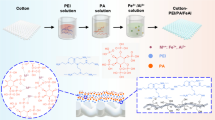
Abstract
Versatile cotton fabrics can be used in a variety of special environments and offer unparalleled advantages in textile products. In this work, bio-based phytic acid (PA), octadecylamine (ODA) and TiO2 (NPs) were used as raw materials to prepare multifunctional cotton fabrics with excellent flame retardant (LOI = 48.5%), hydrophobic (WCA = 152°), UV-blocking (UPF = 2000) and antibacterial (BR = 100%) properties through a facile and scalable dip coating and spraying process. Firstly, the phytic acid was grafted onto the surface of cotton fabric by esterification reaction between its phosphoric acid group and the hydroxyl group of cellulose molecules. Then ODA reacted with the residual phosphoric acid group of phytic acid to form an ODA layer, for further immobilizing TiO2 (NPs) particles on the surface of cotton fabric. The resulting cotton fabric possessed excellent flame resistance (LOI > 36.2%), hydrophobicity (WCA > 138°), UV-blocking (UPF = 2000) and antibacterial abilities (BR > 96.0%) even after 20 washing cycles. Moreover, this modification process did not sacrifice the desired cotton properties, including water vapor permeability, flexibility and tensile strength. This work proposed a simple and scalable pad-dry curing method to construct stable and multifunctional cotton textiles, which have broadened application prospects in many fields.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cotton fabrics are widely used in home decoration, clothing and technical textiles (Yetisen et al. 2016; Ahmed et al. 2020; Karim et al. 2020; Balasubramanian et al. 2021; Li et al. 2021) because of their wide sources, degradability, low cost, breathability, softness and wearing comfort (Flint 1950; Rowland et al. 1976; Park et al. 2004; Klemm et al. 2005). Unfortunately, they have some disadvantages, for example, their moisture-absorbing properties easily lead to the multiplication of bacteria on the surface. When a large number of microorganisms multiply, cross-infection, unpleasant odor and quality deterioration may occur (Zhou and Kan 2015; Cai et al. 2018; Duan et al. 2020; Gao et al. 2021; Wang et al. 2021). Cotton fabrics have a low limiting oxygen index and a low onset ignition temperature (360–425 °C), which make these materials highly flammable. When exposed to flame, cotton fabrics will burn quickly, emitting large amounts of heat and toxic fumes (Xu et al. 2019; Li et al. 2020; Chen et al. 2021). In addition, cotton fabrics also suffer from oxidation aging and performance degradation, when exposed to high UV intensity of the atmosphere for a long time. (Abidi et al. 2009; Yin et al. 2014; Liu et al. 2022a, b). Hence, it is vital to develop multifunctional cotton fabrics with hydrophobic, antibacterial, flame retardant and UV resistance properties to provide a safe environment for human beings.
Halogenated compounds have been the most effective and widely used flame retardants on cotton fabrics in the past decades. But halogenated flame retardants are gradually disappearing from research laboratories, due to their negative impact on the environment and health (Zaikov and Lomakin 2002). The use of renewable resources including nucleic acid (DNA), protein, phytic acid (PA) and other formaldehyde-free, halogen-free, environmentally friendly and efficient bio-based flame retardants has attracted widespread attention (Alongi et al. 2014; Qiu et al. 2018; Li et al. 2019; Miao et al. 2021; Wan et al. 2021; Sykam et al. 2021; Özer and Gaan 2022). For example, DNA coatings were constructed on cotton fabrics using a layer-by-layer technique, resulting in self-extinguishing properties and achieving a 28.0% limiting oxygen index (LOI) (Alongi et al. 2013). Nevertheless, the limited source of DNA hinders its large-scale application. By contrast, the amino acids in whey protein are mostly sulfur-containing amino acids, and casein is a phosphorylated protein. When the surface of cotton fabric was coated with whey protein or casein suspension, the burning rate of cotton fabric was reduced and the burning time was effectively increased (Alongi et al. 2013; Bosco et al. 2013; Faheem et al. 2019; Liu et al. 2020). However, it is difficult to endow cotton fabrics with self-extinguishing properties, due to insufficient content of flame retardance elements in these proteins. Phytic acid (PA), a natural molecule extracted from plant tissues such as grains and rapeseed, has been widely used for flame retardant fabrics, because of its environmental friendliness, biocompatibility and phosphorus-rich nature (one phytic acid molecule has six phosphate groups) (Li et al. 2019, 2020; Liu et al. 2019, 2020; Thota et al. 2019; Sykam et al. 2021). However, PA is limited in making flame retardant fabrics as it degrades cellulose by the acidic effect, resulting in a significant loss of strength in cotton fabrics.
Due to their polyhydroxy structure, cotton fabrics are prone to absorb water, which hinders their application in special fields (Zhou et al. 2018; Yang et al. 2021; Wang et al. 2022; Wu et al. 2022). Good hydrophobicity can reduce the surface contamination of cotton fabrics and facilitate high-value applications in special environments (Wang et al. 2014; Xu et al. 2020). The hydrophobic treatment of cotton fabric surfaces is achieved by a combination of reduced surface energy (Latthe et al. 2014) and surface roughening (Zou et al. 2013), which also has been applied to stain prevention (Xi et al. 2015), self-cleaning (Mishra and Butola 2018), anti-icing (Latthe et al. 2019) and oil–water separation (Zhou et al. 2019). The low surface energy of cotton fabrics is mainly achieved by grafting fluorinated compounds (Zhang et al. 2018) or polysiloxane polymers (Przybylak et al. 2016). However, most of these materials are made from non-renewable or even toxic raw materials and may have serious consequences for human health and the environment, thus running counter to sustainable development goals. Octadecylamine (ODA) can reduce the surface energy of cotton fabrics to achieve hydrophobicity, due to its extra-long alkane chain. (Yan et al. 2020; Lin and Lee 2021; Liu et al. 2022a, b). Moreover, ODA possesses the characteristics of low cost, wide source, degradability and biocompatibility, which have attracted great interest from researchers (Hu et al. 2022). Besides, the micro/nano-structures on the fabric surface are mainly achieved by loading some inorganic nanomaterials, such as ZnO (Athauda et al. 2013), SiO2 (Lin et al. 2018) and TiO2 (Lee et al. 2013; Yang et al. 2018). Due to its UV absorption ability, TiO2 is also widely used in functional textiles for realizing self-cleaning, antibacterial and UV protection properties (Wu and Long 2011; Yu et al. 2013; Shaheen et al. 2019; Raeisi et al. 2021; Sheng et al. 2021; Zhu et al. 2021). However, the weak interface bonding force between inorganic nanomaterials and textile fibers is a major challenge for the further application of TiO2 (NPs).
In this work, we prepared multifunctional cotton fabrics with flame retardant, hydrophobic, UV-resistant and antibacterial properties based on low-cost and bio-based raw materials PA, ODA and TiO2 (NPs). Flame retardant fabric P-CO was prepared by covalently grafting phytic acid onto the surface of cotton fabric through esterification reaction in phytic acid, urea and dicyandiamide system. Furthermore, TiO2 (NPs) particles and ODA were deposited sequentially on the surface of P-CO fabrics by atomization technique. Meanwhile, ODA will react with the residual phosphoric acid group on the surface of P-CO fabrics via amide reaction and ODA layer was formed on its surface. At the same time, the TiO2 (NPs) particles were firmly capsulated by ODA layer on the surface of cotton fabric. The synergistic effects of PA, ODA and TiO2 endowed POT-CO fabrics with efficient flame retardant, hydrophobic, anti-UV and antibacterial properties. To demonstrate the relationship between structure and performance, the surface morphology and chemical structure of POT-CO fabrics were confirmed by SEM, ATR-FTIR, XPS and XRD, and the flame retardancy, hydrophobicity, UV resistance, antibacterial activity and durability of POT-CO fabrics were comprehensively evaluated. Finally, the effects of the modified process on the inherent properties of the original fabrics were also assessed carefully, including tensile strength, water vapor permeability, and flexibility.
Experimental section
Materials
Cotton fabrics (CO, 120 g/m2 weight) were purchased from Shaoxing Qi Dong Textile Co., Ltd (China). Before chemical modification, the cotton samples (5 cm × 5 cm, 4 pieces) were ultrasonically cleaned in a sodium lauryl sulfonate solution (100 mL, 2 wt%) for 60 min, washed with ethanol (100 mL, 95 wt%) for 30 min, and dried at 80 °C. Then, the cotton fabrics were dipped in a sodium hydroxide solution (100 mL, 1 wt%) at 90 °C for 60 min, washed with deionized water (100 mL, 3 times) and dried at 80 °C to obtain alkaline cotton fabrics which were used for further experiments. All reagents, including phytic acid (PA, 70 wt%), urea (AR, 99.0%), dicyandiamide (AR, 98.0%), NaOH (AR, 97.0%), ethanol absolute (AR, 99.5%), sodium lauryl sulfonate (AR, 98.0%), octadecylamine (ODA, AR, 99.8%) and TiO2 (60 nm, 99.8%) were purchased from Shanghai Aladdin Co., Ltd (China) and used without further purification.
Preparation of modified cotton fabrics
Firstly, PA solution (0.3 mol/L, 100 mL) was prepared, and the urea (0.45 mol, 27.00 g) was dissolved in it to obtain finishing solution, while dicyandiamide (0.06 mol,
5.04 g) was added as catalyst. The alkaline cotton fabric was moved into the finishing solution at 70 °C for 60 min with a weight ratio of 1:20 (Wcotton fabric: Wfinishing solution). Then, the cotton fabric was taken out, squeezed to control the wet absorption of the fabric at 120%, and baked at 180 °C for 5 min. Finally, the cotton fabric was washed with deionized water to remove the extra finishing solution and dried thoroughly in the oven at 80 °C to obtain P-CO fabrics. The crease recovery angle of CO and P-CO samples(Fig. S1a) and the pH change of washing leachate solution of P-CO samples (Fig. S1a) proves that the unreacted PA on the surface of P-CO fiber has been completely washed away.
TiO2 nanoparticles were dispersed in ethanol and sonicated for 15 min to dispersed uniformly. Afterwards, the TiO2 ethanol solution (10 mL, 1 wt%) was sprayed on P-CO fabric samples (5 × 5 cm, both sides) by an air-compression-type atomizer for 1 min and heated at 100 °C for 30 min. Octadecylamine ethanol solution (10 mL, 1 wt%, 3 wt%, 5 wt%) was prepared at 60 °C, and then the solution was sprayed on the cotton fabric samples (5 × 5 cm, both sides). The obtained cotton fabric samples in the previous step were experienced under an air-compression-type atomizer for 5 min and heated at 160 °C for 20 min. The final obtained samples were washed in ethanol (three times, 10 min each time) and dried at 80 °C for 2 h to obtain POT-CO-n fabrics (n represented the mass concentration of ODA), and the POT-CO-5 fabric sample with excellent comprehensive performance was used for all performance characterizations. The preparation process of the POT-CO fabrics is shown in Scheme 1.
For comparison, P-CO fabric loaded with ODA (PO-CO fabric) and P-CO fabric loaded with TiO2 (PTCO fabric) were also prepared. PO-CO fabrics were obtained by spraying ODA ethanol solution (10 mL, 5 wt%) on P-CO fabrics for 5 min through an atomizer, followed by heating at 160 °C for 20 min. PT-CO fabrics were obtained by spraying TiO2 ethanol solution (10 mL, 1 wt%) onto P-CO fabrics for 1 min by an atomizer, and then heated at 100 °C for 30 min. The final obtained samples were washed in ethanol (three times, 10 min each time) and dried at 80 °C for 2 h. Also, CO fabric loaded with ODA alone (ODA-CO fabric) and CO fabric loaded with ODA and TiO2 (ODA/TiO TiO2-CO fabric) were prepared, and the whole preparation process conditions were the same as above.
Characterizations
The chemical structures of control cotton fabric and modified cotton fabrics were evaluated by infrared spectroscopy (FTIR, Thermoelectric Corporation of America). The scanning range of the ATR-FTIR spectrometer was 4000–500 cm−1. X-ray diffraction (XRD) was performed in reflection mode on an X-ray diffractometer (Bruker AXS D8 Advance, Germany), and the 2θ ranged from 10° to 80° with a speed of 5°/min. XPS analysis was performed by an AXIS multifunctional X-ray photoelectron spectrometer (ULTRA DLD, Shimadzu Ltd., Japan) at a power of 450 W. Ultra-high resolution field emission scanning electron microscope (SEM, Zeiss, UK) with Energy Dispersive X-Ray Spectroscopy (EDX, Zeiss, UK) was used to observe the surface morphology of the fabric and the surface elemental composition before and after finishing. All samples (2 mm × 2 mm) were coated with gold before SEM testing and EDX was operated with a primary electron beam at an accelerating voltage of 2 kV. The thermal stability of the samples was determined by a thermogravimetric analyzer (TG, Netzsch, Germany) in the air and nitrogen atmosphere, respectively. After chopping the samples, of which 5– 6 mg was prepared for testing in the range of 30–800 ℃ with a heating rate of 10 ℃/min.
The water vapor permeability, tensile strength, flexibility and washing durability of the fabric samples were carried out according to our previous reports (Wang et al. 2021; Xu et al. 2017, 2019). The test methods of the grafting rate (GR), hydrophobicity analysis, antibacterial property, flame retardant property and UV resistant property of the cotton fabrics were also introduced in the “Supporting information”.
Results and discussion
Chemical structure of modified cotton fabrics
The control cotton fabric (CO) and the modified cotton fabric were characterized by FTIR, and the results are shown in Fig. 1a, b. Compared with the CO sample, the P-CO, PO-CO and POT-CO-5 fabrics displayed additional peaks at 1230 and 1056 cm−1, which were attributed to the P=O and P–O–C stretching vibration (Ma et al. 2021), indicating that PA can be grafted on the cotton fabric surface by P(=O)–O–C bonds through the esterification reaction between the −P=O(O-NH4+)2 group and the –OH group of cellulose (Liu et al. 2017; Xu et al. 2019). The band at 1719 cm−1 was attributed to C=O stretching vibration caused by the oxidation of PA during phosphorylation (Lu et al. 2018; Ma et al. 2021). Meanwhile, the new absorption peaks at 2923 and 2850 cm−1 of PO-CO fabrics and POT-CO-5 fabrics were corresponding to the CH3 and CH2 groups of ODA (Yan et al. 2020; Lin and Lee 2021), which indicated that ODA was grafted onto the cotton fabric by amide reaction with the phosphate group on P-CO fabrics. Furthermore, a new broad peak at 850–740 cm−1 in the POT-CO-5 fabric was attributed to the O–Ti–O bond (Pal et al. 2021; Rashid et al. 2022; Refaee et al. 2022), indicating the successful deposition of TiO2 (NPs) on the surface of the cotton fabric.
XRD analysis was also carried out for the control cotton fabric and modified cotton fabric. As shown in Fig. 1c, the peaks at 2θ = 14.7°, 16.5°, 22.7°, and 34.3° were. typical peaks of crystalline structure of cellulose I, which appeared in the XRD spectra of both control cotton fabric and modified cotton fabrics (Xu et al. 2017, 2020; Duan et al. 2020), illustrating that the modification did not destroy the crystalline structure of cellulose. Compared to other samples, the characteristic peaks of TiO2 at 2θ = 25.5° and 48.2° appeared in the POT-CO-5 fabrics (Shaheen et al. 2019; Sheng et al. 2021), which proved that the TiO2 (NPs) were successfully deposited on the surface of the POT-CO-5 fabric. Furthermore, the increment in fabric weight also revealed the successful grafting of PA, TiO2 (NPs) and ODA on the fabric surface, and the GR of POT-CO-5 fabric reached up to 8.5% (Fig. 1d).
To further confirm the chemical structure, the control cotton fabric and the treated cotton fabrics were analyzed by XPS and the results are shown in Fig. 2. From the wide-scan XPS survey spectra (Fig. 2a), new elements (N and P) appeared in all modified cotton fabrics compared to the CO sample, with the additional presence of Ti elements in the POT-CO-5 fabrics. Fig. 2b, c showed the high-resolution C 1s XPS spectra of CO and POT-CO-5, respectively. The former can be deconvoluted into three peaks at 288.05 eV (O–C–O), 286.40 eV (C–OH) and 284.80 eV (C–C/C–H), whereas the latter can be deconvoluted into two peaks at 286.32 eV (C–OH) and 284.50 eV (C–C/C–H) (Wang et al. 2021, 2022; Shen et al. 2022). To confirm the existence of covalent bonds between PA, ODA and cellulose chains of cotton fibers, high-resolution N 1s and P 2p XPS spectra of the POT-CO-5 fabrics were also analyzed. As shown in Fig. 2d, the N 1s peaks of POT-CO-5 fabrics appeared at 400.89, 399.87 and 398.58 eV, attributed to –NH2, –NH– and –N= bonds (Yan et al. 2020; Lin and Lee 2021), respectively. The P 2p peaks of POT-CO-5 fabrics appeared at 134.32 and 133.38 eV (Fig. 2d), which was corresponding to P=O)–O–C bond between phytate and cellulose, and PO22− for the unreacted –P=O(O–NH4+)2 group (Liu et al. 2017; Xu et al. 2019; Ma et al. 2021). The high-resolution Ti 2p XPS spectra of POT-CO-5 fabrics were presented in Fig. 2f. The Ti 2p peaks of POT-CO-5 fabrics were deconvoluted at 465.00 and 457.00 eV, corresponding to the Ti 2p2/3 and Ti 2p1/2 bonds (Shaheen et al. 2019; Raeisi et al. 2021; Sheng et al. 2021), respectively, which proved the successful deposition of TiO2 (NPs) on the POT-CO-5 fabrics surface. The element contents in the fiber surfaces were calculated based on these XPS results (Table S1). The data clearly demonstrated a significant difference between POT-CO-5 fabrics (78.59% C, 11.88% O, 2.55% N, 3.15% P and 3.84% Ti) and the control cotton fabric (54.63% C and 45.37% O). These XPS results suggested the successful grafting of PA, TiO2 and ODA on the surface of cotton fabrics.
The surface morphologies of CO, P-CO, PO-CO, PT-CO and POT-CO-5 fabrics were characterized by SEM observation. As shown in Fig. 3a–c, it can be seen that the control cotton fabric exhibited a smooth and clear morphological structure as stated in previous reports (Xu et al. 2017, 2020; Wang et al. 2021, 2022). The surface of P-CO fabric was slightly rough (Fig. 3d–f) and a thin film appeared on the surface of PO-CO fabric (Fig. 3g–i), which can be attributed to the grafting coating of PA and ODA, respectively. It is worth noting that the surface of PT-CO fabric (Fig. 3j–l) was as smooth as the control cotton fabric, suggesting that the TiO2 (NPs) have been washed off easily from the fiber surfaces. In contrast, many nanoparticles appeared on the surface of POT-CO-5 fabric (Fig. 3m–o), while the fiber surface was covered with a grainy thin film, which indicated the ODA layer successfully immobilized the TiO2 (NPs) on the surface of POT-CO-5 fabric. In order to further analyze the surface structure of the modified cotton fabrics, EDS element mapping tests were performed on both the control and modified cotton fabrics (Fig. S2). Compared to the control cotton fabric, new elements N and P appeared on the P-CO, PO-CO and POT-CO-5 fabrics and were uniformly distributed on the surface of the cotton fabric. The N and P elements on the P-CO fabric were derived from urea and PA, and the N and P elements on the PO-CO and POT-CO-5 fabrics were derived from ODA, urea and PA. Furthermore, the additional presence of uniformly distributed Ti elements on the surface of POT-CO-5 fabric revealed the successful deposition of TiO2 (NPs) on the surface of the modified cotton fabric. In conclusion, SEM and EDS provided strong evidence for the successful modification of cotton fabrics.
Water retardancy
The surface wettability of the modified cotton fabrics was studied by measuring the contact angle of a sessile water droplet on their surfaces. As shown in Fig. 4a–c, when the control cotton fabric was immersed in water, the control cotton fabric readily sank into the water and exhibited the hydrophilic properties of the conventional cotton fabric (Fig. 4d) (Xu et al. 2020). In contrast, when the POT-CO-5 fabric was immersed in water, the POT-CO-5 fabric exhibited water repellency and could float on the water surface (Fig. 4e–g), indicating that the modification of cotton fabric by ODA and TiO2 (NPs) conferred hydrophobic properties (Fig. 4h). Another interesting comparison between control cotton fabric and POT-CO-5 fabric is shown in Fig. 4i, j. When droplets of various commonly used liquids (10 μL) were placed on the fabric samples, the droplets maintained a spherical shape on the surface of the POT-CO-5 fabric, meanwhile the droplets infiltrated into the control cotton fabric. Furthermore, as shown in Fig. 4k, the modified surface of POT-CO-5 fabric showed hydrophobic properties, while the unmodified surface showed hydrophilic properties, which indicated that the mist finishing technology endowed both sides of the modified fabric with different properties. Fig. 4l exhibited the contact angle of all samples, and the POT-CO-5 fabric showed superhydrophobic property, the contact angle of which reached 152°. Hence, the modified cotton fabrics realized good water repellency, which have potential for application in antifouling fabric.
Optical images of CO (a–c) and POT-CO-5 (e–g) fabrics on water surface. SEM images of CO (d) and POT-CO-5 (h) fabrics. Photographs of various solution droplets (10 μL) on CO (i) and POT-CO-5 (j) fabrics. Optical image of modified side and unmodified side of POT-CO-5 fabric (k). Contact angle of all samples (l)
We also observed the self-cleaning ability of the modified cotton fabric. As shown in Fig. 5a–c, the control cotton fabric rapidly absorbed the dye solution and was permeated by the contaminated solution, after immersing in a colored aqueous solution of blue ink. However, the POT-CO-5 fabric maintained a clean surface after immersion in the effluent (Fig. 5d–f). In another experiment, methyl orange (MO) powder was used to simulate the dust on the surface of the fabrics. When MO adhered to the fabric samples and they were rinsed with water droplets, there were significant differences between the control cotton fabric and POT-CO-5 fabric samples. As shown in Fig. 5g–i, the control cotton fabric was immediately wetted and contaminated with MO powder, even when rinsed with a large amount of water. In contrast, on the POT-CO-5 fabric, the fluid water carried away the MO powder quickly, showing a clean surface (Fig. 5j–l). These results indicate that the POT-CO-5 fabrics have excellent stain resistance and can be used in self-cleaning situations such as homes, industrial activities and outdoor decoration. Moreover, the POT-CO-5 fabrics also have a certain photocatalytic degradation ability because of the TiO2 (NPs) fixed on the surfaces (Chimeh et al 2013; Chimeh and Montazer 2015). By placing the POT-CO-5 fabrics under UV irradiation, TiO2 (NPs) on the surface of POT-CO-5 fabrics will degrade the dye molecules to achieve a self-cleaning ability (Fig. S3).
We also tested the contact angles of the POT-CO fabrics after exposuring to daylight simulator (a cabinet equipped with a UVC 4P SE tube lamp, T5 15W G13) for different times (Razaghpour et al. 2022), and the results were shown in Fig.S4. There was a negligible change in the contact angles of the POT-CO fabrics after UV-lighting. Interestingly, the contact angle undergoes a small decrease with the light exposure time increasing, which is attributed to the certain photocatalytic degradation ability of the TiO2 (NPs) that affects the stability of the ODA coating on the fiber surface to some extent.
Flame retardant behavior analysis
Vertical burn test and LOI measurements were performed to evaluate the flame resistance of control cotton fabric and modified cotton fabrics. Fig. 6a, b showed the combustion process of the control cotton fabric and POT-CO-5 fabric in the vertical burn test, and possible flame-retardancy mechanism of POT-CO-5 fabric. It was evident that when the control cotton fabric was exposed to the flame for only 1s, it burned strongly, quickly and completely, leaving only a small char residue. In contrast, POT-CO-5 fabric could not be ignited in the vertical burn test, even when exposed to the flame for 12s, and produced large amounts of char (char length = 3.0 cm) in the ignition area. The LOI values of all samples were shown in Fig. 7a, compared to the CO sample (LOI = 18.0%), POT-CO-5 showed excellent flame resistance (LOI = 48.5%) and much higher than previous reported cellulose-based materials treated by PA or other flame retardants (Fig. 7b; Table S2).
Subsequently, CO sample and POT-CO-5 fabric were tested by microscale combustibility calorimetry (MCC) to further evaluate the combustion and heat release processes. The lower heat release during combustion may reduce harm to people wearing fabrics made from modified cotton, and it may also protect the material underneath the fabric from heat (Chen et al. 2021; Miao et al. 2021). The heat release rate (HRR) of CO sample and POT-CO-5 fabric are shown in Fig. 7c. From the HRR curve we can see the obvious difference between CO sample and POT cotton fabric, compared to the peak of HRR (PHRR) − 287.56 W/g for CO sample, the PHRR of POT-CO-5 fabric is only 31.45 W/g, which is decreased by 89.06%. The difference in HRR between the control cotton fabric and the modified cotton fabric may originate from the fact that the phytate groups in the modified cotton fabric produce dehydrating agents such as polyphosphoric acid and phosphate during pyrolysis, thus accelerating the dehydration/carbonation and heat release from the cotton fiber, resulting in a lower temperature of peak HRR (Liu et al. 2017; Xu et al. 2019; Ma et al. 2021). Moreover, the fiber morphology of POT-CO-5 fabric before and after VFT was also measured by SEM. As shown in Fig. 7d–i, the overall woven structure of POT-CO-5 fabric remained intact after VFT, which indicated that PA could promote the dehydration and carbonization of cellulose to achieve flame retardant effect.
The results of vertical burn test, LOI and microscale combustibility calorimetry tests showed that the flame retardancy of cotton fabrics was significantly improved via impeding combustion and promoting carbonization.
The thermal degradation properties of control and modified cotton fabrics were evaluated by TG and derived thermogravimetric (DTG) in N2 and air atmospheres (Fig. 8). The corresponding data obtained from the TG and DTG curves are shown in Table 1, including 90% (T10%) mass retention temperature, maximum rate degradation temperature (Tmax), maximum decomposition rate (Vmax) and char residue at 800 °C. The T10% of the control cotton fabric in a N2 atmosphere was 343.0 °C. The main pyrolysis phase occurred in the range of 300.5–400.3 °C, and Vmax was 2.6%/°C at 372.7 °C (Tmax). The main pyrolysis phase of the control cotton fabric corresponded to the depolymerization of cellulose to form flammable gases, volatile liquids and solid residues, and finally at 800 °C retained 0.9% of the residue. Compared to the control cotton fabric, both T10% (243.2 °C) and Tmax (284.0 °C) of the POT-CO-5 fabric were significantly reduced, with the main pyrolysis phase occurred in the range of 209.0–298.5 °C, and the char residue at 800 °C increased to 36.5%. Phytic acid is rich in phosphorus and hydroxyl groups, which may produce phosphoric acid and polyphosphoric acid during thermal degradation. The derived phosphoric acid and polyphosphoric acid can promote catalysis, dehydration and carbonization of cellulose, inhibiting the production of levoglucan and leading to the formation of more protective layers of residual carbon (Cheng et al. 2020; Zhou et al. 2020; Ma et al. 2021). The formed carbon residue will impede heat transfer, formation and diffusion of volatiles, resulting in a flame resistance effect for fabrics. Meanwhile, urea and ODA-treated modified cotton fabrics contain certain N elements, which enable the modified cotton fabrics to release inert gases such as NH3 and N2 during combustion, thus preventing the transfer of heat, flame and oxygen. Fig. 8c, d show the thermal oxidation stability of the fabrics in air atmosphere. The thermal degradation behavior of the control cotton fabric in air atmosphere was similar to that in nitrogen atmosphere, with the main thermal decomposition phase occurring from 295.7 to 393.5 °C. The T10%, Tmax and Vmax were 335.8 °C, 357.8 °C and 2.8%/°C, respectively, and the coke residual at 800 °C was 10.3%. Similarly, the decomposition temperature of POT-CO-5 fabric was reduced and the main thermal decomposition stage occurred between 200.3 and 299.3 °C. The T10%, Tmax and Vmax were 246.8 °C, 283.1 °C and 1.8%/°C, respectively, and the coke residue rate at 800 °C was 40.3%. In conclusion, an earlier initial decomposition occurs at lower temperature, which is ascribed to the deposition of PA. The phosphoric acid and polyphosphoric acid derived from PA promotes char residue generation, inhibiting heat and mass transfer. These characteristics lead to the modified cotton fabrics possessing excellent thermal oxidation stability and flame resistance.
UV resistance of the modified cotton fabrics
Figure 9 shows the UV transmittance spectra and UPF values of the CO, P-CO, PO- CO, PT-CO and POT-CO-5 fabrics. As shown in Fig. 9, the CO sample shows a higher transmittance and a lower UPF value (UPF = 37.91) with UV transmittance of 2.21% and 2.52% for UVA and UVB, respectively. This means that UV can easily penetrate through CO samples, resulting in insufficient UV protection of cotton fabrics for human health (Shaheen et al. 2019; Raeisi et al. 2021). In contrast, P-CO, PO-CO, PT-CO and POT-CO-5 fabrics show excellent UV shielding properties, and the UPF values are significantly increased to 657.12, 1463.42, 868.96 and 2000, respectively, while all have low UVA and UVB transmittance (0.05%). PA is a kind of natural antioxidant which imparts UV resistance to the modified cotton fabric (Diouf-Lewis et al. 2017; Kirschweng et al. 2017; Li et al. 2022). Moreover, the UV absorption of TiO2 is carried out by electron leap, and the absorption wavelength of UV light is equal to or less than the wavelength of TiO2 band gap. When TiO2 is exposed to incident light, it absorbs photon energy, allowing the valence band electrons of low energy to cross the band gap into the conduction band of high energy (Wu et al. 2021; Rashid et al. 2022). Therefore, with the synergistic effect of PA and TiO2, POT-CO-5 fabric exhibits excellent UV protection ability (UPF = 2000, UVA = 0.05%, UVB = 0.05%).
Antibacterial effects of the modified cotton fabrics
Figure 10 showed the antibacterial activity of modified cotton fabrics against E. coli and S. aureus. All modified cotton fabrics showed excellent antimicrobial activity compared to the control cotton fabrics. The bacterial inhibition rates (BR) of P-CO and PO-CO fabrics against E. coli and S. aureus were 93.6% and 89.6%, 96.8% and 91.6%, respectively. This is due to the strong acidity of phytic acid, which endows the modified cotton fabrics with antibacterial properties (Li et al. 2021). It is noteworthy that POT-CO-5 fabric achieved 100% antibacterial rate against both E. coli and S. aureus. The antibacterial activity, reflected in the fact that TiO2 can generate ROS, including bactericidal OH. radicals, on its surface under photocatalysis (Sheng et al. 2021; Liu et al. 2022a, b). The synergistic effect with phytic acid at the same time resulted in POT-CO-5 fabric possessing excellent antibacterial properties. Moreover, the inhibition rates of PT-CO fabric against E. coli and S. aureus were only 98.2 and 94.4%, which were lower than those of POT-CO-5 fabric, indicating that the physically coated TiO2 on P-CO fabric could be easily washed off by deionized water. Comprehensively, the POT- CO-5 samples showed superior hydrophobic, fireproof, UV-blocking and antibacterial effects among all samples (Table S3).
Washing fastness of the modified cotton fabrics
As shown in Fig. 11, the durability of the POT-CO-5 fabric for flame retardancy, hydrophobicity, UV resistance, and antimicrobial resistance was evaluated after multiple washing cycles according to the ISO 105-C10 standard method. As shown in Fig. 11a, the LOI of POT-CO-5 fabric was above 36.2% even after 20 washing cycles, and it also showed remarkable fire-retardant property with a self-extinguishing effect by vertical combustion test. Moreover, the water contact angle of POT-CO-5 fabric remained 136°, the UV resistance of UPF value kept at 2000, and the BR rate against both E. coli and S. aureus maintained above 96.0%. These data showed that POT-CO-5 fabric still maintains excellent flame retardant, hydrophobic, anti-UV and antibacterial properties even after 20 washing cycles.
Inherent properties of modified cotton fabrics
Good water vapor permeability, mechanical property, flexibility, and color are the signature features of cotton fabrics. Hence, the corresponding inherent properties of the modified cotton fabrics were tested, and the results were summarized in Fig. 12a–d. As shown in Fig. 12a, normal fluctuations of water vapor permeability were observed when compared the modified fabrics to the CO sample, and the fluctuation range was within the range of the error bar. So, the modification process had rarely impact on the breathability of cotton fabrics. Interestingly, the principle that hydrochloric acid meets ammonia gas to produce white particles was also used for proving the breathability of cotton fabrics. As showed in Fig. 12b, two bottles containing hydrochloric acid.and ammonia respectively were placed next to each other. The bottle containing ammonia was packaged with cotton fabrics (CO fabric and POT-CO-5 fabric), and the bottlecontaining hydrochloric acid was sealed with cap. When the cap of bottle was removed, both had a large amount of white smoke produced, revealing the breathability of the CO fabric and POT-CO-5 fabric. The tensile strength of different modified samples was slightly reduced compared to the CO sample (Fig. 12c). While the POT-CO-5 fabric still had a high strength retention (about 80%), which was sufficient to satisfy the application of the textile fabric. Moreover, there was no significant difference in the maximum loop height between the CO sample and the modified fabrics (Fig. 12d), indicating that their fabric flexibilities were comparable. Interestingly, our modification process shows insignificant color changes on fabric, as shown in Fig. S5. These results demonstrate that the modification process gave an insignificant impact on the desired features of cotton fabric.
Conclusions
In this work, versatile cotton fabrics with excellent flame retardant, hydrophobic, UV-blocking and antibacterial properties were constructed through a simple and scalable pad-dry curing process. Specifically, cotton fabrics were successfully modified with bio-based materials PA, TiO2 (NPs) and ODA in three steps. Firstly, PA molecules were covalently bonded to the cotton fiber surface through an esterification reaction to obtain the flame-retardant cotton fabric P-CO. Then, TiO2 (NPs) and ODA were loaded onto the surface of P-CO fabric by atomization technique. ODA was not only grafted onto the surface of P-CO fabric by amide reaction, but also formed a thin film to fix the titanium dioxide particles on the surface of P-CO fabric. The resulted multifunctional cotton fabric (POT-CO-5) possessed excellent flame retardance (LOI = 48.5%), hydrophobic properties (contact angle = 52°), UV resistance (UPF = 2000) and antibacterial properties (BR = 100%) compared to the untreated cotton fabric. POT-CO-5 fabric also showed excellent resistance to washing, maintaining excellent flame retardance (LOI > 36.2%), hydrophobicity (contact angle > 138°), UV resistance (UPF = 2000) and antimicrobial properties (BR > 96.0%) even after 20 washing cycles. It was worth mentioning that the surface modification process provided cotton fabric versatility without significantly sacrificing desired cotton properties such as water vapor permeability, tensile strength and softness. Due to the combination of excellent multifunctionality and durability, POT-CO-5 fabric have a strong potential for the future application in the field of multifunctional textiles.
Data availability
Data available on request from the authors.
References
Abidi N, Cabrales L, Hequet E (2009) Functionalization of a cotton fabric surface with titania nanosols: applications for self-cleaning and UV-protection properties. ACS Appl Mater Interfaces 1(10):2141–2146. https://doi.org/10.1021/am900315t
Ahmed A, Hossain MM, Adak B, Mukhopadhyay S (2020) Recent advances in 2D mxene integrated smart-textile interfaces for multifunctional applications. Chem Mater 32(24):10296–10320. https://doi.org/10.1021/acs.chemmater.0c03392
Alongi J, Carletto RA, Blasio AD, Carosio F, Bosco F, Malucelli G (2013) DNA: a novel, green, natural flame retardant and suppressant for cotton. J Mater Chem A 1(15):4779. https://doi.org/10.1039/c3ta00107e
Alongi J, Carletto RA, Bosco F, Carosio F, Blasio AD, Cuttica F, Antonucci V, Giordano M, Malucelli G (2014) Caseins and hydrophobins as novel green flame retardants for cotton fabrics. Polym Degrad Stabil 99:111–117. https://doi.org/10.1021/10.1016/j.polymdegradstab.2013.11.016
Athauda TJ, Hari P, Ozer RR (2013) Tuning physical and optical properties of ZnO nanowire arrays grown on cotton fibers. ACS Appl Mater Interfaces 5(13):6237–6246. https://doi.org/10.1021/am401229a
Balasubramanian S, Kulandaisamy AJ, Babu KJ, Das A, Rayappan BJB (2021) Metal organic framework functionalized textiles as protective clothing for the detection and detoxification of chemical warfare agents—a review. Ind Eng Chem Res 60(11):4218–4239. https://doi.org/10.1021/acs.iecr.0c06096
Bosco F, Carletto RA, Alongi J, Marmo L, Blasio AD, Malucelli G (2013) Thermal stability and flame resistance of cotton fabrics treated with whey proteins. Carbohydr Polym 94(1):372–377. https://doi.org/10.1016/j.carbpol.2012.12.075
Cai QQ, Yang SL, Zhang C, Li ZM, Li XD, Shen ZQ, Zhu WP (2018) Facile and versatile modification of cotton fibers for persistent antibacterial activity and enhanced hygroscopicity. ACS Appl Mater Interfaces 10(44):38506–38516. https://doi.org/10.1021/acsami.8b14986
Chen Y, Liu SD, Wan CY, Zhang GX (2021) Facile synthesis of a high efficiency and durability L-citrulline flame retardant for cotton. Int J Biol Macromol 166:1429–1438. https://doi.org/10.1016/j.ijbiomac.2020.11.022
Cheng XW, Tang RC, Guan JP, Zhou SQ (2020) An eco-friendly and effective flame retardant coating for cotton fabric based on phytic acid doped silica sol approach. Prog Org Coat 141:105539. https://doi.org/10.1016/j.porgcoat.2020.105539
Chimeh AE, Montazer M (2015) Fabrication of nano-TiO2/carbon nanotubes and nano- TiO2/nanocarbon black on alkali hydrolyzed polyester producing photoactive conductive fabric. J Text I 107(1):95–106. https://doi.org/10.1080/00405000.2015.1012881
Chimeh AE, Montazer M, Rashidi A (2013) Conductive and photoactive properties of polyethylene terephthalate fabrics treated with nano TiO2/nano carbon blacks. New Carbon Mater 28(4):313–320. https://doi.org/10.1016/s1872-5805(13)60084-0
Diouf-Lewis A, Commereuc S, Verney V (2017) Toward greener polyolefins: antioxidant effect of phytic acid from cereal waste. Eur Polym J 96:190–199. https://doi.org/10.1016/j.eurpolymj.2017.09.014
Duan PP, Xu QB, Zhang XJ, Chen JN, Zheng WS, Li L, Yang J, Fu FY, Diao HY, Liu XD (2020) Naturally occurring betaine grafted on cotton fabric for achieving antibacterial and anti-protein adsorption functions. Cellulose 27(11):6603–6615. https://doi.org/10.1007/s10570-020-03228-0
Faheem S, Baheti V, Tunak M, Wiener J, Militky J (2019) Flame resistance behavior of cotton fabrics coated with bilayer assemblies of ammonium polyphosphate and casein. Cellulose 26(5):3557–3574. https://doi.org/10.1007/s10570-019-02296-1
Flint EA (1950) The structure and development of the cotton fiber. Biol Rev 25(4):414–434. https://doi.org/10.1111/j.1469-185X.1950.tb00767.x
Gao DG, Li XJ, Li YJ, Lyu B, Ren JJ, Ma JZ (2021) Long-acting antibacterial activity on the cotton fabric. Cellulose 28(3):1221–1240. https://doi.org/10.1007/s10570-020-03560-5
Hu DD, Li YD, Weng YX, Peng HQ, Zeng JB (2022) Fabrication of sustainable and durable superwetting cotton fabrics with plant polyphenol for on-demand oil/water separation. Ind Crop Prod 186:115264. https://doi.org/10.1016/j.indcrop.2022.115264
Karim N, Afroj S, Lloyd K, Oaten LC, Andreeva DV, Carr C, Farmery AD, Kim ID, Novoselov KS (2020) Sustainable personal protective clothing for healthcare applications: a review. ACS Nano 14(10):12313–12340. https://doi.org/10.1021/acsnano.0c05537
Kirschweng B, Tátraaljai D, Földes E, Pukánszky B (2017) Natural antioxidants as stabilizers for polymers. Polym Degrad Stabil 145:25–40. https://doi.org/10.1016/j.polymdegradstab.2017.07.012
Klemm D, Heublein B, Fink HP, Bohn A (2005) Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Inter Ed 44(22):3358–3393. https://doi.org/10.1002/anie.200460587
Latthe SS, Terashima C, Nakata K, Fujishima A (2014) Superhydrophobic surfaces developed by mimicking hierarchical surface morphology of lotus leaf. Molecules 19(4):4256–4283. https://doi.org/10.3390/molecules19044256
Latthe SS, Sutar RS, Bhosale AK, Nagappan S, Ha CS, Sadasivuni KK, Liu SH, Xing RM (2019) Recent developments in air-trapped superhydrophobic and liquid-infused slippery surfaces for anti-icing application. Prog Org Coat 137:105373. https://doi.org/10.1016/j.porgcoat.2019.105373
Lee MW, An S, Joshi B, Latthe SS, Yoon SS (2013) Highly efficient wettability control via three-dimensional (3D) suspension of titania nanoparticles in polystyrene nanofibers. ACS Appl Mater Interfaces 5(4):1232–1239. https://doi.org/10.1021/am303008s
Li P, Wang B, Xu YJ, Jiang ZM, Dong CH, Liu Y, Zhu P (2019) Ecofriendly flame-retardant cotton fabrics: preparation, flame retardancy, thermal degradation properties, and mechanism. ACS Sustain Chem Eng 7(23):19246–19256. https://doi.org/10.1021/acssuschemeng.9b05523
Li P, Wang B, Liu YY, Xu YJ, Jiang ZM, Dong CH, Zhang L, Liu Y, Zhu P (2020) Fully bio-based coating from chitosan and phytate for fire-safety and antibacterial cotton fabrics. Carbohydr Polym 237:116173. https://doi.org/10.1016/j.carbpol.2020.116173
Li JH, Tian X, Hua T, Fu JM, Koo M, Chan WM, Poon T (2021) Chitosan natural polymer material for improving antibacterial properties of textiles. ACS Appl Bio Mater 4(5):4014–4038. https://doi.org/10.1021/acsabm.1c00078
Li YC, Wang JZ, Xue BQ, Wang SH, Qi P, Sun J, Li HF, Gu XY, Zhang S (2022) Enhancing the flame retardancy and UV resistance of polyamide 6 by introducing ternary supramolecular aggregates. Chemosphere 287(Pt 2):132100. https://doi.org/10.1016/j.chemosphere.2021.132100
Lin TC, Lee DJ (2021) Cotton fabrics modified with tannic acid/long-chain alkylamine grafting for oil/water separation. J Taiwan Inst Chem E 127:367–375. https://doi.org/10.1016/j.jtice.2021.08.015
Lin DM, Zeng XR, Li HQ, Lai XJ (2018) Facile fabrication of superhydrophobic and flame-retardant coatings on cotton fabrics via layer-by-layer assembly. Cellulose 25(5):3135–3149. https://doi.org/10.1007/s10570-018-1748-9
Liu XH, Zhang QY, Cheng BW, Ren YL, Zhang YG, Ding C (2017) Durable flame retardant cellulosic fibers modified with novel, facile and efficient phytic acid-based finishing agent. Cellulose 25(1):799–811. https://doi.org/10.1007/s10570-017-1550-0
Liu ZL, Shang SM, Chiu K, Jiang SX, Dai FY (2019) Fabrication of conductive and flame-retardant bifunctional cotton fabric by polymerizing pyrrole and doping phytic acid. Polym Degrad Stabil 167:277–282. https://doi.org/10.1016/j.polymdegradstab.2019.06.029
Liu XH, Zhang QY, Peng B, Ren YL, Cheng BW, Ding C, Su XW, He J, Lin SG (2020) Flame retardant cellulosic fabrics via layer-by-layer self-assembly double coating with egg white protein and phytic acid. J Clean Prod 243:118641. https://doi.org/10.1016/j.jclepro.2019.118641
Liu HC, Guo L, Hu SN, Peng F, Zhang XL, Yang HY, Sui XF, Dai YM, Zhou PW, Qi HS (2022a) Scalable fabrication of highly breathable cotton textiles with stable fluorescent, antibacterial, hydrophobic, and uv-blocking performance. ACS Appl Mater Interfaces 14:34049-34–34058. https://doi.org/10.1021/acsami.2c07670
Liu HC, Yin YL, Zhou JL, Yang HY, Guo L, Peng F, Qi HS (2022b) Fabrication of durable fluorescent and hydrophobic cotton fabrics by multiple surface modifications. Ind Crop Prod 175:114238. https://doi.org/10.1016/j.indcrop.2021.114238
Ma YN, Luo XL, Liu L, Zhang C, Shang XL, Yao JM (2021) Eco-friendly, efficient and durable fireproof cotton fabric prepared by a feasible phytic acid grafting route. Cellulose 28(6):3887–3899. https://doi.org/10.1007/s10570-021-03767-0
Miao ZW, Yan DP, Zhang T, Yang F, Zhang SK, Liu W, Wu ZP (2021) High-efficiency flame retardants of a P–N-rich polyphosphazene elastomer nanocoating on cotton fabric. ACS Appl Mater Interfaces 13(27):32094–32105. https://doi.org/10.1021/acsami.1c05884
Mishra A, Butola BS (2018) Development of cotton fabrics with durable uv protective and self-cleaning property by deposition of low TiO2 levels through sol–gel process. Photochem Photobiol 94(3):503–511. https://doi.org/10.1111/php.12888
Özer MS, Gaan S (2022) Recent developments in phosphorus based flame retardant coatings for textiles: synthesis, applications and performance. Prog Org Coat 171:107027. https://doi.org/10.1016/j.porgcoat.2022.107027
Pal S, Mondal S, Pal P, Das A, Pramanik S, Maity J (2021) Fabrication of durable, fluorine-free superhydrophobic cotton fabric for efficient self-cleaning and heavy/light oil-water separation. Colloid Interfac Sci 44:100469. https://doi.org/10.1016/j.colcom.2021.100469
Park CH, Kang YK, Im SS (2004) Biodegradability of cellulose fabrics. J Appl Polym Sci 94(1):248–253. https://doi.org/10.1002/app.20879
Przybylak M, Maciejewski H, Dutkiewicz A, Dąbek I, Nowicki M (2016) Fabrication of superhydrophobic cotton fabrics by a simple chemical modification. Cellulose 23(3):2185–2197. https://doi.org/10.1007/s10570-016-0940-z
Qiu XQ, Li ZW, Li XH, Zhang ZJ (2018) Flame retardant coatings prepared using layer by layer assembly: a review. Chem Eng J 334:108–122. https://doi.org/10.1016/j.cej.2017.09.194
Raeisi M, Kazerouni Y, Mohammadi A, Hashemi M, Hejazi I, Seyfi J, Khonakdar HA, Davachi SM (2021) Superhydrophobic cotton fabrics coated by chitosan and titanium dioxide nanoparticles with enhanced antibacterial and UV-protecting properties. Int J Biol Macromol 171:158–165. https://doi.org/10.1016/j.ijbiomac.2020.12.220
Rashid MM, Tomšič B, Simončič B, Jerman I, Štular D, Zorc M (2022) Sustainable and cost-effective functionalization of textile surfaces with Ag-doped TiO2/polysiloxane hybrid nanocomposite for UV protection, antibacterial and self-cleaning properties. Appl Surf Sci 595:153521. https://doi.org/10.1016/j.apsusc.2022.153521
Razaghpour M, Malek RMA, Montazer M, Mallakpour S (2022) Amino-functionalized cross-linked cellulosic fabric with antibacterial, UV protection, and coloring effects using folic acid. Int J Biol Macromol 219:637–649. https://doi.org/10.1016/j.ijbiomac.2022.07.214
Refaee AA, El-Naggar ME, Mostafa TB, Elshaarawy RFM, Nasr AM (2022) Nano-bio finishing of cotton fabric with quaternized chitosan Schiff base-TiO2–ZnO nanocomposites for antimicrobial and UV protection applications. Eur Polym J 166:111040. https://doi.org/10.1016/j.eurpolymj.2022.111040
Rowland SP, Nelson ML, Welch CM, Hebert JJ (1976) Cotton fiber morphology and textile performance properties. Text Res J 46(3):194–214. https://doi.org/10.1177/004051757604600306
Shaheen TI, Salem SS, Zaghloul S (2019) A new facile strategy for multifunctional textiles development through in situ deposition of sio2/TiO2 nanosols hybrid. Ind Eng Chem Res 58(44):20203–20212. https://doi.org/10.1021/acs.iecr.9b04655
Shen LW, Jiang JJ, Liu J, Fu FY, Diao H, Liu XD (2022) Cotton fabrics with antibacterial and antiviral properties produced by a simple pad-dry-cure process using diphenolic acid. Appl Surf Sci 600:154152. https://doi.org/10.1016/j.apsusc.2022.154152
Sheng CH, Yang LM, Zhang H, Zhang PF, Shen GD (2021) One-step hydrothermal method to prepare superhydrophobic cotton fabric with antibacterial properties. J Eng Fiber Fabric 16:155892502110660. https://doi.org/10.1177/15589250211066095
Sykam K, Försth M, Sas G, Restás Á, Das O (2021) Phytic acid: a bio-based flame retardant for cotton and wool fabrics. Ind Crop Prod 164:113349. https://doi.org/10.1016/j.indcrop.2021.113349
Thota S, Somisetti V, Kulkarni S, Kumar J, Nagarajan R, Mosurkal R (2019) Covalent functionalization of cellulose in cotton and a nylon-cotton blend with phytic acid for flame retardant properties. Cellulose 27(1):11–24. https://doi.org/10.1007/s10570-019-02801-6
Wan CY, Liu MS, Liu SD, Chen Y, Zhang GX, Zhang FX (2021) An efficient and durable DOPO/H3PO4-based flame retardant for cotton fabric. Cellulose 28(11):7421–7434. https://doi.org/10.1007/s10570-021-03981-w
Wang L, Xi GH, Wan SJ, Zhao CH, Liu XD (2014) Asymmetrically superhydrophobic cotton fabrics fabricated by mist polymerization of lauryl methacrylate. Cellulose 21(4):2983–2994. https://doi.org/10.1007/s10570-014-0275-6
Wang LJ, Wen XD, Zhang XJ, Yuan ST, Xu QB, Fu FY, Diao HY, Liu XD (2021) Durable antimicrobial cotton fabric fabricated by carboxymethyl chitosan and quaternary ammonium salts. Cellulose 28(9):5867–5879. https://doi.org/10.1007/s10570-021-03875-x
Wang P, Zhang MY, Qu JH, Wang LJ, Geng JZ, Fu FY, Liu XD (2022) Antibacterial cotton fabric prepared by a grafting to strategy using a QAC copolymer. Cellulose 29(6):3569–3581. https://doi.org/10.1007/s10570-022-04469-x
Wu D, Long M (2011) Realizing visible-light-induced self-cleaning property of cotton through coating N-TiO2 film and loading AgI particles. ACS Appl Mater Interfaces 3(12):4770–4774. https://doi.org/10.1021/am201251d
Wu ZL, Fang KJ, Chen WC, Zhao YR, Xu Y, Zhang CM (2021) Durable superhydrophobic and photocatalytic cotton modified by PDMS with TiO2 supported bamboo charcoal nanocomposites. Ind Crop Prod 171:113896. https://doi.org/10.1016/j.indcrop.2021.113896
Wu Y, Yang S, Fu FY, Zhang JJ, Li JH, Ma TF, Liu XD, Yao JM (2022) Amino acid-mediated loading of Ag NPs and tannic acid onto cotton fabrics: increased antibacterial activity and decreased cytotoxicity. Appl Surf Sci 576:151821. https://doi.org/10.1016/j.apsusc.2021.151821
Xi GH, Wang J, Luo GY, Zhu YH, Fan WC, Huang MQ, Wang HQ, Liu XD (2015) Healable superhydrophobicity of novel cotton fabrics modified via one-pot mist copolymerization. Cellulose 23(1):915–927. https://doi.org/10.1007/s10570-015-0773-1
Xu QB, Xie LJ, Diao H, Li F, Zhang YY, Fu FY, Liu XD (2017) Antibacterial cotton fabric with enhanced durability prepared using silver nanoparticles and carboxymethyl chitosan. Carbohydr Polym 177:187–193. https://doi.org/10.1016/j.carbpol.2017.08.129
Xu F, Zhong L, Zhang C, Wang P, Zhang FX, Zhang GX (2019) Novel high-efficiency casein-based P–N-containing flame retardants with multiple reactive groups for cotton fabrics. ACS Sustain Chem Eng 7(16):13999–14008. https://doi.org/10.1021/acssuschemeng.9b02474
Xu QB, Wang LJ, Fu FY, Liu XD (2020) Fabrication of fluorine-free superhydrophobic cotton fabric using fumed silica and diblock copolymer via mist modification. Prog Org Coat 148:105884. https://doi.org/10.1016/j.porgcoat.2020.105884
Yan XJ, Zhu XW, Ruan YT, Xing TL, Chen GQ, Zhou CX (2020) Biomimetic, dopamine-modified superhydrophobic cotton fabric for oil–water separation. Cellulose 27(13):7873–7885. https://doi.org/10.1007/s10570-020-03336-x
Yang MP, Liu WQ, Jiang C, He S, Xie YK, Wang ZF (2018) Fabrication of superhydrophobic cotton fabric with fluorinated TiO2 sol by a green and one-step sol-gel process. Carbohydr Polym 197:75–82. https://doi.org/10.1016/j.carbpol.2018.05.075
Yang J, Wen XD, Zhang XJ, Hu XY, Fan LN, Jia DX, Xu QB, Fu FY, Diao HY, Liu XD (2021) A lipid coating on cotton fibers with enhanced adsorption capability for fabric functionalization. Cellulose 28(9):5957–5971. https://doi.org/10.1007/s10570-021-03893-9
Yetisen AK, Qu H, Manbachi A, Butt H, Dokmeci MR, Hinestroza JP, Skorobogatiy M, Khademhosseini A, Yun SH (2016) Nanotechnology in textiles. ACS Nano 10(3):3042–3068. https://doi.org/10.1021/acsnano.5b08176
Yin YJ, Guo N, Wang CX, Rao QQ (2014) Alterable superhydrophobic-superhydrophilic wettability of fabric substrates decorated with ion–TiO2 coating via ultraviolet radiation. Ind Eng Chem Res 53(37):14322–14328. https://doi.org/10.1021/ie502338y
Yu M, Wang ZQ, Liu HZ, Xie SY, Wu JG, Jiang HQ, Zhang JY, Li LF, Li JY (2013) Laundering durability of photocatalyzed self-cleaning cotton fabric with TiO2 nanoparticles covalently immobilized. ACS Appl Mater Interfaces 5(9):3697–3703. https://doi.org/10.1021/am400304s
Zaikov GE, Lomakin SM (2002) Ecological issue of polymer flame retardancy. J Appl Polym Sci 86(10):2449–2462. https://doi.org/10.1002/app.10946
Zhang H, Hou CP, Song LX, Ma Y, Ali Z, Gu JW, Zhang BL, Zhang HP, Zhang QY (2018) A stable 3D sol-gel network with dangling fluoroalkyl chains and rapid self-healing ability as a long-lived superhydrophobic fabric coating. Chem Eng J 334:598-610. https://doi.org/10.1016/j.cej.2017.10.036
Zhou CE, Kan CW (2015) Plasma-enhanced regenerable 5,5-dimethylhydantoin (DMH) antibacterial finishing for cotton fabric. Appl Surf Sci 328:410–417. https://doi.org/10.1016/j.apsusc.2014.12.052
Zhou J, Cai DR, Xu QB, Zhang YY, Fu FY, Dia HY, Liu XD (2018) Excellent binding effect of l-methionine for immobilizing silver nanoparticles onto cotton fabrics to improve the antibacterial durability against washing. RSC Adv 8(43):24458–24463. https://doi.org/10.1039/c8ra04401e
Zhou QQ, Yan BB, Xing TL, Chen GQ (2019) Fabrication of superhydrophobic caffeic acid/Fe@cotton fabric and its oil–water separation performance. Carbohydr Polym 203:1–9. https://doi.org/10.1016/j.carbpol.2018.09.025
Zhou QQ, Chen JY, Zhou TC, Shao JZ (2020) In situ polymerization of polyaniline on cotton fabrics with phytic acid as a novel efficient dopant for flame retardancy and conductivity switching. New J Chem 44(8):3504–3513. https://doi.org/10.1039/c9nj05689k
Zhu H, Cai S, Liao GF, Gao ZF, Min XH, Huang Y, Jin SW, Fan X (2021) Recent advances in photocatalysis based on bioinspired superwettabilities. ACS Catal 11(24):14751–14771. https://doi.org/10.1021/acscatal.1c04049
Zou HL, Lin SD, Tu YY, Liu GJ, Hu JW, Li F, Miao L, Zhang GW, Luo HS, Liu F, Hou CM, Hu ML (2013) Simple approach towards fabrication of highly durable and robust superhydrophobic cotton fabric from functional diblock copolymer. J Mater Chem A 1(37):11246. https://doi.org/10.1039/c3ta12224g
Acknowledgements
This work was financially supported by the the Natural Science Foundation of Zhejiang Province (No. LZ22E030004), Special Support Program for High-Level Talents of Zhejiang Province, Outstanding Talent Project (No. 2021R51003), the National Natural Science Foundation of China (Nos. 51873195), the Foundation of Zhejiang Provincial Innovation Center of Advanced Textile Technology (CXZX2022004), and the Science Foundation of Zhejiang Sci-Tech University (22212128-Y).
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. MC: data curation, writing-original draft. PA, YX, YL, PW, WH: investigation. SZ and FF: supervision. XL and SX: writing-review and editing. all authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval and informed consent
The authors have no statement about ethical approval and informed consent. Because, this research does not involve human and animal.
Consent for publication
All authors had read and agreed to the published version of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, M., Ayaz, P., Xiao, Y. et al. Hydrophobic, fireproof, UV-blocking and antibacterial cotton fabric activated by bio-based PA/ODA/TiO2. Cellulose 30, 4713–4733 (2023). https://doi.org/10.1007/s10570-023-05146-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05146-3