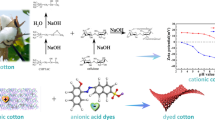
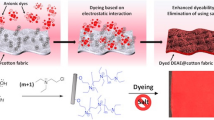
Abstract
The linkage between the dye and the cellulose is generally responsible for obtaining good washing and rubbing fastness properties of dyed materials. For reactive dyes this linkage is formed in reaction between reactive group of the dye with hydroxyl group of the cellulose. This reaction can go through nucleophilic substitution or an addition mechanism. Introducing the cationic groups to the chain of cellulose in the modification process completely changes the cotton surface charge from negative to partially or totally positive. Electrostatic interaction between the cation modifiers and sulfo group of anionic dye leads to the formation a strong ionic bond and rapid exhaustion the dye from the bath without addition of electrolytes. It was found and experimentally confirmed that when the cotton was cationised with 3-chloro-2-hydroxypropyltrimethylammonium chloride ([CHPTA]+Cl−) reactive dye creates a covalent bond with hydroxyl group located in modification agent instead of with hydroxyl group in a glucopyranose ring. This reaction can be done without salt and alkali and at room temperature. The analysis using particle optimisation with MM + molecular mechanics and quantum-chemical calculations PM3 by the method of all valence orbitals confirmed the experimental results of high activity of the nucleophile being formed on the hydroxyl group in the chain of modificator.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With a total consumption of about 50% of all textile fibres, cotton is still a dominant one, mainly due to its unique properties, including hydrophilicity, biodegradability, and relatively inexpensive. After scouring and bleaching, cotton is almost pure cellulose (Acharya et al. 2014). Cellulose can be dyed with many classes of dyes, such as direct, sulphur, vat and reactive dyes. Among these colorant compounds, reactive dyes have become the most popular and most important group of dyes since began to be used in industry. They offer a wide range of colors and high resistance to washing fastness. Each year, the textile industry uses over 400,000 tonnes of the reactive dyes for dyeing and printing (Liu et al. 2019) and the greatest amounts of them are used for dyeing from the bath exhaustion method (Lewis 2014). The application of reactive dyes to cellulosic materials requires the use of very large amounts of salts such as NaCl and Na2SO4. It is estimated that every year only in Europe, 200,000–250,000 tonnes of these compounds are discharged into wastewater (Aktek and Malekul Millat 2017), causing an increase in the salinity of water in the environment. This amount is increased by the salts formed in the dyeing process from the added alkalis necessary for the covalent bonding of the dye with the fibre.
Much research has been done to eliminate the use of such large amounts of salt and alkali. Nippon Kayaku has developed a range of Kayacelon React dyes for neutral fixation based on a triazine ring in which chlorine atom was replaced by nicotinic acid, achieving the goal of non-alkaline fixation (Lewis et al. 2008). New solutions in reactive dyes were investigated by introducing cationic groups into the chemical structure of the dye (Lewis 2014; Zhang and Zhang 2015), increasing the sorption on the fibre. A number of new reactive dyes containing 2, 3 and even 4 reactive groups (the same or different) were developed. These solutions are still insufficient for end users, due to the salt consumption and the washability of hydrolysed form of the dye.
Chemical modification of cellulose is generally performed in reaction with the functional hydroxyl groups present in the fibre (Wang and Lewis 2002; Montazer et al. 2007). However it leads to the reduction of the total number of free-hydroxyl groups mainly on the surface and in amorphic part of the fibres proportionally to the substitution degree by modifier agent. Cationisation of cellulose is most often associated with the introduction of tetraalkylammonium (quaternary) groups into its chemical structure. Such derivatives have a permanent positive charge with a high electron density regardless of pH (Heinze et al. 2018). Depending on the degree of cationisation, cellulose becomes a more or less cationic polymer capable of ionic reactions with anionic substances. Cellulose, after cationic modification, can be dyed with all kind of anionic dyes, for example with direct, reactive, acid and solubilized sulphur dyes (Atiq et al. 2019). Depend on the type of the applied dyes, different kind of linkage between cellulose and the dyes can be create. The energy of ionic bond energy of the sulfonate group with the cationic polymer is in the range of 550–1000 kJ/mol (Oakes et al. 2004). It is much higher than the energy of the hydrogen bond 21–30 kJ/mol, or the van der Walls intermolecular bond 2–5.5 kJ/mol (Stiepanow 1980). The kind of created linkage between cellulose and the dyes have great influence on the color fastness of dyeings.
In the past decades, nearly a thousand publications (Correia et al. 2020) involved with the cationisation of cotton fibres and conditions their utilising for dyeing, printing and other functional applications were published. There is a commonly known mechanism of reaction all groups of reactive dyes with cellulose (Łukoś and Ornaf 1966, Venkataraman 1972, Clark 2011 and Burkinshaw 2016). However there are only a few speculative reports about the reaction mechanism of reactive dyes with cationised cellulose (Lewis 2014, Aktek and Malekull Millat 2017, Arivithamani and Dev 2017, Niu et al. 2020). None of these reports present how covalent bond were formed, their fastness for hydrolysis and other technical parameters.
The basic aim of the experiments carried out was to establish in which way the reactive dyes react with cationised cellulose. For this purpose, two modifiers with similar chemical structures and with the same cationic group capable to reaction with cellulose were selected. However only one modifier had possibility to introduce an additional hydroxyl group in the cationisation process. After cationisation this group was in close proximity to the cationic group but out of the glucopyranose ring of cellulose. Five reactive dyes with different reactive groups were chosen for the experimental studies. The most environmentally friendly conditions for dyeing cationised cellulose, i.e. room temperature and the bath without electrolytes and alkalis were used. It was assumed that Coulomb interactions between the positive ammonium group of modified cellulose and the negative sulfo groups of reactive dyes would cause chemical adsorption to form a very strong ionic bonds leading to complete exhaustion of the dyes from the bath. Chemical adsorption under these conditions eliminates the need to use electrolytes and elevated temperature. It was also assumed that during dyeing without alkali in room temperature no cellulosan anions would be form on the hydroxyl groups in the glucopyranose ring of cellulose. As a result, the bond between the reactive dye and the dissociated hydroxyl group in the modifier will be formed. After dyeing, a water rinsing instead of the alkali washing was applied.
To confirm creation of the covalent bond of the reactive dye with the hydroxyl group of the modifier, the methods of testing the resistance of the bond to acid and alkali hydrolysis, extraction chemically unbounded dye with DMF and electron density analysis were used.
Experimental
Materials
The cotton plain fabric after classical alkali scouring and bleaching with a surface weight of 180 g/m2 (16 warps and 22 wefts) was used. Cationising agents: 3-chloro-2-hydroxypropyltrimethylammonium chloride and 2-chloroethyl-trimethylammonium chloride were purchased from Sigma Aldrich and ADAMA Poland, respectively. Reactive dyes: RR 24:1 from Boruta-Zachem Poland, RR 274 from Swisscolor Poland, RB 19 from Biliński Factory Poland, RB 160 from Kalpactive India and RR 221 from Kisco South Korea were purchased respectively. All dyes were applied without further purification. Ready for use polyelectrolyte standard solutions, PES-Na (MW 21.800 g/mol) and poly-DADMAC (MW 107.000 g/mol) were purchased from BTG Instruments AB Sweden. Tanaterge Advance (non-ionic detergent) was purchased from Tanachem Poland. Other chemicals and solvents were used as laboratory grade purity. Chemical structures and data of cationising agents and dyes are shown in Tables 1 and 2.
Methods
Cationisation of cellulose
Ugolini Redkrome–model RED P (Italy) laboratory dyeing machine, heated by infrared ray radiators, equipped with 150/400 ml cups was used for cationising cotton fabric samples.
Cationisation with [ClCh] + Cl ‒
Cotton fabric samples of 15 g were introduced into 400 ml Ugolini cups to 60 mL [ClCh]+Cl− 66.5% (0.28 mol), and then 24 g of NaOH (0.6 mol) dissolved in 150 ml distilled water was added. Then the bath was heated to 97.5 ± 1 °C.
(2 °C/min, rotation right/left 40 rpm), for five hours. Next, the bath was cooled to 50 °C and the cationised cotton material was washed with cold tap water, neutralised with 0.5% acetic acid, again rinsed to a neutral pH and dried at room temperature. Reaction of cellulose cationisation with [ClCh]+Cl− was shown on Fig. 1.
Cationisation with [CHPTA] + Cl ‒
Cotton fabric samples of 15 g were introduced into 400 mL Ugolini cups contained 285 mL bath with 4.86 g NaOH (0.12 mol) and 1 g/l Tanaterge Advance, and then five min wetted. Next, 15 mL [CHPTA]+Cl− 60% (0.055 mol) was added, and the bath was heated to 70 °C (2 °C/min with rotation right/left 40 rpm) and continued at that temperature for 90 min. After that, the bath was cooled to 50 °C and cationised samples were rinsed and washed with warm and cold tap water, neutralised with 0.5% acetic acid and again rinsed to reach a neutral pH. Cationised samples were dried at room temperature. Reaction of cellulose cationisation with [CHPTA]+Cl− was shown on Fig. 2.
Nitrogen content
The Nitrogen content before and after modification the cotton samples by the classical Kjeldahl method in the Institute of Technical Biochemistry, Faculty of Biotechnology and Food Sciences, Technical University of Lodz (Poland) was determined (Pruś et al. 2019 and 2021). Specimens ca. 1.5 g cotton samples were mineralised in concentrated sulphuric acid with the addition of a selenium mixture in a Büchi K-424/435 apparatus. Then solution were alkalinised with a concentrated sodium hydroxide. The ammonia formed was saturated in a boric acid solution in the Büchi K-314 apparatus. The distillate was titrated with an hydrochloric acid solution against a Tashiro indicator using a Schott Geracle Titronic digital burette to change the colour from green to violet. The Nitrogen content was calculated according to Eq. (1):
where \(v\)– mL of hydrochloric acid used for titration distillate, c–concentration of hydrochloric acid 0.1 M [mol/L], m–weight of the sample for analysis [g].
Specific charge measurement
Muetek PCD 03 pH Particle Charge Detector (Muetek GmbH Germany) for measurement of the potential on the surface of cotton samples was used according to the previously developed recipe (Pruś et al. 2019 and 2021). The values of surface charges were calculated by Eq. (2):
where \(v_{0}\)–mL of polyelectrolyte PES-Na for titration of 10 mL polyelectrolyte poly- DADMAC (blind test), \(v_{1}\)– mL of polyelectrolyte PES-Na for titration of 10 mL of filtrate after treatment, c–polyelectrolyte concentration of poly-DADMAC, \(v_{c}\)– mL of polyelectrolyte poly-DADMAC used for treatment, \(v_{a}\)– mL of filtrate used for titration, m–test sample weight [g].
Dyeing of cellulose
Ugolini Redkrome–model RED P (Italy) laboratory dyeing machine, heated by infrared ray radiators, equipped with 150/400 mL cups and simply 2- roll padder with 100% pick-up for dyeing in exhaustion method and cold pad batch (CPB) method were used respectively.
Conventional dyeing
For uncationised cotton samples, conventional methods of dyeing were used. Selected dyes were applied using methods and recipes recommended by their producers (Table 3).
All samples after conventional dyeing process were washed with 1 g/L Na2CO3 and 1 g/L Tanaterge Advance at 90 °C for 15 min and next rinsed with warm and cold tap water for removing unfixed dye. Dyed samples were dried at room temperature.
Eco-friendly dyeing
Cationised cotton samples in eco-friendly dyeing conditions (LR = 1:20, temp. 25 ± 1 °C, rotation right/left 40 rpm, distilled water, pH neutral, without salt and alkalis) were dyed for 30 min with 1% owf (RR 221 or RB 160) and 0.9% owf (RR 24:1 or RR 274 or RB 19) respectively. After eco-friendly dyeing samples were rinsed with cold distilled water to remove not fixed dye and dried at room temperature. For comparison, noncationised cotton samples were dyed under the same conditions and with the same amounts of dyes.
Estimation of the type of bond between dye and cellulose
Hydrolysis of covalent bond between dye and cationised cellulose
In order to confirm the place of the covalent bond formed between the [Cell-O-HPTA]+Cl− and reactive dye in eco-friendly conditions the dyed material was hydrolysed under acid or alkali conditions. Two dyes were selected for these experiments: RR 24:1 (monochlorotriazine type) and RB 19 (vinylsulfone type) for acid and alkali hydrolysis respectively.
Acid hydrolysis
Four pieces of approx. 2 g of samples [Cell-O-HPTA]+Cl− dyed with 2% owf RR 24:1 were introduced into the bath (LR = 1:30) containing 1 mL/L H2SO4 96% and 2 g/L Na2SO4 at room temperature, then heated to boil, boiled for 6 h, then cooled to room temperature. The hydrolysis process was then repeated in a fresh bath for the same samples for next three hours. The samples after hydrolysis, were rinsed to neutral pH and dried at room temperature.
Alkali hydrolysis
Four pieces of approx. 2 g of samples [Cell-O-HPTA]+Cl− dyed with 2% owf RB 19 were introduced into the bath (LR = 1:30) with 20 g/L Na2CO3 at room temperature then heated to boil, boiled for 1.5 h, next cooled to room temperature. The hydrolysis process was then repeated in a fresh bath with the same samples for next 1.5 h. The samples after hydrolysis, were rinsed to neutral pH and dried at room temperature.
Two pieces the samples after acid or alkali hydrolysis were re-dyed respectively with the same dye and at the same eco-friendly dyeing conditions, rinsed in water and dried at room temperature.
Resistance of dyed cotton to DMF extraction
Cotton samples after dyeing, re-dyeing and hydrolysis treatment were extracted with DMF (LR = 1:40) for 15 min at boiling temperature, then cooled down to 70 °C, rinsed in warm and cold distilled water and dried at room temperature.
Resistance of dyed cotton samples to DMF extraction was estimated based on the K/S measurement. The color strength of noncationised and cationised cotton samples before and after DMF extraction as well as before and after hydrolysis of the bonds was measured based on the evaluation of colorimetric measurements using a Datacolor 850 spectrophotometer (Datacolor, USA). K/S values were calculated in accordance with Eq. (3):
where K–light absorption coefficient, S–light scattering coefficient, R–decimal fraction of the reflectance of dyed fabric, R0–decimal fraction of the reflectance undyed fabric.
Resistance to DMF extraction was calculated according to Eq. (4):
where:\(\left( \frac{K}{S} \right)_{0}\)- value measured for dyed samples before DMF extraction,\(\left( \frac{K}{S} \right)_{1}\)- value measured for dyed samples after DMF extraction.
Result and discussion
The research was aimed at confirming the hypothesis that reactive dyes in eco-friendly conditions can form a covalent bond with the hydroxyl group of the cationic modifier instead of the hydroxyl group of the glucopyranose ring. Two cationic modifiers and five reactive dyes with different reactive systems were examined. The concentration of dyes was used at such a level that it was possible to conduct a wide analysis of the test results. All obtained experimental data are presented in Tables 4, 5 and 6. The tables contain average data for four series of experiments. Percentage deviations were ± 0.5%, ± 1.5% and ± 2% for the measurements of nitrogen content, the amount of charge on the fibre surface, and the measurements of color strenght and resistance to DMF extraction, respectively.
Cationisation of cellulose
The results of the cationisation cellulose experiments with selected modification agents in comparing to the starting material are presented in Table 4.
Cationisation cellulose with [ClCh]+Cl− create the product with a small degree of introduced quaternary groups, all of which were found on the cotton surface (Table 4). This is confirmed by the value of ΔN (5 × 10–6 eq/g) introduced by cationisation, which is almost consistent with the increase in the value of the positive surface charge \((\hspace{0.17em}=\) 4.26 × 10–6 eq/g).
Cationisation cellulose with [CHPTA]+Cl− create the product with a much higher degree of introduced Nitrogen (ΔN = 111 × 10–6 eq/g) in the form of quaternary groups, but only ca. 20% of this value was found in positive surface charge form (\(\Delta Q_{surf}^{ + }\) = 19.02 × 10−6 eq/g). The rest of the introduced ammonium groups calculated by Nitrogen with the Kjeldahl method analysis were not available for titrating with PES-Na anionic polyelectrolyte, which is typical for measuring the charge on the cotton surface.
The most important for dyeing are the positively charged ammonium groups on the surface of the fibre. They form strong ionic bonds with the sulfo groups of the anionic dyes. Although cationisation of cellulose with [ClCh]+Cl− was less effective than with [CHPTA]+Cl−, it was enough, however, to conduct comparative tests to determine the binding site of the reactive dye with cationised cellulose.
Dyeing of cationised cellulose in an eco-friendly conditions
To confirm the hypothesis that reactive dyes under eco-friendly conditions can form a covalent bond with the hydroxyl group of the cationic modifier, comparative tests of dyeing non-cationised cellulose were performed in the same conditions and also in the conventional dyeing method. The reaction mechanisms have been proposed for selected reactive dyes with cationised cellulose (Figs. 3, 4, 5, 6 and 7).
Proposed mechanism of the first step of dyeing [Cell-O-HPTA]+Cl− with triazine dyes: (I—ionic formation bonds on the fibre; II—dissociation of a hydroxyl group in ionic pair: cationised cotton + reactive dye on the fibre) where R—different chemical substituents in the chemical structure of RR 24:1, RB 160, RR 221 and RR 274, X- halogen, nicotinic acid
[Cell-O–Ch] + Cl − dyeing
In the case of [Cell-O–Ch] + Cl− the amount of introduced dye mainly corresponds to the cationic group’s presence. Formation of the linkage with reactive dyes and cellulose cationised with [ClCh] + Cl− was showed in Fig. 3.
[Cell-O-HPTA] + Cl − dyeing
[Cell-O-HPTA]+Cl− in eco-friendly dyeing conditions with reactive dyes firstly form strong ionic bonds with positive quaternary ammonium group and next covalent bonds with the dissociated hydroxyl group being in adjacent β-position. The occurring possible reactions were showed in Figs. 4, 5, 6 and 7.
where R—different chemical substituents in the chemical structure of RR 24:1, RB 160, RR 221 and RR 274, X- halogen, nicotinic acid.
where R—different chemical substituents in the chemical structure of RR 24:1, RB 160, RR 221 and RR 274, X- halogen, nicotinic acid.
Cell-OH dyeing
In the case of Cell-OH dyed at the same eco-friendly conditions. the amount of introduced dye to the fibre correspond to its substantivity and the durability to the formation of physicochemical bonds such as Van der Waals forces, hydrogen bridges, etc.
Conventional dyeing of Cell-OH
Reactive dyes in the conventional dyeing process were bonded covalently on substitution or addition mechanism with hydroxyl groups belonging to the cellulose polymer in an alkaline medium. Only RR 221 (dinicotinic-triazine form reactive dye) was applied on cotton from the buffered bath at neutral pH with 1 g/L recommended buffer prepared from Na2HPO4 and KH2PO4. The obtained DMF resistance values (Table 5) are generally in line expected for dyeing’s after the final washing treatment. Some differences may be the result of the apparatus used and the application conditions.
Estimation of the type of bond between dye and cellulose
Covalent bonds of cellulose with reactive dyes show the expected high resistance to DMF solvent (Venkataraman 1972). Non-covalently bound particles of reactive dyes with cellulose fibres (including hydrolysed forms of dyes) are easily removed by extraction with this solvent. The higher the resistance to DMF extraction, the smaller the K/S change in color.
Resistance of dyed cellulose to DMF extraction
The RDMF [%] parameter as resistance to DMF extraction was used to estimate the type/kind of reactive dye binding with dyed cellulose. Summary values of RDMF [%] for noncationised and cationised cotton samples dyed in different conditions presented Table 5.
Cell-OH and [Cell-O–Ch] + Cl − dyeings
It can be seen from Table 5 that Cell-OH and [Cell-O–Ch]+Cl− do not form the covalent bond with the dye in the dyeing process in environmentally friendly conditions. After DMF extraction only below 10% (RR 24:1, RB 19 and RR 274) and below 35% (RB 160 and RR 221) of the dyes remained on noncationised cotton samples. The resistances to DMF extraction of the dyeing’s on cationised with [ClCh]+Cl− cellulose ranged from 11.48 to 50% and were slightly higher than on noncationised cellulose. These differences are due to the different chemical structures of the reactive dyes used and their substantivity to cellulose. Under ecological dyeing conditions, the hydroxyl groups in the glucopyranose chain do not dissociate to the extent that would allow the formation of an active nucleophile for a chemical reaction with reactive dye. The greater amount of RR 221 remaining on the cationised and noncationised cellulose after DMF extraction compared to other dyes was related probably with the chemical bonding of the absorbed dye to the cellulose hydroxyl groups during extraction. This assumption was confirmed in a special experiment. Noncationised cotton sample was padded in the bath contained 30 g/l RR 221 at 50 °C (this temperature was used for better solubility of the dye) then squeezed to ca. 100%, dried at room temperature and divided into three parts. One part was extracted in the boiling and the second in cold DMF, respectively, until the next portions of DMF were colorless. Then the samples were rinsed with cold tap water and dried at room temperature.
The measured K/S (Fig. 8) for all three parts confirmed the speculative explanation that the RR 221 dye (Kayacelon type dye), under boiling extraction conditions can form a chemical bond with the cellulose hydroxyl group.
[Cell-O-HPTA] + Cl − dyeings
The bonding degree of reactive dyes applied in the eco-friendly conditions on [Cell-O-HPTA]+Cl− was similar to the bonding degree of the dyes with cellulose in the conventional method. This was confirmed by similar values of resistance to DMF extraction (Table 5). It confirmed that the reactive dyes are covalently bonded with the modified cellulose. The difference is only in the place of bonding. The hydroxyl group in the modificator chain of cationised cellulose can probably easily dissociate in water at neutral pH, forms a typical nucleophile anion ready for the substitution or addition reaction with a reactive part of the dye molecule. To confirm that the dissociated form of the hydroxyl group in the modifier chain is necessary for the chemical reaction between the reactive dye and [Cell-O-HPTA]+Cl in eco-friendly conditions the dyeing in aprotic solvents was done. When aprotic solvents (DMF, DMSO) were used as a dyeing bath instead of water with the same dyes, the dyeing/fixation process was not observed. There was no dissociation of the hydroxyl groups in the aliphatic chain of the cationised cellulose, hence the reactive dyes do not bond covalently. When such solvent bath was diluted with water to 1:1, the dyeing process started immediately, and an increase in the color shade was observed. After 30 min of dyeing the final effect was comparable with samples dyed only in water bath and also with similar fastness for extraction with boiling DMF.
Table 5 presented some unexpected RDMF [%] values for the samples of [Cell-O-HPTA]+Cl− dyed with dyes RB 19 and RR 274. It is likely that in the case of RB 19 in eco-friendly conditions, a strong ionic bond could be formed between an ammonium group of cationic cellulose and both anionic groups of the dye (β-sulfatoethylsulfonyl group or/and sulfo group). As a result of the ionic bond formation through β-sulfatoethylsulfonyl group, in eco-friendly condition, the dye probably cannot covalently bond with hydroxyl group of cationic cellulose modifier. Additionally, such a linkage is not resistant to DMF extraction.
In the case of the RR 274, the situation is more complicated. RR 274 is a heterobifunctional dye which in eco-friendly conditions may react in different ways even with cross-linking. Dyeing of cationised cotton in these conditions favours aggregation of a dye molecules mainly on its surface and the resulting that dyeing’s are therefore somewhat cloudy. Dye aggregates on surface of cationic cellulose fibres can be broken during DMF extraction leads to an increase of color strength and increase of degree of bonding up to 100%. Those phenomena require further research.
Hydrolysis of reactive bond reactive dye with cationised cellulose
The linkages formed between reactive dye molecules and those of the fibre should be stable under all or most of the conditions to which the dyeing is likely to be subjected during normal use (Beech 1970). Ether and pseudoester bonds formed between reactive dyes and cellulose are rather fast for acid and alkali hydrolysis. This fastness mainly depends on the chemical construction of the used dyes. Hydrolysis reactions of dye-fiber bonds on a cationised fabric have not been described so far. It was assumed that during such hydrolysis the dye-modificator bond would be broken without breaking the cellulose-(modificator + dye) bond. In such a case, it would be possible to re-dyeing the cationised cellulose under eco-friendly conditions with reactive dye in reaction with the restored hydroxyl group of the modificator.
The obtained results (Table 6) fully confirmed that dye molecules were bonded with a hydroxyl group belonging to the modification agent and that the hydrolysis reaction (bond cleavage) for ether/pseudoester bonds takes at the same place. It is clear that if the cleavage of the ether bond occurred between the modifying agent and the cellulose, the re-dye of the noncationised cotton under eco-friendly conditions would be impossible.
Density electron calculations
O(6), O(2) and O(3) are the reactive oxygen atoms in the cellulose molecule. The quantum-chemical PM3 MO method was used to calculate the electron densities on these atoms, which will allow us to predict the direction of the cellulose etherification and modification reaction with the use of the quaternary ammonium salts of [ClCh]+Cl−, [CHPTA]+Cl−, and also in the final the stage where the chemical bond with the reactive dye is formed. A fragment of the cellulose molecule Cell = (Glu)3, consisting of three glucopyranose molecules linked by glycoside bonds, was used as a model compound for the calculations.
The structures of the analysed molecules in the ground state was optimised with the use of molecular mechanics MM + (option with charge analysis) applying Hyperchem v.8.06 programme (Hypercube, Inc. Gainesville, Florida, US) and next with the semi-empirical method of quantum-chemical calculations PM3 (Stewart 1989), taking into account the length of all bonds, angles between them and torsion angles from following the eigenvector procedure (convergence criterion 0.02 kcal/mol).
The electron densities for all oxygen atoms were calculated, assuming that the etherification reaction could take place on each of the -OH groups of the glucopyranose ring.
The calculations showed that the most probable derivatives (Table 7) are ether bonds with the tested modifiers formed with the hydroxyl group on the C(6) carbon (Fig. 9). Such derivatives were accepted for further analysis.
As a result of the formation of these bonds, the electron densities on the remaining O(2) and O(3) oxygen atoms in the glucopyranose ring change, and these changes are so large that in some cases, their reactivity is even greater than in the unsubstituted Cell = (Glu)3 molecule. The oxygen with the highest electron density as a nucleophile should react to form a chemical bond in the SN2 reaction with a dye containing, e.g., cyanuric chloride as a reactive group. Then, in the case of [Cell-O(6)-Ch]+Cl‒ derivatives, the reaction should take place on the O(3) oxygen atom:
and for the [Cell-O(6)-HPTA]+Cl‒ derivatives on the oxygen atom O(β):
However, it is known that oxygen atoms in hydroxyl groups in positions 2 and 3 in cellulose are very strongly involved in intermolecular hydrogen bonds (not included in quantum calculations), which significantly reduces their ability to perform chemical reactions, especially in neutral conditions. According to Nishiyama, Langan and Chanzy (2002) hydrogen bonds for cellulose I include two intramolecular bonding, namely, O(2)H⋯O(6) bonding and O(3)H⋯O(5) bonding and one intermolecular bonding, O(6)H⋯O(3).
A completely different situation was found in the hydroxyl group with the O(β) oxygen atom in the [Cell-O(6)-HPTA]+Cl− derivative, which does not participate in intramolecular hydrogen bonds and can easily undergo a dissociation reaction with the formation of a nucleophile and react with the reactive system of the dyes to form a strong covalent bond.
Additional calculations were performed using benzenesulfonic acid (PhSO3H) as an acid dye model containing a sulfo group capable of ion-pairing with a cellulose fragment, containing the introduced cationisation modifier. These derivatives (Fig. 10) showed further changes in the electron density at the oxygen atoms, but also marked changes on the quaternary group (Table 8).
The formation of an ionic bond between [Cell-O(6)-Ch]+Cl− and benzenesulfonic acid causes only changes in the value of the positive charge on the quaternary group without changes on the cellulose oxygen atoms in the glucopyranose ring.
(Table 8). In the case of a similar reaction of [Cell-O(6)-HPTA]+Cl− with benzenesulfonic acid, apart from a marked increase in the positive charge on the quaternary group, a significant change in the negative charge on the oxygen atoms is also observed. Lowering the electron density at the O(β) oxygen causes an increase in dissociation of that hydroxyl group.
The presented results of electron density calculations confirmed the results of experimental studies obtained in the process of dyeing [Cell-O-HPTA]+Cl− with reactive dyes in an aqueous bath, at ambient temperature without the addition of electrolytes and alkali. The dye is covalently bound to the hydroxyl group in the modifier. Cationic modification of cellulose with [ClCh]+Cl− allows it to be dyed with reactive dyes under the same conditions, only with the formation of ionic bonds as for acid dyes. As already mentioned in the experimental section, the extraction treatment with DMF allows distinguishing between these bonds.
Conclusion
Existing reactive systems depend on two basic elements: an electron-deficient “reactive” carbon centre in the dye and an electron-rich “nucleophile centre” in the fibre (Niu et al. 2020). Studying the processes of dyeing modified cellulose by cationisation with [CHPTA]+Cl−, it was found that in a water bath without the addition of electrolytes and alkali at room temperature, reactive dyes of various classes formed covalent bonds according to the substitution/addition mechanism with nucleophile hydroxyl group located in the modifier chain. These stable bonds of the reactive dyes were confirmed by extraction treatment in boiling DMF, acid/alkali hydrolysis of the formed ether/pseudoester linkage and effective re-dyeing under the same conditions as the basic process. The results of dyeing on noncationised and cationised cellulose with [ClCh]+Cl− (it does not contain hydroxyl groups in its structure) were compared. The analysis using particle optimisation with MM + molecular mechanics and quantum-chemical calculations by the method of all valence orbitals confirmed the experimental results of high activity of the nucleophile being formed on the hydroxyl group in the modifier chain.
Abbreviations
- Cell-OH:
-
Cellulose
- [ClCh]+Cl− :
-
2-Chloroethyltrimethylammonium chloride
- [Cell-O–Ch]+Cl− :
-
Cellulose cationised with [ClCh]+Cl−
- [Cell-O(2)-Ch]+Cl− :
-
Cellulose cationised [ClCh]+Cl− on hydroxyl group in position 2 glucopyranose ring
- [Cell-O(3)-Ch]+Cl− :
-
Cellulose cationised [ClCh]+Cl− on hydroxyl group in position 3 glucopyranose ring
- [Cell-O(6)-Ch]+Cl− :
-
Cellulose cationised [ClCh]+Cl− on hydroxyl group in position 6 glucopyranose ring
- [Cell-O(6)-Ch]+PhSO3 − :
-
Ionic pair [Cell-O(6)-Ch]+Cl− and benzenesulfonic acid
- [CHPTA]+Cl− :
-
3-Chloro-2-hydroxypropyltrimethylammonium chloride (CHPTAC)
- [Cell-O-HPTA]+Cl− :
-
Cellulose cationised with [CHPTA]+Cl−
- [Cell-O(2)-HPTA]+Cl− :
-
Cellulose cationised [CHPTA]+Cl− on hydroxyl group in position 2 glucopyranose ring
- [Cell-O(3)-HPTA]+Cl− :
-
Cellulose cationised [CHPTA]+Cl− on hydroxyl group in position 3 glucopyranose ring
- [Cell-O(6)-HPTA]+Cl− :
-
Cellulose cationised [CHPTA]+Cl− on hydroxyl group in position 6 glucopyranose ring
- [Cell-O(6)-HPTA]+PhSO3 − :
-
Ionic pair [Cell-O(6)-HPTA]+Cl− and benzenesulfonic acid
- DMF:
-
N,N-Dimethylformamide
- DMSO:
-
Dimethyl sulfoxide
- [EPTA]+Cl− :
-
2,3-Epoxypropyltrimethylammonium chloride
- Glu:
-
Glucopyranose ring
- Kayacelon React:
-
Brand name of reactive dyes with nicotinic group
- LR:
-
Liquor ratio
- MCT:
-
Monochlorotriazine type of reactive dyes
- owf:
-
On weight fibre
- PhSO3H:
-
Benzenesulfonic acid
- PES-Na:
-
Polystyrene sulfonic acid natrium salt
- poly-DADMAC:
-
Polydiallyldimethylammonium chloride
- RB 19:
-
Remazol brilliant blue R (reactive blue 19)
- RB 160:
-
Kalpactive blue HE-BR (reactive blue 160)
- RR 24:1:
-
Helaktyn red D-BN (reactive red 24:1)
- RR 221:
-
Papizolon red HT-3BN (reactive red 221)
- RR 274:
-
Eriofast red 2B (reactive red 274)
- rpm:
-
Round per minute
- VS:
-
Vinylosulfone type of reactive dyes
References
Acharya S, Abidi N, Rajbhandari R, Meulewaeter F (2014) Chemical cationisation of cotton fabric for improved dyeuptake. Cellulose 21:4693–4706. https://doi.org/10.1007/s10570-014-0457-2
Aktek T, Mallekut Millat AKM (2017) Salt free dyeing of cotton fiber–a critical review. Int J Text Sci 6(2):21–33. https://doi.org/10.5923/j.textile.20170602.01
Arivithamani N, Dev VRG (2017) Cationisation of cotton for industrial scale salt-free reactive dyeing of garments. Clean Technol Envir 19:2317–2326. https://doi.org/10.1007/s10098-017-1425-y
Atiq MS, Rehman A, Iqbal K, Safdar F, Basit A, Ashraf M, Maqsood HS, Khan A (2019) Salt free sulphur black dyeing of cotton fabric after cationisation. Cell Chem Technol 53(1–2):155–161
Beech WF (1970) Fibre-Reactive Dyes, Logos Press Limited. ICI Dyestuff Division, London
Burkinshaw SM (2016) Physico-chemical aspects of textile coloration. J. Wiley and Sons in association Society Dyers and Colourists. ISBAN 978-1-118-72569-6
Clark M (2011) Handbook of textile and industrial dyeing principles, procedures and types of dyes, vol 1. Woodhead Publishing Limited, Sawston
Correia J, Rainert KT, Oliveira FR, Curto Valle R, Valle JAB (2020) Cationization of cotton fiber-an integrated view o cationic agents, processes variables, properties, market and future prospects. Cellulose 27:8527–8550. https://doi.org/10.1007/s10570-020-03361-w
Heinze T, Koschella A, El Seoud OA (2018) Cellulose derivatives–synthesis, structure, and properties. Springer, pp 444. ISSN 2364-1878. https://doi.org/10.1007/978-3-73168-1
Kucharska M, Wyrębska Ł, Kwiecień A, Gosławski S (2009) Eriofast–reactive dyes for dyeing of polyamide. XXV seminar of the Polish chemists colourists, Tarnów, pp 52–69. ISBN 978-83-927176-1-4
Lewis DM (2014) Developments in the chemistry of reactive dyes and their application processes. Color Technol 130:382–412. https://doi.org/10.1111/cote.12114
Lewis DM, Broadbent PJ, Vo LTT (2008) Covalent fixation of reactive dyes on cotton under neutral conditions. AATCC Rev 8(1):35–41
Liu L, Mu B, Li W, Yang Y (2019) Semistable emulsion system based on spent cooking oil for pilot-scale reactive dyeing with minimal discharges. ACS Sustain Chem Eng 7:13698–13707. https://doi.org/10.1021/acssuschemeng.9b01003
Łukoś A, Ornaf W (1966) Barwniki reaktywne - budowa i zastosowanie. WPLiS, Warszawa (in polish)
Montazer M, Malek RMA, Rahimi A (2007) Salt free reactive dyeing of cationised cotton. Fib Polym 8(6):608–612. https://doi.org/10.1007/BF02875997
Nishiyama Y, Langan P, Chanzy H (2002) Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J Am Chem Soc 124(31):9074–9082. https://doi.org/10.1021/ja0257319
Niu T, Wang X, Wu C, Sun D, Zhang X, Chen Z, Fang L (2020) Chemical modification of cotton fabrics by a bifunctional cationic polymer for salt-free reactive dyeing. ACS Omega 5:15409–15416. https://doi.org/10.1021/acsomega
Oakes J, Graton P, Dixon S (2004) Solubilisation of dyes by surfactant micelles. Part 3: durability of dye fixatives. Color Technol 120:276–283. https://doi.org/10.1111/j.1478-4408.2004.tb00231.x
Pruś S, Kulpiński P, Matyjas-Zgondek E (2019) Changes in the specific charge amount on the surface of cotton fibres during the alkali pre-treatment process. Fibres Text East Eur 274(14136):30–37. https://doi.org/10.5604/01.3001.0013.1817
Pruś S, Kulpiński P, Matyjas-Zgondek E (2021) Comparison of the effects of the cationization of raw, bio- and alkali- scoured cotton knitted fabric with different surface charge density. Autex Res J 2:255–264. https://doi.org/10.2478/aut-2020-0049
Stewart JJP (1989) Optimization of parameters for semiempirical methods I. Method Comput Chem 10:209. https://doi.org/10.1002/jcc.540100208
Stiepanow BI (1980) Podstawy Chemii i Technologii Barwników Organicznych. WNT Warszawa, pp 242. ISBN 83-204-0202-6 (in polish)
Venkataraman K (1972) The chemistry of synthetic dyes reactive dyes, vol 6. Acadamic Press, New York
Wang H, Lewis DM (2002) Chemical modification of cotton to improve fibre dyeability. Color Technol 118:159–167. https://doi.org/10.1111/j.1478-4408.2002.tb00094.x
Zhang Y, Zhang W (2015) Clean dyeing of cotton fiber using a novel nicotinic acid quaternary triazine cationic reactive dye: salt-free, alkali-free, and non-toxic by product. Clean Technol Envir 17:563–569. https://doi.org/10.1007/s10098-014-0821-9
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pruś, S., Kulpiński, P., Matyjas-Zgondek, E. et al. Eco-friendly dyeing of cationised cotton with reactive dyes: mechanism of bonding reactive dyes with CHPTAC cationised cellulose. Cellulose 29, 4167–4182 (2022). https://doi.org/10.1007/s10570-022-04521-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-022-04521-w