any strong effect; (2) subsequent grafting of tested chemical compounds depends on chemicals, the most significant changes were obtained after curcumin grafting; (3) the most promised antibacterial activity against both of colonies (E. coli and S. epidermidis) were obtained for cellulose activated by UV irradiation at 254 nm wavelength for 10 min and subsequently grafted with curcumin. From other interesting results can be concluded that curcumin, due to its bigger molecule, bound mostly on the polymer surface while the smaller molecules (alanine and leucine) penetrated the cellulose pores and tended to clog them. This fact strongly affects the antibacterial activity of modified cellulose, leucine or alanine grafted on the cellulose had slight visible effect on the antibacterial activity against both of colonies.
References
Alonso D, Gimeno M, Olayo R, Vázquez-Torres H, Sepúlveda-Sánchez JD, Shirai K (2009) Cross-linking chitosan into UV-irradiated cellulose fibers for the preparation of antimicrobial-finished textiles. Carbohydr Polym 77(3):536–543
Andrade JD (1985) Surface and interfacial aspects of biomedical polymers i: surface chemistry and physics. Plenum Press, New York
Bačáková L, Švorčík V (2008) Cell colonization control by physical and chemical modification of materials. In: Kimura D (ed) Cell growth processes: new research. Nova Science Publishers, Inc. New York
Benkocká M, Knapová T, Braborec J, Matoušek J, Černá H, Londesborough MGS, Švorčík V, Kolská Z (2015) Chemicaly activated and grafted substrates and their analyses. Chem Listy 109(12):960–964
Benkocká M, Lupínková S, Knapová T, Kolářová K, Matoušek J, Slepička P, Kolská Z (2019) Antimicrobial and photophysical properties of chemically grafted ultra-high-molecular-weight polyethylene. Mater Sci Eng C Mater Biol Appl 96:479–486
Borcia C, Borcia G, Dumitrascu N (2011) Surface treatment of polymer by plasma and UV radiation. Rom J Phys 56:224–232
Chen ZJ, Lu X, Chan CM, Mi YL (2006) Manipulating the surface properties of polyacrylamide with nitrogen plasma. Eur Polym J 42(11):2914–2920
Chen LC, Chiang WD, Chen WC, Chen HH, Huang YW, Chen WJ, Lin SB (2012) Influence of alanine uptake on Staphylococcus aureus surface charge and its susceptibility to two cationic antibacterial agents, nisin and low molecular weight chitosan. Food Chem 135(4):2397–2403
Czernicka L, Grzegorczyk A, Marzec Z, Antosiewicz B, Malm A, Kukula-Koch W (2019) Antimicrobial potential of single metabolites of curcuma longa assessed in the total extract by thin-layer chromatography-based bioautography and image analysis. Int J Mol Sci 20(4):898
Fan H, Li G, Yang F, Yang L, Zhang S (2011) Photodegradation of cellulose under UV light catalysed by TiO2. J Chem Technol Biotechnol 86(8):1107–1112
Fischbach C, Tessmar J, Lucke A, Schnell E, Schmeer G, Blunk T, Göpferich A (2001) Does UV irradiation affect polymer properties relevant to tissue engineering? Surf Sci 491(3):333–345
Goddard JM, Hotchkiss JH (2007) Polymer surface modification for the attachment of bioactive compounds. Prog Polym Sci 32(7):698–725
Guruvenket S, Rao GM, Komath M, Raichur AM (2004) Plasma surface modification of polystyrene and polyethylene. Appl Surf Sci 236:278–284
Hegemann D, Brunner H, Oehr C (2003) Plasma treatment of polymers for surface and adhesion improvement. Nucl Instrum Methods Phys Res B 208:281–286
Heitz J, Švorčík V, Bačáková L et al (2003) Cell adhesion on polytetrafluoroethylene modified by UV-irradiation in an ammonia atmosphere: cell adhesion on polytetrafluoroethylene. J Biomed Mater Res 67A:130–137
Izui S, Sekine S, Maeda K, Kuboniwa M, Takada A, Amano A, Nagata H (2016) Antibacterial activity of curcumin against periodontopathic bacteria. J Periodontol 87(1):83–90
Kaur S, Modi NH, Panda D, Roy N (2010) Probing the binding site of curcumin in Escherichia coli and Bacillus subtilis FtsZ—a structural insight to unveil antibacterial activity of curcumin. Eur J Med Chem 45(9):4209–4214
Knapová T, Matoušek J, Fajstavr D, Švorčík V, Kolská Z (2020) Antimicrobial effect of polymers grafted with cinnamaldehyde. Mater Lett 277:128274
Kolářová K, Vosmanská V, Rimpelová S, Švorčík V (2013) Effect of plasma treatment on cellulose fiber. Cellulose 20(2):953–961
Kolská Z, Řezníčková A, Nagyová M, Slepičková Kasálková N, Sajdl P, Slepička P, Švorčík V (2014) Plasma activated polymers grafted with cysteamine improving surfaces cytocompatibility. Polym Degrad Stabil 101:1–9
Kolská Z, Polanský R, Prosr P, Zemanová M, Ryšánek P, Slepička P, Švorčík V (2018) Properties of polyamide nanofibers treated by UV-A radiation. Mater Lett 214:264–267
Kotál V, Švorčík V, Slepička P, Sajdl P, Bláhová O, Šutta P, Hnatowicz V (2007) Gold coating of poly(ethylene terephthalate) modified by argo plasma. Plasma Process Polym 4:69–76
Kovač A, Konc J, Vehar B, Bostock JM, Chopra I, Janežič D, Gobec S (2008) Discovery of new inhibitors of d-Alanine:d-Alanine ligase by structure-based virtual screening. J Med Chem 51(23):7442–7448
Kraus E, Orf L, Baudrit B, Heidemeyer P, Bastian M, Bonenberger R, Starostina I, Stoyanov O (2016) Analysis of the low-pressure plasma pretreated polymer surface in terms of acid-base approach. Appl Surf Sci 371(15):365–375
Lupínková S, Benkocká M, Braborec J, Matoušek J, Kolářová K, Londesborough MGS, Kolská Z (2016) Analyses of chemically modified polymer surfaces. Czech Chem Soc Symp Ser 14:19–22
Lupínková S, Výborný K, Benkocká M, Kolská Z, Slepičková Kasálková N, Švorčík V (2014) Analysis of polymer surfaces of activated plasma and subsequently grafted vicinal compounds. Chem Listy 108:237–240
Lupínková S, Kaimlová M, Kormunda M, Kolská Z (2021) Chitosan-capped sulfur microparticles grafted on UV-treated PET surface. Surf Interface Anal 53:108–117
Marcone GL, Binda E, Rosini E et al (2019) Antibacterial properties of D-amino acid oxidase: impact on the food industry. Front Microbiol 10:2786
Martelli G, Giacomini D (2018) Antibacterial and antioxidant activities for natural and synthetic dual-active compounds. Eur J Med Chem 158:91–105
Mun SH, Joung DK, Kim YS, Kang OH, Kim SB, Seo YS, Kwon DY (2013) Synergistic antibacterial effect of curcumin against methicillin-resistant Staphylococcus aureus. Phytomedicine 20(8–9):714–718
Nechifor M (2016) Factors influencing the photochemical behavior of multicomponent polymeric materials. In: Rosu D and Visakh PM (eds.) Photochemical behavior of multicomponent polymeric-based materials. Springer International Publishing, Switzerland
Nelson DL, Cox MM (2005) Lehninger principles of biochemistry. Worth Publishers, New York
Neubertová V, Knapová T, Kormunda M, Kolská Z (2018) Modification of polymer foils by UV radiation and chemical grafting. Chem Listy 112(5):224–328
Newman DJ, Cragg GM (2016) Natural products as sources of new drugs from 1981 to 2014. J Nat Prod 79(3):629–661
Novotna K, Havelka P, Sopuch T, Kolarova K, Vosmanska V, Lis V, Svorcik V, Bacakova L (2013) Cellulose-based materials as scaffolds for tissue engineering. Cellulose 20(5):2263–2278
Rahmani S, Mohammadi Z, Amini M, Isaei E, Taheritarigh S, Rafiee Tehrani N, Rafiee Tehrani M (2016) Methylated 4-N, N dimethyl aminobenzyl N, O carboxymethyl chitosan as a new chitosan derivative: synthesis, characterization, cytotoxicity and antibacterial activity. Carbohydr Polym 149:131–139
Rossiter SE, Fletcher MH, Wuest WM (2017) Natural products as platforms to overcome antibiotic resistance. Chem Rev 117(19):12415–12474
Sarra-Bournet C, Turgeon S, Mantovani D, Laroche G (2006) Comparison of atmospheric-pressure plasma versus low-pressure RF plasma for surface functionalization of PTFE for biomedical applications. Plasma Process Polym 3(6–7):506–515
Shin J, Xiaojing L, Chikthimmah N, Lee YS (2016) Polymer surface modification using UV treatment for attachment of natamycin and the potential applications for conventional food cling wrap (LDPE). Appl Surf Sci 386(15):276–284
Silovská T, Matoušek J, Fajstavr D, Švorčík V, Kolská Z (2020) Antimicrobial effect of polymers grafted with cinnamaldehyde. Mater Lett 277:128274
Silva LN, Zimmer KR, Macedo AJ, Trentin DS (2016) Plant natural products targeting bacterial virulence factors. Chem Rev 116(16):9162–9236
Slepička P, Trostová S, Slepičková Kasálková N, Kolská Z, Sajdl P, Švorčík V (2011) Surface modification of biopolymers by argon plasma and thermal treatment. Plasma Process Polym 9(2):197–206
Slepickova Kasalkova N, Žáková P, Stibor I, Slepička P, Kolská Z, Karpíšková J, Švorčík V (2019) Carbon nanostructures grafted biopolymers for medical applications. Mater Technol 34(7):376–385
Švorčík V, Kotál V, Slepička P, Bláhová O, Špírková M, Sajdl P, Hnatowicz V (2006) Mofificatiion of surface properties of polyethylene by Ar plasma discharge. Nucl Instrum Methods Phys Res B 244(2):365–372
Švorčík V, Ročková K, Ratajová E, Heitz J, Huber N, Bäuerle D, Bačáková L, Dvořánková B, Hnatowicz V (2004) Cell proliferation on UV-excimer lamp modified and grafted polytetrafluoroethylene. Nucl Instrum Methods Phys Res B 217:307–313
Taniguchi M, Ochiai A, Takahashi K, Nakamichi S, Nomoto T, Saitoh E, Kato T, Tanaka T (2016) Effect of alanine, leucine, and arginine substitution on antimicrobial activity against candida albicans and action mechanism of a cationic octadecapeptide derived from α-amylase of rice. PeptideScience 106(2):219–229
Teow SY, Liew K, Ali SA, Khoo ASB., Peh SC (2016) Antibacterial action of Curcumin against Staphylococcus aureus: a brief review. J Trop Med 2016: Article No. UNSP 2853045
Vosmanská V, Kolářová K, Rimpelová S, Švorčík V (2014) Surface modification of oxidized cellulose haemostat by argon plasma treatment. Cellulose 21(4):2445–2456
Vosmanská V, Kolářová K, Rimpelová S, Kolská Z, Švorčík V (2015) Antibacterial wound dressing: plasma treatment effect on chitosan impregnation and in situ synthesis of silver chloride on cellulose surface. RSC Adv 5(23):17690–17699
Vaughn AR, Haas KN, Burney W, Andersen E, Clark AK, Crawford R, Sivamani RK (2017) Potential role of Curcumin against biofilm-producing organisms on the skin: a review. Phytother Res 31(12):1807–1816
Vosmanská V, Barb RA, Kolářová K, Rimpelová S, Heitz J, Švorčík V (2016) Effect of VUV-excimer lamp treatment on cellulose fiber. Int J Polym Anal Char 21(4):337–347
Wang H, Brown HR (2004) UV grafting of methacrylic acid and acrylic acid on high-density polyethylene in different solvents and the wettability of grafted high-density polyethylene. II. Wettability. J Polym Sci a Polym Chem 42(2):263–270
Yousaf A, Farrukh A, Oluz Z, Tuncel E, Duran H, Dogan AY, Tekinay T, Rehman H, Yameenm B (2014) UV-light assisted single step route to functional PEEK surfaces. React Funct Polym 83:70–75
Žáková P, Slepičková Kasálková N, Slepička P, Kolská Z, Karpíšková J, Stibor I, Švorčík V (2017) Cytocompatibility of polyethylene grafted with triethylenetetramine functionalized carbon nanoparticles. Appl Surf Sci 422:809–816
Acknowledgments
This work was supported by the GACR Project No. GACR 20-01641S, by the Grant Agency of Health Ministry No. NU20-08-00208, by ERDF/ESF Project UniQSurf—Centre of biointerfaces and hybrid functional materials (No. CZ.02.1.01/0.0/0.0/17_048/0007411) and by the Research Infrastructure NanoEnviCz, supported by the Ministry of Education, Youth and Sports of the Czech Republic under Project No. LM2018124.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Neubertová, V., Slepičková Kasálková, N., Vokatá, B. et al. Influence of UV irradiation and subsequent chemical grafting on the surface properties of cellulose. Cellulose 29, 1405–1418 (2022). https://doi.org/10.1007/s10570-022-04426-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-022-04426-8